Abstract
Analysis of pmoA and 16S rRNA gene clone libraries of methanotrophic bacteria in Lake Constance revealed an overall dominance of type I methanotrophs in both littoral and profundal sediments. The sediments exhibited minor differences in their methanotrophic community structures. Type X methanotrophs made up a significant part of the clone libraries only in the profundal sediment and were also found only there as a prominent peak by T-RFLP analyses.
Freshwater lakes contribute 6 to 16% of total global methane emission (2). Aerobic oxidation of methane is an important process controlling its release to the atmosphere and takes place in freshwater lakes, usually at the oxic-anoxic boundary layer of the sediment-water interface, where both methane and oxygen are available (9, 13). Lake Constance is a prealpine lake and the second largest one in Europe. Various aspects of methane oxidation in Lake Constance have been studied in great detail (3, 5-7, 9, 16, 19) over the years. In Lake Constance sediment, oxygen penetrates only a few millimeters into the upper sediment (9, 10). In the littoral zone, large amounts of methane are produced (5 to 95 mmol m−2 day−1) (20) in the deeper anoxic sediment layers, whereas in the profundal sediment, much less methane (around 1.4 mmol m−2 day−1) (9) is produced. Much of the methane produced in the profundal sediment and in the littoral sediment is oxidized aerobically at the sediment-water interface, i.e., 93% and 79%, respectively (3, 9). In the littoral zone only, a significant amount of methane is lost by ebullition (20). Unlike the profundal sediment, the littoral sediment is subject to changing environmental factors such as wind and waves, seasonal water level fluctuations, and day-night cycles of light, temperature, and oxygen.
The aerobic methane-oxidizing bacteria (MOB) from the littoral zone of Lake Constance have been studied by culture-independent techniques such as pmoA clone library analysis (7, 16) and terminal restriction fragment length polymorphism (T-RFLP) of the pmoA genes (16). Both types of studies concluded that the littoral sediment is dominated by type I MOB, particularly by Methylobacter-like bacteria. The MOB communities in the profundal zone have also been studied by T-RFLP of the partial pmoA gene (16) using the A189f-A682r primer set, and these studies concluded that the profundal sediment is dominated by type X methanotrophs, but no pmoA clone libraries were done, leaving the community of the MOB unexplored. Our aim in the present study was to find out whether different conditions (in the profundal versus the littoral sediment) within a single lake have a significant effect on the community structure of methanotrophs.
Littoral sediment samples were collected at different sites at a 2- to 5-m water depth. Two independent samples were collected at the littoral garden (site 1, 47°41′35‴N, 9°12′06‴E) in June 2006 and one in front of the Limnological Institute (site 2, 47° 41′53‴N, 9°11′26‴E) in March 2007; these sites are ∼500 m apart. Profundal sediment samples were collected at three different sites, on the Northern shore between Birnau and Nussdorf (site 1, 90-m depth, 47°44′20‴N, 9°12′17‴E) in August 2005 and June 2006, near Mainau Island (site 2, 80-m depth, 47°42′17‴N, 9°12′32‴E) in February 2007, and directly in front of Mainau Island (site 3, 50- to 60-m depth, 47°42′02‴N, 9°12′14‴E) in October 2006. Sediment cores were sliced immediately into sections of 0.5 cm for the uppermost 5 cm and of 1 to 2 cm from 5 to 20 cm. DNA was extracted from 250 mg of wet sediment mass in every case, using a Power Soil DNA isolation kit (Mo-Bio Laboratories, CA). DNA from one of the littoral samples collected at site 1 was extracted by a different extraction method (Fast DNA spin kit; QBiogene) to check for the effects of different extraction procedures. DNA concentrations were determined photometrically, and the exact quantification was done using SYBR green dye after dilution to 5 ng/μl, followed by pmoA-based quantitative real-time PCR (Q-PCR) assay using the A189f-mb661r primer set (12) to determine the abundance of pmoA gene copies in different sediment slices. Q-PCR revealed the highest numbers of MOB at 0 to 2.5 cm in the littoral sediment (average value of 2.1 × 107 copies g [fresh weight]−1) and at 0 to 2 cm in the profundal sediment (average value of 2.3 × 107 ± 0.2 × 107 copies g [fresh weight]−1) (M. Rahalkar and B. Schink, unpublished data). Although methane oxidation is believed to take place mainly in the upper 1-cm layer of the sediment, the deeper layers in both the littoral and the profundal sediment have also been shown to contain high numbers of aerobic methane-oxidizing bacteria (9, 18, 20). Thus, to explore the entire diversity of methanotrophs, we used DNA from the upper 2.5- and 2-cm layers of littoral and profundal sediments, respectively, for construction of clone libraries. DNA samples from these sections were pooled and were used to amplify partial 16S rRNA genes of type I and type II MOB using the primer sets MethT1df-MethT1br and 27f-MethT2r, respectively (21). Around 10 to 15 ng of the pooled DNA from the samples was used for amplification in type I MOB- and type II MOB-specific PCR. No PCR products for type II methanotrophs were obtained in any of the samples. PCR products were obtained in all cases for type I MOB 16S rRNA gene-specific PCR. Bands were cut, subjected to gel elution using a QIAGEN PCR purification kit (QIAGEN, Germany), and cloned with a TA cloning kit (Invitrogen, Germany). A total of 100 clones from the type I 16S rRNA gene clone library were screened for the littoral sediment (sites 1 and 2) and 116 clones for the profundal sediment (site 1 and 3) using MspI digestion, followed by RFLP analysis of the clones. Representatives of each clone type were sequenced partially to obtain a sequence of 750 to 850 bp using the M13f and M13r or MethT1f primer at GATC Biotech AG (Konstanz, Germany), and one representative clone from each group was sequenced completely (920 bp). Similarly, pmoA clone libraries were constructed for the littoral sediment (site 2) and profundal sediment (site 1 and 3), and clone library data obtained in earlier studies (7) were used for comparison. A total of 77 positive clones for profundal site 1, 35 clones for profundal site 3, and 35 clones for littoral site 2 were analyzed by RFLP, using MspI and HaeIII enzymes as described before (7). Representative unique clones were sequenced using the primer M13f. Phylogenetic analyses were done using ARB (14) as described before (7). Type I 16S rRNA gene sequences having around 800 bp were used for the construction of phylogenetic trees using ARB (14).
T-RFLP analysis of the amplified pmoA gene was used to elucidate the differences and similarities between littoral and profundal sediments and within different sites and samples. pmoA products were obtained as described above with 5′ 6-carboxyfluorescein-labeled A189f primer (Thermo Electron Corporation), purified using a MiniElute kit (QIAGEN), and quantified photometrically. Around 100 ng of DNA from each sample was used for digestion with the restriction endonucleases MspI and RsaI (3 U each; Fermentas). A 10-μl reaction was performed, 1 or 2 μl of which was loaded in the sequencer (Applied Biosystems) and mixed with ROX (Applied Biosystems) as the internal standard (0.25 μl) in a total volume of 10 μl. Peaks were analyzed by the programs GeneScan (Applied Biosystems) and Peak Scanner software version 1.0 (Applied Biosystems).
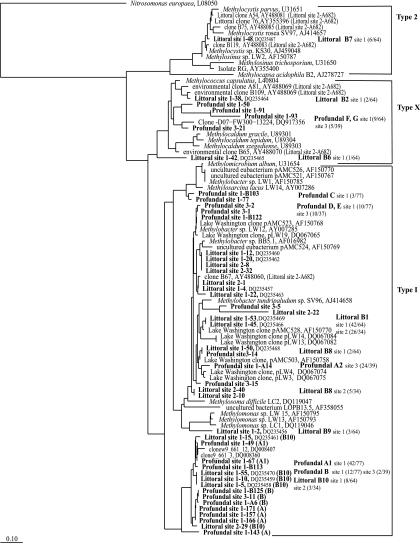
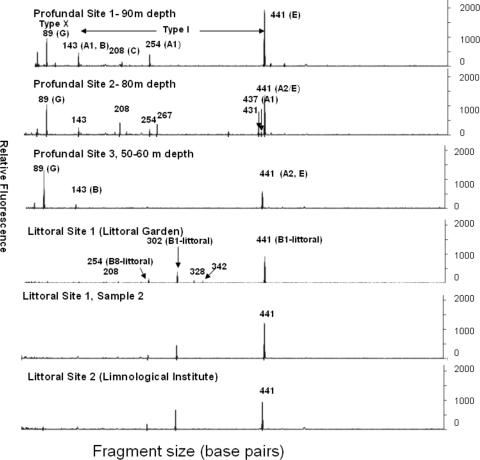
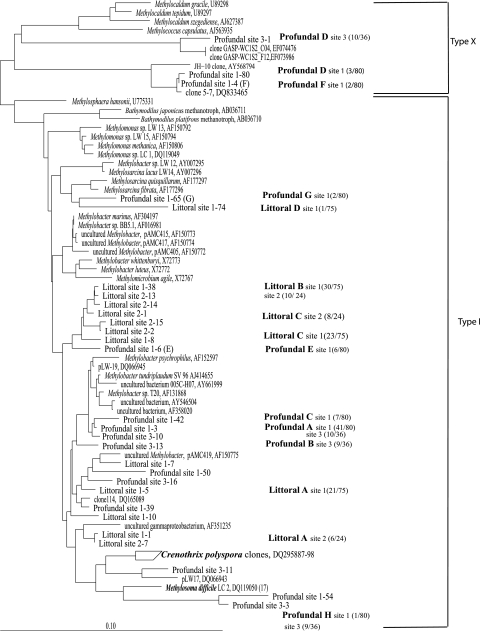
In our study, the littoral sediments at both sites were dominated by type I methanotrophs, as had been observed before. Littoral sediment (site 1) was shown to be dominated by type I methanotrophs related to Methylobacter-like spp. in our earlier studies (7). Littoral site 2 showed a community composition very similar to that of littoral site 1 (7), dominated by Methylobacter-related methanotrophs. All phylotypes grouped close to the phylotypes from site 1 (7) and to the clones obtained for the same site using the primer set A189f-A682r from site 2 (16) (Fig. 1). T-RFLP analyses of the pmoA gene from littoral sites 1 and 2, which are around 500 m apart and have different sediment textures, also resulted in virtually identical profiles (Fig. 2). The dominant peaks were 441 bp and 302 bp, which represented the dominant clone group, B1. The peak at 254 bp represented the clone group B8. Peaks specific for type X methanotrophs and type II methanotrophs (244 bp) were absent in the littoral samples. Two sediments from the same site that were sampled independently with two different DNA extraction methods also showed nearly identical profiles (Fig. 2). Type I 16S rRNA gene clone libraries also showed the dominance of type I methanotrophs all related to Methylobacter spp. (Fig. 3). At site 1, the dominant clone types A and B were related to Methylobacter psychrophilus and Methylobacter tundripaludum. The dominant pmoA clone group, B1, from the littoral sediment is also closely related to representatives of M. psychrophilus and M. tundripaludum; thus, the dominating methanotroph in the littoral sediment is probably a Methylobacter species. Another clone group, littoral type I C (30% clones), was related to other Methylobacter-related clones, and only one clone was distantly related to Methylosarcina-like methanotrophs. Littoral site 2 also showed the presence of these three dominant clone groups, and all the sequences from these clone groups grouped very close to clones from site 1 and to Methylobacter spp.
FIG. 1.
Phylogenetic dendrogram based on the derived amino acid sequences of pmoA genes from the littoral (sites 1 and 2) and the profundal (sites 1 and 3) sediments of Lake Constance. Sequences of clones for littoral sediment site 1 are from our previous studies (7). The RFLP groups are indicated as profundal A to G, and the clone frequencies of each site are in parentheses. National Center for Biotechnology Information accession numbers from other studies are given along with the names or clone numbers. The tree was constructed by the neighbor-joining method using the PAM correction as implemented in the ARB software and was based on 144 amino acids. The bar represents 10% divergence.
FIG. 2.
T-RFLP analyses of pmoA genes amplified from littoral (site 1 [two samples] and site 2) and profundal sediment (sites 1 to 3) using the MspI-RsaI restriction enzyme pair. The fragment sizes are labeled, and the clone groups are in parentheses.
FIG. 3.
Neighbor-joining analysis of 16S rRNA gene sequences of type I methanotrophic clones from profundal and littoral sediments of Lake Constance. The clones are prefixed with “Littoral site” and “Profundal site,” and other sequences from cultured methanotrophs and clones are also shown. Around 800 nucleotides were considered for the tree construction. The RFLP groups are indicated as Littoral A to D and Profundal A to H, and the clone frequencies are given in parentheses. The National Center for Biotechnology Information accession numbers of clones and strains from other studies are given near each name. The bar represents 10% divergence.
The profundal sediment was dominated by type I methanotrophs as well, in contrast to earlier studies, which had indicated the presence of only type X methanotrophs (19). At site 1, pmoA clones from the dominant clone group A1 clustered with the B10 group from the littoral sediment and two clones from the Snake River Plain Aquifer (8) (Fig. 1). Clones from the next dominant group B from site 1 also clustered with these two groups. Amino acid similarities of sequences from the A1 group with other Methylobacter spp. or clones were 82% or less, whereas with the Snake River Plain Aquifer clones and clones from the B10 group, the similarities were more than 95%. Profundal C was related to Methylosarcina spp., especially to Methylosarcina lacus, isolated from a similar habitat, i.e., profundal sediment of Lake Washington. Groups Profundal D and E were related to the Methylobacter-like sequences from group B1, which is the dominant group in the littoral sediment (7), and to Methylobacter sp. strain LW12. Profundal G and F were moderately related to Methylococcus and Methylocaldum (type X methanotrophs). Profundal site 3 showed a different community composition with respect to the dominant species compared to site 1. In this case, the clones of the dominant RFLP group (profundal A2) all clustered close to the pmoA sequences of Methylobacter spp. and group B1 that are dominant in littoral sediment. A considerable number of clones belonged to profundal group G and grouped close to Methylococcus. The other phylotypes clustered close to other clones from profundal site 1, e.g., clone group B. T-RFLP analyses showed minor differences in the profundal sites 1, 2, and 3 (Fig. 2). Profundal sites 1 and 2 (90 m and 80 m), which were far from each other but had similar depths, showed similarities in most peaks, whereas profundal site 3, which was shallower (50 to 60 m) and closer to site 2, showed less-prominent peaks. The major peaks were 441 bp and 89 bp in all the three cases, the 441-bp peak corresponded to clone groups D and E (at site 1) and profundal A2 (at site 3). The other dominant peak was 89 bp, which corresponded to one of the clones belonging to clone group G, type X methanotrophs (clone 91, site 1). The other peaks were 143 bp, representing profundal groups A1 and B, and 254 bp, representing profundal group A2. There was also a prominent peak in profundal sites 1 and 2 at 208 bp, which could be seen as a pseudopeak associated with profundal group C. According to the T-RFLP patterns, the type X methanotrophs also appeared as prominent peaks, whereas in the pmoA clone libraries they were underrepresented (13% at site 1 and 16% at site 3). Further, type I 16S rRNA gene clone libraries also reflected the dominance of type I methanotrophs along with the presence of type X methanotrophs in the profundal sediment (Fig. 3). The profundal site 1 clone library was dominated by Methylobacter psychrophilus/M. tundripaludum-related methanotrophs (a total of 63% A and C). B and E clone groups were distantly related to Methylobacter spp. (95 to 96% similarity) and contributed to a total of 31% of clones. Other clone groups were related to type X methanotrophs (D and F), Crenothrix polyspora (H), and Methylosarcina (G). Profundal site 3 clone library also showed a dominance of Methylobacter psychrophilus/M. tundripaludum-related methanotrophs represented by clone groups A (28%) and B (25%) and other Methylobacter clones (14%). A considerable number of clones were related to clones belonging to type X methanotrophs (28%), and a few were related to Crenothrix sequences.
In general, the methanotrophic community in Lake Constance as a whole was found to be dominated by type I methanotrophs related to Methylobacter spp. No PCR products were obtained by specifically amplifying the type II MOB-specific 16S rRNA genes, indicating that type II MOB were not dominant in the littoral or the profundal sediments. No peaks specific for type II methanotrophs were obtained in the T-RFLP analysis. These results were consistent with the fluorescence in situ hybridization data, which indicated that numbers of type II methanotrophs were lower in the littoral and profundal sediments by almost 1 order of magnitude (M. Rahalkar and J. Deutzmann, unpublished data).
We showed for the first time that type I methanotrophs were present and dominant in the profundal sediment of Lake Constance. This was in contrast to an earlier T-RFLP study that showed that Lake Constance profundal sediment was dominated by type X methanotrophs (16). We propose that this is probably because only primers A189f and A682r were used in the earlier case (16), and pmoA genes of type I methanotrophs were not amplified by this primer pair. In our study, we used a primer pair (A189-mb661) specific for the pmoA gene (1) which has also been shown to amplify a larger part of the methanotrophic communities (4).
In spite of differences in environmental factors, such as desiccation, waves, and exposure to changing oxygen, temperature, and light conditions, in the littoral and profundal sediments, the overall methanotrophic communities appeared to be more or less similar, except that the dominant species and the abundance of species could be different. The littoral sites were very alike in community composition, whereas profundal sediments collected at different sites and different depths exhibited differences in the dominant species. The obvious and important observation was that most of the clones obtained in our study grouped with isolates and clones obtained directly from Lake Washington, e.g., Methylobacter sp. strain LW12 and pAMC clones (1), or from stable isotope probing experiments done on Lake Washington, e.g., pLW clones (15). In Lake Washington, Methylobacter-related sequences were dominant in the stable isotope probing and the mRNA approach (15) as well as in fluorescence in situ hybridization-based cell sorting (11), which confirms the significance of this group in a similar habitat.
Only type II methanotrophs have been cultivated from the littoral sediment of Lake Constance by traditional approaches (6). New approaches, such as gradient cultivation, led to the isolation of type I methanotrophs (7, 17). With our present cultivation-independent study, we broadened our knowledge about the methanotrophic community structure in littoral and profundal sediments, which will be useful for future cultivation approaches.
Nucleotide sequence accession numbers.
All sequences used in the trees have been deposited in GenBank, and the accession numbers are EF101316 to EF101340 and EF587721 to EF587751.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (SFB 454) and research funds of Universität Konstanz.
We thank Andreas Brune for fruitful discussions, summer course students in 2005 and 2006 for their help in DNA extractions, and Joerg Deutzmann and Alfred Sulger and colleagues for sample collection. We express our gratitude to Axel Meyer for allowing us to use the sequencer for T-RFLP and Elke Hespeler for excellent assistance and cooperation in this respect.
Footnotes
Published ahead of print on 4 May 2007.
REFERENCES
- 1.Auman, A. J., S. Stolyar, A. M. Costello, and M. E. Lidstrom. 2000. Molecular characterisation of methanotrophic isolates from freshwater lake sediment. Appl. Environ. Microbiol. 66:5259-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastviken, D., J. Cole, M. Pace, and L. Tranvik. 2004. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochem. Cycles 18:B4009. [Google Scholar]
- 3.Bosse, U., P. Frenzel, and R. Conrad. 1993. Inhibition of methane oxidation by ammonium in the surface layer of littoral sediment. FEMS Microbiol. Ecol. 13:123-134. [Google Scholar]
- 4.Bourne, D. G., I. R. McDonald, and J. C. Murrell. 2001. Comparison of pmoA PCR primer sets as tools for investigating methanotroph diversity in three Danish soils. Appl. Environ. Microbiol. 67:3802-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussmann, I. 2005. Methane release through suspension of littoral sediment. Biogeochemistry 74:283-302. [Google Scholar]
- 6.Bussmann, I., M. Pester, A. Brune, and B. Schink. 2004. Preferential cultivation of type II methanotrophic bacteria from littoral sediments (Lake Constance). FEMS Microbiol. Ecol. 47:179-189. [DOI] [PubMed] [Google Scholar]
- 7.Bussmann, I., M. Rahalkar, and B. Schink. 2006. Cultivation of methanotrophic bacteria in opposing gradients of methane and oxygen. FEMS Microbiol. Ecol. 56:331-344. [DOI] [PubMed] [Google Scholar]
- 8.Erwin, D. P., I. K. Erickson, M. E. Delwiche, F. S. Colwell, J. L. Strap, and R. L. Crawford. 2005. Diversity of oxygenase genes from methane- and ammonia-oxidizing bacteria in the Eastern Snake River Plain Aquifer. Appl. Environ. Microbiol. 71:2016-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frenzel, P., B. Thebrath, and R. Conrad. 1990. Oxidation of methane in the oxic surface layer of a deep lake sediment (Lake Constance). FEMS Microbiol. Ecol. 73:149-153. [Google Scholar]
- 10.Gerhardt, S., A. Brune, and B. Schink. 2005. Dynamics of redox changes of iron caused by light-dark variations in littoral sediment of a freshwater lake. Biogeochemistry 74:323-339. [Google Scholar]
- 11.Kalyuzhnaya, M. G., R. Zabinsky, S. Bowermann, D. R. Baker, M. E. Lidstrom, and L. Chistoserdova. 2006. Fluorescence in situ hybridization-flow cytometry-cell sorting-based method for separation and enrichment of type I and type II methanotroph populations. Appl. Environ. Microbiol. 72:4293-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb, S., C. Knief, S. Stubner, and R. Conrad. 2003. Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl. Environ. Microbiol. 69:2423-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuivila, K. M., J. W. Murray, and A. H. Devol. 1988. Methane cycling in the sediments of Lake Washington. Limnol. Oceanogr. 33:571-581. [Google Scholar]
- 14.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nercessian, O. G., E. Noyes, M. G. Kaluyzhnaya, M. E. Lidstrom, and L. Chistoserdova. 2005. Bacterial populations active in metabolism of C1 compounds in the sediment of Lake Washington, a freshwater lake. Appl. Environ. Microbiol. 71:6885-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pester, M., M. W. Friedrich, B. Schink, and A. Brune. 2004. pmoA-based analysis of methanotrophs in a littoral lake sediment reveals a diverse and stable community in a dynamic environment. Appl. Environ. Microbiol. 70:3138-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahalkar, M., I. Bussmann, and B. Schink. Methylosoma difficile gen. nov., sp. nov., a novel methanotroph enriched by gradient cultivation from littoral sediment of Lake Constance. Int. J. Syst. Evol. Microbiol. 57:1073-1080. [DOI] [PubMed]
- 18.Rothfuss, F., M. Bender, and R. Conrad. 1997. Survival and activity of bacteria in a deep, aged lake sediment (Lake Constance). Microb. Ecol. 33:69-77. [DOI] [PubMed] [Google Scholar]
- 19.Schulz, S., and R. Conrad. 1995. Effect of algal deposition on acetate and methane concentrations in the profundal sediment of a deep lake (Lake Constance). FEMS Microbiol. Ecol. 16:251-260. [Google Scholar]
- 20.Thebrath, B., F. Rothfuss, M. J. Whiticar, and R. Conrad. 1993. Methane production in littoral sediment of Lake Constance. FEMS Microbiol. Ecol. 102:279-289. [Google Scholar]
- 21.Wise, M. G., J. V. McArthur, and L. J. Shimkets. 1999. Methanotroph diversity in landfill soil: isolation of novel type I and type II methanotrophs whose presence was suggested by culture-independent 16S ribosomal DNA analysis. Appl. Environ. Microbiol. 65:4887-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]