Abstract
The removal of plants and soil to bedrock to eradicate exotic invasive plants within the Hole-in-the-Donut (HID) region, part of the Everglades National Park (Florida), presented a unique opportunity to study the redevelopment of soil and the associated microbial communities in the context of short-term primary succession and ecosystem restoration. The goal of this study was to identify relationships between soil redevelopment and activity and composition of methanogenic assemblages in HID soils. Methane production potentials indicated a general decline in methanogenic activity with restoration age. Microcosm incubations strongly suggested hydrogenotrophic methanogenesis as the most favorable pathway for methane formation in HID soils from all sites. Culture-independent techniques targeting methyl coenzyme M reductase genes (mcrA) were used to assess the dynamics of methanogenic assemblages. Clone libraries were dominated by sequences related to hydrogenotrophic methanogens of the orders Methanobacteriales and Methanococcales and suggested a general decline in the relative abundance of Methanobacteriales mcrA with time since restoration. Terminal restriction fragment length polymorphism analysis indicated methanogenic assemblages remain relatively stable between wet and dry seasons. Interestingly, analysis of soils across the restoration chronosequence indicated a shift in Methanobacteriales populations with restoration age, suggesting genotypic shifts due to site-specific factors.
The Hole-in-the-Donut (HID) region is a 4,000-ha region within Everglades National Park (ENP), in Florida. Once consisting of oligotrophic sawgrass (Cladium jamaicense Crantz) prairies and short hydroperiod pinelands, the HID was subjected to agricultural land use practices for 60 years (10, 20). Preagriculture, HID soils were characterized as shallow, poorly drained, and low in nutrient marls (19). Intensive rock plowing destroyed underlying limestone bedrock and created coarsely textured, well-drained soil suitable for vegetable production (19). When farming ceased in 1976, the HID was left abandoned as a relatively high-nutrient and short-hydroperiod environment. Farmland within the HID was invaded by dense stands of Schinus terebinthifolius Raddi (Brazilian pepper), an exotic shrub native to South America. S. terebinthifolius Raddi was intentionally introduced to Florida as an ornamental in 1898 (1) and is thought to have entered ENP in the 1940s (2, 20). HID restoration efforts initiated by ENP in 1996 are based on the complete removal of all plants and of much of the soil down to bedrock. Plots within the HID are cleared one at a time, so plots representing a chronosequence of times since being cleared can be studied simultaneously. Several years will be required to completely clear the S. terebinthifolius Raddi-affected area (10). Following clearing, individual plots are left undisturbed to allow the natural restoration of microbial communities and colonization by native wetlands plants. This staggered approach to clearing provides an excellent opportunity to study the development of soil, microbial communities, and ecosystem processes over a short-term chronosequence in this wetland.
Soil development is a critical first step preceding plant colonization on bare substrate. Soil formation results from complex interactions between physical, chemical, and biological factors. Subsequently, soil will become the direct link between the biotic and abiotic factors that drive primary succession (33). In a developing system such as the HID, establishment of anaerobic microbial communities occurs in concert with soil profile development and is related to organic matter accumulation and the development of anaerobic food webs. Methanogenesis is responsible for terminal anaerobic carbon mineralization in most freshwater wetlands (27), and the development of methanogenesis and methanogenic guilds is likely to be an important factor in the recovery of highly disturbed wetlands such as the HID. An added complexity of this system is the existence of wet and dry seasons, with sites being submerged during the wet season and not submerged during the dry season.
The overall objective of this study was to investigate the development of methanogenic guilds with time since restoration in the HID by using the gene encoding methyl coenzyme M reductase (mcrA) as an indicator of the structure and composition of methanogens along the restoration gradient. The methanogen-specific mcrA has been used as a molecular marker to study the distribution of methanogens in many terrestrial environments, including nutrient-impacted regions of the Florida Everglades (4, 5).
To our knowledge, this is the first study to monitor the composition and activity of microbial assemblages during the restoration of a highly disturbed freshwater wetland ecosystem. The short-term chronosequence created by complete soil removal allowed us to characterize those communities initially colonizing bare substrate and monitor their development with soil accretion and changes in geochemical processes. Differences between wet and dry season communities were also assessed.
MATERIALS AND METHODS
Site characteristics, sample collection, and biogeochemical characterization.
Samples were collected in April and November 2004. Plots of 20 by 20 m2 were established in sites cleared in 1989, 1997, 2000, and 2003 (R89, R97, R00, and R03, respectively) and in an undisturbed (not farmed) site (UND). The range of elevations for the five plots was 0.5 to 0.6 m. Within each sampling area, 2- by 2-m2 grids were used to establish 81 sampling nodes, which were evaluated with respect to soil depth, ground coverage, and elevation. Nine nodes were chosen based on relative range of soil depth within each site, three from each depth range (shallow, intermediate, and deep). Sampling nodes were marked for future sampling efforts. Soil samples were taken with a plastic coring device; however, due to nonuniform soil cover in recently restored sites, grab samples were collected where necessary. Individual samples from each depth range were combined to make three representative soil samples, which were used for molecular and geochemical analyses. Soil samples were kept on ice and transported to the laboratory within 72 h of collection, where they were manually mixed and large roots were removed from them. Subsamples for DNA analysis were stored at −70°C until analysis. Biogeochemical analyses were conducted at the Wetland Biogeochemistry Laboratory, University of Florida (11, 34, 35). Values for select parameters are presented in Table 1.
TABLE 1.
Geochemical parameters of dry (April 2004) and wet (November 2004) season soils
| Study time and site | Soil depth (cm)a | % Moistureb | Mean amt (SD) in g kg−1 of:
|
LOI (%)f | MBC (mg kg−1)g | ||
|---|---|---|---|---|---|---|---|
| TCc | TNd | TPe | |||||
| April 2004 | |||||||
| UND | 5.9 (1.5-10) | 43.7 (10.4) | 159.4 (10) | 6.6 (0.8) | 0.2 (0.0) | 17.03 | 3,881.2 (829) |
| R89 | 4.5 (3-6.5) | 28.3 (7.4) | 164.5 (16) | 7.7 (1.6) | 0.7 (0.2) | 23.96 | 5,003.8 (1,323) |
| R97 | 5.2 (2-8) | 39.1 (18.2) | 169.4 (8) | 8.2 (0.8) | 1.0 (0.2) | 24.84 | 4,806.3 (1,340) |
| R00 | 2.7 (1-5.5) | 36.8 (12.2) | 161.1 (17) | 6.7 (0.9) | 0.6 (0.1) | 20.88 | 4,185.3 (1,472) |
| R03 | 1.6 (0.5-2.5) | 13.5 (9.1) | 139.9 (9.7) | 4.0 (0.9) | 1.0 (0.1) | 15.90 | 2,161.3 (579) |
| November 2004 | |||||||
| UND | 10.1 (2-15) | 49.6 (4.5) | 192.5 (8.8) | 7.2 (0.9) | 0.1 (0.2) | 16.71 | 1,925.1 (516) |
| R89 | 5.4 (1-17) | 56.7 (8.9) | 340.9 (17.2) | 9.2 (1.5) | 0.8 (0.2) | 14.38 | 3,109.9 (1,009) |
| R97 | 4.6 (3-11) | 53.4 (5.8) | 323.7 (15.5) | 9.0 (1.4) | 1.0 (0.2) | 14.19 | 3,237.5 (1,303) |
| R00 | 3.3 (1-4) | 54.8 (0.8) | 234.3 (11.3) | 7.4 (0.8) | 0.6 (0.1) | 23.90 | 2,343.7 (494) |
| R03 | 1.2 (0-3) | 52.2 (7.0) | 194.1 (7.7) | 5.2 (0.6) | 0.9 (0.2) | 18.86 | 1,752.5 (680.8) |
Values in parentheses represent the range of soil depths measured over 81 sample nodes, as described in Materials and Methods.
Values in parentheses are standard deviations of the mean values for determinations based on three soil samples.
TC, total carbon.
TN, total nitrogen.
TP, total phosphorus.
LOI, loss on ignition.
Values are the means (± the standard deviations) of the results from the indicated soil samples. MBC, microbial biomass carbon.
Methane production potentials.
Soil samples were collected in November 2004 from the UND, R89, R97, R00, and R03 sites, and 2 grams of soil from each site was mixed with 25 ml of anoxic modified basal carbonate yeast extract Trypticase (BCYT) medium (6, 30) under a N2 stream in 50-ml anaerobic culture bottles that were later closed with butyl rubber stoppers and aluminum crimp seals. Modified BCYT contains 0.01 g/liter Trypticase-peptone and induced negligible methanogenesis in a previous study (6). Tubes were preincubated for 10 days prior to the addition of electron donors. Acetate and formate (20 mM each) were added from N2-sparged sterile stock solutions. The bottles were fitted with three-way Luer stopcocks (Cole-Parmer, Vernon Hills, IL) for gas sampling and incubated in the dark at 25°C without shaking. Methane in the headspace was measured by gas chromatography as previously described (6). All determinations were carried out in triplicate with bottles with soil samples from each site (three bottles per site). Headspace pressure was measured using a digital pressure indicator (DPI 705; Druck, New Fairfield, CT).
Nucleic acid extraction and PCR amplification.
Nucleic acids were extracted from 0.25 g of soil with a Power Soil DNA isolation kit (MoBio, Carlsbad, CA) according to the manufacturer's instructions. PCR amplification was conducted using the primer set designed by Luton et al. (23) and consists of primers mcrA-f (5′-GGTGGTGTMGGATTCACACARTAYGCWACAGC-3′) and mcrA-r (5′-TTCATTGCRTAGTTWGGRTAGTT-3′), which amplify a fragment of 465 to 490 bp of mcrA. Each 20 μl of PCR mixture contained 7 μl of distilled water, 1 μl of each primer (10 pmol μl−1), 10 μl of HotStarTaq Master Mix (QIAGEN, Valencia, CA), and 1 μl of diluted DNA solution.
PCR amplification was carried out in a GeneAmp PCR system 9700 (PerkinElmer Applied Biosystems, Norwalk, CT). The initial enzyme activation and DNA denaturation were performed for 15 min at 95°C, followed by five cycles of 30 s at 95°C, 30 s at 55°C, and a 30-s extension at 72°C, and the temperature ramp rate between the annealing and extension segment was set to 0.1°C s−1 because of the degeneracy of the primers (23). After this, the ramp rate was set to 1°C s−1, and 30 cycles were performed with the following conditions: 30 s at 95°C, 30 s at 55°C, and a 30-s extension at 72°C, and a final extension of 72°C for 7 min. PCR conditions for terminal restriction fragment length polymorphism (T-RFLP) analysis were identical, except the annealing temperature was decreased to 53°C. PCR products were analyzed by electrophoresis through 2% agarose gels to confirm amplification of expected size product. To account for the spatial patchiness of soils and attempt to more fully characterize diversity, bulk nucleic acid extracts from all soil samples from within a site were combined prior to PCR.
Cloning and RFLP analysis.
Fresh PCR amplicons were ligated into pCRII-TOPO cloning vector and transformed into chemically competent Escherichia coli TOP10F′ cells according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Positive colonies were screened by PCR amplification with the primer set and PCR conditions described above. PCR product from positive clones was digested with RsaI restriction enzyme. Each 10-μl reaction consisted of 5 U of enzyme, 1× restriction enzyme buffer, 0.6 μg of bovine serum albumin, 5 μl of PCR amplicon, and water to volume. Digests were analyzed by electrophoresis through 4% agarose gels.
Sequencing and phylogenetic analysis.
Representative clones from the most frequently occurring restriction patterns from each library were sequenced at the DNA Sequencing Core Laboratory at the University of Florida using internal vector primers. DNA sequences of mcrA generated from each treatment were translated into putative amino acid sequences and aligned manually in Se-Al version 2.0a11 (http://evolve.zoo.ox.ac.uk/). Sequences were then aligned with Clustal_X version 1.81 (29) to objectify gapping within the alignment. Phylogenetic trees were built with a neighbor-joining analysis using a Jukes and Cantor correction method as implemented in the TREECON software package (31). Bootstrap analysis was performed with 100 resamplings of the amino acid sequences.
T-RFLP analysis.
A total of 35 dry season samples and 26 wet season samples contained enough mcrA amplicons to be used in T-RFLP. No samples from the wet season UND samples and only eight from the wet season R03 samples were amplified. Approximately 100 to 150 ng of PCR product was digested with RsaI. The enzymatic digestion reaction consisted of 5 units of restriction enzyme (Promega, Madison, WI), 1× restriction enzyme buffer, 0.6 μg bovine serum albumin, and deionized water to a final volume of 10 μl. Enzymatic digestions were incubated at 37°C overnight. One and one-half microliters of digested product was used for terminal restriction fragment (T-RF) detection by the DNA Sequencing Core Laboratory at the University of Florida. Briefly, digested products were mixed with 2.5 μl deionized formamide, 0.5 μl ROX-labeled GeneScan 500-bp internal size standard (Applied Biosystems, PerkinElmer Corporation, Norwalk, CT), and 0.5 μl of loading buffer (50 mM EDTA, 50 mg/ml blue dextran). Samples were denatured by heating them at 95°C for 3 min, and they were subsequently transferred to ice until the loading of the gel. One microliter was electrophoresed through a 36-cm, 5% polyacrylamide gel containing 7 M urea at 3 kV on an ABI PRISM 377 genetic analyzer (Applied Biosystems). T-RFLP profiles were analyzed with GeneScan version 2.1 (Applied Biosystems). T-RF size (in base pairs) was calculated using internal standards. Peak sizes in base pairs and peak areas were exported to Excel 97 SR-1 (Microsoft Corporation, Redmond, WA) for data analysis.
Diversity indices.
Clone libraries were analyzed by analytic rarefaction employing RarefactWin (version 1.3; S. Holland, Stratigraphy Lab, University of Georgia, Athens; http://www.uga.edu/∼strata/software/). Cumulative expected phylotypes were calculated for each clone library according to the methods of Castro et al. (5). Rarefaction curves were fit to a hyperbolic model with the formula y = ax/(b + x) using Datafit software version 8.0.32 (Oakdale Engineering, Oakdale, PA), where y represents the number of phylotypes and x is the number of individuals. Coverage values were determined by comparison of obtained versus cumulative expected phylotypes. Shannon-Weaver values were calculated using default parameters of the program DOTUR (28).
Nucleotide sequence accession numbers.
GenBank accession numbers for partial mcrA sequences are DQ662544 to DQ662598.
RESULTS AND DISCUSSION
Methane production in HID soils.
Observed rates of methane production did not correlate with measured geochemical parameters (Table 1) (correlation statistics not presented) or show clear trends associated with time since clearing.
Intrinsic (no added carbon or electron donors) methane production rates were highest in R97 and R03 soils, methane production potentials in UND soils were the lowest of all study sites, and data suggest a general decline in methanogenic activity in older sites (Table 2). UND soil produced the least methane, with rates approximately 30 times lower than intrinsic rates reported for oligotrophic soils of the Everglades Water Conservation Area 2A (5). Additions of acetate to microcosms led to insignificant increases in methane production after 10 days. R03 showed the greatest rate of methane production, although the average rate was only 1.2-fold higher than that observed in unamended soils. UND soils were unaffected by acetate addition, and rates suggest a general decline in acetoclastic methanogenesis with restoration age. In general, less than 2% of the acetate added to all samples was converted to methane over the 10-day incubation period, suggesting an alternative pathway for consumption of acetate under methanogenic conditions in these soils. Syntrophic acetate oxidation is a major pathway for acetate consumption in soils of the northern Everglades (6, 7) and may be important in HID soils.
TABLE 2.
Potential methanogenesis rates and accumulated CH4 in wet season soils
| Site | No addition
|
Formate
|
Acetate
|
|||
|---|---|---|---|---|---|---|
| Ratea | Totalb | Ratea | Totalb | Ratea | Totalb | |
| UND | 0.1 (0.0) | 41 (16) | 0.4 (0.1) | 198 (37) | 0.1 (0.1) | 52 (21) |
| R89 | 1.1 (0.3) | 513 (112) | 26.6 (1.6) | 8,843 (3951) | 1.5 (0.1) | 700 (34) |
| R97 | 11.4 (4.7) | 4,193 (283) | 36.0 (11.1) | 17,274 (3066) | 7.3 (1.0) | 3,782 (141) |
| R00 | 3.4 (1.3) | 1,619 (425) | 22.6 (13.9) | 10,844 (3843) | 6.6 (12.0) | 3,178 (701) |
| R03 | 7.4 (2.2) | 1,052 (744) | 50.4 (14.7) | 24,183 (4077) | 8.8 (3.3) | 2,998 (926) |
Potential methanogenesis rates (in nanomoles per gram soil per hour). Standard errors of the means are shown in parentheses for determinations with three replicate soil samples.
Average total methane accumulated in headspace of three replicate samples per site after 10 days of incubation, expressed as nanomoles of methane accumulated.
Hydrogen has been shown to be an important electron donor for methanogenesis in other regions of the Everglades (5, 8). Formate is commonly used as an analogue for H2-CO2 in anaerobic mineralization studies (12) and was added to HID soil microcosms to estimate the activity and population sizes of hydrogenotrophic methanogens. Methane production potentials in formate-amended soils were 4 to 17 times higher than in unamended soils and 4 to 20 times higher than in acetate-amended soils. Approximately 18 to 50% of added formate was converted to methane over the 10-day incubation period, and production rates and total substrate conversion percentage values were strongly correlated, indicating the dominance of hydrogenotrophic methanogenesis in HID soils. Further, these data may underestimate actual hydrogenotrophic production potentials, as only 60% of hydrogenotrophic methanogens are able to utilize formate for methane production (16). Correspondingly, most probable numbers of hydrogenotrophic methanogens were recently reported to be 100 to 1,000 times higher than those of acetoclastic methanogens in the northern Everglades (8). Relatively low numbers of acetoclastic methanogens may be due to consumption of acetate by syntrophic acetate oxidation (7), which is more energetically favorable than acetoclastic methanogenesis in some subtropical climates (25).
Phylogenetic characterization of methanogenic assemblages in HID soils.
PCR amplification of mcrA from soils sampled in undisturbed and cleared sites during the dry season (April 2004) yielded the expected ca. 465- to 490-bp mcrA fragments. Clone libraries constructed from dry season soils indicated considerable diversity of methanogens in HID soils. The number of obtained and expected phylotypes was highest in UND soils and lowest in R00 soils (Table 3). Coverage of expected mcrA diversity within each clone library was ascertained by comparison of observed versus expected phylotypes for each library. Values ranged from 45 to 76% and were highest in the R97 and R00 libraries and lowest in the UND library (Table 3). Both measures of sampling coverage indicate that our clone libraries do not fully represent mcrA diversity in HID soils.
TABLE 3.
Expected and observed phylotypes and diversity indices for dry season mcrA clone libraries for HID soils
| Site | No. of expected phylotypesa | No. of observed phylotypesb | Coverage (%)c | Shannon's H′ |
|---|---|---|---|---|
| UND | 35.35 (1.60) | 16 (31) | 45 | 2.09 |
| R89 | 21.41 (0.13) | 14 (39) | 67 | 2.01 |
| R97 | 13.62 (0.53) | 10 (39) | 76 | 1.68 |
| R00 | 13.29 (0.43) | 10 (39) | 76 | 2.10 |
| R03 | 19.20 (1.32) | 12 (37) | 63 | 1.76 |
Value of constant a from the equation y = ax/(b + x) (standard error).
Value in parentheses is the total number of clones screened.
Expressed as the percentage of expected phylotypes obtained within each library.
mcrA sequences obtained from dry season soils formed seven clades distributed among the orders Methanobacteriales, Methanococcales, Methanomicrobiales, Methanosarcinales, and two clades sharing similarity with genes from uncultured organisms (Fig. 1). MCR-1 sequences share ca. 90% DNA sequence similarity to Methanosarcina; these sequences were obtained from UND, R89, and R00 soils, but not in significant quantities. Related sequences were reported from nutrient-impacted regions of the Florida Everglades (5). MCR-2 sequences were most abundant in UND soils but comprised a small percentage of R03 and R00 sequences; they shared highest similarity with sequences from uncultivated methanogens found in rice paddy (21) and Everglades soils (5), sharing between 87 and 94% similarity with putative hydrogenotrophs in rice cluster I (21). Clones in MCR-3, present in R89, R00, and R03 libraries, were most similar to uncultured Methanosaeta spp. obtained from permanently flooded riparian soils (18). Methanosaeta spp. may be considered specialists able to generate methane only from catabolism of acetate (3). Cluster MCR-4 branched deeply within cultured Methanomicrobiales and contained sequences obtained from UND, R89, and R03 soils. MCR-4-like sequences have also been obtained from eutrophic Everglades soils (5) and a peat bog (17); our clones share ca. 85% similarity with sequences belonging to fen cluster methanogens, a potentially novel group of uncertain function (14, 15). Cluster MCR-5 sequences branch deeply within the Methanococcales and were obtained from all study sites; an increase in MCR-5 abundance was observed in more-established sites (Fig. 2). These sequences are closest to those from uncultivated organisms obtained from rice roots (9). Previous characterization of methanogenic assemblages in the Florida Everglades did not recover sequences clustering with Methanococcales (4, 5). Cluster MCR-6 sequences, present in UND and R97, clustered outside of cultured Methanococcales and shared greatest similarity with clones from other regions of the Everglades (5). Clones associated with MCR-7 were found in all sites and formed a distinct clade within Methanobacteriales. Sequence distributions suggest a general decrease in MCR-7 relative abundance as restoration progresses (Fig. 2). Methanobacteriales mcrA clones comprised a significant portion of clone libraries constructed from other regions of the Everglades (5).
FIG. 1.
Neighbor-joining mcrA tree for representative clones from April 2004 soils. Scale bar represents 10% sequence divergence. Numbers at nodes represent percentage of bootstrap resamplings based on 100 replicates. Values greater than 85 are shown, while black dots (•) on nodes represent values between 50 and 85.
FIG. 2.
Distribution of mcrA sequences obtained from dry season soils within designated phylogenetic clusters.
T-RFLP analysis of methanogenic assemblage structure.
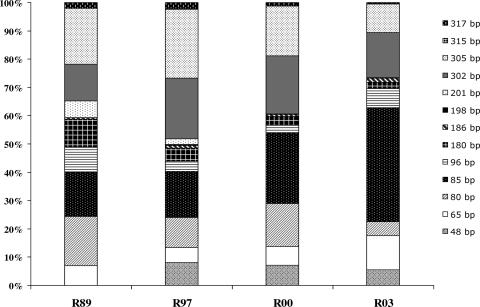
Compositions of methanogenic assemblages at each of the nine nodes selected per site were evaluated with T-RFLP for both the wet and dry seasons. The likely phylogenetic affiliations, determined by in silico digestion of clones, for individual T-RFs are presented in Table 4. In silico analyses of mcrA clones indicated that most observed T-RFs are likely associated with known phylogenetic groups of methanogens, although a few are associated with unknown groups. Averages of T-RF relative frequencies for UND, R89, R97, R00, and R03 dry and wet season soil samples are presented in Fig. 3 and 4, respectively. PCR amplification of mcrA in UND samples was generally poor, such that only dry season T-RFLPs were included in the analysis. Dominant T-RFs for each site were obtained consistently from replicate soil samples.
TABLE 4.
Phylogenetic affiliation of mcrA T-RFs for HID soil samples
| Observed T-RF (bp) | Theoretical T-RF (bp) | Cluster | Order |
|---|---|---|---|
| 48 | 48 | MCR-1 | Methanosarcinales |
| MCR-7 | Methanobacteriales | ||
| 65 | 65 | MCR-5 | Methanococcales |
| 80 | 80 | MCR-1 | Methanosarcinales |
| MCR-5 | Methanococcales | ||
| 85 | 84 | MCR-7 | Methanobacteriales |
| 96 | 95 | MCR-2 | Methanosarcinales |
| MCR-4 | Methanomicrobiales | ||
| MCR-6 | Uncultured | ||
| Not obtained | 175 | MCR-6 | Uncultured |
| Not obtained | 177 | MCR-3 | Methanosarcinales |
| MCR-4 | Methanomicrobiales | ||
| 180 | 179 | MCR-3 | Methanosarcinales |
| MCR-4 | Methanomicrobiales | ||
| 186 | 188 | MCR-7 | Methanobacteriales |
| 198 | —a | ||
| 201 | — | ||
| 302 | 302 | MCR-5 | Methanococcales |
| 305 | 305 | MCR-7 | Methanobacteriales |
| 315 | — | ||
| 317 | — | ||
| Not obtained | 438 | MCR-5 | Methanococcales |
| 458-487 | No cut | Not obtained |
—, unknown phylogenetic affiliation.
FIG. 3.
Community dynamics for mcrA in dry season HID soils determined by T-RFLP analysis. y-axis values represent percentages of total fluorescence.
FIG. 4.
Community dynamics for the mcrA gene in wet season HID soils determined by T-RFLP analysis. y-axis values represent percentages of total fluorescence.
Seasonal structure of methanogenic assemblages.
Thirteen T-RFs were obtained from both dry (Fig. 3) and wet (Fig. 4) season samples. T-RFs representative of MCR-7, clustering with the hydrogenotrophic Methanobacteriales, and MCR-5, clustering with the hydrogenotrophic Methanococcales, dominated all samples. Methanobacteriales T-RFs comprised between 35 and 55% of total fluorescence within each site for both wet and dry seasons, and no significant seasonal changes in the relative abundance of MCR-7 T-RFs were observed within sites.
T-RFLP analysis did not identify shifts in composition of methanogenic assemblages between seasons. Significant shifts in soil moisture between seasons may lead to the development of hot spots of methanogenesis in dry soils. Methanogenesis has been detected in extremely dry soils (26), and rewetting events have been correlated to observed shifts in dominant organisms (24). Microorganisms inhabiting seasonally water-stressed soils may be more resistant to moisture fluctuations (13), however, and slow-growing organisms, such as methanogens, may be less affected by dry-wet cycles (32).
Shifts in methanogenic assemblages with restoration age.
The relative abundances of MCR-5 T-RFs (65 and 302 bp) differ slightly within sites; however, significant variation between sites was not evident. Their combined abundance suggests that Methanococcales populations remain stable in soils from all sites.
The dynamics within MCR-7 (T-RFs of 85 and 305 bp) proved interesting. The relative abundance of the T-RF of 85 bp decreased with successional stage and showed an approximately linear decrease with restoration age. The decreased abundance of the T-RF of 85 bp in R89 and R97 soils corresponded with the increased abundance of the MCR-7 T-RF of 305 bp. Shifts in MCR-7 T-RFs are evident in both seasons but more pronounced in dry season profiles. Assuming these T-RFs are exclusively associated with Methanobacteriales mcrA, these data suggest a shift within Methanobacteriales populations with restoration age. T-RFLP analysis of mcrA genes obtained from riparian soils also reported shifts in the abundance of Methanobacteriales; T-RFs differing by approximately 100 bp were obtained in significantly different quantities in soils subjected to differing periods of inundation (18).
At best, T-RFLP may be employed as a semiquantitative measure of community structure. Interpretation of shifts in T-RF abundance may not indicate significant changes in assemblage composition. Furthermore, different efficiencies of labeled and unlabeled primers required the use of different annealing temperatures during PCR for cloning and T-RFLP analysis, possibly leading to discrepancies between T-RFLP profiles and clone libraries. Such discrepancies have been described previously for mcrA PCR-cloning and T-RFLP analyses (4, 22).
Putative hydrogenotrophic mcrA genes were most frequently observed in clone libraries and T-RFLP profiles. This is consistent with the highest methane production resulting from formate addition in all sites. However, the exact proportion of Methanobacteriales and Methanococcales in HID restoration sites is not reflected in T-RFLP results, as the degenerate primers employed in this study do not provide quantitative recovery of all phylogenetic lineages (22). Further, it has been suggested that the primer set employed for this study is biased toward hydrogenotrophic orders of methanogens and underrepresents Methanosaeta spp. and Methanosarcina spp (5, 23).
In summary, our results indicate that a diverse assemblage of methanogenic archaea colonize recently restored sites. Seasonal T-RFLP profiles indicate that methanogenic assemblage structures remained constant in composition across the two seasons. This is consistent with previous studies reporting temporal stability of prokaryotic communities (4, 13, 21). Shifts within certain methanogenic groups in association with restoration age were evident. Both molecular and functional assessments suggest hydrogenotrophic methanogens are responsible for most of the methane production observed along the chronosequence.
Acknowledgments
This project was funded by a grant from the U.S. Department of the Interior.
We thank Mark Clark for assistance with sampling design and Kanika Sharma, Mark Clark, and Kevin Grace for assistance with sampling. The late Michael Norland is acknowledged for access to the site and facilitation of sample collection.
Footnotes
Published ahead of print on 20 April 2007.
REFERENCES
- 1.Austin, D. F. 1978. Exotic plants and their effects in southeastern Florida. Environ. Conserv. 5:25-34. [Google Scholar]
- 2.Bancroft, L. 1973. Exotic control plan. Everglades National Park, Homestead, FL.
- 3.Boone, D. R., W. B. Whitman, and P. Rouviére. 1993. Diversity and taxonomy of methanogens, p. 35-80. In J. G. Ferry (ed.), Methanogens: ecology, physiology, biochemistry and genetics, vol. 1. Chapman and Hall, New York, NY. [Google Scholar]
- 4.Castro, H., S. Newman, K. R. Reddy, and A. Ogram. 2005. Distribution and stability of sulfate-reducing prokaryotic and hydrogenotrophic methanogenic assemblages in nutrient-impacted regions of the Florida Everglades. Appl. Environ. Microbiol. 71:2695-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro, H. F., K. R. Reddy, and A. V. Ogram. 2004. Phylogenetic characterization of methanogenic assemblages in eutrophic and oligotrophic areas of the Florida Everglades. Appl. Environ. Microbiol. 70:6559-6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan, A., and A. Ogram. 2006. Fatty acid-oxidizing consortia along a nutrient gradient in the Florida Everglades. Appl. Environ. Microbiol. 72:2400-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan, A., and A. Ogram. 2006. Phylogeny of acetate-utilizing microorganisms in soils along a nutrient gradient in the Florida Everglades. Appl. Environ. Microbiol. 72:6837-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan, A., A. Ogram, and K. R. Reddy. 2004. Syntrophic-methanogenic associations along a nutrient gradient in the Florida Everglades. Appl. Environ. Microbiol. 70:3475-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin, K.-J., T. Lueders, M. W. Friedrich, M. Klose, and R. Conrad. 2004. Archaeal community structure and pathway of methane formation on rice roots. Microb. Ecol. 47:59-67. [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple, G. H., R. F. Doren, N. K. O'Hare, M. R. Norland, and T. V. Armentano. 2003. Plant colonization after complete and partial removal of disturbed soils for wetland restoration of former agricultural fields in Everglades National Park. Wetlands 22:1015-1029. [Google Scholar]
- 11.D'Angelo, E. M., and K. R. Reddy. 1999. Regulators of heterotrophic microbial potentials in wetland soils. Soil Biol. Biochem. 31:815-830. [Google Scholar]
- 12.Dolfing, J., and W. G. B. M. Bloemen. 1985. Activity measurements as a tool to characterize the microbial composition of methanogenic environments. J. Microbiol. Methods 4:1-12. [Google Scholar]
- 13.Fierer, N., J. P. Schimel, and P. A. Holden. 2003. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 45:63-71. [DOI] [PubMed] [Google Scholar]
- 14.Galand, P. E., H. Saarnio, H. Fritze, and K. Yrjälä. 2002. Depth related diversity of methanogen archaea in Finnish oligotrophic fen. FEMS Microbiol. Ecol. 42:441-449. [DOI] [PubMed] [Google Scholar]
- 15.Galand, P. E., H. Fritze, R. Conrad, and K. Yrjälä. 2005. Pathways for methanogenesis and diversity of methanogenic archaea in three boreal peatland ecosystems. Appl. Environ. Microbiol. 71:2195-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, J. L., B. K. C. Patel, and B. Ollivier. 2000. Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe 6:205-226. [DOI] [PubMed] [Google Scholar]
- 17.Juottonen, H., P. E. Galand, E.-S. Tuittila, J. Laine, H. Fritze, and K. Yrjälä. 2005. Methanogen communities and bacteria along an ecohydrological gradient in a northern raised bog complex. Environ. Microbiol. 7:1547-1557. [DOI] [PubMed] [Google Scholar]
- 18.Kemnitz, D., K.-J. Chin, P. Bodelier, and R. Conrad. 2004. Community analysis of methanogenic archaea within a riparian flooding gradient. Environ. Microbiol. 6:449-462. [DOI] [PubMed] [Google Scholar]
- 19.Li, Y., and M. Norland. 2001. The role of soil fertility in invasion of Brazilian Pepper (Schinus terebinthifolius) in Everglades National Park, Florida. Soil Sci. 166:400-405. [Google Scholar]
- 20.Loope, L. L., and V. L. Dunevitz. 1981. Impact of fire exclusion and invasion of Schinus terebinthifolius on limestone rockland pine forests of southeastern Florida. National Park Service, Homestead, FL.
- 21.Lueders, T., K.-J. Chin, R. Conrad, and M. Friedrich. 2001. Molecular analyses of methyl-coenzyme M reductase α-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ. Microbiol. 3:194-204. [DOI] [PubMed] [Google Scholar]
- 22.Lueders, T., and M. Friedrich. 2003. Evaluation of PCR amplification bias by terminal restriction fragment length polymorphism analysis of small-subunit rRNA and mcrA genes by using defined template mixtures of methanogenic pure cultures and soil DNA extracts. Appl. Environ. Microbiol. 69:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luton, P. E., J. M. Wayne, R. J. Sharp, and P. W. Riley. 2002. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 148:3521-3530. [DOI] [PubMed] [Google Scholar]
- 24.Nannipieri, P., J. Ascher, M. T. Ceccherini, L. Landi, G. Pietramellara, and G. Renella. 2003. Microbial diversity and soil functions. Eur. J. Soil Sci. 54:655-670. [Google Scholar]
- 25.Nusslein, B., K-J. Chin, W. Eckert, and R. Conrad. 2001. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ. Microbiol. 3:460-470. [DOI] [PubMed] [Google Scholar]
- 26.Peters, V., and R. Conrad. 1995. Methanogenic and other strictly anaerobic bacteria in desert soil and other oxic soils. Appl. Environ. Microbiol. 61:1673-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schimel, J. P., and J. Gulledge. 1998. Microbial community structure and global trace gases. Global Change Biol. 4:745-758. [Google Scholar]
- 28.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Touzel, J. P., and G. Albagnac. 1983. Isolation and characterization of Methanococcus mazei strain MC3. FEMS Microbiol. Lett. 16:241-245. [Google Scholar]
- 31.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 32.Van Gestel, M., R. Merckx, and K. Vlassak. 1993. Microbial biomass responses to soil drying and rewetting: the fate of fast- and slow-growing microorganisms in soils from different climates. Soil Biol. Biochem. 25:109-123. [Google Scholar]
- 33.Walker, L. R., and R. del Moral. 2003. Primary succession and ecosystem rehabilitation. Cambridge University Press, Cambridge, United Kingdom.
- 34.White, J. R., and K. R. Reddy. 1999. Influence of nitrate and phosphorous loading on denitrifying enzyme activity in Everglades wetland soils. Soil Sci. Soc. Am. J. 63:1945-1954. [Google Scholar]
- 35.Wright, A. L., and K. R. Reddy. 2001. Heterotrophic microbial activity in northern Everglades wetland soils. Soil Sci. Soc. Am. J. 65:1856-1864. [Google Scholar]