Abstract
The effectiveness of high-temperature, short holding time (HTST) pasteurization and homogenization with respect to inactivation of Mycobacterium avium subsp. paratuberculosis was evaluated quantitatively. This allowed a detailed determination of inactivation kinetics. High concentrations of feces from cows with clinical symptoms of Johne's disease were used to contaminate raw milk in order to realistically mimic possible incidents most closely. Final M. avium subsp. paratuberculosis concentrations varying from 102 to 3.5 × 105 cells per ml raw milk were used. Heat treatments including industrial HTST were simulated on a pilot scale with 22 different time-temperature combinations, including 60 to 90°C at holding (mean residence) times of 6 to 15 s. Following 72°C and a holding time of 6 s, 70°C for 10 and 15 s, or under more stringent conditions, no viable M. avium subsp. paratuberculosis cells were recovered, resulting in >4.2- to >7.1-fold reductions, depending on the original inoculum concentrations. Inactivation kinetic modeling of 69 quantitative data points yielded an Ea of 305,635 J/mol and an lnk0 of 107.2, corresponding to a D value of 1.2 s at 72°C and a Z value of 7.7°C. Homogenization did not significantly affect the inactivation. The conclusion can be drawn that HTST pasteurization conditions equal to 15 s at ≥72°C result in a more-than-sevenfold reduction of M. avium subsp. paratuberculosis.
Heat treatment is the process most frequently used to ensure the quality and safety of milk and milk products. A decade ago, Mycobacterium avium subsp. paratuberculosis-positive PCR signals in pasteurized retail milk were reported (7). The possible survival of this bacterium under the pasteurization conditions currently used was suggested (18). M. avium subsp. paratuberculosis is the causal agent of Johne's disease, which is a chronic intestinal infection of cattle and other ruminants and shows some similarities to Crohn's disease, a severe chronic inflammation of the gastrointestinal tracts of humans, and an association of M. avium subsp. paratuberculosis with this human disease has been suggested (references 6, 11, and 22 and references therein). Many alternative causes for the multifactorial disease have been suggested, including familial environmental risk factors for Crohn's disease related to a Western lifestyle, diet, bacteria, and good standards of domestic hygiene and the development of the cold chain (references 11, 13, and 15 and references therein). However, in a recent case-control study the consumption of pasteurized milk could not be associated with an increased risk of Crohn's disease (1). Several research groups have studied the inactivation of M. avium subsp. paratuberculosis under pasteurization conditions. A number of industrial and pilot scale turbulent-flow pasteurization experiments have been performed (8, 9, 12, 17, 20, 21, 24). The interpretation and comparison of the experiments and results remain complicated because of the different experimental conditions used. Specific critical factors influencing heat inactivation results have been discussed (12, 14, 16). Many studies have used laboratory strains of M. avium subsp. paratuberculosis to inoculate milk for inactivation studies. However, the strains from culture collections may have adapted to the laboratory environment and changed their heat resistance and clumping behavior.
It is very important to maintain a turbulent flow and the resulting low residence time distribution (RTD) during pasteurization to ensure that the entire milk volume gets the same treatment. In this study, industrial conditions to achieve high-temperature, short holding time (HTST) pasteurization were simulated on a pilot scale with 22 different time-temperature combinations, ranging from 60 to 90°C at holding times of 6 to 15 s. The heat resistance of M. avium subsp. paratuberculosis in milk was determined with and without additional homogenization, and kinetic parameters were derived.
MATERIALS AND METHODS
Preparation of M. avium subsp. paratuberculosis-contaminated milk.
Milk and feces from cows with clinical signs of Johne's disease (paratuberculosis) were obtained from the Centraal Instituut voor Dierziekte Controle, Lelystad, The Netherlands. M. avium subsp. paratuberculosis-containing feces was diluted 1:2 with PFZ (peptone physiological salt solution, 8.5 g/liter NaCl, 1 g/liter peptone). Large particles were removed by brief simple filtering with a hair mesh, and the resulting liquid was stored for 1 to 2 days at 4°C. On the day of the pasteurization experiment, the liquid fecal preparation was used to contaminate M. avium subsp. paratuberculosis-negative raw milk from cows at the experimental farm de Ossenkamp (Wageningen, The Netherlands). The final concentration of viable M. avium subsp. paratuberculosis cells was determined by the culture method on each day of the pasteurization experiment. The initial concentration of M. avium subsp. paratuberculosis in milk varied between experiments and ranged from 102 to 3.5 × 105 cells per ml.
Pasteurization.
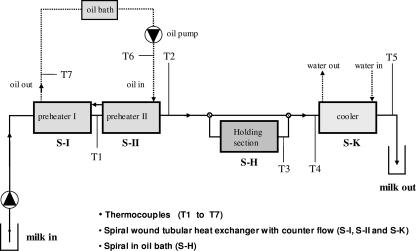
A pilot plant scale turbulent-flow pasteurizer (Fig. 1) was used to determine inactivation of M. avium subsp. paratuberculosis at temperatures ranging from 60 to 90°C and holding times ranging from 6 to 15 s (see Table 1). The pasteurizing system was operated at turbulent flow at 30 liters/h, resulting in a Reynolds number of 3,405. The holding section tube was exchanged to modify the holding times. Between temperature and holding time changes, the equipment was cleaned with steam. All experiments started with the most stringent and ended with the least stringent time-temperature conditions. The temperature was monitored at seven points in the process (Fig. 1). Additional homogenization of raw milk was used prior to heating with a Rannie type 12.50 homogenizer at 150/40 bar and 15 to 18°C.
FIG. 1.
Pilot scale indirect pasteurizer.
TABLE 1.
n-Fold reductions after pasteurization by different time-temperature combinations
| Temp (°C) |
n-Fold reductiona at:
|
||
|---|---|---|---|
| 6 s | 10 s | 15 s | |
| 60 | 0.0-0.5 | 0.4-0.5 | 0.0-1.5 |
| 62 | 0.5-1.1 | 0.0-1.1 | 0.0-0.7 |
| 64 | 0.5 | 0.0-1.1 | 0.6-1.6 |
| 66 | 0.0-1.5 | 0.5-2.1 | 1.0-2.8 |
| 68 | 0.6->5.7 | 3.1->7.1 | 4.1->7.1 |
| 70 | 3.1->7.1 | >4.2->7.1 | >4.2->7.1 |
| 72 | >4.7->7.1 | NDb | >4.2->7.1 |
| 80 | >4.2->7.1 | ND | ND |
| 90 | >4.2->7.1 | ND | ND |
The range of reduction determined is dependent on the inoculum concentration. No recovery of viable cells with a given time-temperature combination results in a larger-than (>) reduction.
ND, not determined.
Quantitative analysis of M. avium subsp. paratuberculosis in milk.
Samples of 40 ml milk, before homogenization and before and after subsequent heat treatment, were obtained aseptically and prepared for culturing. First, the milk samples were cleared by adding 8 ml 18% sodium citrate to disrupt the milk matrix, leaving a clear solution. Subsequently, bacterial cells were collected by centrifugation for 1 h at 6,000 × g (Heraeus Megafuge 1.0 R; Meyvis, Etten-Leur, Netherlands). The pellet was resuspended in 0.75 ml PFZ, centrifuged for 10 min at 8,000 × g (Eppendorf 5417R), resuspended in 0.5 ml 0.75% (wt/vol) hexadecylpyridinium chloride (HPC; Sigma-Aldrich Chemie, Zwijndrecht, The Netherlands) in brain heart infusion broth (BHI; Difco), and incubated for 5 h at 37°C. Subsequently, the cells were collected by centrifugation for 10 min at 8,000 × g (Eppendorf 5417R) and resuspended in 1.5 ml PFZ. A most-probable-number (MPN) method was applied by using triplicate dilution series in mycobacteria growth indicator tubes (MGIT; Becton Dickinson) containing 4 ml Middlebrook 7H9 broth, 500 μl OADC enrichment (oleic acid, bovine albumin, dextrose, catalase), 100 μl PANTA antibiotic mixture (polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin, all from Becton Dickinson), and 16 μl mycobactin J (Synbiotics Europe, Lyon, France) in ethanol at 0.5 mg/ml.
The tubes were inoculated with 0.5 ml of each sample. Growth after 16 to 50 weeks of incubation at 37°C was confirmed by using a two-target IS900-based real-time PCR (21). Based on the number of M. avium subsp. paratuberculosis culture-positive tubes found, the MPN was determined, with an estimated detection limit of 0.023 cells/ml.
Heat inactivation kinetics.
Inactivation kinetics were determined by assuming a first-order inactivation reaction of the microbial population C in CFU per milliliter over time t in seconds (equation 1), in combination with an Arrhenius-type equation describing the temperature dependence of the specific inactivation rate k (per second) (equation 2) as follows:
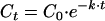 |
(1) |
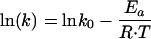 |
(2) |
where Ct is the microbial population after time t (in CFU per milliliter), C0 is the microbial population in untreated milk (in CFU per milliliter), k is the inactivation constant (1/s), t is the heating time (seconds), lnk0 is a model constant (theoretical inactivation rate at 0 K) also named the preexponential factor with notation A, Ea is the activation energy (in Joules per mole), R is the gas constant (8.314 J/mol·K), and T is the temperature (K). In this inactivation model, lnk0 and the inactivation energy Ea (Joules per mole) are model constants characteristic for the microorganisms. Model constants lnk0 and Ea can be translated into parameters for the D, z concept of Bigelow (3).
To determine the kinetic parameters Ea and lnk0, only data points with a concentration above the detection limit and a log10 Ct/C0 ratio of >0.5 were used. Model constants were then determined by taking into account heating and cooling trajectories with NIZO Premia (5, 23) and minimizing the square difference between measured and simulated points.
RESULTS
Milk and feces from cows with clinical signs of Johne's disease (paratuberculosis) only sporadically showed a high concentration of M. avium subsp. paratuberculosis. The concentration of M. avium subsp. paratuberculosis in harvested milk was estimated to be much lower than 1 cell/10 ml and was too low for direct use in the pasteurization experiments. Most of the cows with clinical signs of Johne's disease yielded concentrations of M. avium subsp. paratuberculosis not detectable by real-time PCR, i.e., lower than 4 × 102 cells/g feces. Sampling six cows with clinical signs of Johne's disease, three cows once every day for 3 days and three cows once, resulted in 11 samples with fewer than 4 × 102 M. avium subsp. paratuberculosis cells/g feces and one fecal sample (from a cow sampled three times) that contained more than 105 cells/g feces. Samples containing more than 105 M. avium subsp. paratuberculosis cells/g feces were used to contaminate raw milk for use in the pasteurization experiments. The final concentration of viable M. avium subsp. paratuberculosis cells varied between experiments and ranged from 102 to 3.5 × 105 cells per ml.
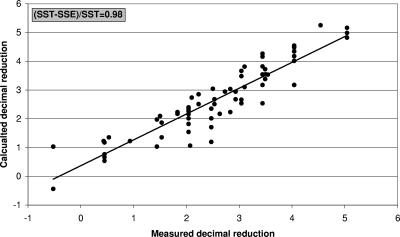
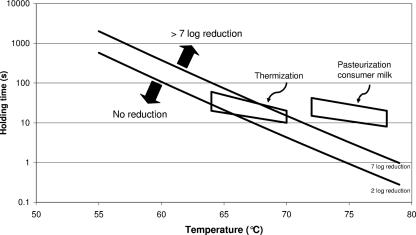
Heat inactivation of M. avium subsp. paratuberculosis was determined in eight independent pasteurization experiments, and a total of 136 observations were obtained (Table 1). No survival was found at 72°C and a holding time of 6 s and at 70°C for 10 and 15 s or under more stringent conditions, resulting in >4.2- to >7.1-fold reductions, depending on the original inoculum concentrations. Because the detection limit was 0.023 cell/ml and the maximum inoculum concentration was 3.5 × 105 cells per ml, the maximum reduction it was possible to measure was 7.1-fold. When fewer cells were present, the maximum reduction measured was correspondingly lower. No significant reduction (maximum 1.5-fold reduction) was found at temperatures between 60 and 66°C and a holding time of 6 s. At other time-temperature combinations, partial inactivation was found, resulting in up-to-4.1-fold reductions. The quantitative data points were used to determine inactivation kinetics. The kinetic parameters determined were Ea = 305,635 J/mol and lnk0 = 107.2. These kinetics correspond to a D value of 1.2 s at 72°C and a Z value of 7.7°C. In Fig. 2, n-fold reductions calculated with these kinetic parameters are plotted against the n-fold reductions measured. The sum of the square of the differences between measured and calculated data points was 18. This corresponds to a determination coefficient (R2) of 0.98. Inactivation curves of M. avium subsp. paratuberculosis (two- to sevenfold reduction) and the heat inactivation processes used in the dairy industry such as heat treatment at moderate temperature and pasteurization are shown in Fig. 3. The model simulations showed that under the international standard HTST pasteurization conditions, as used in the Dutch dairy industry, 15 s at ≥72°C, more-than-sevenfold reductions of M. avium subsp. paratuberculosis are achieved. Homogenization was found to increase the MPN count with approximately a factor 10 (n = 3 samples). However, the inactivation rate observed did not reveal any effect of homogenization on M. avium subsp. paratuberculosis inactivation kinetics at different temperatures (Fig. 4).
FIG. 2.
Measured versus calculated n-fold reductions. SSE, sum of the square of the differences between measured and calculated data points; SST, difference between the sum of squares and the square of the sum of the data points divided by the number of data points.
FIG. 3.
Temperature-time combinations needed for inactivation of M. avium subsp. paratuberculosis and during various dairy production processes.
FIG. 4.
Pasteurization inactivation constant of M. avium subsp. paratuberculosis versus temperature with (+) or without (○) homogenization.
DISCUSSION
In this study, the effectiveness of heat inactivation of M. avium subsp. paratuberculosis under turbulent-flow conditions in milk contaminated with naturally infected feces was quantified and modeled. For the experiments, raw milk was inoculated with naturally contaminated feces. M. avium subsp. paratuberculosis contamination levels in raw milk of cows with advanced clinical signs of Johne's disease were too low to be detected by using M. avium subsp. paratuberculosis-specific PCR and culturing. The concentration of M. avium subsp. paratuberculosis in the milk of these cows was estimated to be lower than 1 cell/10 ml. This supports the results of a study that estimated the levels of M. avium subsp. paratuberculosis in raw milk as lower than 8 cells/50 ml (25). This is too low to ascertain heat inactivation kinetics in a reliable way. Fecal shedding of M. avium subsp. paratuberculosis has been estimated to be up to (2, 24) or greater than 108 CFU/g (see references in references 4 and 19), an overestimation of >100 times compared to our findings of 105 to 106, which are close to the 105.5 CFU/g used in a modeling approach (19). Feces of M. avium subsp. paratuberculosis-infected cows, being regarded as the most important source of M. avium subsp. paratuberculosis contamination of milk, were used as the inoculum for the experiments. Still, only a small number (e.g., 1 out of 12) of the fecal samples were found to contain a number of M. avium subsp. paratuberculosis cells sufficient to contaminate a large volume (>50 liters) of milk.
In comparison to batch and laboratory scale studies (6), in the past 5 years several studies have been published (8, 9, 12, 17, 20, 21, 24) describing (semi)industrial turbulent-flow heat inactivation of M. avium subsp. paratuberculosis in milk (Table 2). Results are conflicting because of the use of culture collection versus field strains, their history, the presence of clumps, the heating methods used for pasteurization, and recovery and culturing of survivors, as well as the determination of initial and postpasteurization quantities of viable M. avium subsp. paratuberculosis cells (8, 9, 12, 17, 20, 21, 24). Only limited information is available about the effect of homogenization on the survival of M. avium subsp. paratuberculosis during heat treatment (9). Overall, a significant reduction of M. avium subsp. paratuberculosis after pasteurization is reported (8, 9, 12, 17, 20, 21, 24). Some studies (8, 9, 12, 17) have reported survival at high temperatures (>72°C). However, Grant et al. (8) reported survival of M. avium subsp. paratuberculosis cells after industrial pasteurization. A key difference between the studies of Pearce et al. and Grant et al. is that heated M. avium subsp. paratuberculosis cells were allowed to recover for 24 h at 4°C before testing commenced in the former study. Moreover, a counterflow of not-yet-pasteurized milk was used for cooling in the latter study. Hammer et al. (12) reported a sixfold overall reduction of M. avium subsp. paratuberculosis but also found low numbers of M. avium subsp. paratuberculosis cells surviving heat treatment even at 90°C. Possible reasons for the high heat resistance reported are cell clumps, phagocytosis, and metabolic activity. The heat resistance reported remains unexplained, as does the possible heat activation of M. avium subsp. paratuberculosis that was suggested (12). Hypothetical underestimation of survival caused by inactivation during the decontamination procedures was expected to be limited in the present study since the same procedure was used before and after pasteurization, although stressed cells do not necessarily behave in the same way as unstressed cells.
TABLE 2.
Important parameters of raw milk turbulent-flow pasteurization experiments, sample preparation methods, and growth media among studiesa
| Reference | Reduction (n-fold) at 72°C, 15 s | Survival temp (°C), time (s) | Additional reduction by homogenization | Inoculum | No. of CFU/ml inoculated milk | Heat exchanger | Heat regeneration | Reynolds no. | RTD | Quantification | Sample vol (ml) | Collection 1 | Decontamination, pellet resuspension | Collection 2 | Pellet resuspension | Growth medium for detection | Incubation time (wk) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This study | >4.2->7.1 | 68, 6 | Not at 6, 10 or 15 s pasteurization holding time | Feces | 3.0 × 103-4.5 × 103 | Spiral | No | 3,405 | Mono pump | Yes | 40 milk + 8 18% sodium citrate | 6,000 × g, 60 min, wash in 0.75 ml PFZ, 8,000 × g, 10 min | 0.5 ml 0.75% HPC in BHI, 5 h, 37°C | 8,000 × g, 10 min | PFZ | Inoculation into MGIT | >48 |
| 21 | >4 | 68, 20 | ND | Strains | 103 | Spiral | No | 3,405 | Plunger pump | Yes | 40 milk + 8 18% sodium citrate | 6,000 × g, 60 min, wash in 0.75 ml PFZ, 8,000 × g, 10 min | 0.5 ml 0.75% HPC in BHI, 5 h, 37°C | 8,000 × g, 10 min | PFZ | Inoculation into MGIT | >48 |
| 17 | >4->6 | 72, 15 | ND | Laboratory-grown strains | 4.7 × 103-1.4 × 105 | Plate, holding section tube | Yes | 62,112 | + | Yes | 3 × 50 | 1,500 × g, 20 min | 5 ml 0.75% HPC 4 h, RT; 30 ml 0.75% HPC, 18-24 h, 37°C | 1,500 × g, 15 min | 1 ml PBS | 0.2 ml in BACTEC 12B | >28 |
| 9 | 4-5.2 | 82.5, 60 | At 25 s, not at 15 s | Laboratory-grown strains | 101-105 | Plate | Yes | Turbulent flow | − | No | 3 × 50 | − | − | 4,000 rpm, 15 min | 1 ml VAN-BHI | 0.2 ml in BACTEC 12B, 0.2 ml in BACTEC 12B PANTA, 0.05 ml in HEYM | 18 |
| 24 | 5-7.7 | 72, 15; 74, 15 | ND | 3 laboratory-grown strains | 105, 108 | Slug flow, laboratory scale pasteurizer | Not specified | Not specified | Not specified | Yes | 2 × 50 | 1,172 × g, 30 min, 4°C | 1 ml, 10× diluted | − | 4.5 ml PBS-0.05% Tween 20 | 0.5 ml in BACTEC 12B, 2 × 0.25 ml on HEYM | Up to 52 |
| 12 | 3-6 | 72, 18; 90, 60 | ND | Laboratory-grown strains | ∼101-105 | Plate, holding section spiral | Yes | 3,461, 1,420 | + | No | 100 | 140,000 × g, 10 min | NALC | − | − | 2 × 0.2 ml on HEYM | Up to 52 |
| 8 | Not reported | 73, 25 | Yes | “Naturally” | Unknown | Combination | Yes | 39,226 | − | No | 50 | 2,500 × g, 15 min | 10 ml 0.75% HPC, 5 h, 21°C | 2,500 × g, 15 min | 2 ml 0.85% NaCl | 3 × 0.33 ml on HEYM, 3 × 0.33 ml in Dubos-NALC | 18 |
| 20 | >4 | 69, 15 | ND | Feces | 2 × 102-3.2 × 102 | Plate | No | 11,400 | + | Yes | 50 | 7,000 × g, 10 min, 21°C | 1% HPC, 50 min, 21 °C | 7,000 × g, 10 min, 15°C | 0.75 ml PBS | Inoculation of HEYM-BACTEC | 20 |
| 20 | >7 | 69, 15 | ND | 5 strains | 0.7 × 103-16 × 103 | Plate | No | 11,400 | + | Yes | 50 | 7,000 × g, 10 min, 21°C | 1% HPC, 50 min, 21 °C | 7,000 × g, 10 min, 15°C | 0.75 ml PBS | Inoculation of HEYM-BACTEC | 20 |
Abbreviations: HEYM, Herrold's egg yolk medium; Dubos medium, see reference 12; BACTEC, radiometric culture method (Becton Dickinson); +, detailed description; −, not described or influenced by plunger pump. NALC was used to decontaminate the sample only when contamination of the primary sample occurred (see reference 12); RT, room temperature; ND, not done; NALC, see reference 12.
In most studies, the inactivation of M. avium subsp. paratuberculosis in raw milk at inoculum levels of 101 to 108 CFU/ml is reported as n-fold reductions at relevant time-temperature combinations such as 15 s at 72°C (Table 2). However, mainly laboratory-grown collection strains are used, with the disadvantage that the strains may have adapted to the laboratory environment, including changed heat resistance and clumping behavior. This would result in under- or overestimation of the inactivation achieved in dairy practice. The use of field isolates of M. avium subsp. paratuberculosis, for example, directly from cow manure, such as in the present study, mimics practical worst-case scenarios.
In recent studies, including the present one, quantification of the number of cells surviving heat treatment has been done. This approach has the benefit that derived data can be modeled and inactivation kinetics parameters can be obtained, as is shown in this study.
Spiral and plate heat exchangers and combinations have been used in recent raw-milk turbulent-flow pasteurization experiments. In most cases, heat regeneration was used in a counterflow between processed and unprocessed product. This introduces the possibility of contamination, but some studies showed that the chance of this was minimal in their setups. We opted for independent heating and cooling without regeneration, thus excluding the possibility of postpasteurization contamination with live M. avium subsp. paratuberculosis cells from raw milk.
It is very important to maintain turbulent flow and the resulting low RTD during pasteurization to ensure that all of the particles in the milk get the same treatment. Most studies (Table 2) report a Reynolds number indicating a turbulent flow, and some studies make note of the RTD in the system while other do not mention this point at all. We used a turbulent flow and a mono pump to ensure a low RTD.
Both BACTEC and Herrold's egg yolk media have been used as growth media for detection of surviving M. avium subsp. paratuberculosis cells after processing. The MGIT system has several advantages over BACTEC. (i) It is nonradioactive and therefore avoids 14C disposal issues, (ii) MGIT are smaller, (iii) syringes and needles are not required for the addition of supplements or specimens, and (iv) MGIT can be read manually on small UV devices or a transilluminator so there is no need for an expensive automated reader, while similar detection capabilities have been reported (10). We used MGIT medium without a detection apparatus but used real-time PCR detection for confirmation of growth.
Incubation times ranged from 18 to 52 weeks in the earlier studies. In order to minimize false-negative detection and allow sublethally damaged cells ample time to start growing, final detection was performed after >48 weeks.
Enumeration of M. avium subsp. paratuberculosis before and after homogenization revealed an increase in the MPN count with a factor of approximately 10, as determined in a small number of samples (three out of three). This could indicate that homogenization increased the number of particles containing cultivable cells, by (stress) activation or disruption of clumps. However, the inactivation rate observed did not reveal any effect of homogenization on M. avium subsp. paratuberculosis inactivation kinetics based on MPN counts at different temperatures.
In conclusion; heat inactivation experiments with a pilot scale pasteurizer mimicking industrial conditions showed a significant reduction in M. avium subsp. paratuberculosis. The model simulations supported the conclusions that under the international standard HTST pasteurization conditions, as used in the Dutch dairy industry, 15 s at ≥72°C, a more-than-sevenfold reduction of M. avium subsp. paratuberculosis cell numbers is achieved. There is no significant additional effect of homogenization of M. avium subsp. paratuberculosis-containing raw milk on M. avium subsp. paratuberculosis inactivation kinetics.
Acknowledgments
Douwe Bakker (ID-Lelystad, The Netherlands) is gratefully acknowledged for providing milk and fecal samples from M. avium subsp. paratuberculosis-infected cows. Jan Hoolwerf, Jos Meeuwisse, Arjen Wagendorp, and the operators of the NIZO food research pilot plant are gratefully acknowledged for operating the pilot scale pasteurizer and for excellent technical assistance. Moreover, we thank P. Hammer and L. Pearce for useful discussions of M. avium subsp. paratuberculosis inactivation studies.
Footnotes
Published ahead of print on 11 May 2007.
REFERENCES
- 1.Abubakar, I., D. J. Myhill, A. R. Hart, I. Lake, I. Harvey J. M. Rhodes, R. Robinson, A. J. Lobo, C. S. J. Probert, and P. R. Hunter. 2007. A case-control study of drinking water and dairy products in Crohn's disease—further investigation of the possible role of Mycobacterium avium paratuberculosis. Am. J. Epidemiol. 165:776-783. [DOI] [PubMed] [Google Scholar]
- 2.Ayele, W. Y., M. Machackova, and I. Pavlik. 2001. The transmission and impact of paratuberculosis infection in domestic and wild ruminants. Vet. Med. (Prague) 46:205-224. [Google Scholar]
- 3.Bigelow, W. D. 1921. The logarithmic nature of thermal death time curves. J. Infect. Dis. 29:528-536. [Google Scholar]
- 4.Chiodini, R. J., H. J. Van Kruiningen, and R. S. Mekal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74:218-262. [PubMed] [Google Scholar]
- 5.De Jong, P., M. Verschueren, M. M. M. Vissers, J. Straatsma, and F. Smit. 2002. Hybrid modelling for development and optimisation of food production chains including costs and food quality, p. 13-17. In B. O'Connor and D. Thiel (ed.), Proceedings of the 2nd International Conference on Simulation in Food and Bio Industries. SCS Europe, Ghent, Belgium.
- 6.Gould, G., P. Franken, P. Hammer, B. Mackey, and F. Shanahan. 2004. Mycobacterium avium subsp. paratuberculosis (M. avium subsp. paratuberculosis) and the food chain. Report prepared under the responsibility of the ILSI Europe Emerging Pathogen Task Force. ILSI Press, Washington, DC. http://europe.ilsi.org/publications/Report+Series/Mycobacterium.htm.
- 7.Grant, I. R., H. J. Ball, and M. T. Rowe. 1998. Effect of high-temperature, short-time (HTST) pasteurization on milk containing low numbers of Mycobacterium paratuberculosis. Lett. Appl. Microbiol. 26:166-170. [DOI] [PubMed] [Google Scholar]
- 8.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale high temperature short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows’ milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant, I. R., A. G. Williams, M. T. Rowe, and D. D. Muir. 2005. Efficacy of various pasteurization time-temperature conditions in combination with homogenization on inactivation of Mycobacterium avium subsp. paratuberculosis in milk. Appl. Environ. Microbiol. 71:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant, I. R., R. B. Kirk, E. Hitchings, and M. T. Rowe. 2003. Comparative evaluation of the MGIT™ and BACTEC culture systems for the recovery of Mycobacterium avium subsp. paratuberculosis from milk. J. Appl. Microbiol. 95:196-201. [DOI] [PubMed] [Google Scholar]
- 11.Grant, I. R. 2005. Zoonotic potential of Mycobacterium avium ssp. paratuberculosis: the current position. J. Appl. Microbiol. 98:1282-1293. [DOI] [PubMed] [Google Scholar]
- 12.Hammer, P., C. Kiesner, H. G. Walte, K. Knappstein, and P. Teufel. 2002. Heat resistance of Mycobacterium avium ssp. paratuberculosis in raw milk tested in a pilot plant pasteurizer. Kiel. Milchwirtsch. Forschungsber. 54:275-303. [Google Scholar]
- 13.Hugot, J.-P., C. Alberti, D. Berrebi, E. Bingen, and J.-P. Cézard. 2003. Crohn's disease: the cold chain hypothesis. Lancet 362:2012-2015. [DOI] [PubMed] [Google Scholar]
- 14.Klijn, N., A. A. P. M. Herrewegh, and P. de Jong. 2001. Heat inactivation data for Mycobacterium avium subspecies paratuberculosis: implications for interpretation. J. Appl. Microbiol. 91:697-704. [DOI] [PubMed] [Google Scholar]
- 15.Korzenik, J. R. 2005. Past and current theories of etiology of IBD: toothpaste, worms, and refrigerators. J. Clin. Gastroenterol. 39:S59-S65. [DOI] [PubMed] [Google Scholar]
- 16.Lund, B. M., G. W. Gould, and A. M. Rampling. 2002. Pasteurization of milk and the heat resistance of Mycobacterium avium subsp. paratuberculosis: a critical review of the data. Int. J. Food Microbiol. 77:135-145. [DOI] [PubMed] [Google Scholar]
- 17.McDonald, W. L., K. J., O'Riley, C. J. Schroen, and R. J. Condron. 2005. Heat inactivation of Mycobacterium avium subsp. paratuberculosis in milk. Appl. Environ. Microbiol. 71:1785-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millar, D., J. Ford, J. Sanderson, S. Withey, M. Tizard, T. Doran, and J. Hermon-Taylor. 1996. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows’ milk in England and Wales. Appl. Environ. Microbiol. 62:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nauta, M. J., and J. W. van der Giessen. 1998. Human exposure to Mycobacterium paratuberculosis via pasteurized milk: a modeling approach. Vet. Rec. 143:293-296. [DOI] [PubMed] [Google Scholar]
- 20.Pearce, L. E., H. T. Truong, R. A. Crawford, G. F. Yates, S. Cavaignac, and G. W. de Lisle. 2001. Effect of turbulent-flow pasteurization on survival of Mycobacterium avium subsp. paratuberculosis added to raw milk. Appl. Environ. Microbiol. 67:3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rademaker, J. L. W., J. Meeuwisse, and M. C. te Giffel. 2003. Heat inactivation of Mycobacterium avium subspecies paratuberculosis, p. 317-319. In R. A. Juste, M. V. Geijo, and J. M. Garrido (ed.) Proceedings of the 7th International Colloquium on Paratuberculosis, Bilbao, Spain. The International Association for Paratuberculosis, Madison, WI.
- 22.Shanahan, F., and J. O'Mahony. 2005. The mycobacteria story in Crohn's disease. Am. J. Gastroenterol. 100:1537-1538. [DOI] [PubMed] [Google Scholar]
- 23.Smit, F., P. De Jong, J. Straatsma, and M. Verschueren. 2001. NIZO-Premia enables knowledgement in the food industry. Part 1. Background and application of the system. Voedingsmiddelentechnologie 34:23-26. [Google Scholar]
- 24.Stabel, J. R., and A. Lambertz. 2004. Efficacy of pasteurization conditions for the inactivation of Mycobacterium avium subsp. paratuberculosis in milk. J. Food Prot. 67:2719-2726. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney, R. W., R. H. Whitlock, and A. E. Rosenberger. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]