Abstract
Long-chain fatty acid (LCFA) degradation is a key step in methanogenic treatment of wastes/wastewaters containing high concentrations of lipids. However, despite the importance of LCFA-degrading bacteria, their natural diversity is little explored due to the limited availability of isolate information and the lack of appropriate molecular markers. We therefore investigated these microbes by using RNA-based stable isotope probing. We incubated four methanogenic sludges (mesophilic sludges MP and MBF and thermophilic sludges TP and JET) with 13C-labeled palmitate (1 mM) as a substrate. After 8 to 19 days of incubation, we could detect 13C-labeled bacterial rRNA. A density-resolved terminal restriction fragment length polymorphism fingerprinting analysis showed distinct bacterial populations in 13C-labeled and unlabeled rRNA fractions. The bacterial populations in the 13C-labeled rRNA fractions were identified by cloning and sequencing of reverse-transcribed 16S rRNA. Diverse phylogenetic bacterial sequences were retrieved, including those of members of the family Syntrophaceae, clone cluster MST belonging to the class Deltaproteobacteria, Clostridium clusters III and IV, phylum Bacteroidetes, phylum Spirochaetes, and family Syntrophomonadaceae. Although Syntrophomonadaceae species are considered to be the major fatty acid-degrading syntrophic microorganisms under methanogenic conditions, they were detected in only two of the clone libraries. These results suggest that phylogenetically diverse bacterial groups were active in situ in the degradation of LCFA under methanogenic conditions.
Lipid is a one of the major organic fractions of wastes/wastewaters, and lipid-rich wastes/wastewaters are widely found in certain food processing industries, such as dairy, edible oil, and slaughterhouses (20). Because lipids have a high theoretical methane yield in comparison with other organic substances, methanogenic treatment has been applied to lipid-rich wastes/wastewaters but resulted in low organic loading rates (see, for example, references 16 and 50) compared to that seen for other types of wastes/wastewaters. This is at least partly due to the acute toxicity of long-chain fatty acids (LCFA), which are the main constituent and hydrolysate of lipids in the anaerobic consortium. LCFA can cause substrate toxicity in anaerobic microorganisms (see, for example, references 18 and 44) and tend to adsorb onto the biomass and flow out of the reactor.
Under methanogenic conditions, LCFA degradation requires a syntrophic association of LCFA-degrading anaerobes and hydrogenotrophic methanogens, because the oxidation of LCFA is thermodynamically unfavorable in such environments unless the consumption of reducing equivalents (hydrogen and/or formate) is coupled with oxidation (37). Due to the syntrophic metabolism and toxicity of LCFA, isolation of LCFA-degrading syntrophs is difficult. Thus, information on LCFA-degrading bacteria in pure culture is based on Syntrophomonas species (10, 21, 36, 47, 54) and on Thermosyntropha lipolytica (49) of the family Syntrophomonadaceae. Syntrophus aciditrophicus (15) is also an LCFA degrader; however, this strain is more often identified as a benzoate degrader. Due to the dearth of knowledge of LCFA-degrading syntrophs and the lack of appropriate molecular markers, culture-independent studies have focused only on species of the family Syntrophomonadaceae (9, 30). Consequently, the natural diversity of syntrophic LCFA-degrading bacteria has not been well explored.
The recent development of stable isotope probing (SIP) enables metabolic function and taxonomic identity to be examined concurrently (27, 33). To date, many studies of DNA-based SIP or RNA-based SIP (RNA-SIP) have been applied to investigate the natural diverse and active populations that conduct particular metabolic processes in a specific environment (for reviews, see references 5 and 53). Methanogenic syntrophic LCFA degradation has not yet been studied in this way, but because there is no appropriate molecular marker for LCFA-degrading syntrophs, SIP provides a potentially fruitful tool for identifying potential LCFA degraders in methanogenic environments. Since anaerobic syntrophic substrate-oxidizing microorganisms grow slowly, RNA-SIP may be especially useful for identification of LCFA-degrading syntrophic anaerobes. Furthermore, incubation periods for RNA-SIP are shorter than those for DNA-SIP, so enrichment bias and unintended labeling of intermediate products or dead biomass may be minimized. In this study, we used RNA-SIP with 13C-palmitate as a substrate to explore the microorganisms involved in LCFA degradation in four methanogenic sludges derived from treatment of lipid-containing wastes/wastewaters. A density-resolved bacterial terminal restriction fragment length polymorphism (T-RFLP) fingerprinting and sequence analysis indicated that not only species belonging to the family Syntrophomonadaceae but also other groups of bacteria could be involved in LCFA degradation.
MATERIALS AND METHODS
Sources of methanogenic sludge.
The four methanogenic sludges used in this study are listed in Table 1. Methanogenic granular sludges (sludges MP and TP) were taken from two lab-scale multistaged upflow anaerobic sludge blanket reactors that were operated in parallel at two different temperatures (19). Mesophilic anaerobic digester sludge (sludge MBF) and thermophilic digester sludge (sludge JET) were taken from commercial plants. The granular sludges taken from the two upflow anaerobic sludge blanket reactors had been stored at 4°C for over 2 years; all other digester sludges were used immediately after sampling.
TABLE 1.
Samples from anaerobic sludges used in this study
| Sample name | Sludge type | Reactor operation temp (°C) | Waste/wastewater | Influent CODcra (mg/liter) | Influent lipids (mg·CODcr/liter) | Location |
|---|---|---|---|---|---|---|
| MP | Granular | 35 | POMEb | 2,000 | 430 | Our laboratory |
| TP | Granular | 55 | POME | 3,000 | 650 | Our laboratory |
| JET | Digester | 55 | Municipal solid waste | 93,500c | —d | Niigata, Japan |
| MBF | Digester | 35 | POME | 81,000 | 10,000 | Selangor, Malaysia |
Chemical oxygen demand as determined using dichromate (CODcr).
POME, palm oil mill effluent.
Data from the digester plant were used because we could not obtain raw influent material.
—, concentration of lipids was not determined because we could not obtain raw influent material.
Sludge incubation.
Seventy-milliliter serum vials containing 20 ml of basal medium prepared as described previously (41) were used for sludge incubation. About 0.8 mg volatile suspended solid (VSS) of sludge was mixed with medium, and each vial was sealed with a butyl rubber septum after the headspace was flushed with a mixture gas of N2-CO2 (80/20 [vol/vol]) and a 0.5-ml reductant solution (mixture of 12 g·liter−1 Na2S·9H2O and cysteine·HCl) was added. Incubation was carried out anaerobically at 37°C (for mesophilic sludges) or 55°C (thermophilic sludges) without shaking. Calcium chloride (1 mM) was added to the culture to reduce the inhibitory effect of LCFA. Two granular sludges, MP and TP, were preincubated with 1 mM sodium palmitate to activate the methanogenic microbial consortium after prolonged storage at 4°C. Following preincubation, the headspace of the vial was flushed with a mixture gas of N2-CO2 (80/20 [vol/vol]) to remove methane gas, and the sludge (∼5 ml) was sampled. The 13C labeling of microbial populations was performed using 13C-palmitate ([1,2,3,4-13C4] palmitic acid potassium salt; Isotec, Miamisburg, Ohio) at a concentration of 1 mM (0.02 mmol). When the palmitate was mostly converted to methane, the sludge (∼5 ml) was sampled. This 13C-palmitate incubation step was repeated to obtain the second incubated sludge. Methane produced in control experiments (without the addition of palmitate) was subtracted from the gross methane produced from incubation with palmitate.
RNA extraction, cesium trifluoroacetate (CsTFA) fractionation, and quantification of rRNA.
Total RNA extraction from the collected sludge and RNA purification were conducted by the method described previously (42). Equilibrium density gradient centrifugation was performed based on methods reported by Manefield et al. (27) and Lueders et al. (23), with the following modification of the centrifugation conditions. Density gradient centrifugation was performed in 5-ml polyallomer Quick-Seal tubes in an NVT65.2 rotor (both Beckman Coulter) at 41,500 rpm and 20°C for 48 h. Gradients were fractionated from below by displacement with water by using a syringe pump (Harvard Apparatus). RNA was precipitated by isopropanol from fractionated gradients, and rRNA was quantified by real-time reverse transcription-PCR (RT-PCR) performed with a LightCycler (Roche) by using the QuantiTect SYBR green RT-PCR kit (QIAGEN) as described in a previous report (11).
T-RFLP fingerprinting.
RNA extracted from density-resolved gradient fractions was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) with 16S rRNA-targeted prokaryote-specific primer 1490R (5′-GGHTACCTTGTTACGACTT-3′, Escherichia coli position 1491 to 1509) slightly modified from that reported by Weisburg et al. (52). T-RFLP fingerprinting of bacterial communities was conducted as published elsewhere (11).
Cloning, sequencing, and phylogenetic analysis.
RNA from selected fractions was reverse transcribed as described above and bacterial 16S rRNA gene clone libraries were constructed as described elsewhere (11). Fifty clones were randomly picked from each clone library and subjected to RFLP analysis with the HaeIII and MspI restriction endonucleases. We categorized the clones having the same electrophoresis pattern in the RFLP analysis into a phylotype. The sequences of clones were determined as described previously (32). Chimeric sequences were searched using the Chimera_Check program at Ribosomal Database Project (26) and the Bellerophon program (13). Sequence data were aligned with the ARB program package (22), and the aligned data were manually corrected based on information about primary and secondary structures. The phylogenetic trees based on 16S rRNA gene sequences were constructed by the neighbor-joining method implemented in the ARB program. Bootstrap resampling analysis for 1,000 replicates was performed to estimate the confidence of tree topologies.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequence data obtained in this study were deposited in the GenBank/EMBL/DDBJ databases under accession numbers AB290356 to AB290407.
RESULTS
Incubation of 13C-palmitate and RNA distribution in centrifugation gradient.
Because of prolonged storage at 4°C, the two granular sludges TP and MP were preincubated with 1 mM palmitate. The palmitate was completely consumed and converted to methane in 2 and 3 weeks, respectively. After the preincubation, RNA extracted from the sludges was used as an unlabeled control RNA for the original microbial consortium. In the first round of incubation with 13C-palmitate, methanogenesis was faster than the preincubation, and 13C-palmitate was converted to methane in 6 and 11 days for sludges TP and MP, respectively. The average methanogenesis rates were 42 μmol·mg−1 VSS·day−1 and 23 μmol·mg−1 VSS·day−1 for sludges TP and MP, respectively. In the second round of 13C-palmitate incubation, methanogenesis was faster and incubation was finished within 4 and 8 days for sludges TP and MP, yielding methanogenesis rates of 63 μmol·mg−1 VSS·day−1 and 31 μmol·mg−1 VSS·day−1, respectively.
For digest sludges MBF and JET, RNA extracted from unincubated sludges was used as an unlabeled control RNA. 13C-palmitate was consumed in the first round within 8 and 4 days and in the second round within 4 and 2 days for sludges MBF and JET, respectively. The average methanogenesis rates were 31 μmol·mg−1 VSS·day−1 (first incubation) and 63 μmol·mg−1 VSS·day−1 (second incubation) and 63 μmol·mg−1 VSS·day−1 (first incubation) and 125 μmol·mg−1 VSS·day−1 (second incubation) for sludges MBF and JET, respectively. Each experiment was repeated twice, and the observed methanogenesis rates were nearly identical.
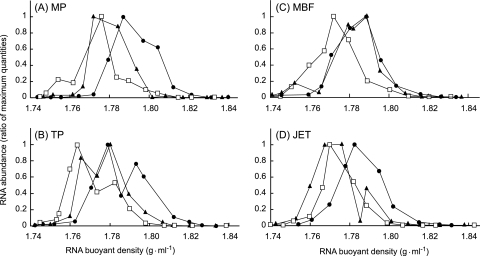
After isopycnic centrifugation and fractionation, bacterial rRNA content in each fraction was quantified by quantitative RT-PCR with bacterial universal primers shown in Fig. 1. The unlabeled control rRNA gradient showed peaks at a buoyant density (BD) of around 1.77 to 1.78 g·ml−1, which is characteristic of unlabeled bacterial rRNAs in CsTFA (23) (Fig. 1). In sludge MP, the rRNA gradient profile for the first incubation sludge had a gradual increase of intermediate (∼1.80 g·ml−1) rRNA, but the peak BD was almost the same as that of control rRNA (Fig. 1A). After the second incubation, the entire rRNA BD was shifted toward the heavier fraction, and 13C-labeled rRNAs (>1.80 g·ml−1) were detected (Fig. 1A). In sludge TP, after the first incubation with 13C-palmitate, the rRNA profile was gradually shifted toward a heavier BD, and a minute tailing of 13C-enriched rRNA was detected. After the second incubation, the entire rRNA BD was shifted toward the heavier fraction, similar to that for sludge MP (Fig. 1B). In contrast to what was observed for the other sludges, all of the rRNA of sludge MBF had completed a substantial shift toward a heavier BD even after the first incubation with 13C-palmitate (Fig. 1C). After the first incubation, the rRNA gradient profile of sludge JET had not changed; however, after the second incubation, the entire rRNA BD was shifted toward the heavier fraction (Fig. 1D). An archaeal rRNA BD profile had almost the same pattern as bacterial rRNA BD profiles in all four sludges (data not shown).
FIG. 1.
CsTFA density gradient centrifugation profiles of bacterial rRNA extracted from sludges MP (A), TP (B), MBF (C), and JET (D). Open squares, RNA from unlabeled sludge; closed triangles, RNA from first 13C-palmitate incubation; closed circles, RNA from second 13C-palmitate incubation. The amount of bacterial rRNA content in each gradient fraction was quantified by quantitative RT-PCR using a bacterial domain-specific primer set.
Fingerprinting of density-resolved rRNA.
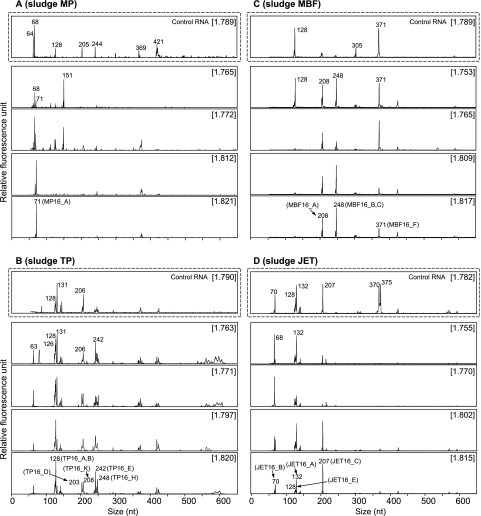
We used T-RFLP fingerprinting of bacterial rRNA template resolved by gradient centrifugation to trace specific microorganisms that incorporated 13C into their rRNA. Based on a comparison of the T-RFLP fingerprint profile of control RNA with that of RNA taken from sludges incubated with 13C-palmitate, representative 13C-labeled and unlabeled fractions of 13C-treated sludges are shown in Fig. 2. In the control RNA, T-RFLP fingerprints of 13C-labeled fractions (>1.81 g·ml−1) could not be obtained. The T-RFLP fingerprints were nearly identical in different gradient fractions; therefore, typical profiles are shown for the control RNA of every sample (Fig. 2, dashed boxes).
FIG. 2.
Bacterial T-RFLP fingerprinting of density-resolved RNA from representative 13C-labeled and unlabeled fractions of sludges MP (A), TP (B), MBF (C), and JET (D). Electropherograms of sludges MP, TP, and JET were generated from gradient fractions of RNA from the second 13C-palmitate incubation as the template, whereas sludge MBF was generated from gradient fractions of RNA from the first 13C-palmitate incubation as the template. Dashed boxes show T-RFLP fingerprints of density-resolved unlabeled control RNA. CsTFA BDs (g·ml−1) of gradient fractions are given in brackets. The numbers at the T-RF peaks indicate the T-RF lengths. Representing phylotypes of T-RFs identified by clone analysis are indicated in parenthesis.
For sludge MP, electropherograms generated from gradient fractions of RNA taken from the second 13C-palmitate incubation sludge are shown in Fig. 2A. The 71-bp terminal restriction fragment (T-RF) became predominant only in the 13C-labeled fractions. The 68- and 151-bp T-RFs were dominant in the unlabeled fractions, but the 68-bp T-RF was a minor component and the 151-bp T-RF was not detectable in 13C-labeled fractions. For sludge TP, gradient fractions of RNA taken after the second 13C-palmitate incubation showed broadly similar patterns (Fig. 2B). However, specific differences were detectable; most strikingly, the major T-RFs of 131, 126, and 206 bp in the unlabeled fractions were markedly reduced in abundance in 13C-labeled fractions. The 248-bp T-RF was dominant in the 13C-labeled fraction (1.820 g·ml−1) but minor in the unlabeled fractions. Also, 203- and 208-bp T-RFs became detectable in 13C-labeled fractions. These two T-RFs (203 and 208 bp) were not detected in the control RNA profile (Fig. 2B, dashed box) or in RNA of the first 13C-palmitate incubation sludge (data not shown). Electropherograms generated from RNA gradient fractions of MBF sludge after the first 13C-palmitate incubation are shown in Fig. 2C. In comparison to the unlabeled fractions, 208- and 248-bp T-RFs had specifically increased in relative abundance in 13C-labeled fractions. The 128-bp T-RF was detectable only in the unlabeled fraction (1.753 g·ml−1), and the 371-bp T-RF was reduced in relative fluorescence intensity toward 13C-labeled fractions (Fig. 2C). T-RFLP fingerprints from gradient fractions of RNA taken from second 13C-palmitate incubation of sludge JET are shown in Fig. 2D. The 207-bp T-RF had increased in relative abundance specifically in 13C-labeled fractions, whereas the 68-bp T-RF was reduced in relative abundance toward heaver fractions. The 132-bp T-RF was detectable throughout the fractions and also in fingerprints generated from unlabeled control RNA (Fig. 2D, dashed box).
Sequence analysis of 13C-labeled bacterial 16S rRNA.
To phylogenetically identify the bacterial populations in the 13C-labeled rRNA fractions and to assign the T-RFs to the individual populations, we constructed clone libraries from the 13C-labeled RNA fractions and analyzed 50 clones for each clone library. The phylogenetic affiliations of all analyzed bacterial clones are summarized in Table 2, and the positions of selected phylotypes, represented by at least four clones, are shown in Fig. 3.
TABLE 2.
Phylogenetic affiliation and numbers of bacterial 16S rRNA clones retrieved from clone libraries generated from 13C-labeled RNA fractions
| Phylogenetic groupa | No. of clones
|
T-RF length of clones (bp)b | |||
|---|---|---|---|---|---|
| MP | TP | MBF | JET | ||
| Bacteroidetes | 3 | 9 | 6 | 206, 207, 370, 371 | |
| Firmicutes | |||||
| Clostridium | 30 | 18 | 68, 70, 71 | ||
| Syntrophomonas | 1 | 374 | |||
| Syntrophothermus | 1 | 4 | 210, 208 | ||
| Tepidanaerobacter | 1 | 2 | 68 | ||
| Desulfotomaculum | 2 | 244 | |||
| Deltaproteobacteria | |||||
| Syntrophaceae | 1 | 18 | 210, 208 | ||
| Geobacteraceae | 1 | 210 | |||
| Clone MST group | 4 | 17 | 248 | ||
| Deferribacteres | |||||
| Deferribacteraceae | 2 | 249 | |||
| Synergistes | 2 | 130 | |||
| Chlorobi | 1 | 73 | |||
| Thermotoga | 7 | 128 | |||
| Acidobacteria | 1 | 72 | |||
| Spirochaetes | 4 | 8 | 3 | 244, 305, 373 | |
| Coprothermobacter | 13 | 132 | |||
| Anaerobaculum | 5 | 6 | 128 | ||
| OP8 | 1 | 92 | |||
| OP9 | 1 | 133 | |||
| OP10 | 2 | 132 | |||
| OP11 | 4 | 382 | |||
| Marine group A | 1 | 5 | 209, 207 | ||
| EM3 | 5 | 203 | |||
| WS3 | 1 | 132 | |||
| WCHB1-27 | 1 | 248 | |||
| NKB19 | 1 | 210 | |||
| Unidentified | 1 | 2 | 72, 132 | ||
Phylogenetic groups with cultivated representatives are named according to the taxonomic outline of Bergey's Manual of Systematic Bacteriology (6). Candidate phyla are named based on review by Hugenholtz (14).
Phylotypes that are represented by at least three clones and whose sequence lengths matches the lengths of specific T-RFs are highlighted in boldface, and others had their T-RF lengths predicted using sequence data.
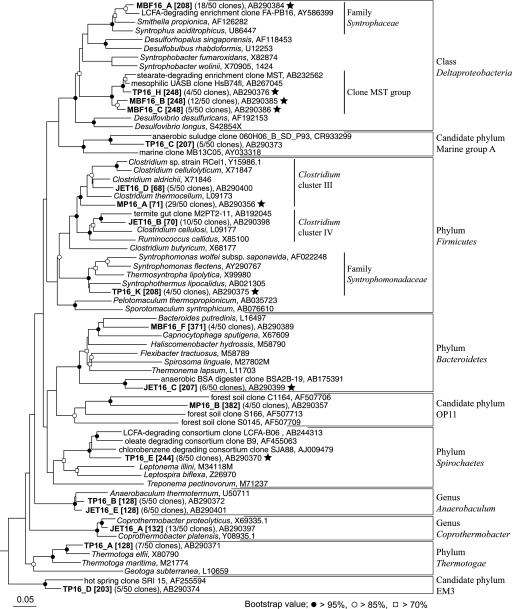
FIG. 3.
Phylogenetic placement of representative bacterial 16S rRNA phylotypes from 13C-labeled RNA fractions. The measured T-RF lengths of phylotypes digested with MspI are shown in brackets. The scale bar represents the number of nucleotide changes per sequence position. The symbols at each branch point show the bootstrap values obtained with resampling analysis (1,000 resamples). Stars indicate putative palmitate degraders inferred from our results. BSA, bovine serum albumin.
The clone library MP was generated from the 1.821-g·ml−1 RNA fraction from the second 13C-palmitate incubation sludge MP. Of 50 clones analyzed from the MP library, 30 clones (phylotype MP16_A) were affiliated with the Clostridium cluster III but did not show specific affiliations with cultured Clostridium species within this group (Fig. 3). Four clones (phylotype MP16_B) belong to candidate phylum OP11, 4 clones clustered with phylum Spirochaetes, and the remaining 10 clones belonged to diverse phylogenetic groups (Table 2). In silico prediction of the T-RF length of phylotype MP16_A indicated 72 bp, whereas our experimentally measured fragment length was 71 bp. Small differences between measured T-RFs and those predicted using sequence data have been reported frequently and seem to be sequence dependent, but they have yet to be explained (3, 17). Therefore, we postulate that the predominant 71-bp T-RF in the 13C-enriched fractions corresponds to phylotype MP16_A, and we show the experimentally measured T-RFs in the following text and in Fig. 3 and Table 2.
The clone library TP was generated from the 1.820-g·ml−1 RNA fraction from the second 13C-palmitate incubation sludge, TP. Phylogenetic analysis of clones from the TP library found diverse groups of bacteria (Table 2), but none of them predominated. In these clones, phylotype TP16_K was affiliated with the family Syntrophomonadaceae, including the known LCFA-degrading syntrophs (55); also, phylotype TP16_H was clustered with clone MST, which could represent a novel LCFA oxidizer, as reported in our previous study (11) (Fig. 3). The 248- and 208-bp T-RFs were assigned to phylotypes TP16_H and TP16_K, respectively. Based on comparative T-RFLP fingerprinting analysis and phylogenetic analysis, at least these two phylotypes (TP16_H and TP16_K) might be degrading palmitate directly. The major 128-bp T-RF corresponded to phylotypes TP16_A and TP16_B, which were assigned to Thermotoga species and Anaerobaculum species, respectively. The peak observed at 242 bp did not retrieve a corresponding phylotype but for the phylotype TP16_E was most similar to a T-RF of 244 bp. Phylotype TP16_E was affiliated with the phylum Spirochaetes but distantly related to other described species. It clustered with clones SJA88, B9, and LCFA-B06 (Fig. 3). The phylotype TP16_D, which corresponded to a 203-bp T-RF, was affiliated with an uncultivated phylum, EM3 (Fig. 3).
The RNA fraction of 1.817 g·ml−1 from the first 13C-palmitate incubation sludge MBF was used for cloning. Seventy percent of all clones formed three phylotypes (MBF16_A [18 clones], MBF16_B [12 clones], and MBF16_C [5 clones]), and their T-RFs were 208 bp (for MBF16_A) and 248 bp (for MBF16_B and MBF16_C) (Table 2 and Fig. 3). The phylotype MBF16_A clustered with the family Syntrophaceae and was closely related to clone FA-PB16, which was retrieved from an LCFA-degrading methanogenic enrichment (8). Phylotypes MBF16_B and MBF16_C clustered with the clone MST group within the class Deltaproteobacteria (Fig. 3). Based on our current results and previous findings (8, 11, 15), microbes detected as phylotypes MBF16_A, MBF16_B, and MBF16_C could be degrading palmitate in sludge MBF.
The 1.815-g·ml−1 RNA fraction from the second 13C-palmitate incubation sludge JET was used for construction of clone library JET. The most abundant phylotype, JET16_A (13 clones), which corresponded to a 132-bp T-RF, was closely related to Coprothermobacter spp. (Fig. 3). The phylotype JET16_B (10 clones), which corresponded to a 70-bp T-RF, belonged to Clostridium cluster IV. Phylotype JET16_C deeply branched with phylum Bacteroidetes, and phylotype JET16_E was closely related to Anaerobaculum spp. Their corresponding 207- and 128-bp T-RFs were retrieved from six clones (Table 2 and Fig. 3). The phylotype JET16_D (five clones), which clustered with Clostridium cluster III but corresponded to a T-RF of 68 bp, could be detected as only a minor portion in T-RFLP analysis (Fig. 2D). These four T-RFs (132, 70, 128, and 68 bp) were observed predominantly in unlabeled gradient fractions or control RNA fractions (Fig. 2D). We therefore speculated that they represented microbes found in high abundance in sludge JET, because a certain amount of unlabeled rRNA is known to contaminate 13C-labeled rRNA gradient fractions due to limited separation efficiency (23, 27). Hence, the phylotype JET16_C, which corresponded to the strongest 207-bp T-RF in 13C-labeled fractions (Fig. 2D), may be closely linked to the degradation of palmitate in sludge JET (Fig. 3).
DISCUSSION
We applied an RNA-SIP method to methanogenic sludges to investigate the LCFA-assimilating syntrophic microorganisms by use of 13C-palmitate. We could effectively label the microcosms in incubation periods shorter (8 to 19 days) than those reported for other SIP studies targeting anaerobic syntrophic substrate-oxidizing microorganisms; for example, propionate-oxidizing microorganisms in paddy soil required 7 weeks of incubation for detection of the 13C-labeled rRNA (24), and Chauhan and Ogram also performed incubation for 7 weeks to analyze propionate or butyrate oxidizers in freshwater marsh soil (1). One likely reason for this short incubation period is the high microbial activity of methanogenic sludges relative to what is seen for soil microorganisms. The methanogenesis rates in our experiments were in the range of 23 to 63 μmol·mg−1 VSS·day−1 for mesophilic sludges and 42 to 125 μmol·mg−1 VSS·day−1 for thermophilic sludges, at least 1 order of magnitude higher than rates reported for soil experiments (1, 24). Preincubation was prerequisite for obtaining high microbial activities for sludges TP and MP. This short incubation is one of the key points in avoiding a possible cross-feeding of label into a microbial consortium not involved in syntrophic LCFA oxidation. Moreover, we used comparative T-RFLP fingerprint analysis along the gradient fraction to detect peaks that increased in relative dominance or became detectable in heavier fractions. This T-RFLP quality control procedure confirms the interpretation of our sequence results (24, 25). In addition, we used partially 13C-labeled palmitate, which has four carbon atoms on the carboxyl end labeled with 13C, to avoid an unintended labeling of short-chain fatty acid-oxidizing microorganisms that cannot degrade LCFA. Since LCFA degradation is via β-oxidation, which proceeds by the removal of two carbon units from the carboxyl end of LCFA, these 13C-labeled carbons could be mineralized only by LCFA degraders. During the β-oxidation, cross-feeding of intermediately formed 13C-acetate could label the non-LCFA degrader. However, acetate is immediately converted to methane by aceticlastic methanogens under methanogenic conditions (48), and concomitantly archaeal rRNA was also becoming heavier, suggesting that 13C-acetate was assimilated by methanogenic Archaea. In addition to acetate utilization by aceticlastic methanogen, it is known that acetate oxidation is also performed by a syntrophic association with acetate-oxidizing syntrophic bacteria and hydrogenotrophic methanogens (37). However, the syntrophic acetate oxidation tends to occur only under certain conditions, such as high ammonium or volatile fatty acid concentrations (38, 40).
Although some species belonging to the family Syntrophomonadaceae are typically known as LCFA-degrading syntrophic microorganisms under methanogenic conditions, they were detected in our studies only in sludges MP and TP. Only one clone of each species was retrieved from the clone library MP (Table 2). In sludge TP, a variety of clones were retrieved, but a Syntrophothermus sp. was the only known palmitate-degrading microorganism identified. In many enrichment studies, species belonging to the family Syntrophomonadaceae have been readily enriched (11, 29, 45, 46), but their natural abundance was less than 2% of the total small subunit rRNA (9, 28), as determined by membrane hybridization with probe Synm700 targeted for mesophilic, syntrophic fatty acid-oxidizing syntrophic bacteria within the family Syntrophomonadaceae. Recently, Menes and Travers designed a 16S rRNA-targeted probe for detection of fatty acid-oxidizing bacterial members within the family Syntrophomonadaceae (including both LCFA and short-chain fatty acid degraders) and applied it to several sludges. However, quantification was possible only for lipid-rich wastewater treated sludge (3% of EUB338 probe-positive cells), and other sludges were below the detection limit (30). Given that the effect of enrichment bias is well known and that other bacterial groups are also involved in LCFA degradation (8, 11, 15, 43), non-Syntrophomonadaceae bacteria also may have a major role in LCFA degradation in anaerobic environments.
Syntrophaceae sequence was derived predominantly from sludge MBF (Table 2). S. aciditrophicus is known to utilize LCFA, and Grabowski et al. also reported that a bacterial clone closely related to the genus Syntrophus may be involved in stearate and heptadecanoate degradation (8). Therefore, it is apparent that this bacterial group could be involved in the degradation of LCFA. Although acetate utilization has not been shown for described Syntrophus spp. so far, very recently Chauhan and Ogram reported that Syntrophus-like species might utilize acetate syntrophically (2). If so, we could not conclude a distinct metabolic function of Syntrophaceae bacteria in sludge MBF. In addition to the Syntrophaceae sequence, clones closely related to clone MST belonging to the class Deltaproteobacteria were also highly retrieved from sludge MBF. Clone MST probably represents an LCFA-degrading microorganism, as indicated by our previous study (11). The single branch affiliated with the clone MST group was retrieved from different anaerobic sludges, and many of these microorganisms may be involved in LCFA degradation; thus, it is conceivable that this branch of bacteria represents a new group of LCFA degrader under methanogenic conditions. However, we need further studies to obtain concrete evidence for this hypothesis.
In sludge TP, Syntrophothermus species and the sequence related to clone MST might be involved in palmitate degradation. A large number of sequences belonging to the genera Thermotoga and Anaerobaculum were also retrieved from the thermophilic sludge. However, we could not conclude that they contributed to palmitate degradation. In addition, there are no reports of isolates belonging to these genera having LCFA-degrading ability (34, 35). Therefore, we speculate that these species might be LCFA tolerant. Some species affiliated with these genera are frequently isolated from petroleum reservoir fluids or oil-producing wells (34, 35), suggesting that they are oil tolerant and thus might also tolerate LCFA. Moreover, many uncultivated groups of bacteria were detected in sludge TP (Table 2 and Fig. 3), but it is hard to speculate on their role in environment. One of them, phylotype TP16_E, which was retrieved mostly from sludge TP, clustered with clones SJA88, B9, and LCFA-B06 (Fig. 3) within the phylum Spirochaetes. The clones B9 and LCFA-B06 were obtained from LCFA-degrading methanogenic consortia (31, 43), and so this group of bacteria might play an important role in LCFA degradation or simply be LCFA tolerant, but further analysis will be necessary.
Clostridium spp. are known for degradation of a variety of substrates under anaerobic conditions. Recently, Chauhan and Ogram reported a Clostridium sequence, derived from their DNA-SIP study using butyrate, which may represent a novel lineage of butyrate-oxidizing bacteria (1). Based on our results from sludge MP, it is reasonable to postulate that the Clostridium sequence of phylotype MP16_A may also represent uncultivated syntrophic LCFA-degrading microbes. Of course, some Clostridium species utilize acetate in syntrophic association with hydrogen-utilizing methanogens under methanogenic conditions (12, 39). However, such syntrophic acetate oxidation was minimized in our experimental conditions as stated above, and this could be demonstrated by SIP of 13C-acetate for the sludge. Additionally, this result might be affected by longer periods of sludge storage resulting in the survival of spore-forming bacteria.
In the clone library JET, Clostridium sequences were mostly derived (Table 2), but their T-RFs were not dominant in 13C-labeled fractions. The second-most-abundant phylotype, JET16_A, was of Coprothermobacter spp. However, isolates belonging to this genus were not reported as LCFA degraders (4) and also might not have been involved in palmitate degradation in our study. Furthermore, this species was predominant in the sludge, as revealed by our previous 16S rRNA gene-based cloning analysis (unpublished data), and hence this might be not the result of 13C-palmitate assimilation by Coprothermobacter spp. but rather due to the limited separation efficiency of 13C-labeled RNA from unlabeled RNA (23, 27). The 207-bp T-RF was enriched in 13C-labeled RNA fractions (Fig. 2D) and identified as phylotype JET_C (Fig. 3). This sequence is affiliated with the phylum Bacteroides and is most closely related to clone BSA2B, which was retrieved from a bovine serum albumin-fed methanogenic batch reactor (51). Because these clones are distinct from other cultured strains, their metabolic functions could not be elucidated from their phylogenetic positions. Because none of the known LCFA-oxidizing bacteria were sequenced from sludge JET, phylotype JET_C might represent an LCFA-utilizing bacterium.
In conclusion, we have identified potential LCFA degraders in methanogenic environments, including many microbes belonging to uncultivated phylogenetic groups. Despite the limited number of bacteria known to utilize palmitate under methanogenic conditions to date, many unrecognized bacteria were detected. It is not possible to determine the physiology of these uncultivated microorganisms by using only the present SIP data. The best way to clearly demonstrate LCFA-utilizing ability in these candidate LCFA-degrading microorganisms will be to isolate them, but this will be difficult. Application of other methods, such as fluorescent in situ hybridization-microautoradiography (7), combined with our sequence data could help to define the metabolic functions and the as-yet-uncultivated potential of LCFA-degrading microorganisms.
Acknowledgments
We thank Eiji Masai at Nagaoka University of Technology for use of the ultracentrifuge.
This study was financially supported by the New Energy and Industrial Technology Development Organization (NEDO), Japan Society for the Promotion of Science, and the 21st Century COE program “Global Renaissance by Green Energy Revolution” subsidized by the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Footnotes
Published ahead of print on 4 May 2007.
REFERENCES
- 1.Chauhan, A., and A. Ogram. 2006. Fatty acid-oxidizing consortia along a nutrient gradient in the Florida Everglades. Appl. Environ. Microbiol. 72:2400-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan, A., and A. Ogram. 2006. Phylogeny of acetate-utilizing microorganisms in soils along a nutrient gradient in the Florida Everglades. Appl. Environ. Microbiol. 72:6837-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cupples, A. M., and G. K. Sims. 2007. Identification of in situ 2,4-dichlorophenoxyacetic acid-degrading soil microorganisms using DNA-stable isotope probing. Soil Biol. Biochem. 39:232-238. [Google Scholar]
- 4.Etchebehere, C., M. E. Pavan, J. Zorzopulos, M. Soubes, and L. Muxi. 1998. Coprothermobacter platensis sp. nov., a new anaerobic proteolytic thermophilic bacterium isolated from an anaerobic mesophilic sludge. Int. J. Syst. Bacteriol. 48:1297-1304. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich, M. W. 2006. Stable-isotope probing of DNA: insights into the function of uncultivated microorganisms from isotopically labeled metagenomes. Curr. Opin. Biotechnol. 17:59-66. [DOI] [PubMed] [Google Scholar]
- 6.Garrity, G. M., J. A. Bell, and T. G. Lilburn. 2004. Taxonomic outline of the prokaryotes. Bergey's manual of systematic bacteriology, 2nd ed., release 5.0. Springer-Verlag, New York, NY. doi: 10.1007/bergeysoutline200405. [DOI]
- 7.Ginige, M. P., P. Hugenholtz, H. Daims, M. Wagner, J. Keller, and L. L. Blackall. 2004. Use of stable-isotope probing, full-cycle rRNA analysis, and fluorescence in situ hybridization-microautoradiography to study a methanol-fed denitrifying microbial community. Appl. Environ. Microbiol. 70:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski, A., D. Blanchet, and C. Jeanthon. 2005. Characterization of long-chain fatty-acid-degrading syntrophic associations from a biodegraded oil reservoir. Res. Microbiol. 156:814-821. [DOI] [PubMed] [Google Scholar]
- 9.Hansen, K. H., B. K. Ahring, and L. Raskin. 1999. Quantification of syntrophic fatty acid-beta-oxidizing bacteria in a mesophilic biogas reactor by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 65:4767-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatamoto, M., H. Imachi, S. Fukayo, A. Ohashi, and H. Harada. Syntrophomonas palmitatica sp. nov., a novel anaerobic, syntrophic long-chain fatty acid-oxidizing bacterium isolated from a methanogenic sludge. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 11.Hatamoto, M., H. Imachi, A. Ohashi, and H. Harada. 2007. Identification and cultivation of anaerobic, syntrophic long-chain fatty acid-degrading microbes from mesophilic and thermophilic methanogenic sludges. Appl. Environ. Microbiol. 73:1332-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattori, S., Y. Kamagata, S. Hanada, and H. Shoun. 2000. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 50:1601-1609. [DOI] [PubMed] [Google Scholar]
- 13.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 14.Hugenholtz, P. 2002. Exploring prokaryotic diversity in the genomic era. Genome Biol. 3:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson, B. E., V. K. Bhupathiraju, R. S. Tanner, C. R. Woese, and M. J. McInerney. 1999. Syntrophus aciditrophicus sp. nov., a new anaerobic bacterium that degrades fatty acids and benzoate in syntrophic association with hydrogen-using microorganisms. Arch. Microbiol. 171:107-114. [DOI] [PubMed] [Google Scholar]
- 16.Jeganathan, J., G. Nakhla, and A. Bassi. 2006. Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ. Sci. Technol. 40:6466-6472. [DOI] [PubMed] [Google Scholar]
- 17.Kitts, C. L. 2001. Terminal restriction fragment patterns: a tool for comparing microbial communities and assessing community dynamics. Curr. Issues Intest. Microbiol. 2:17-25. [PubMed] [Google Scholar]
- 18.Koster, I. W., and A. Cramer. 1987. Inhibition of methanogenesis from acetate in granular sludge by long-chain fatty acids. Appl. Environ. Microbiol. 53:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucivilize, P., A. Ohashi, and H. Harada. 2003. Process performance and sludge behaviors of multi-staged UASB reactor for treatment of palm oil mill effluent (POME). Environ. Eng. Res. 40:441-449. (In Japanese.) [Google Scholar]
- 20.Li, Y. Y., H. Sasaki, K. Yamashita, K. Seki, and I. Kamigochi. 2002. High-rate methane fermentation of lipid-rich food wastes by a high-solids co-digestion process. Water Sci. Technol. 45(12):143-150. [PubMed] [Google Scholar]
- 21.Lorowitz, W. H., H. Zhao, and M. P. Bryant. 1989. Syntrophomonas wolfei subsp. saponavida subsp. nov., a long-chain fatty-acid-degrading, anaerobic, syntrophic bacterium; Syntrophomonas wolfei subsp. wolfei. nov.; and emended descriptions of the genus and species. Int. J. Syst. Bacteriol. 39:122-126. [Google Scholar]
- 22.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüâmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lueders, T., M. Manefield, and M. W. Friedrich. 2004. Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ. Microbiol. 6:73-78. [DOI] [PubMed] [Google Scholar]
- 24.Lueders, T., B. Pommerenke, and M. W. Friedrich. 2004. Stable-isotope probing of microorganisms thriving at thermodynamic limits: syntrophic propionate oxidation in flooded soil. Appl. Environ. Microbiol. 70:5778-5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madsen, E. L. 2006. The use of stable isotope probing techniques in bioreactor and field studies on bioremediation. Curr. Opin. Biotechnol. 17:92-97. [DOI] [PubMed] [Google Scholar]
- 26.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manefield, M., A. S. Whiteley, R. I. Griffiths, and M. J. Bailey. 2002. RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon, K. D., D. Zheng, A. J. Stams, R. I. Mackie, and L. Raskin. 2004. Microbial population dynamics during start-up and overload conditions of anaerobic digesters treating municipal solid waste and sewage sludge. Biotechnol. Bioeng. 87:823-834. [DOI] [PubMed] [Google Scholar]
- 29.Menes, R. J., A. Fernandez, and L. Muxi. 2001. Physiological and molecular characterisation of an anaerobic thermophilic oleate-degrading enrichment culture. Anaerobe 7:17-24. [Google Scholar]
- 30.Menes, R. J., and D. Travers. 2006. Detection of fatty acid beta-oxidizing syntrophic bacteria by fluorescence in situ hybridization. Water Sci. Technol. 54(2):33-39. [DOI] [PubMed] [Google Scholar]
- 31.Pereira, M. A., K. Roest, A. J. M. Stams, M. Mota, M. Alves, and A. D. L. Akkermans. 2002. Molecular monitoring of microbial diversity in expanded granular sludge bed (EGSB) reactors treating oleic acid. FEMS Microbiol. Ecol. 41:95-103. [DOI] [PubMed] [Google Scholar]
- 32.Qiu, Y. L., Y. Sekiguchi, H. Imachi, Y. Kamagata, I. C. Tseng, S. S. Cheng, A. Ohashi, and H. Harada. 2004. Identification and isolation of anaerobic, syntrophic phthalate isomer-degrading microbes from methanogenic sludges treating wastewater from terephthalate manufacturing. Appl. Environ. Microbiol. 70:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radajewski, S., P. Ineson, N. R. Parekh, and J. C. Murrell. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403:646-649. [DOI] [PubMed] [Google Scholar]
- 34.Ravot, G., M. Magot, M. L. Fardeau, B. K. Patel, G. Prensier, A. Egan, J. L. Garcia, and B. Ollivier. 1995. Thermotoga elfii sp. nov., a novel thermophilic bacterium from an African oil-producing well. Int. J. Syst. Bacteriol. 45:308-314. [DOI] [PubMed] [Google Scholar]
- 35.Rees, G. N., B. K. Patel, G. S. Grassia, and A. J. Sheehy. 1997. Anaerobaculum thermoterrenum gen. nov., sp. nov., a novel, thermophilic bacterium which ferments citrate. Int. J. Syst. Bacteriol. 47:150-154. [DOI] [PubMed] [Google Scholar]
- 36.Roy, F., E. Samain, H. C. Dubourguier, and G. Albagac. 1986. Syntrophomonas sapovorans sp. nov., a new obligately proton reducing anaerobe oxidizing saturated and unsaturated long chain fatty acids. Arch. Microbiol. 145:142-147. [Google Scholar]
- 37.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnürer, A., P. H. Frans, and B. H. Svensson. 1994. Mesophilic syntrophic acetate oxidation during methane formation by a triculture at high ammonium concentration. Arch. Microbiol. 162:70-74. [Google Scholar]
- 39.Schnürer, A., B. Schink, and B. Svensson. 1996. Clostridium ultunense sp. nov., a mesophilic bacterium oxidizing acetate in syntrophic association with a hydrogenotrophic methanogenic bacterium. Int. J. Syst. Bacteriol. 46:1145-1152. [DOI] [PubMed] [Google Scholar]
- 40.Schnürer, A., G. Zellner, and B. H. Svensson. 1999. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol. Ecol. 29:249-261. [Google Scholar]
- 41.Sekiguchi, Y., Y. Kamagata, K. Nakamura, A. Ohashi, and H. Harada. 2000. Syntrophothermus lipocalidus gen. nov., sp. nov., a novel thermophilic, syntrophic, fatty-acid-oxidizing anaerobe which utilizes isobutyrate. Int. J. Syst. Evol. Microbiol. 50:771-779. [DOI] [PubMed] [Google Scholar]
- 42.Sekiguchi, Y., Y. Uyeno, A. Sunaga, H. Yoshida, and Y. Kamagata. 2005. Sequence-specific cleavage of 16S rRNA for rapid and quantitative detection of particular groups of anaerobes in bioreactors. Water Sci. Technol. 52(1-2):107-113. [PubMed] [Google Scholar]
- 43.Shigematu, T., Y. Tang, Y. Mizuno, H. Kawaguchi, S. Morimura, and K. Kida. 2006. Microbial diversity of mesophilic methanogenic consortium that can degrade long-chain fatty acids in chemostat cultivation. J. Biosci. Bioeng. 102:535-544. [DOI] [PubMed] [Google Scholar]
- 44.Shin, H., S. H. Kim, C. Y. Le, and S. Y. Nam. 2003. Inhibitory effects of long-chain fatty acids on VFA degradation and beta-oxidation. Water Sci. Technol. 47(10):139-146. [PubMed] [Google Scholar]
- 45.Sobieraj, M., and D. R. Boone. 2002. Syntrophomonadaceae. In M. Dworkin (ed.), The prokaryotes. Springer-Verlag, New York, NY. http://link.springer-ny.com/link/service/books/10125/.
- 46.Sousa, D. Z., M. A. Pereira, A. J. M. Stams, M. M. Alves, and H. Smidt. 2007. Microbial communities involved in anaerobic degradation of unsaturated or saturated long-chain fatty acids. Appl. Environ. Microbiol. 73:1054-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sousa, D. Z., H. Smidt, M. M. Alves, and A. J. M. Stams. 2007. Syntrophomonas zehnderi sp. nov., an anaerobe that degrades long chain fatty acids in co-culture with Methanobacterium formicicum. Int. J. Syst. Evol. Microbiol. 57:609-615. [DOI] [PubMed] [Google Scholar]
- 48.Speece, R. E. 1996. Anaerobic biotechnology for industrial wastewaters. Archae Press, Nashville, TN.
- 49.Svetlitshnyi, V., F. Rainey, and J. Wiegel. 1996. Thermosyntropha lipolytica gen. nov., sp. nov., a lipolytic, anaerobic, alkalitolerant, thermophilic bacterium utilizing short- and long-chain fatty acids in syntrophic coculture with a methanogenic archaeum. Int. J. Syst. Bacteriol. 46:1131-1137. [DOI] [PubMed] [Google Scholar]
- 50.Tagawa, T., H. Takahashi, Y. Sekiguchi, A. Ohashi, and H. Harada. 2002. Pilot-plant study on anaerobic treatment of a lipid- and protein-rich food industrial wastewater by a thermophilic multi-staged UASB reactor. Water Sci. Technol. 45(10):225-230. [PubMed] [Google Scholar]
- 51.Tang, Y., T. Shigematsu, S. Morimura, and K. Kida. 2005. Microbial community analysis of mesophilic anaerobic protein degradation process using bovine serum albumin (BSA)-fed continuous cultivation. J. Biosci. Bioeng. 99:150-164. [DOI] [PubMed] [Google Scholar]
- 52.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteley, A. S., M. Manefield, and T. Lueders. 2006. Unlocking the ‘microbial black box’ using RNA-based stable isotope probing technologies. Curr. Opin. Biotechnol. 17:67-71. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, C., X. Liu, and X. Dong. 2004. Syntrophomonas curvata sp. nov., an anaerobe that degrades fatty acids in co-culture with methanogens. Int. J. Syst. Evol. Microbiol. 54:969-973. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, H., D. Yang, C. R. Woese, and M. P. Bryant. 1993. Assignment of fatty acid-beta-oxidizing syntrophic bacteria to Syntrophomonadaceae fam. nov. on the basis of 16S rRNA sequence analyses. Int. J. Syst. Bacteriol. 43:278-286. [DOI] [PubMed] [Google Scholar]