Abstract
Background
We previously reported that cancer-related psychological stress is associated with reduced natural killer (NK) cell lysis. We hypothesized that reduced NK cell cytotoxicity in patients with increased levels of stress would correlate with alterations in the expression of inhibitory NK cell receptors (killer immunoglobulin-like receptors, or KIRs). The specific aim of this study was to examine KIR expression in patients with high or low levels of psychologic stress and correlate alterations in KIR expression with NK cell function.
Materials and Methods
227 patients underwent baseline evaluation of cancer-related psychological stress and were randomized to psychosocial intervention versus observation. From this population, two groups were defined based on pre-treatment measurements of NK lytic activity, stress levels, and the availability of cryopreserved peripheral blood mononuclear cells (PBMC). Group I (n = 9) had low stress by the Impact of Events Scale (IES), and high NK cell lysis at the 50:1 effector: target ratio (NK50 = 52–89%). Group II (n = 8) had high stress and low NK50 (27–52%). Lymphokine activated killer (LAK) activity, antibody dependent cellular cytotoxicity (ADCC), and expression of cytokine receptors, adhesion molecules, and killer immunoglobulin-like receptors (KIRs) were assessed in PBMC.
Results
Incubation of PBMC with NK-stimulatory cytokines (IL-2, IL-12, or IL-15) led to significant increases in cytotoxic activity regardless of IES/NK50 scores. There were no significant group differences in NK cell surface expression of the IL-2 receptor components CD25 and CD122, antibody-dependent lysis of HER2/neu-positive SKBr3 cells treated with an anti-HER2/neu monoclonal antibody, expression of adhesion molecules (CD2, CD11a, CD18) and markers of activation (CD69), or expression of the KIRs CD158a, NKG2a, NKB1, and CD161. However, levels of CD158b were significantly higher in Group I after incubation in media alone or with IL-2, and CD94 expression was significantly lower in Group I after incubation with IL-2.
Conclusions
In this study of a small subset of breast cancer patients chosen from a previous clinical trial of psychosocial intervention for breast cancer, impaired NK lysis in breast cancer patients with high levels of psychological stress was associated with alterations in surface expression of killer immunoglobulin-like receptors. However, immune effectors retained the ability to lyse antibody-coated targets and to initiate lymphokine-activated killer activity, irrespective of stress levels or baseline NK50.
Keywords: Breast cancer, Killer immunoglobulin-like receptors (KIR), Natural killer (NK) cells, Psychologic stress
INTRODUCTION
Studies suggest that one-third of all oncology patients experience significant distress associated with cancer diagnosis and treatment [1]. Manifestations may include depression, fatigue, perturbation in intimate relationships and social support networks, destabilization of financial situations, non-compliance with treatment, cessation of positive health behaviors, and pursuance of negative health behaviors, e.g. cigarette smoking [1–9].
The level of psychological stress attributable to the cancer diagnosis can be quantitated with various patient-reported tools, or by clinician assessment using instruments such as the Stress Response Rating Scale [10–12]. The Impact of Events Scale (IES), developed by Horowitz et al. in the late 1970’s, is a patient-reported instrument that quantitates stress-related intrusive thoughts, denial of thoughts, and avoidance behavior [13]. It has been extensively validated in studies of patients with diverse serious illnesses, including cancer [8, 13–14]. Significant reductions in cancer-associated psychological distress can be achieved with psychosocial interventions aimed at decreasing stress levels, strengthening social support networks, and improving coping and life management skills [15–17].
A large body of literature suggests that psychological stress associated with cancer diagnosis and treatment contributes to impaired immunity [18–25]. The exact mechanisms are unknown, but there is solid evidence for the impairment of natural killer (NK) cell and T cell function in response to acute and chronic psychological stress. Surgery also has been associated with impaired immunity in the immediate postoperative period, an effect that is compounded by the use of adjuvant chemotherapy and radiation, which have short-term effects on the generation and activity of immune effectors [26–29].
We previously reported that cancer-related stress correlated with impaired immunity in patients with invasive breast cancer [15]. This study of 116 patients after surgical resection of Stage II or Stage III breast cancer demonstrated decreased NK cell toxicity and T cell blastogenesis in patients exhibiting high levels of cancer-related psychological stress [15]. We subsequently conducted a randomized phase III trial testing the effects of psychological and behavioral interventions aimed at stress reduction in 227 women with surgically treated Stage II or Stage III breast cancer [16]. Patients were randomized to receive either psychosocial intervention or assessment only. As predicted, patients in the intervention group demonstrated psychological and behavioral improvements over time [16]. T cell proliferation in response to phytohemagglutinin (PHA) and concavalin A (con A) remained stable or increased for patients in the intervention group, whereas both responses declined for patients in the assessment group. This finding was statistically significant across multiple concentrations of PHA and con A [16].
The purpose of the present study was to further investigate the finding that cancer-related psychological distress is associated with decreased NK cell cytotoxic activity [15]. To study this phenomenon, we identified two subsets of patients based on characteristics present upon pre-study evaluation. Patients at opposite ends of the spectrum for preexisting levels of psychologic stress and NK cell lysis (and for whom adequate numbers of cryopreserved cells were available) were divided into two groups: Group I included patients (n = 9) with a low stress level and high NK cell lysis, and Group II included patients (n = 8) with a high stress level and low NK cell lysis.
MATERIALS AND METHODS
Patients
Subjects for this study were 17 women drawn from the Stress and Immunity in Breast Cancer (SIBC) study conducted at The Ohio State University [16]. The SIBC study enrolled 227 women with Stage II (205 patients, 90%) or Stage III (22 patients, 10%) breast cancer treated surgically within the previous three months (breast conservation therapy, 43%; modified radical mastectomy, 57%; mean number of days after surgery = 50.8 ± 17.5). Prior to psychosocial intervention, cancer-related psychological stress was evaluated using the IES. Patients were then randomized: the intervention group received psychological and behavioral interventions aimed at decreasing stress, and the control group received assessment only [16]. The present investigation utilized data from trial participants for whom banked PBMC were available. Recruitment for the larger trial was conducted from May of 1994 through May of 2000, but PBMC banking was introduced relatively late during this process. Beginning in June, 1998, the amount of blood collected from each participant was increased in order to begin banking PBMC. Fifty-eight participants were recruited into the trial after this change was implemented. Of these 58, insufficient blood was obtained for three participants. Thus, banked PBMC were available for 55 participants. Dividing the groups at the median of IES and NK50 yielded four subgroups from whom the two subgroups of high stress/low NK50, and low stress/high NK50 were chosen for this study (see Table 1). Due to the need for large numbers of cryopreserved PBMC to complete the proposed studies, it was not possible to examine additional trial patients. There were no statistically significant differences between the groups with respect to age; time since cancer diagnosis; common risk factors such as menopausal status, parity, family history of breast cancer, and personal history of atypical hyperplasia; and tumor characteristics such as histologic type, histologic grade, tumor stage, and status of estrogen and progesterone receptors (see Fig 1 and Table 1). Each participant gave informed consent, completed the baseline IES exam, and had 60 mL of venous blood drawn prior to treatment under this Institutional Review Board-approved study.
Table 1.
Patient subgroups.
| Low NK50 NK50 ≤ 52% | High NK50 NK50 ≥ 52% | |
|---|---|---|
| Low stress IES ≤ 28 | N = 11 | n= 18 (present study, n =9; 50%) |
| High stress IES > 28 | N = 17 (present study, n = 8; 47%) | n = 9 |
Figure 1. Patient characteristics.
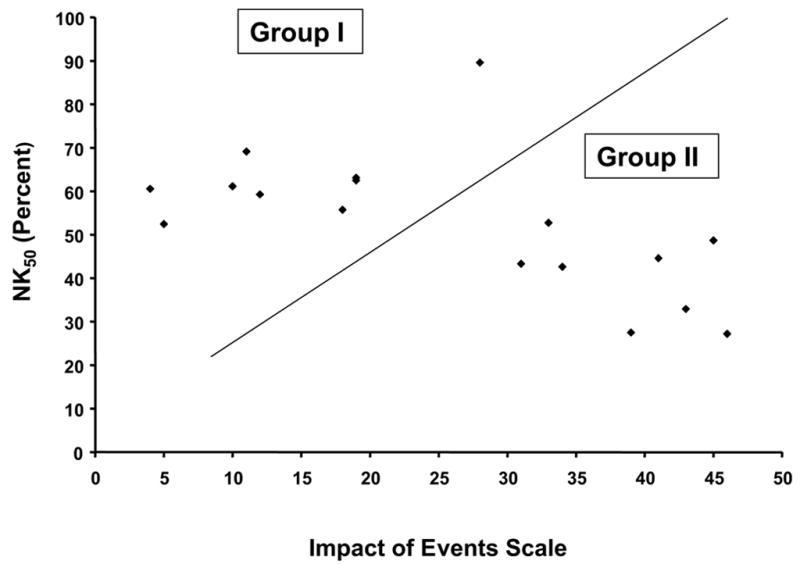
Schematic view of individual patient values for the Impact of Events Scale (IES) and natural killer cell lysis of K562 cells at an effector:target ratio of 50:1 (NK50) for patients in Group I (low stress, high NK lysis) and Group II (high stress, low NK lysis). Each individual patient is represented by a black diamond.
Blood separation procedures
Peripheral blood mononuclear cells (PBMC) were isolated from venous blood using Ficoll density gradient centrifugation (Amersham Pharmacia Biotech, Inc., Piscataway, NJ), washed in calcium- and magnesium-free Dulbecco’s Phosphate-Buffered Saline (PBS, Invitrogen Corporation, Grand Island, NY), and counted on a Z1 Coulter Particle Counter (Beckman Coulter Corp., Miami, FL). Aliquots of PBMC were resuspended at a final concentration of 1 × 107 cells/mL in RPMI-1640 supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 2-mercaptoethanol (Sigma, St. Louis, MO), 100X antibiotic-antimycotic stock (Gibco BRL, Grand Island, NY), HEPES (Gibco), sodium bicarbonate (Gibco), and L-glutamine (Gibco) (complete medium), and used for the studies described herein.
NK cytotoxicity assay
To assess NK cell cytotoxicity, a microtiter chromium 51 (51Cr)-release assay was performed as described previously [19]. Target cells for this assay were K562 cells, an NK-sensitive human myeloid cell line. Briefly, PBMC were resuspended in complete medium at a density of 10 × 106 cells/mL and seeded in triplicate into 96-well V-bottom microtiter plates (Corning Incorporated, Corning, NY) in a volume sufficient to provide an effector-to-target (E:T) cell ratio of 100:1, 50:1, 25:1, 12.5:1, 6.25:1, or 3.125:1. Complete medium was added to a total volume of 100 μL. K562 cells were harvested, labeled with 51Cr, and washed. Labeled K562 cells (5 × 103 cells in 100 μL medium) were added to each well. Plates were centrifuged at 300 g × 5 minutes, incubated for 5 hours (5% CO2, 37°C), and centrifuged again at 300 g × 5 minutes. Supernatant (100 μL) was harvested and counted using a Beckman 5500 gamma counter. Minimum and maximum 51Cr release were determined using target cells incubated in complete medium or 5% SDS detergent solution, respectively. For the purpose of analysis, data obtained at an E:T ratio of 50:1 were used. Cytotoxicity was calculated as follows:
Experimental 51Cr release - minimum release/maximum release- minimum release
Lymphokine activated killer (LAK) assay
PBMC were viably thawed and plated in complete medium at a density of 5 × 104 cells/well in 96-well U-bottom plates (Corning Incorporated, Corning, NY) in the presence of IL-2, IL-12, IL-15 (each at 20 ng/well) or control, and incubated for 24 hours (5% CO2, 37°C). These cytokines were chosen because of their important roles in NK cell function [30]. IL-2, IL-12, and IL-15 are all known activators of NK cell proliferation, target cell lysis, and cytokine production. In addition, these cytokines are clinically available, so that the results obtained with these factors are potentially applicable to the clinical situation. For this assay, cells from the estrogen receptor-positive, human breast adenocarcinoma cell line MCF-7 were used as targets. Following incubation with cytokine, target MCF-7 cells were labeled with 200 μCi of 51Cr for 1 hour, washed, enumerated, and added to the wells (E:T ratio = 50:1). Plates were centrifuged at 300 g for 5 minutes, incubated for 4 hours, and centrifuged again. Supernatant (100 μl) was harvested and counted on a Beckman 5500 gamma counter. Minimum and maximum release were determined using target cells incubated in complete medium or 5% SDS, respectively. Percent specific lysis was calculated as previously stated.
Antibody dependent cellular cytotoxicity (ADCC) assay
PBMC were thawed, resuspended in complete medium, and plated at a density of 1 × 104 cells/well in 96-well V-bottom plates. Aliquots of the HER2/neu-positive breast cancer line SKBr3 or the MDA-468 HER2/neu-negative breast cancer cell line were labeled with 200 μCi of 51Cr, incubated with 0.05 mg of trastuzumab or control antibody for one hour (5% CO2, 37°C), washed, and enumerated. Target cells (5 × 103 cells/well) were mixed with effector cells to obtain an E:T ratio of 50:1. Plates were incubated for 18 hours (5% CO2, 37°C), centrifuged, supernatant was analyzed, and percent specific lysis was calculated.
Monoclonal antibody sources
The following murine monoclonal anti-human antibodies were obtained from Beckman Coulter Co. (Fullerton, CA): CD11a-PE, CD18-PE, CD44-FITC, CD56-PC5, CD94-PE, CD122-FITC, CD158a-PE, CD158b-PE, NKG2A-PE, and appropriate fluorochrome-conjugated isotype control antibodies. The following monoclonal antibodies were purchased from BD PharMingen (San Diego, CA): CD2-FITC, CD25-FITC, CD69-FITC, CD161-PE (NKR-P1A), and NKB1-FITC (DX-9). TCRζ-FITC antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A summary of the functions of the different cell markers assayed in this study is presented in Table 3.
Table 3.
Cell Markers.
| Name | Function |
|---|---|
| CD11a | Forms leukocyte function-associated molecule with CD18 |
| CD18 | Forms leukocyte function-associated molecule with CD11a |
| CD122 | Interleukin-2 receptor β chain |
| CD158a | Killer immunoglobulin-like receptor |
| CD158b | Killer immunoglobulin-like receptor |
| CD16 (FcγRIII) | Low affinity receptor for Fc region of IgG |
| CD161 | Killer immunoglobulin-like receptor |
| CD2 | Adhesion molecule involved in T cell activation |
| CD25 | Interleukin-2 receptor α chain |
| CD44 | Adhesion molecule mediating leukocyte attachment and homing |
| CD56 | NK cell marker |
| CD69 | Marker of NK cell activation |
| CD94 | Common subunit of C-type lectin receptor family |
| NKB1 | Killer immunoglobulin-like receptor |
| NKG2a | Killer immunoglobulin-like receptor |
| TCRζ | Component of the T cell receptor; associates with CD16 |
Excerpted in part from Kuby Immunology, 4th Edition, eds. Goldsby et al. New York, NY: W.H. Freeman and Company, 2000.
Flow Cytometry
Dual parameter flow cytometry for CD56 and the above mentioned cell surface markers was employed for characterization of the NK cell population [31]. PBMC were thawed and plated in complete medium at a density of 5 × 104 cells/well in 96-well U-bottom plates (Corning Incorporated, Corning, NY) in the presence of IL-2, IL-12, IL-15 (each at 20 ng/well) or control, and incubated for 24 hours (5% CO2, 37°C). Cells were then placed into tubes with appropriate amounts of pre-titrated monoclonal antibodies (MAb), and incubated in the dark on ice for 15 minutes. Cells were washed in PBS, fixed in 2% formalin, and analyzed on a Coulter XL flow cytometer using at least 10,000 PBMC gated on the lymphocyte population, as determined by light scatter properties. Mean fluorescence intensity (MFI) and the percentage of positively stained cells were calculated for each condition.
Statistical analysis
To test for differences between Groups I and II, the Wilcoxon-Mann-Whitney test was used as a nonparametric equivalent to the t test. Based on the relative ranks of the cases, the test statistic U was analyzed to determine whether the groups were significantly different on each variable of interest. This conservative alternative to the t test was chosen because the small sample size yielded skewed distributions on six of the variables, and we wished to avoid the possibility that influential data points or outliers would affect the outcome. The alpha level was set at p = 0.05.
RESULTS
Patient selection
The patients evaluated in this study were chosen from among 227 women with surgically treated Stage II or III breast cancer participating in a randomized Phase III clinical trial of a psychological intervention for stress reduction [16]. In order to determine whether the group of patients reported in this study was representative of the entire group of 227 patients in the trial, we compared the demographic and prognostic information between the group reported herein and the trial population as a whole. There were no statistically significant differences between the patients in the trial and the subgroup reported herein with respect to age, marital status, race, stage of disease, hormone receptor status, size of tumor, or number of positive lymph nodes (all p values > 0.15). Thus, the patients in the present study appear to be a representative sample of the entire trial population. These patients were subdivided into two groups based upon pre-treatment measurements of lytic ability of NK cells in the peripheral blood, and upon the degree of psychological stress related to the cancer diagnosis (measured by the Impact of Events Scale, as described above). Group I consisted of 9 women with low levels of cancer-related stress (mean IES = 14) and high NK50 (mean = 63.7% lysis). Group II consisted of 8 women with high cancer-related stress levels (mean IES = 39) and low NK50 (mean = 40.0% lysis) (p < 0.05 for both conditions, Fig 1a, 1b). There were no statistically significant group differences in age, cancer stage, time since diagnosis, or the presence of risk factors for breast cancer (Table 2). Importantly, there was no difference in the total number of circulating NK cells between the two groups of patients in the present study.
Table 2.
Patient Information.
| Characteristic | Group I | Group II |
|---|---|---|
|
Age (years)
Mean Range |
52.78 40 − 68 |
52.25 33 − 70 |
|
Race
Caucasian African American |
7 2 |
8 0 |
|
Psychosocial support (patient-reported)
Present Absent |
7 2 |
7 1 |
| Average age of menarche (years) | 12.0 | 12.1 |
|
Menopausal status
Pre-menopausal Peri-menopausal Post-menopausal |
2 3 4 |
2 1 5 |
|
Parity
Nulliparous At least one previous pregnancy |
2 7 |
0 8 |
|
Family history of breast cancer
None One second-degree relative Two second-degree relatives Mother, unilateral disease Sister, unilateral disease |
4 0 2 2 1 |
6 1 0 1 0 |
|
History of benign breast disease
None Atypical hyperplasia |
7 2 |
6 2 |
|
Prior use of oral contraceptive or estrogen replacement therapy
Yes No |
7 2 |
7 1 |
|
Histologic type
Infiltrating ductal NOS (not otherwise specified) Lobular with infiltrating ductal NOS |
8 1 |
8 0 |
|
Histologic grade
G1, well differentiated G2, moderately differentiated G3, poorly differentiated Not assessed |
1 2 5 1 |
1 4 2 1 |
|
TNM stage
IIA IIB IIIA |
6 2 1 |
5 2 1 |
|
Estrogen receptor status
Positive Negative |
5 4 |
2 6 |
|
Progesterone receptor status
Positive Negative |
5 4 |
3 5 |
Lyphokine activated killer (LAK) activity
We wished to determine whether the diminished NK cell activity in patients with high levels of psychological stress translated into reduced sensitivity of PBMC to exogenous cytokines. Cells were incubated for 24 hours in IL-2, IL-12, or IL-15 (20 ng/well) and tested against the MCF-7 cell line at an effector-to-target ratio of 50:1. For both Groups I and II, there was a significant increase in LAK activity following incubation with IL-2, IL-12, or IL-15, as compared to control (all p values < 0.001) (Fig 2a). Observed levels of LAK activity for Groups I and II after incubation in IL-2 were 51.7 ± 17.6% and 38.6 ± 13.4%, respectively, as compared to 12.7 ± 16.2% and 3.4 ± 0.2% after incubation in PBS, respectively (Fig 2a). For Groups I and II respectively, LAK activity was 29.1 ±17.2% and 17.2 ± 5.7% after incubation in IL-12, and 51.5 ± 17.1% and 39.3 ± 12.8% after incubation in IL-15 (Fig 2a). For any given condition (media, IL-2, IL-12, or IL-15), the percent lysis was not significantly different between Group I and Group II (p values > 0.39), although the PBMC from Group II patients had an overall lower level of LAK activity. Similarly, surface expression of CD25, CD122 (components of the IL2-R), and CD44 (an adhesion molecule expressed on both hematopoietic and non-hematopoietic cells) were equivalent in Groups I and II after incubation in media alone or in IL-2 (Fig 2b). These findings suggest that there were no significant group differences with regard to the ability of patient NK cells to develop into functional LAK effectors in response to NK-stimulatory cytokines.
Figure 2. Lymphokine activated killer (LAK) activity.
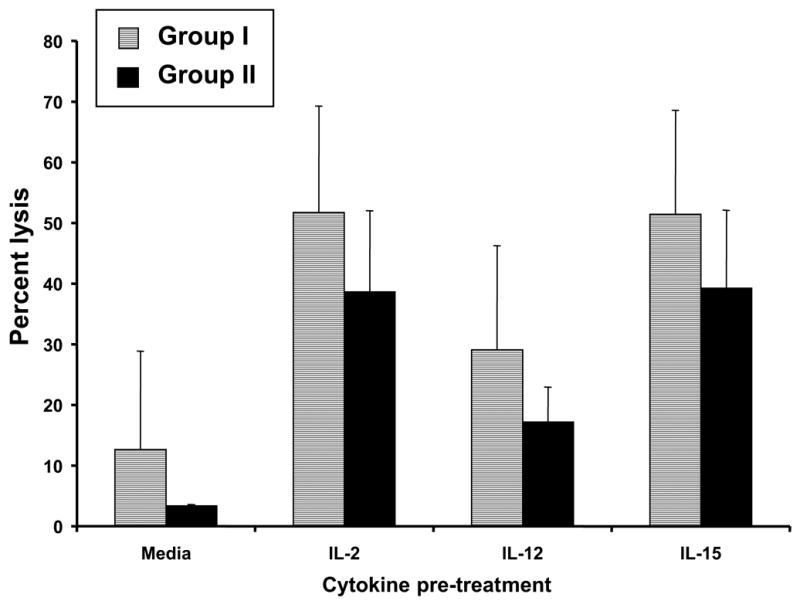
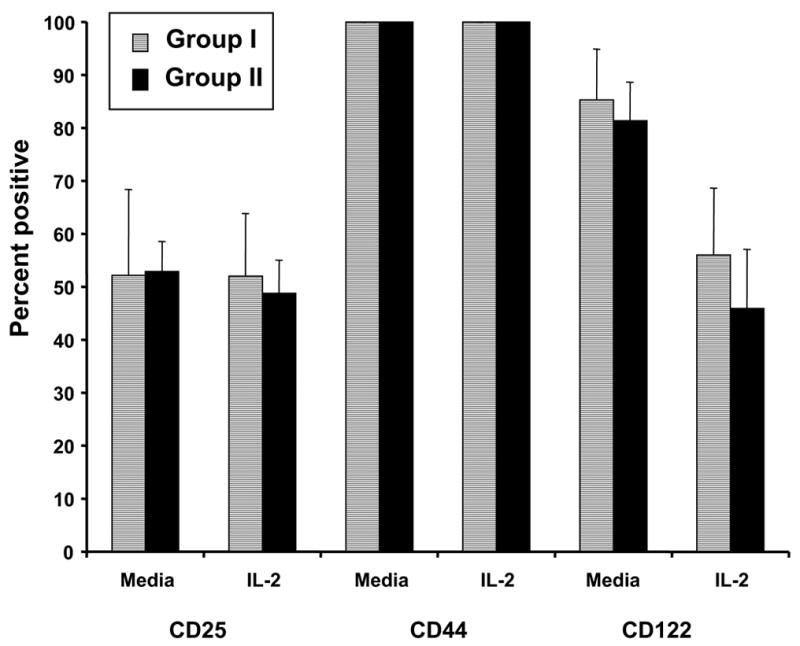
a) Patient PBMC were analyzed for their ability to conduct lymphokine activated killing against the MCF-7 cell line in the presence of media alone, IL-2, IL-12, or IL-15 via a standard 51Cr release assay. Percent specific lysis at the E:T ratio of 50:1 is shown. b) Levels of expression of the T cell markers CD25, CD44, and CD122 after incubation in media alone or in IL-2, quantitated by flow cytometric analysis, are shown. Error bars represent the standard deviation.
Antibody dependent cellular cytotoxicity (ADCC) assay
NK cells have the capacity to lyse Ab-coated targets due to their expression of a low affinity activating receptor for IgG (FcRγIIIa). Cryopreserved PBMC were tested for their ability to lyse antibody-coated breast cancer target cells. Cells from the HER-2/neu-positive cell line SKBr3 were incubated in the presence or absence of an anti-HER-2/neu mAb (trastuzumab; Herceptin®), labeled with Cr51, and incubated with effector cells (PBMC). In the absence of trastuzumab, lysis of SKBr3 cells was equivalent in Groups I and II (Figure 3). As expected, the capacity of patient PBMC to lyse SKBr3 cells was markedly enhanced in the presence of trastuzumab, but Groups I and Group II were not significantly different in this regard (Figure 3). We also examined NK cells for expression of CD16 (FcRγIIIa) and TCRζ, since these molecules are critical mediators of ADCC. These proteins were equivalently expressed in the two groups (data not shown).
Figure 3. Antibody dependent cellular cytotoxicity (ADCC).
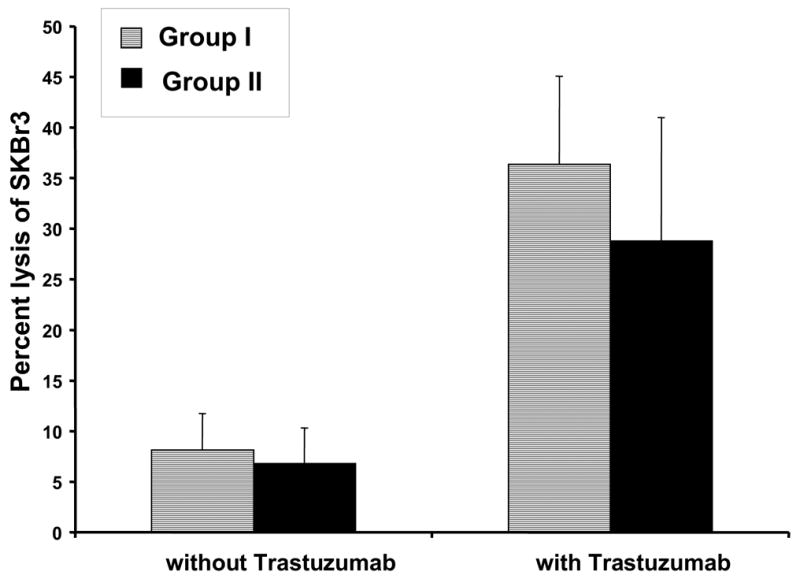
Patient PBMC were analyzed for their ability to conduct ADCC against the HER2/neu-positive breast cancer line SKBr3, in the absence or presence of Trastuzumab, via a standard 51Cr release cytotoxicity assay. Shown is the average percentage of specific lysis for patients in Group I and Group II at an E:T ratio of 50:1. ADCC against the MDA-468 HER2/neu-negative breast cancer cell line was less than 5% in the presence or absence of Ab (not shown). Error bars represent the standard deviation.
Comparison of NK cell receptor expression
NK cells of patients in Groups I and II were assessed for their expression of surface molecules that might impact their ability to conduct natural cytotoxicity (e.g. adhesion molecules and KIR’s). Dual parameter flow cytometry for CD56 and cell surface markers of NK cell lytic activity was used to characterize the effects of stress on the NK cell phenotype [31]. Cells were examined after 24 hour culture in media or in media supplemented with IL-2, IL-12, or IL-15. No differences were seen in the expression of CD2, CD11a, CD18, or CD69 in the presence of these cytokines (data not shown). Next, we wanted to determine whether differences in KIR expression between Groups I and II could explain the impaired NK cell lysis observed in patients with high levels of psychological stress. Cells of patients in Group I showed significantly higher levels of CD158b (KIR2DL1, an inhibitory KIR) than cells of patients in Group II after incubation in medium or medium plus IL-2 (p = 0.03 and 0.02, respectively) (Fig 4). Levels of CD158a between the two groups did not achieve statistical significance (p = 0.055). In contrast, levels of CD94 (the common subunit of the C-type lectin NKG2 receptor family) were significantly lower in Group I than in Group II after incubation in IL-2 (p = 0.03) (Fig 4). Expression of the KIRs NKB1 and CD161 was not significantly different between Groups I and II. Notably, there is considerable inter-patient variation in the KIR expression, and although the results are statistically significant, the clinical significance may be small. Subsequent studies employing larger numbers of patients would help to further elucidate this phenomenon.
Figure 4. Killer cell immunoglobulin-like receptors.
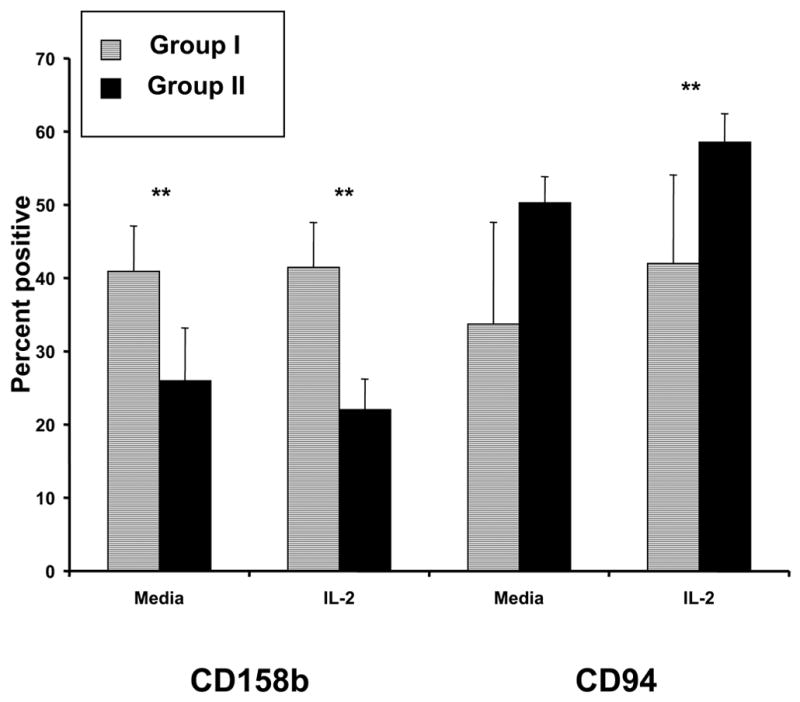
PBMC were thawed, incubated in medium alone or in the presence of IL-2 for 24 hours, and assayed for levels of the KIRs CD158a, CD158b, CD94, NKG2a, NKB1, and CD161 by flow cytometric analysis. Levels are expressed as percent positive cells ± standard deviation.
DISCUSSION
The goal of this study was to further characterize the impaired NK cell function that had been previously identified in patients with high levels of psychologic stress. Two groups of patients were chosen from a randomized clinical trial of psychological intervention in breast cancer patients: those having low stress and high NK cell lysis, and those having high stress and low NK cell lysis. PBMC from both groups of patients exhibited similar increases in LAK activity after incubation with exogenous cytokines (IL-2, IL-12, or IL-15). Antibody-dependent cellular cytotoxicity was also not significantly different between the patients with low levels of stress and those with high levels of stress. NK cells in the two patient groups had similar levels of CD16 and TCRζ (mediators of ADCC) and similar expression of CD2, CD11a, CD18, and CD69 (adhesion molecules and activation markers). Importantly, cells of patients in the low-stress group had significantly higher levels of the KIR CD158b [32]. Also, lower levels of the common lectin-type receptor subunit CD94 were found in the low-stress group as compared to the high-stress group. These findings may provide a potential explanation for the impaired NK lysis that has been observed among patients with high levels of cancer-related psychologic stress.
The aim of the present study was to examine the previously observed phenomenon of reduced NK cytotoxicity in the presence of psychological stress among a subgroup of patients enrolled in a trial of psychosocial intervention versus observation for patients with Stage II or Stage III breast cancer. The relationship between chronic stress and NK cytotoxicity has been well documented [15,33]. However, individuals vary in the degree of physiologic response to psychological stress [34–35]. Because this was an exploratory study, our goal was to examine only patients exhibiting a physiological response to stress. Thus, we selected individuals who appeared to be exhibiting the physiological effects of psychological stress (i.e., the high stress/low NK group) and compared this group to individuals who did not appear to be suffering from excessive stress (the low stress/high NK group). This study design was chosen to maximize statistical power in view of the necessarily small sample size. Importantly, the small sample size and corresponding low power may have prevented the detection of various differences (unrelated to stress or the physiologic response to stress) between the two groups of patients. If such differences exist, they may be responsible for the differential expression of KIRs by cytokine-treated PBMCs. One way to test this hypothesis would be to show that the patient characteristics are at least weakly related to group assignment and that the same patient characteristics are related to the outcome variables. Therefore, we first visually examined the two groups’ patient and disease characteristics (as described in Table 2). We identified three variables that appeared to be weakly related to group assignment: family history of breast cancer (none vs. some), histologic grade, and hormone receptor status. Using analysis of variance, we tested whether these patient characteristics were related to the three variables in which significant differences were observed between the study groups (i.e., CD158b in media, CD158b with IL-2, and CD94 in IL-2). The ANOVAs revealed no statistically significant effects based on patient characteristics (all p values > 0.47). Of course, these ANOVAs also have the problem of low power. However, if group differences in patient characteristics were responsible for the statistically significant effects reported in the present study, they would demonstrate strong relationships with the outcome variables. Thus, it appears unlikely that the results reported here were artifacts of group differences in patient characteristics.
Several previous reports have demonstrated an effect of psychologic stress on the immune response, particularly NK cell activity [18,20,21,25]. In general, acute psychologic stress leads to the upregulation of the immune response, but chronic psychologic stress, such as is experienced due to the diagnosis of chronic illness, is associated with impairment of immune parameters, such as decreased T cell mitogenesis, impaired NK cell activity, and decreased PBMC production of IgG2a, IL-2, and IFN-γ [36]. Potential mechanisms for the effect of stress on NK function include processes leading to decreased total numbers of NK cells or to impaired function of existing NK cells. Importantly, the number of NK cells was equivalent between the two groups of patients in the present study. A focus of recent research has been the relationship between chronic stress and cellular senescence. Epel et al. reported that high levels of perceived psychologic stress led to higher oxidative stress, lower telomerase activity, and accelerated telomere shortening in the PBMC of healthy, post-menopausal women [37]. In a previous study, we reported that high levels of stress in patients with breast cancer were associated with decreased NK cell lytic activity [16]. Importantly, interventions aimed at decreasing psychologic stress may ameliorate these effects. Fawzy et al. have reported that a six-week psychosocial intervention in patients with early-stage malignant melanoma led to alterations in the immune system including increased percentages of NK cells, increased NK cell cytolytic activity, and decreased percentage of CD4+ helper T cells [38]. Patients in the intervention group demonstrated a significant increase in survival as compared to the control group, even at ten years after the intervention [39]. Similarly, our previous Phase III randomized trial demonstrated that breast cancer patients receiving a planned psychological intervention for stress reduction exhibited significant improvement in immune function (e.g., T cell proliferation) as compared to patients receiving assessment only [16].
Killer immunoglobulin-like receptors are expressed on the surface of all NK cells and on some T cells, and are of two main types: members of the Ig superfamily (such as CD158) are considered to be inhibitory to NK lysis, and members of the lectin-type superfamily (defined as CD94 associated with a specific NKG2 subunit) are considered to be activating [40,41]. However, it is becoming increasingly clear that the role of KIR’s in NK cytotoxicity is more complex than was once thought. The role of the common lectin-type subunit CD94 is regulated in part by the other moieties with which it associates: CD94/NKG2A has an inhibitory effect on NK function, while the other CD94/NKG2 complexes (e.g., CD94/NKG2C) lead to NK activation [12]. KIR2DL2 (CD158b) is known as an inhibitory KIR [42]. Recent transfection studies with the YTINDY NK-like cell line have shown that cells transfected with CD158b (but not those transfected to express CD94/NKG2A C-type lectin) exhibited decreased MHC class I-mediated cytotoxicity [43]. Likewise, Guerra et al. suggested that CD158b negatively modulates the lytic activity of tumor-infiltrating T lymphocytes in patients with renal cell carcinoma [44].
Interestingly, in the present study, patients in Group II (high stress and low NK lysis) had increased levels of CD94 and decreased levels of CD158b as compared to patients in Group I. These findings suggest that CD94 might be mediating an overall negative effect on NK cell cytotoxicity. Support for this hypothesis is provided by the recent study of Nguyen et al. which found that NK cells generated after stem cell transplant exhibited an immature phenotype characterized by decreased expression of KIRs and increased expression of CD94/NKG2A. This phenotype was associated with decreased in vitro cytotoxicity against the K562 cell line [45]. Furthermore, these cells also exhibited decreased cytotoxicity against primary mismatched AML blasts that could be restored by blockade of CD94/NKG2A. These findings provide a possible explanation for the association of increased CD94 and impaired NK cell function that was observed in patients of Group II (high stress, low NK cell lysis). Another potential explanation for the seemingly contradictory finding of decreased levels of CD158b in patients in Group II as compared to patients in Group I is the presence of disparate levels of other regulatory molecules (not measured) between the patients. These findings highlight the complexity of the interactions between KIRs, specifically CD158, and other effectors of the immune response to malignant cells. It should be noted that in order to compare the KIR expression with several conditions (cytokine or media), multiple comparisons were performed. There were 10 statistical comparisons performed on KIR variables (of which three tests were significant) and 30 comparisons performed with other variables (of which none was significant). Thus, there is a possibility that the observed differences seen between the two groups in KIR expression may be attributable to the performance of multiple comparisons, rather than representing a true significant difference between the groups. Interpretation of significant results in the context of multiple comparisons includes examining the consistency or pattern of results. Of note, all significant group differences were observed in KIR expression. However, not all KIR variables were significantly different between groups. The results of this study require replication.
In summary, the presence of high levels of psychologic stress in cancer patients may contribute to impaired immunity. The present study of a small group of breast cancer patients suggests that the observed impairment of NK lysis among patients with high levels of cancer-related psychologic stress may be due to dysregulation of NK cell inhibitory and activating receptors. Further studies in larger numbers of patients are warranted.
LIST OF ABBREVIATIONS
- ADCC
antibody dependent cellular cytotoxicity
- BCT
breast conservation therapy
- Con A
Concavalin A
- E
T, effector-to-target ratio
- IES
Impact of Events Scale
- HPA
hypothalamic-pituitary-adrenal axis
- IL-2
Interleukin-2
- KIR
killer cell immunoglobulin-like receptor
- LAK
lymphokine activated killing
- Mab
monoclonal antibody
- NK cells
natural killer cells
- NK50
NK cell lysis at an effector:target ratio of 50:1
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
Footnotes
Supported by American Cancer Society grant No. PBR-89; the Longaberger Company-American Cancer Society Grant for Breast Cancer Research grant No. PBR-89A; the US Army Medical Research Acquisition Activity grant Nos. DAMD17-94-J-4165, DAMD17-96-1-6294, and DAMD 17-97-1-7062; National Institute of Mental Health grant No. R01MH51487; National Cancer Institute grant No. R0192704, P01 CA95426; K24 CA93670; and General Clinical Research Center grant No. M01-RR0034. KAV is an NRSA T32 fellow (5 T32 CA009338-27).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49(5):389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen BL. Biobehavioral outcomes following psychological interventions for cancer patients. J Consult Clin Psychol. 2003;70(3):590–610. doi: 10.1037//0022-006X.70.3.590. Erratum in J. Consult. Clin. Psychol 71(3):481, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herschbach P, Keller M, Knight L, et al. Psychological problems of cancer patients: a cancer distress screening with a cancer-specific questionnaire. Br J Cancer. 2004;91(3):504–11. doi: 10.1038/sj.bjc.6601986. [Epub ahead of print 2004 Jul 6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamitsu Y, Shimoda K, Abe H, Rani T, Kodama M, Okawa M. Differences in emotional distress between breast tumor patients with emotional inhibition and those with emotional expression. Psychiatry Clin Neurosci. 2003;57:289–94. doi: 10.1046/j.1440-1819.2003.01119.x. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery GH, David D, Goldfarb AB, et al. Sources of anticipatory distress among breast surgery patients. J Behav Med. 2003;26:153–64. doi: 10.1023/a:1023034706298. [DOI] [PubMed] [Google Scholar]
- 7.Morasso G, Costantini M, Baracco G, Borreani C, Capelli M. Assessing psychological distress in cancer patients: validation of a self-administered questionnaire. Oncology. 1996;53(4):295–302. doi: 10.1159/000227576. [DOI] [PubMed] [Google Scholar]
- 8.Sundin EC, Horowitz MJ. Horowitz’s Impact of Event Scale: evaluation of 20 years of use. Psychosom Med. 2003;65:870–6. doi: 10.1097/01.psy.0000084835.46074.f0. [DOI] [PubMed] [Google Scholar]
- 9.van’t Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: a meta-analytical review of 58 studies after 1980. Psychosom Med. 1997;59(3):280–93. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin PJ, Ennis M, Bordeleau LJ, et al. Health-related quality of life and psychosocial status in breast cancer prognosis: analysis of multiple variables. J Clin Oncol. 2004;22(20):4184–92. doi: 10.1200/JCO.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 11.Thomas BC, Mohan VN, Thomas I, Pandey M. Development of a distress inventory for cancer: preliminary results. J Postgrad Med. 2002;48(1):16–20. [PubMed] [Google Scholar]
- 12.Weiss DS, Horowitz MJ, Wilner N. The Stress Response Rating Scale: a clinician’s measure for rating the response to serious life events. Br J Clin Psychol. 1984;23(Pt 3):202–15. [PubMed] [Google Scholar]
- 13.Horowitz M, Wilner N, William A. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. Br J Psychiatry. 2002;180:205–9. doi: 10.1192/bjp.180.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90(1):30–3. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22(17):3570–80. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56(1):1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 18.Bonilla F, Alvarez-Mon M, Merino F, et al. Natural killer activity in patients with breast cancer. Eur J Gynaecol Oncol. 1990;11(2):103–9. [PubMed] [Google Scholar]
- 19.Esterling BA, Keicolt-Glaser JK, Bodnar JC, Glaser R. Chronic stress, social support, and persistent alterations in the natural killer cell response to cytokines in older adults. Health Psychol. 1994;13:291–8. doi: 10.1037//0278-6133.13.4.291. [DOI] [PubMed] [Google Scholar]
- 20.Esterling BA, Kiecolt-Glaser JK, Glaser R. Psychosocial modulation of cytokine-induced natural killer cell activity in older adults. Psychosom Med. 1996;58:264–72. doi: 10.1097/00006842-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Garner WL, Minton JP, James AG, Hoffmann CC. Human breast cancer and impaired NK cell function. J Surg Oncol. 1983;24(1):64–6. doi: 10.1002/jso.2930240115. [DOI] [PubMed] [Google Scholar]
- 22.Hebert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993;113(3):472–86. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 23.Kiecolt-Glaser JK, Marucha WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–6. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 24.Schedlowski M, Jacobs R, Stratmann G, et al. Changes in natural killer cells during acute psychological stress. J Clin Immunol. 1993;13:119–26. doi: 10.1007/BF00919268. [DOI] [PubMed] [Google Scholar]
- 25.Uchida A, Kolb R, Micksche M. Generation of suppressor cells for natural killer activity in cancer patients after surgery. J Natl Cancer Inst. 1982;68(5):735–41. [PubMed] [Google Scholar]
- 26.Beitsch P, Lotzova E, Hortobagyi G, Pollock R. Natural immunity in breast cancer patients during neoadjuvant chemotherapy and after surgery. Surg Oncol. 1994;3(4):211–9. doi: 10.1016/0960-7404(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 27.McCulloch PG, MacIntryre A. Effects of surgery on the generation of lymphokine-activated killer cells in patients with breast cancer. Br J Surg. 1993;80(8):1005–7. doi: 10.1002/bjs.1800800824. [DOI] [PubMed] [Google Scholar]
- 28.Pollock RE, Lotzova E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991;126(3):338–42. doi: 10.1001/archsurg.1991.01410270082013. [DOI] [PubMed] [Google Scholar]
- 29.Pollock RE, Lotzova E, Stanford SD. Surgical stress impairs natural killer cell programming of tumor for lysis in patients with sarcomas and other solid tumors. Cancer. 1992;70(8):2192–2202. doi: 10.1002/1097-0142(19921015)70:8<2192::aid-cncr2820700830>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Farag SS, Caligiuri MA. Human natural killer cell development biology. Blood Rev. 2006;20(3):123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Carson WE, III, Shapiro CL, Crespin T, et al. Cellular immunity in breast cancer patients completing taxane treatment. Clin Cancer Res. 2004;10:3401–9. doi: 10.1158/1078-0432.CCR-1016-03. [DOI] [PubMed] [Google Scholar]
- 32.Snyder MR, Weyand CM, Goronzy JJ. The double life of NK receptors: stimulation or co-stimulation? Trends Immunol. 2004;25(1):25–32. doi: 10.1016/j.it.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Light KC, Girdler SS, Sherwood A, et al. High stress responsivity predicts later blood pressure only in combination with positive family history and high life stress. Hypertension. 1999;33(6):1458–64. doi: 10.1161/01.hyp.33.6.1458. [DOI] [PubMed] [Google Scholar]
- 35.Kirschbaum C, Prussner JC, Stone AA, et al. Persistent high cortisol response to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57(5):468–74. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Moynihan JA. Mechanisms of stress-induced modulation of immunity. Brain Behav Immun. 2003;17(Suppl):S11–6. doi: 10.1016/s0889-1591(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 37.Epel ES, Blackburn EH, Line H, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fawzy FI, Kemeny ME, Fawzy NW, et al. A structured psychiatric intervention for cancer patients. II. Changes over time in immunological measures. Arch Gen Psychiatry. 1990;47(8):729–735. doi: 10.1001/archpsyc.1990.01810200037005. [DOI] [PubMed] [Google Scholar]
- 39.Fawzy FI, Canada AL, Fawzy NW. Malignant melanoma: effects of a brief, structured psychiatric intervention on survival and recurrence at 10-year follow-up. Arch Gen Psychiatry. 2003;60(1):100–3. doi: 10.1001/archpsyc.60.1.100. [DOI] [PubMed] [Google Scholar]
- 40.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–14. doi: 10.1016/s0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 41.Sun PD. Structure and function of natural-killer-cell receptors. Immunol Res. 2003;27(2):539–48. doi: 10.1385/IR:27:2-3:539. [DOI] [PubMed] [Google Scholar]
- 42.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–47. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 43.Lin Chua H, Brahmi Z. Expression of p58.2 or CD94/NKG2A inhibitory receptors in an NK-like cell line, YTINDY, leads to HLA Class I-mediated inhibition of cytotoxicity in the p58.2- but not the CD94/NKG2A-expressing transfectant. Cell Immunol. 2002;219(1):57–70. doi: 10.1016/s0008-8749(02)00578-6. [DOI] [PubMed] [Google Scholar]
- 44.Guerra N, Guillard M, Angevin E, et al. Killer inhibitory receptor (CD158b) modulates the lytic activity of tumor-specific T lymphocytes infiltrating renal cell carcinoma. Blood. 2000;95(9):2883–9. [PubMed] [Google Scholar]
- 45.Nguyen S, Dhedin N, Vernant JP, et al. VNK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105(10):4135–42. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]


