Abstract
The genome sequences of three Helicobacter pylori strains revealed an abundant number of putative restriction and modification (R-M) systems within a small genome (1.60 to 1.67 Mb). Each R-M system includes an endonuclease that cleaves a specific DNA sequence and a DNA methyltransferase that methylates either adenosine or cytosine within the same DNA sequence. These are believed to be a defense mechanism, protecting bacteria from foreign DNA. They have been classified as selfish genetic elements; in some instances it has been shown that they are not easily lost from their host cell. Possibly because of this phenomenon, the H. pylori genome is very rich in R-M systems, with considerable variation in potential recognition sequences. For this reason the protective aspect of the methyltransferase gene has been proposed as a tool for typing H. pylori isolates. We studied the expression of H. pylori methyltransferases by digesting the genomic DNAs of 50 strains with 31 restriction endonucleases. We conclude that methyltransferase diversity is sufficiently high to enable the use of the genomic methylation status as a typing tool. The stability of methyltransferase expression was assessed by comparing the methylation status of genomic DNAs from strains that were isolated either from the same patient at different times or from different stomach locations (antrum and corpus). We found a group of five methyltransferases common to all tested strains. These five may be characteristic of the genetic pool analyzed, and their biological role may be important in the host/bacterium interaction.
Microorganisms with spiral morphology in human and animal stomach have been known for over a century, but for many years, the stomach environment was considered too harsh for bacteria, and those observations were neglected (11, 19). After the isolation and cultivation of Helicobacter pylori from a human gastric biopsy specimen, achieved by Barry Marshall and Robin Warren in 1982, the scientific community finally accepted the idea that there are bacteria living in the human stomach (24). Soon after this important discovery, similar microorganisms were isolated from the stomachs of various wild and domestic mammals, such as cheetahs, dogs, pigs, and cats. Marshall and Warren discovered that several human gastric pathologies are induced by H. pylori (gastritis, or ulcers of the duodenum or of the stomach) and that the bacterium is associated with inflammation of the stomach mucous membrane. Later, several epidemiological studies found a strong correlation between gastric cancer and H. pylori. Since 1994, the World Health Organization has also classified H. pylori as a group 1 carcinogen (15, 41).
More than half of the human population is infected with H. pylori. In North America, as well as in northern and western Europe, 5 to 15% of children and 10 to 60% of adults are infected with H. pylori. The prevalence is much higher elsewhere (3). Although only a small percentage develop disease, this is a very large number of patients.
For these reasons, H. pylori research has been of high priority in human medical research over the last 2 decades, with more than 23,500 papers listed in the PubMed database. Despite the intensive work on this microorganism, there are many unanswered questions about its basic biology. There are also some controversies: these include the mode of transmission of H. pylori, whether the human stomach is the only reservoir, and whether to classify it as a human pathogen or a commensal (7, 8).
In 1997 the first H. pylori genome was sequenced. The sequenced strain, NTCC 26695, was isolated from a patient with superficial gastritis. Remarkably, the genome had an extraordinarily large number of putative restriction-and-modification (R-M) systems: 26 putative methyltransferases (MTases) were identified (22, 38, 44). Two years later, a second strain, J99, obtained from a patient with duodenal ulcer, was sequenced. It was possible for the first time to compare two microbial genomes (1). Again, a large number of R-M systems were found. The 26 putative R-M systems of strains J99 are very similar to the 26 R-M systems of strain NTCC 26695: their genomic positions are very close, and a large number of these systems have more than 85% sequence similarity. H. pylori strains were believed to exhibit a large degree of genomic and allelic diversity, but it was found that the overall genomic organization, gene order, and predicted proteomes of the two strains were quite similar (1, 12). Recently, a third H. pylori strain, HPAG1, isolated from a patient with chronic atrophic gastritis, was sequenced, and 30 putative R-M systems were identified (31, 38). Definitively, the presence of large numbers of R-M systems is characteristic of H. pylori.
An R-M system is defined by the association of at least two genes: one codes for a restriction endonuclease (REase) that recognizes a specific DNA sequence and cuts both strands; the other gene codes for a cognate MTase that methylates the same DNA sequence, thus protecting it from being cleaved by the companion REase (39). According to the selfish gene theory, once a type II R-M system is acquired by a bacterial genome, the deletion or inactivation of the type II MTase implies cell death (17, 18, 20, 28). This is because the cell loses the ability to protectively methylate all recognition sites in the newly synthesized chromosome, while unmethylated sites are cut by the residual REase still present in the bacterial cytoplasm. Due to the pressure of the REase on the expression of the cognate MTase, type II R-M systems tend to be maintained after acquisition. This last observation and the diversity of R-M systems in H. pylori led to the suggestion that the MTase from R-M systems could be used to type H. pylori strains (48). Typing methods are useful for understanding the natural history, epidemiology, mode of transmission, reservoir, and clinical implications of bacterial infection. However the typing methods used so far for H. pylori have not been very successful because of its genomic and allelic diversity. Indeed, no single molecular method has yet emerged as an internationally accepted basis for typing. A typing method for H. pylori will be useful for understanding many of the unclear and controversial items listed above, for appreciating population genetic structure, and for understanding the evolution of the microorganism (7, 8, 33).
In this study, we evaluated the diversity of MTase expression among 50 H. pylori strains isolated from Portuguese patients, and we assessed the use of genomic methylation status for typing H. pylori isolates. MTase expression is variable enough to be useful in tracing epidemics but stable enough to enable identification of a suitable set of restriction enzymes for the test.
MATERIALS AND METHODS
H. pylori strains.
The present study was performed on a collection of H. pylori strains containing 1,021 isolates from Portuguese patients obtained between 1990 and 2001 and maintained by the Portuguese National Institute of Health (INSA) in Lisbon.
We used a first subset of 21 strains, X1, randomly chosen (P1 to P21; see Table S1 in the supplemental material). The second subset of strains, X2, had 28 strains, isolated from 11 patients after biopsies performed with a gap of months to years (usually before and after antibiotic therapy) (P22.x to P32.x; see Table S2 in the supplemental material) or from biopsies performed on the same day from stomach antrum and corpus (patients A, B, and K). Some of these strains had already been typed by randomly amplified polymorphic DNA (RAPD), PCR-restriction fragment length polymorphism (RFLP) (ureA-ureB), and pulsed-field gel electrophoresis (32). This set allowed us to compare the utility of our method with that of existing methods. One strain, P33, was isolated from an asymptomatic patient (wife of patient E). A final reference strain, CCUG 15818, was isolated from a 65-year-old Australian male with a duodenal ulcer in 1983.
In total, 51 strains were used in this study, but only 32 were considered independently: all strains from Table S1 and the first strain isolated from each of the 11 patients with repeated observations (P22.1, …, P32.1; see Table S2 in the supplemental material).
The Kolmogorov-Smirnov (two-sided) test and the Welch-Satterthwaite test were used to confirm that both samples (X1 and X2) belonged to the same population, so that they could be analyzed together.
H. pylori culture.
The biomass from H. pylori strains was obtained from strains cultured on H. pylori selective agar (Wilkins-Chalgren agar supplemented with 10% horse blood, vancomycin [10 mg liter−1], cefsulodin [5 mg liter−1], trimethoprim [5 mg liter−1], and cycloheximide [100 mg liter−1] [Biogerm, Porto, Portugal]) and incubated at 37°C for 48 h in an anaerobic jar (Oxoid or BBL) with a gas generator system (CampyGen; Oxoid) (26, 27).
Genomic DNA extraction.
The genomic DNA was extracted using the Pitcher method with minor modifications. The modifications included a final wash with 70% (vol/vol) ethanol and treatment with RNase A (Sigma) to a final concentration of 0.5 mg ml−1 for 1 h at 37°C, after resuspension of the genomic DNA in 100 μl sterile double-distilled water (35).
REase choice.
We chose 31 REases (New England Biolabs, Inc.) to evaluate the methylation status of the 51 strains (see Table S3 in the supplemental material). We verified the presence of the corresponding recognition sequences in the genomes of H. pylori strains NTCC 26695, J99, and HPAG1 (Table 1). This ensures that a lack of DNA digestion would strongly suggest that the corresponding recognition site is methylated (34).
TABLE 1.
No. of recognition sites per sequenced genomes and comparison of resistance to REase cleavage in different studies
| REase | Recognized sequencea | No. of recognition sites per sequenced genomeb
|
% of strains resistant to cleavage (recognition site methylated)
|
|||||
|---|---|---|---|---|---|---|---|---|
| 26695 | J99 | HPAG1 | Present study (n = 51) | A (n = 122)c | B (n = 19)d | C (n = 16)e | ||
| BseRI | GAGGAG | 149 | 141 | 128 | 100 | |||
| EagI | CGGCCG | 57 | 61 | 53 | 100 | |||
| HhaI | GCGC | 5,924 | 6,035 | 5,638 | 100 | 100 | ||
| Hinp1I | GCGC | 5,924 | 6,035 | 5,638 | 100 | |||
| NaeI | GCCGGC | 150 | 167 | 139 | 100 | |||
| NgoMIV | GCCGGC | 150 | 167 | 139 | 100 | |||
| NlaIII | CATG | 7,172 | 7,335 | 6,687 | 100 | 100 | 100 | 100 |
| DpnI | GmATC | 5,348 | 5,396 | 4,839 | 98 | |||
| DpnII | GATC | 5,348 | 5,396 | 4,839 | 98 | |||
| BssHII | GCGCGC | 282 | 303 | 266 | 96 | |||
| AseI | ATTAAT | 484 | 424 | 426 | 90 | |||
| TacI | TCGA | 303 | 336 | 275 | 88 | 86 | 63 | |
| HpaII | CCGG | 1,731 | 1,808 | 1,654 | 82 | 67 | ||
| HpyCH4V | TGCA | 5,662 | 5,110 | 5,206 | 82 | 71 | ||
| MspI | CCGG | 1,731 | 1,808 | 1,654 | 82 | |||
| HpyCH4IV | ACGT | 282 | 500 | 278 | 65 | 74 | ||
| HpyCH4III | ACNGT | 557 | 587 | 503 | 55 | 51 | ||
| DdeI | CTNAG | 3,024 | 2,998 | 2,665 | 37 | 45 | 32 | 38 |
| Hpy188I | TCNGA | 1,396 | 1,244 | 1,209 | 37 | 21 | 84 | 13 |
| FauI | CCCGC | 1,657 | 1,637 | 1,535 | 24 | |||
| HaeIII | GGCC | 1,607 | 1,598 | 1,540 | 24 | 38 | 21 | 13 |
| BstUI | CGCG | 2,478 | 2,664 | 2,358 | 14 | |||
| Hpy99I | CGWCG | 247 | 267 | 244 | 14 | 62 | ||
| Sau96I | GGNCC | 837 | 865 | 803 | 14 | |||
| AciI | CCGC | 7,038 | 7,033 | 6,610 | 8 | |||
| BssKI | CCNGG | 1,385 | 1,435 | 1,289 | 8 | |||
| ScrFI | CCNGG | 1,385 | 1,435 | 1,289 | 8 | |||
| DraI | TTTAAA | 4,905 | 4,692 | 4,338 | 6 | |||
| Fnu4HI | GCNGC | 2,487 | 2,313 | 2,267 | 2 | |||
| FokI | GGATG | 1,353 | 1,328 | 1,235 | 0 | 2 | 16 | 6 |
| Sau3AI | GATC | 5,348 | 5,396 | 4,839 | 0 | |||
Genomic DNA methylation.
The genomic DNA was digested with REases, according to the manufacturer's instructions, in a volume of 50 μl. An excess of REase units was always used to ensure complete cleavage of the DNA. The DNA digestion status was observed after electrophoresis in 0.7% agarose gel (agarose LE; SeaKem, FMC) for 30 min and recorded (Polaroid 665 film or Kodak EDAS290). An undigested control of the genomic DNA was always present. The results were recorded on a binary matrix, where “0” indicates digestion (DNA is unmethylated) and “1” indicates no digestion (suggesting an active MTase).
Quality control.
We set up two types of data quality control. First, we used four pairs of isoschizomers that differ with regard to which modified base prevents cleavage. One of each pair serves as an internal control for the presence of sites in the sequence and the ability of the DNA to be digested at all. These pairs were MspI and HpaII, BssKI and ScrFI, HhaI and HinP1I, and NaeI and NgoMIV. Second, we did an REase replication assay, reisolating genomic DNA from seven randomly selected strains and repeating some of the DNA digestions (3.4% of the assays were repeated). The activity of each REase was verified by digesting a standard DNA from a sequenced phage or plasmid at the start and finish of each experimental series.
Data analysis.
Kolmogorov-Smirnov and Welch-Satterthwaite statistical tests were used to determine whether samples X1 and X2 could have been drawn from the same population. The nonparametric Kolmogorov-Smirnov test determines whether the datasets, X1 and X2, differ significantly, and the Welch-Satterthwaite test is used to compare means without any a priori consideration of variances. The software NTSYS v.2.0 (Exeter Software) was used to construct a dendrogram from the binary matrix, using the unweighted pair group method with arithmetic average (UPGMA method) and the Jaccard coefficient. The Jaccard coefficient reflects the percentage of methylases common to two strains. To evaluate the data, the following parameters were determined: (i) the cophenetic correlation coefficient, which represents the goodness of fit of the dendrogram to the initial data; (ii) the discriminatory method capacity, which is the strain frequency per cluster and should be less than 5% (nj/N < 0.05) (13); and (iii) Simpson's index of diversity, which reflects the capacity of the method to distinguish unrelated strains (25, 36).
RESULTS
Quality control.
Validation of the data by control experiments yielded high confidence that the results reflect the state of modification of the genome of each strain. The four pairs of isoschizomers that are differentially sensitive to modification revealed the same methylation status in all tested strains. The replication assay (repeating DNA isolation and digestion) of 3.4% of total assays (54/1,581) showed 100% agreement with the first result obtained. For comparison, we calculated the reproducibility of methylation expression in two published studies. Ando et al. (2) included 10 common strains and 10 common REases previously used by Xu et al. (48). The reproducibility between these two studies was 97%, which clearly shows the stability of MTase expression in H. pylori during laboratory manipulation.
All strains in Table S1 (see the supplemental material) are obviously independent, as they were randomly selected. Could we consider any of the strains sequentially isolated from each patient independent (Table S2)? To answer this question we used two statistical tests, Kolmogorov-Smirnov (two-sided) and Welch-Satterthwaite (6). These tests results allowed us to consider 32 strains independent, all of which were from Table S1 (sample X1), and one of the multiple strains isolated from each of the 11 patients with repeated observations (sample X2p, that is, a partial set of sample X2 randomly chosen). The nonparametric Kolmogorov-Smirnov test showed that the two data sets, X1 and X2p, do not differ significantly (α = 0.05, 0.16 < Dα = 0.51); i.e., both samples belong to the same population. To compare means without any a priori consideration of variances (X1 MTase mean = 14.62, standard deviation [SD] = 2.03; X2p MTase mean = 14.45, SD = 1.81), we used the Welch-Satterthwaite test. The result supported the same conclusion (α = 0.05, 0.23 < t0.97523= 2.07), i.e., there is an equality of mean values.
Simultaneous digestion by DpnI and DpnII.
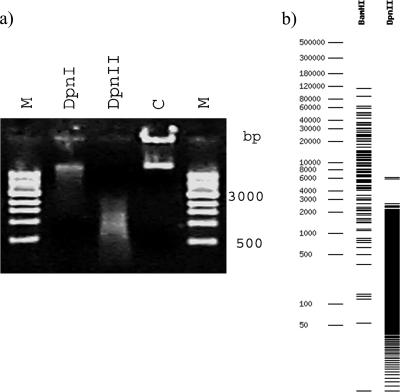
A peculiar result was obtained with the digestion of genomic DNA of strain P12 with DpnI and DpnII: DpnI cuts the sequence GATC only when the adenine residue is methylated (21), while DpnII does not cut GATC when that adenine residue is methylated (46). Theoretically, these enzymes should always give opposite results. In strain P12, both REases cut the DNA (Fig. 1a). This reaction was carried out several times, but always with the same outcome. A possible explanation for this result is that this strain has an adenine MTase that methylates a sequence that overlaps GATC, like M.SolI (GGmATCC), and the M.MboI isoschizomer is absent (GmATC) (14). We did not test this hypothesis because SolI is not commercially available. However, the simulation of a SolI digestion pattern for H. pylori NCTC 26695 supports this hypothesis (Fig. 1b). Another explanation could be the presence of a regulatory protein that sequesters GATC sites and thus inhibits methylation, such as SeqA in Escherichia coli (23). However, simultaneous DpnI/DpnII digestion is an infrequent, but reproducible, observation, supporting the explanation of the presence of a rare MTase (Table 1). For this reason, the result of digestion of strain P12 by DpnI was considered negative (complete digestion results are in Table S4 in the supplemental material).
FIG. 1.
(a) Strain P12 genomic DNA digested with DpnI and DpnII. Digestion with DpnI is clearly partial, because part of the DNA is the same size as the control (lane C). M, 1-kb DNA ladder (molecular size marker; New England Biolabs, Inc.). (b) Simulation of H. pylori NCTC 26695 genomic DNA digestion with BamHI and DpnII. BamHI digestion simulates the digestion pattern of DpnI if the DNA was methylated with M.SolI.
Dendrogram analysis.
Of the 27 REases tested, isoschizomers and DpnI excluded, 7 gave the same result in all strains and thus are uninformative as a typing tool. These results were excluded from dendrogram analysis. A first dendrogram was constructed for the enlarged set of independent strains, with NTSYS version 2.0 software, using the UPGMA method and the Jaccard coefficient, for 32 strains [(X1 + X2p) × 19 REases] (Fig. S1 in the supplemental material). The dendrogram has a cophenetic correlation factor of 0.79, which revealed a very good fit to the data matrix. Simpson's index of diversity was 100%, referring to the discriminatory power as the average probability that the typing method will identify as different types two unrelated strains randomly sampled in the microbial population; the frequency of each group for a similarity level of 100% was less than 5% (nj/N = 0) as suggested by Maslow et al. (25). The typeability, which is the proportion of strains that are assigned a type by the typing system, revealed that the 32 strains were considered different. When strains were chosen from set X2 by randomization, we obtained similar results (data not shown).
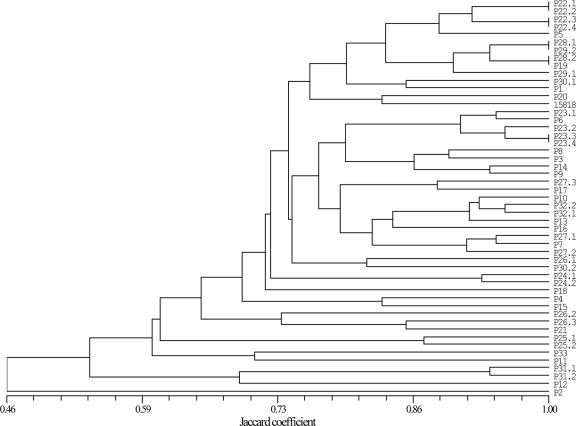
The inclusion of all strains (51 strains × 19 REases) is more suitable for evaluating the performance of the typing method, especially in the present case, where quite similar strains were used in order to determine the discriminatory power of the method. The global dendrogram, constructed with NTSYS version 2.0 software with the UPGMA method and the Jaccard coefficient, had a cophenetic correlation factor of 0.79, which shows a very good fit of the basic dendrogram to the main matrix (Fig. 2). The Simpson index of diversity was excellent (0.996), and the frequency in each group for a similarity level of 100% was less than 5% (nj/N = 0.039).
FIG. 2.
Global dendrogram constructed with all strains (51 strains × 19 REases), using the UPGMA method and the Jaccard coefficient.
The global dendrogram analysis confirms that the method has a good discriminatory power. In the five pairs of strains isolated from two different locations in the same patient, antrum strains P22.1, P22.3, P23.1, P23.3, and P32.1 and corpus strains P22.2, P22.4, P23.2, P23.4, and P32.2, only three pairs, P22.1/P22.2, P22.3/P22.4, and P23.3/P23.4, have exactly the same methylation profile. This could be explained either by evolution in situ or by mixed infection.
Visual analysis of both dendrograms suggests that there are at least two clusters, with several subclusters (Fig. 2; also, see Fig. S1 in the supplemental material). It was also observed that there are two pairs of strains with similar methylation status that were not sequentially isolated from the same patient: P28.1/P29.2 and P28.2/P19.
The typing method based on the genomic methylation status is an excellent tool for H. pylori with respect to all performance criteria (42): i.e., (i) typeability (0.9; should be similar to 1); (ii) reproducibility (100%); (iii) stability (see below); (iv) discriminatory power (0.966; should be >0.95); (v) test population; (vi) epidemiological concordance (sequenced isolates show similar status of genomic methylation); (vii) typing system concordance (comparing with other methods showed similar results); and (viii) convenience criteria (simple, rapid, and inexpensive). These characteristics make the genomic methylation status a powerful method for typing H. pylori strains.
DISCUSSION
Diversity of MTase expression.
We have observed a high number of expressed MTases, with an average of 14.6 distinct resistant recognition sequences (inferred to represent distinct MTases) per independent strain (SD, 1.9) and a maximum and minimum of 18 and 11 active MTases, respectively. A diversity of MTases expressed among independent tested strains was also observed. Both the large number and the diversity are in agreement with results published elsewhere (4, 5, 22, 43, 45, 48). Among the tested MTases, eight gave the same result for all tested strains: five MTases were common to all (M.BseRI, M.EagI, M.HhaI, M.NlaIII, and M.NaeI), and two MTases were absent in all strains (M.FokI and M.Sau3AI). So far, only M.NlaIII (iceA or hpyIM) has been described as a conserved H. pylori MTase (49). The other nonmonotonous MTases can be classified into four groups accordingly to their frequency: (i) rare (M.AciI, M.DraI, M.Fnu4HI, and M.ScrFI), present in 2 to 8% of the strains; (ii) infrequent (M.BstUI, M.DdeI, M.FauI, M.HaeIII, M.Hpy99I, and M.Sau96I), present in 14 to 37%; (iii) frequent (M.Hpy188I, M.HpyCH4III, M.HpyCH4IV, M.HpyCH4V, and M.MspI), present in 55 to 82%; and (iv) very frequent (M.AseI, M.BssHII, M.DpnII, and M.TacI), present in >88% (Table 1). Inspection of Table 1 shows that in the other studies there are also four major groups of MTases. Most of them agree with the ones we found except for FokI, Hpy188I, and Hpy99I. The TacI results are in agreement with only one study (43) (Table 1). A possible explanation for this observation might be that some MTases are geographic markers. The other studies worked with groups of strains isolated from mixed geographic origins (Europe, Asia, North America, and Africa) (2, 43, 48).
It is also interesting that the numbers of recognition sites of the studied REases in the three sequenced H. pylori genomes are remarkably similar (Table 1). This is in agreement with the observation that the overall genomic organization, gene order, and predicted proteomes of the two first sequenced strains are quite similar (12).
Maintenance of the expressed methylases.
We performed a retrospective cohort study, treating sample X2 (Table S2 in the supplemental material) as a historical cohort, where all sequentially isolated strains available from the INSA collection and/or strains isolated from the stomach antrum and corpus were selected to determine the genomic methylation status. We observed a conservation of MTase expression of 94.9% (Table 2), revealing that after acquisition, the R-M system tends to be maintained and expressed (17, 18, 20, 28, 30).
TABLE 2.
Observed changes in the methylation status of sequential isolates from the same patient
| Patient | Theoretical maximal no. of changesa | No. of observed changesa | Strains | Observed changed methylationb,c | Same methylation status? | Same profile with different typing methodsd?
|
|
|---|---|---|---|---|---|---|---|
| RAPD (% similarity) | PCR-RFLP (% similarity) | ||||||
| A | 168 (28 × 6) | 1 | P22.1 and P22.2 | None | Yes | Yes | Yes |
| P22.3 and P22.4 | None | Yes | Yes | Yes | |||
| P22.1/P22.2 and P22.3/P22.4 | +M.TacI | No | Yes | Yes | |||
| B | 168 (28 × 6) | 4 | P23.1 and P23.2 | M.HpyCH4III, M.DraI | No | Yes | Yes |
| P23.3 and P23.4 | None | Yes | Yes | Yes | |||
| P23.1 and P23.3/P23.4 | +M.HpyCH4III | No | No (93) | Yes | |||
| P23.2 and P23.3/P23.4 | −M.DraI | No | No (93) | Yes | |||
| C | 28 | 1 | P24.1 and P24.2 | +M.FauI | No | No (97) | Yes |
| D | 28 | 2 | P25.1 and P25.2 | +M.HpyCH4III, −M.Sau96I | No | No (90) | Yes |
| E | 56 (28 × 2) | 9 | P26.1 and P26.2 | −M.DdeI, +M.Hpy188I, −M.HpyCH4IV, −M.BssHII, −M.Sau96I | No | No (41) | No (55) |
| P26.2 and P26.3 | −M.HpyCH4V, +M.BssHII, −M.FauI, +M.Sau96I | No | NT | NT | |||
| F | 56 (28 × 2) | 6 | P27.1 and P27.2 | −M.HpyCH4IV, +M.HpyCH4V | No | NT | NT |
| P27.2 and P28.3 | +M.Hpy188I, −M.HpyCH4III, +M.HpyCH4IV, −M.HpyCH4V | No | NT | NT | |||
| G | 28 | 1 | P28.1 and P28.2 | +M.Hpy99I | No | NT | NT |
| H | 28 | 2 | P29.1 and P29.2 | +M.Hpy99I, +M.HpyCH4V | No | NT | NT |
| I | 28 | 5 | P30.1 and P30.2 | +M.MspI, +M.ScFI, +M.FauI, +M.TacI, +M.Sau96I | No | NT | NT |
| J | 28 | 1 | P31.1 and P31.2 | −M.Sau96I | No | NT | NT |
| K | 28 | 1 | P32.1 and P32.2 | M.AciI | No | Yes | Yes |
| Total | 644 | 33 | |||||
The MTase alteration represented 5.1% (33/644) of the results, and the maintenance of the expressed MTase was 94,9% among the sequential isolated strains.
Isoschizomers not included.
+, MTase acquisition; −, MTase loss; no plus or minus sign, impossible to determine if the MTase alteration was an acquisition or loss.
“Yes” alone represents 100% similarity. When strains do not have the same profile, the similarity percentage is shown. NT, not tested. Data are from reference 32.
We had also observed that strains isolated from stomach antrum and corpus of the same patient at the same moment had, in most cases, the same expressed methylases, except P23.1 and P23.2, with two observed differences, and P32.1 and P32.2, with one observed difference (Table 2; also, see Table S2 in the supplemental material). The differences in the methylation status between strains from stomach antrum and corpus may reveal an infection of the same patient with different H. pylori strains. Mixed infection with different H. pylori strains had already been described (9, 16, 47) and may provide the opportunity for horizontal gene transfer (10, 37).
Strains isolated from patients A to E and K were previously typed using PCR-RFLP (gene ureA-ureB digested with HaeIII) and RAPD (32). The strains from patients A and K were not differentiated, although genomic methylation revealed minor differences (Table 2). The other strains were discriminated with at least one method. Strains from patients B, C, and D were not identified as being different by PCR-RFLP but were discriminated with RAPD. Methylation analysis also discriminated these strains. Strains from patient E were considered different with RAPD, PCR-RFLP, and genomic methylation (Table 2).
The strains isolated from a couple (patients E and L) showed that the strain from the asymptomatic wife (P33) had a genomic methylation status different from that of all strains isolated from her husband (P26.1 to P26.3 [see Table S2 in the supplemental material]), as they were considered different when analyzed with PCR-RFLP and RAPD (32).
The results from the sequentially isolated strains demonstrate that the discriminatory power of the methylation status is superior to those of PCR-RFLP and RAPD, which are considered to have high discriminatory power and typeability (33). The stability of MTase expression in vivo (94.9%) in addition to the diversity of R-M systems present makes the use of genomic methylation status a quite attractive typing method. The 1-year follow-up of murine gastric colonization by three unrelated H. pylori strains did not show any temporal phase variation associated with R-M systems (40). Moreover, our follow-up of 10 patients during a period of 6 months to 4 years did not reveal substantial alteration in the MTase expression. Nevertheless, we cannot rule out the possibility that the patients were subject to reinfection and/or mixed colonization, which could have introduced a bias in this study.
Although MTase expression is quite stable, we found minor changes in methylation status in 5.1% of the epidemiologically related strains, reflecting mainly acquisitions of MTases, 63.3% (19/30). As expected for these strains, MTase acquisition was almost two-thirds higher than MTase loss. The MTases may be acquired by horizontal gene transfer, as H. pylori organisms have access to DNA from bacteria that are introduced with food, because H. pylori is a naturally competent bacterium (29).
The loss of expression of a type II R-M system is more difficult due to the postsegregational killing effect (17, 18, 20, 28). However, it is possible that after REase loss, the orphan MTase suffers mutation, truncation, or deletion (43). In fact, among the sequenced strains there are several examples of orphan MTases: NCTC 26695 has two, J99 three, and HPAG1 five orphan MTases (38).
The typing method based on genomic methylation status is an excellent tool for H. pylori. An on-line database with the typing results from different origins would be easy to design and implement, allowing interlaboratory result comparison. This typing method can be applied to species with a high number of R-M systems, such as Campylobacter upsaliensis, Neisseria gonorrhoeae, Neisseria meningitidis, Mycoplasma pulmonis, Thermoplasma volcanium, Methanococcus jannaschii, Methanosarcina mazei, Anabaena variabilis, and Xylella fastidiosa, among others (38).
Supplementary Material
Acknowledgments
We thank Lurdes Monteiro for the H. pylori strains, Dinis Pestana for valuable help on the statistical analysis, Elisabeth Raleigh for critical reading of the manuscript, Graça Malheiro for English review of the manuscript, Rick Morgan for many insightful discussions, and Carol Polisson for her assistance in our work.
This work was partially supported by New England Biolabs, Inc.
Footnotes
Published ahead of print on 4 May 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Ando, T., Q. Xu, M. Torres, K. Kusugami, D. A. Israel, and M. J. Blaser. 2000. Restriction-modification system differences in Helicobacter pylori are a barrier to interstrain plasmid transfer. Mol. Microbiol. 37:1052-1065. [DOI] [PubMed] [Google Scholar]
- 3.Ando, T., Y. Goto, O. Maeda, O. Watanabe, K. Ishiguro, and H. Goto. 2006. Causal role of Helicobacter pylori infection in gastric cancer. World J. Gastroenterol. 14:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aras, R. A., T. Takata, T. Ando, A. van der Ende, and M. J. Blaser. 2001. Regulation of the HpyII restriction-modification system of Helicobacter pylori by gene deletion and horizontal reconstitution. Mol. Microbiol. 40:369-382. [DOI] [PubMed] [Google Scholar]
- 5.Aras, R. A., A. J. Small, T. Ando, and M. J. Blaser. 2002. Helicobacter pylori interstrain restriction-modification diversity prevents genome subversion by chromosomal DNA from competing strains. Nucleic Acids Res. 30:5391-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett, V. 2002. Sample survey—principles and methods, p. 184. Hodder Arnold, London, United Kingdom.
- 7.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Investig. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eslick, G. D. 2006. Helicobacter pylori infection causes gastric cancer? A review of the epidemiological, meta-analytic, and experimental evidence. World J. Gastroenterol. 12:2991-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghose, C., G. I. Perez-Perez, L. J. van Doorn, M. G. Dominguez-Bello, and M. J. Blaser. 2005. High frequency of gastric colonization with multiple Helicobacter pylori strains in Venezuelan subjects. J. Clin. Microbiol. 43:2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gressmann, H., B. Linz, R. Ghai, K. P. Pleissner, R. Schlapbach, Y. Yamaoka, C. Kraft, S. Suerbaum, T. F. Meyer, and M. Achtman. 2005. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet. 1:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han, S. R., H. C. E. Zschausch, H. G. Meyer, T. Schneider, M. Loos, S. Bhakdi, and M. J. Maeurer. 2000. Helicobacter pylori: clonal population structure and restricted transmission within families revealed by molecular typing. J. Clin. Microbiol. 38:3646-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock, R. E., R. Alm, J. Bina, and T. Trust. 1998. Helicobacter pylori: a surprisingly conserved bacterium. Nat. Biotechnol. 16:216-217. [DOI] [PubMed] [Google Scholar]
- 13.Hunter, P. R., and M. A. Gaston. 1988. Numerical Index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang, H. Y., and J. Yim. 1994. SolI, a novel isoschizomer of BamHI isolated from Streptoverticillium olivoverticillatum. Nucleic Acids Res. 22:2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer. 1994. IARC monographs on the evaluation of carcinogenic risk to humans, no. 61. Schistosomes, liver flukes and Helicobacter pylori. IARC, Lyon, France. [PMC free article] [PubMed]
- 16.Israel, D. A., N. Salama, U. Krishna, U. M. Rieger, J. C. Atherton, S. E. Falkow, and R. M. Peek, Jr. 2001. Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc. Natl. Acad. Sci. USA 98:14625-14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, I. 1998. Selfishness and death: raison d'être of restriction, recombination and mitochondria. Trends Genet. 14:368-374. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, I., A. Nobusato, N. Kobayashi-Takahasi, and I. Uchiyama. 1999. Shaping the genome—restriction-modification systems as mobile genetic elements. Curr. Opin. Genet. Dev. 9:649-656. [DOI] [PubMed] [Google Scholar]
- 19.Konturek, J. W. 2003. Discovery by Jaworski of Helicobacter pylori and its pathogenetic role in peptic ulcer, gastritis and gastric cancer. J Physiol. Pharmacol. 54(Suppl. 3):23-41. [PubMed] [Google Scholar]
- 20.Kusano, K., T. Naito, N. Handa, and I. Kobayashi. 1995. Restriction-modification systems as genomic parasites in competition for specific sequences. Proc. Natl. Acad. Sci. USA 92:11095-11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacks, S., and B. Greenberg. 1975. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J. Biol. Chem. 250:4060-4066. [PubMed] [Google Scholar]
- 22.Lin, L.-F., J. Posfai, R. J. Roberts, and H. Kong. 2001. Comparative genomics of the restriction-modification systems in Helicobacter pylori. Proc. Natl. Acad. Sci. USA 98:2740-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low, D. A., N. J. Nathan, and M. J. Mahan. 2001. Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 69:7197-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 25.Maslow, J. N., M. E. Mulligan, and R. D. Arbeit. 1993. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin. Infect. Dis. 17:153-162. [DOI] [PubMed] [Google Scholar]
- 26.Mégraud, F. 1996. Diagnostic bactériologique standart de l'infection à Helicobacter pylori, p. 249-266. In F. Mégraud and H. Lamouliatte (ed.), Helicobacter pylori, vol. I. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 27.Monteiro, L., and F. Mégraud. 1999. Par quels moyens rechercher Helicobacter pylori avant et après éradication? Gastroenterol. Clin. Biol. 23:C3-C19. [PubMed] [Google Scholar]
- 28.Naito, T., K. Kusano, and I. Kobayashi. 1995. Selfish behavior of restriction-modification systems. Science 267:897-899. [DOI] [PubMed] [Google Scholar]
- 29.Nobusato, A., I. Uchiyama, and I. Kobayashi. 2000. Diversity of restriction-modification gene homologues in Helicobacter pylori. Gene 259:89-98. [DOI] [PubMed] [Google Scholar]
- 30.Nobusato, A., I. Uchiyama, S. Ohashi, and I. Kobayashi. 2000. Insertion with long target duplication: mechanism for gene mobility suggested from comparison of two related bacterial genomes. Gene 259:99-108. [DOI] [PubMed] [Google Scholar]
- 31.Oh, J. D., H. Kling-Backhed, M. Giannakis, J. Xu, R. S. Fulton, L. A. Fulton, H. S. Cordum, C. Wang, G. Elliott, J. Edwards, E. R. Mardis, L. G. Engstrand, and J. I. Gordon. 2006. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc. Natl. Acad. Sci. USA 103:9999-10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oleastro, M. 2000. Master thesis. Universidade Técnica de Lisboa, Lisboa, Portugal.
- 33.Owen, R. J., D. Taylor, G. Wang, and L. J. van Doorn. 2001. Heterogeneity and subtyping, p. 363-378. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori physiology and genetics. ASM Press, Washington, DC. [PubMed]
- 34.Peterson, J. D., L. A. Umayam, T. M. Dickinson, E. K. Hickey, and O. White. 2001. The Comprehensive Microbial Resource. Nucleic Acids Res. 29:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 36.Priest, F., and B. Austin. 1993. Modern bacterial taxonomy, 2nd ed., p. 14-49. Chapman & Hall, London, United Kingdom.
- 37.Raymond, J., J. M. Thiberge, C. Chevalier, N. Kalach, M. Bergeret, A. Labigne, and C. Dauga. 2004. Genetic and transmission analysis of Helicobacter pylori strains within a family. Emerg. Infect. Dis. 10:1816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts, R. J., T. Vincze, J. Posfai, and D. Macelis. 2005. REBASE—restriction enzymes and DNA methyltransferases. Nucleic Acids Res. 33:D230-D232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts, R. J., M. Belfort, T. Bestor, A. S. Bhagwat, T. A. Bickle, J. Bitinaite, R. M. Blumenthal, S. K. Degtyarev, D. T. Dryden, K. Dybvig, K. Firman, E. S. Gromova, R. I. Gumport, S. E. Halford, S. Hattman, J. Heitman, D. P. Hornby, A. Janulaitis, A. Jeltsch, J. Josephsen, A. Kiss, T. R. Klaenhammer, I. Kobayashi, H. Kong, D. H. Kruger, S. Lacks, M. G. Marinus, M. Miyahara, R. D. Morgan, N. E. Murray, V. Nagaraja, A. Piekarowicz, A. Pingoud, E. Raleigh, D. N. Rao, N. Reich, V. E. Repin, E. U. Selker, P. C. Shaw, D. C. Stein, B. L. Stoddard, W. Szybalski, T. A. Trautner, J. L. Van Etten, J. M. Vitor, G. G. Wilson, and S. Y. Xu. 2003. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 31:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salaun, L., S. Ayraud, and N. Saunders. 2005. Phase variation mediated niche adaptation during prolonged experimental murine infection with Helicobacter pylori. Microbiology 151:917-923. [DOI] [PubMed] [Google Scholar]
- 41.Smith, M. G., G. L. Hold, E. Tahara, and E. M. El-Omar. 2006. Cellular and molecular aspects of gastric cancer. World J. Gastroenterol. 12:2979-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Struelens, M. J. 1996. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin. Microb. Infect. 2:2-11. [DOI] [PubMed] [Google Scholar]
- 43.Takata, T., R. Aras, D. Tavakoli, T. Ando, A. Z. Olivares, and M. J. Blaser. 2002. Phenotypic and genotypic variation in methylases involved in type II restriction-modification systems in Helicobacter pylori. Nucleic Acids Res. 30:2444-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 45.Vitkute, J., K. Stankevicius, G. Tamulaitiene, Z. Maneliene, A. Timinskas, D. E. Berg, and A. Janulaitis. 2001. Specificities of eleven different DNA methyltransferases of Helicobacter pylori strain 26695. J. Bacteriol. 183:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vovis, G. F., and S. Lacks. 1977. Complementary action of restriction enzymes Endo R.DpnI and Endo R.DpnII on bacteriophage f1 DNA. J. Mol. Biol. 115:525-538. [DOI] [PubMed] [Google Scholar]
- 47.Wong, B. C., W. H. Wang, D. E. Berg, F. M. Fung, K. W. Wong, W. M. Wong, K. C. Lai, C. H. Cho, W. M. Hui, and S. K. Lam. 2001. High prevalence of mixed infections by Helicobacter pylori in Hong Kong: metronidazole sensitivity and overall genotype. Aliment. Pharmacol. Ther. 15:493-503. [DOI] [PubMed] [Google Scholar]
- 48.Xu, Q., R. D. Morgan, R. J. Roberts, and M. J. Blaser. 2000. Identification of type II restriction and modification systems in Helicobacter pylori reveals their substantial diversity among strains. Proc. Natl. Acad. Sci. USA 97:9671-9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, Q., R. M. Peek, Jr., G. G. Miller, and M. J. Blaser. 1997. The Helicobacter pylori genome is modified at CATG by product of hpyIM. J. Bacteriol. 179:6807-6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.