Abstract
Presently, the only effective treatment for celiac disease is a life-long gluten-free diet. In this work, we used a new mixture of selected sourdough lactobacilli and fungal proteases to eliminate the toxicity of wheat flour during long-time fermentation. Immunological (R5 antibody-based sandwich and competitive enzyme-linked immunosorbent assay [ELISA] and R5 antibody-based Western blot), two-dimensional electrophoresis, and mass spectrometry (matrix-assisted laser desorption ionization-time of flight, strong-cation-exchange-liquid chromatography/capillary liquid chromatography-electrospray ionization-quadrupole-time of flight [SCX-LC/CapLC-ESI-Q-TOF], and high-pressure liquid chromatography-electrospray ionization-ion trap mass spectrometry) analyses were used to determine the gluten concentration. Assays based on the proliferation of peripheral blood mononuclear cells (PBMCs) and gamma interferon production by PBMCs and intestinal T-cell lines (iTCLs) from 12 celiac disease patients were used to determine the protein toxicity of the pepsin-trypsin digests from fermented wheat dough (sourdough). As determined by R5-based sandwich and competitive ELISAs, the residual concentration of gluten in sourdough was 12 ppm. Albumins, globulins, and gliadins were completely hydrolyzed, while ca. 20% of glutenins persisted. Low-molecular-weight epitopes were not detectable by SCX-LC/CapLC-ESI-Q-TOF mass spectrometry and R5-based Western blot analyses. The kinetics of the hydrolysis of the 33-mer by lactobacilli were highly efficient. All proteins extracted from sourdough activated PBMCs and induced gamma interferon production at levels comparable to the negative control. None of the iTCLs demonstrated immunoreactivity towards pepsin-trypsin digests. Bread making was standardized to show the suitability of the detoxified wheat flour. Food processing by selected sourdough lactobacilli and fungal proteases may be considered an efficient approach to eliminate gluten toxicity.
Celiac disease (CD) is an inflammatory disorder of the small intestine that affects genetically predisposed individuals when they ingest gluten from any Triticum species and similar proteins of barley and rye and their crossbred varieties.
Genes encoding HLA-DQ2 and HLA-DQ8 predispose to CD by causing the preferential presentation of Pro-rich gluten peptides that have undergone deamidation by tissue transglutaminase (tTG) to mucosal CD4+ T cells (37). Gliadin-reactive CD4+ T cells have a key role in the damage of the intestinal mucosa that culminates with villus atrophy and crypt hyperplasia (12). Several studies have demonstrated the presence of gliadin-reactive T cells within the celiac mucosa, and T-cell clones have been established and used to identify specific gliadin-derived immunogenic peptides (3, 23). Overall, the lamina propriae of CD patients contain significantly increased levels of gamma interferon (IFN-γ) after challenge with gliadin (22). The high level of secretion of the Th1 cytokine IFN-γ by peripheral blood mononuclear cells (PBMCs) in response to incubation with toxic peptides demonstrates a transient, disease-specific, HLA-DQ2-restricted, CD4+-T-cell response to a single dominant epitope (1). Importantly, gliadin-induced IFN-γ production contributes to the onset and maintenance of the chronic inflammatory response at the epithelial layer and the lamina propria (27), presumably by attracting γδ CD8+ and αβ CD4+ T cells and activating the Janus kinase-signal transducer and activator of transcription 1 pathway.
The prevalence of CD worldwide is increasing; it is estimated to be 0.5 to 2.0% in most of the European countries and the United States (31). Presently, the only treatment for CD consists of a life-long gluten-free diet (GFD). The Codex Alimentarius Commission of the World Health Organization and the FAO distinguishes gluten-free foods as those consisting of ingredients with a gluten level of <20 ppm or those which have been rendered gluten free with a gluten level of <200 ppm (16).
Several attractive targets for new CD treatments are under investigation. Complementary strategies aiming to interfere with the activation of gluten-reactive CD4+ T cells include the inhibition of intestinal tTG activity to prevent the selective deamidation of gluten immunogenic peptides (25) and the blockage of the binding of gluten epitopes to the HLA-DQ2 and HLA-DQ8 molecules (21). Other treatments involve cytokine therapy (32), selective adhesion molecule inhibitors (37), and peptide degradation by prolyl endopeptidases (PEPs) of microbial origin (24, 29, 34, 35). Beyond therapeutic treatments, there have been recent efforts to use the knowledge on the toxicity of gluten sequences for developing wheat that is free of such sequences (18). In the last decades, cereal food technology has changed dramatically by influencing the dietary habitudes of entire populations previously naïve to gluten exposure. Cereal baked goods are presently manufactured by fast processes in which long-time fermentations by sourdough, a cocktail of acidifying and proteolytic lactic acid bacteria with or without Saccharomyces cerevisiae, have been almost totally replaced by the use of chemical and/or baker's yeast leavening agents. In this technology, cereal components (e.g., proteins) are not degraded during manufacture (17). Recent studies (6, 7, 10, 11) showed that the manufacture of wheat and rye breads or pasta with flours made tolerable to CD patients by using selected sourdough lactobacilli may markedly decrease the toxicity of prolamin epitopes but that the concentration of gluten in these foods remains above 6,000 ppm.
This paper describes the use of a more complex formula, consisting of sourdough lactobacilli selected for their peptidase systems and fungal proteases active specifically towards gluten, than the above-cited studies for the manufacture of wheat bread having a concentration of gluten of <20 ppm. Wheat protein hydrolysis was determined by complementary techniques such as two-dimensional electrophoresis (2DE), matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS), strong-cation-exchange (SCX)-liquid chromatography (LC)/capillary LC (CapLC)-electrospray ionization (ESI)-quadrupole-time of flight (Q-TOF) MS, R5 antibody-based enzyme-linked immunosorbent assay (ELISA), and R5-based Western blot analyses. In vitro assays of PBMC-lymphocyte proliferation and IFN-γ production from PBMCs and intestinal T-cell lines (iTCLs) confirmed the absence of toxicity of hydrolyzed wheat proteins.
MATERIALS AND METHODS
Microorganisms and enzymes.
Lactobacillus alimentarius 15 M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B (defined as pool 1) were previously selected based on their capacity to hydrolyze gliadins (10). L. sanfranciscensis LS3, LS10, LS19, LS23, LS38, and LS47 (defined as pool 2) were selected based on their peptidase systems, with particular reference to activities towards Pro-rich peptides (8). Strains were propagated for 24 h at 30°C in MRS broth (Oxoid, Basingstoke, Hampshire, United Kingdom) with the addition of fresh yeast extract (5%, vol/vol) and 28 mM maltose at a final pH of 5.6. When used for wheat sourdough fermentations, Lactobacillus cells were cultivated until the late exponential phase of growth was reached (ca. 12 h).
Proteases of Aspergillus oryzae (500,000 hemoglobin units on the tyrosine basis/g; enzyme 1 [E1]) and A. niger (3,000 spectrophotometric acid protease units/g; enzyme 2 [E2]), routinely used for bakery applications, were supplied by BIO-CAT Inc. (Troy, VA).
Wheat sourdough fermentation.
The characteristics of the wheat (Triticum aestivum cv. Appulo) flour used in this study were as follows: moisture content, 12.8%; protein content (approximately equal to the organic nitrogen content multiplied by 5.70), 10.3% (dry weight); fat content, 1.8% (dry weight); ash content, 0.6% (dry weight); and total soluble carbohydrate content, 1.5% (dry weight). The following formulas were used for sourdough fermentation: sourdough 1 (S1), 80 g of wheat flour and 320 g of tap water containing 5 × 108 CFU/g of pool 1 (final density in the dough); S2, the S1 formula with the addition of 5 × 108 CFU/g of pool 2; S2E1, the S2 formula with the addition of 200 ppm of E1; S2E2, the S2 formula with the addition of 200 ppm of E2; and S2E12, the S2 formula with the addition of 200 ppm of both E1 and E2. Doughs were incubated for 48 h at 37°C with stirring (ca. 200 rpm). A chemically acidified dough (CAD) without a bacterial and enzyme inoculum was acidified to pH 3.5 by a mixture of lactic and acetic acids (molar ratio, 4:1) and used as the control. For each condition, four independent fermentations were carried out.
Extraction of wheat flour proteins and electrophoresis.
Wheat flour proteins were selectively extracted by following the method of Weiss et al. (46). For Tricine-sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), immunological, and MS analyses, protein extraction was carried out directly with 60% ethanol to include both peptides and proteins that were hydroalcoholically soluble. The protein concentration was determined by the Bradford method (5). The organic nitrogen concentration was determined using the Kjeldahl method. Free amino acids were analyzed by a series 30 amino acid analyzer (Biochrom Ltd., Cambridge Science Park, United Kingdom) (10).
2DE of aliquots of ca. 30 μg of protein from extracted fractions was performed with the Immobiline-polyacrylamide system (6, 10). Isoelectric focusing on immobilized pH gradient strips (Immobiline strips; Amersham Pharmacia Biotech, Uppsala, Sweden) was carried out to provide a linear gradient of pH 6.0 to 11.0 for gliadin fractions or a nonlinear gradient of pH 3.0 to 10.0 for albumin-globulin and glutenin fractions by using an IPGphor system. Gels were silver stained and spot intensities were normalized as reported by Bini et al. (4). Four gels from independent fermentations were analyzed.
Aliquots of 10 to 20 μl (ca. 10 μg of protein) of extracted fractions were also analyzed by Tricine-SDS-PAGE according to the method of Schägger and von Jagow (33).
Immunological and MALDI-TOF MS analysis.
Immunological analyses were carried out by using R5 antibody-based sandwich and competitive ELISAs and R5 antibody-based Western blotting. The R5 monoclonal antibody and the horseradish peroxidase-conjugated R5 antibody were used for gluten analysis. The R5-based sandwich ELISA (43) was performed with the Transia plate detection kit by following the instructions of the manufacturer (Diffchamb, Västra Frölunda, Sweden). The R5-based competitive ELISA (15) was performed at the gluten unit of the Centro National de Biotecnologia (Madrid, Spain). For R5-based Western blot analysis, after one-dimensional SDS-PAGE or Tricine-SDS-PAGE, proteins were electrotransferred onto polyvinylidene difluoride membranes, the membranes were incubated directly with R5-horseradish peroxidase, and the blots were developed by immunodetection with the ECL Western blotting analysis system (Amersham Pharmacia) (43).
MALDI-TOF MS analysis was carried out with a Voyager De Pro workstation (PerSeptive Biosystems, United Kingdom). Eight microliters of 50 mM octyl-d-glucopyranoside detergent and 25 μl of saturated sinapic acid in 30% (vol/vol) acetonitrile solution, containing 0.1% (vol/vol) trifluoroacetic acid (TFA), were added to 100 μl of gliadin ethanol extracts. The matrix-sample mixture and the experimental conditions used were described previously (19). A standard of European gliadins was also included in the analyses.
Off-line bidimensional SCX-LC/CapLC followed by ESI-Q-TOF MS analysis of gliadin peptides.
The SCX-LC apparatus consisted of a binary LC pump (model no. 1525μ; Waters, Milford, MA), an SCX BIO BASIC column (ThermoElectron, San Jose, CA), and a Rheodyne six-port injector. SCX-LC separations were performed at a 0.2-ml/min flow rate by using a binary gradient with (i) a 60% water-40% acetonitrile (vol/vol) mixture (mixture A) and (ii) mixture A containing 300 mM ammonium acetate (mixture B; elution program, 0 to 40% mixture B in 50 min, return to initial conditions in 10 min, and column reconditioning for 20 min). UV detection was performed at 240 nm by using a UV-VIS detector (model no. 2487; Waters). The UV trace, processed by the MassLynx 4.0 software, was employed as a guide for the collection of fractions to be further separated and analyzed by CapLC-ESI-Q-TOF MS. This stage of analysis was performed under the conditions described by De Angelis et al. (6).
Kinetics of hydrolysis and high-pressure liquid chromatography (HPLC)-ESI MS analysis of the 33-mer.
A mixture containing the microbial pools 1 and 2, 2 mM 33-mer peptide, and 0.05% (wt/vol) NaN3 in 1 ml of 200 mM phosphate buffer, pH 7.5, was incubated for 48 h at 37°C with stirring (150 rpm). Aliquots were taken at intervals, and TFA (0.1% [vol/vol] final concentration) was added. After centrifugation, supernatants corresponding to extracellular solutions were collected. Harvested cells were resuspended in 100 μl of 200 mM phosphate buffer, pH 7.5, and sonicated to obtain the cytoplasmic extracts. Extracellular solutions and cytoplasmic extracts were stored at −80°C until MS analysis.
Reverse-phase HPLC coupled with ESI-ion trap MS was adopted for the analysis of the 33-mer either in the extracellular solutions or in the cytoplasmic extracts. The HPLC apparatus consisted of a Waters 600 MS chromatographic pump and a Supelcosil LC-18-DB column (250 by 2.1 mm in diameter; Supelco, Bellefonte, PA) connected to an LCQ ion trap mass spectrometer (ThermoElectron) through its ESI interface; the divert/inject six-port valve embedded in the spectrometer was used for loop injection (injection volume, 40 μl) of samples.
HPLC separations were performed at a flow rate of 0.16 ml/min by using gradient elution with (i) water and (ii) acetonitrile, both containing 0.1% (vol/vol) TFA, according to the following program: 0 to 70% (vol/vol) acetonitrile in 35 min, isocratic elution with 70% acetonitrile for 5 min, return to 0% acetonitrile in 5 min, and column reconditioning for 20 min. The LCQ spectrometer, completely controlled by the Xcalibur software (ThermoElectron), was operated in the positive ion mode; MS chromatograms in the total ion current (m/z range, 50 to 2,000) and selected ion monitoring (for an m/z of 1,955.5, corresponding to the doubly charged ion [M + 2H]2+ of the 33-mer) modes were recorded for each sample.
Pepsin-trypsin (PT) digests and patients.
Protein fractions selectively extracted from CAD and sourdoughs were subjected to sequential PT hydrolysis steps to simulate in vivo digestion (6). After digestion, the PT digests were heated at 100°C for 30 min to inactivate enzymes and then freeze dried.
Six newly diagnosed CD patients (age range, 18 to 54 years) were included in the study. CD was diagnosed according to European Society for Paediatric Gastroenterology, Hepatology and Nutrition criteria (14). All six patients were on gluten-containing diets at the time of enrollment. A sample of peripheral blood was taken from each patient on the day of diagnosis by gastrointestinal endoscopy.
For the generation of T-cell lines, biopsy specimens from the small intestines of six additional patients (age range, 18 to 49 years) undergoing treatment for CD were obtained. All patients expressed the HLA-DQ2 phenotype.
Culture of PBMCs, MTT assay, and IFN-γ evaluation.
PBMCs were isolated from 6 ml of heparinized blood by Lympholyte-H (Cederlane, Hornby, Ontario, Canada) gradient centrifugation and cultured at a density of 1.0 × 106 cells per ml in 96-well culture plates in RPMI 1640 culture medium supplemented with 10% fetal bovine serum, streptomycin (50 ng/ml), and penicillin (100 g/ml; GIBCO-Invitrogen Ltd., Paisley, United Kingdom). After 24 h, PBMCs were treated in triplicate (independent experiments) with the PT digest of glutenins or gliadins (0.5 mg/ml). After 24 h of stimulation, PBMCs were harvested for the MTT [3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide] assay, and the free supernatant was collected and stored at −80°C for IFN-γ evaluation.
To assess the effect of treatments on the PBMC-lymphocyte proliferation, the MTT assay (MTT-based cell growth determination kit; Sigma, St. Louis, MO) was carried out. After 24 h of incubation, measurements were carried out by an ELISA reader (Bio-Rad, Hercules, CA) at 570 nm by using a 630-nm-pore-size filter as a reference. The proliferative response in the presence of the culture medium alone (negative control) was taken as 100%, and the percentage of inhibition in response to each PT digest was calculated.
The concentrations of IFN-γ in PBMC supernatant samples were determined by commercial ELISA kits according to the instructions of the manufacturer (Biosource, Camarillo, CA). Standards were run on each plate. Samples from the different patients were run at the same time. The level of IFN-γ production in the presence of the culture medium alone was taken as 100%, and the responses of PBMCs treated with PT digests were compared to this standard.
Generation of gliadin-specific iTCLs and T-cell specificity assays.
Jejunal explants were digested with collagenase A as described previously (42). T-cell lines were established by repeated stimulation (every 7 days) of intestinal cells with 50 μg of tTG-deamidated gliadin/ml and autologous irradiated PBMCs in complete culture medium containing 10% human serum. Gliadin-specific iTCLs were kept as long-term cell cultures by cyclic restimulation (every 14 days) with phytohemagglutinin and feeder cells and interleukin-2.
iTCLs were tested for the recognition of gluten-derived fractions from both CAD and sourdoughs by the detection of IFN-γ production by ELISAs. tTG-treated deamidated PT digests of albumin-globulin, gliadin, and glutenin fractions were used as antigens. Intestinal cells (0.3 × 105) were plated in 200 μl of 10% human serum in the presence of irradiated (11,000 rad) autologous or HLA-matched B lymphoblastoid cell lines transformed with Epstein-Barr virus and antigens (50 μg/ml). A tTG-PT-gliadin from T. aestivum cv. Sagittario was used as a positive control. After 48 h, cell supernatants (50 μl) were collected for the assessment of IFN-γ production; thereafter, cells were labeled with 0.5 μCi of [3H]thymidine (Amersham Pharmacia)/well and harvested after 16 h. IFN-γ production was analyzed using a sandwich ELISA manufactured in-house with a pair of anti-IFN-γ monoclonal antibodies (purified and biotinylated; Pharmingen- BD). All experiments were performed in duplicate. T-cell lines were considered antigen specific when IFN-γ production increased more than twofold with respect to that in the medium alone.
Bread making.
Baker's yeast bread was made by short fermentation: a mixture of 125 g of wheat flour and 64 ml of tap water (containing 1.5% baker's yeast) was allowed to ferment for 2 h at 37°C and baked at 250°C for 15 min. Sourdough bread was made using the same formula and conditions described for S2E12. After fermentation for 48 h at 37°C, water was removed by spray drying, the flour was milled, and the following formula was used for bread making: 125 g of fermented wheat flour (moisture content, ca. 12%), 100 ml of tap water (containing 1.5% baker's yeast), 6% (wt/wt) cornstarch (Clearam CH 20; product no. E1442; CHIMAB Spa, Pordenone, Italy), and 3% (wt/wt) xanthan gum (product no. E415; CHIMAB Spa). Compared to that in the baker's yeast bread formula, the amount of water was increased to permit the optimal performance of the structuring agents. The dough was mixed continuously with a high-speed mixer (60 × g; dough mixing time, 5 min; Chopin and Co., Boulogne-sur-Seine, France), allowed to ferment for 2 h at 37°C, and baked at 250°C for 15 min.
Four independent baking tests were carried out, and loaf volumes (as determined by rapeseed displacement) and weights were measured.
RESULTS
Immunological analyses.
Cell numbers of lactic acid bacteria in sourdoughs at the end of fermentation ranged from 3 × 109 to 5 × 109 CFU/g of dough, and the pH was ca. 3.5. The total bacterial count in the CAD did not exceed 103 CFU/g of dough.
After fermentation, samples were analyzed by an R5-based sandwich ELISA (Table 1). Compared to that in CAD, the concentration of gluten in the sourdough progressively decreased in the order of S1, S2, S2E1, S2E2, and S2E12. The concentration of residual gluten in S2E12 was 12 ppm. The same level of gluten was found by an R5-based competitive ELISA. This sourdough was fermented with microbial pool 1, consisting of L. alimentarius 15 M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B (10); microbial pool 2, consisting of L. sanfranciscensis LS3, LS10, LS19, LS23, LS38, and LS47 (8); and the addition of proteases from A. oryzae (E1) and A. niger (E2). None of the other possible combinations not included in Table 1 gave a concentration of gluten of less than 20 ppm. Relative to the European gliadin standard and the CAD, no traces of gliadins in S2E12 were detectable by R5-based Western blotting (Fig. 1). The same was found regarding hydrolysis end products.
TABLE 1.
Concentrations of gluten in CAD and sourdoughs started with different mixtures of lactobacilli and enzymes as estimated by R5-based sandwich ELISAa
| Dough or sourdough | Gluten concn (ppm) |
|---|---|
| CAD | 74,592 ± 320 |
| S1 | 20,315 ± 112 |
| S2 | 12,362 ± 86 |
| S2E1 | 4,895 ± 92 |
| S2E2 | 1,055 ± 45 |
| S2E12 | 12 ± 2 |
CAD and sourdoughs were fermented for 48 h at 37°C. Details about sourdough compositions, initial cell densities, and enzyme concentrations are given in Materials and Methods. Data are the means from four independent fermentations each analyzed twice.
FIG. 1.
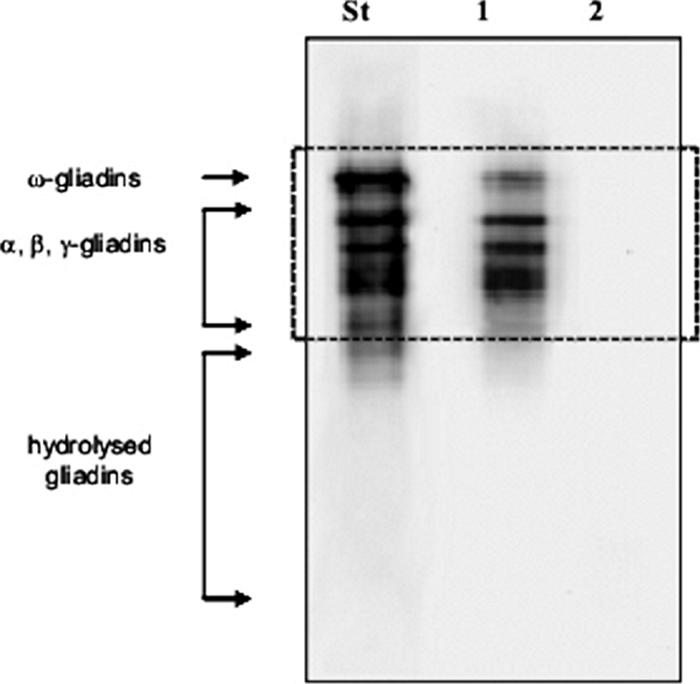
R5-based Western blot analyses of the European gliadin reference (St), CAD (lane 1), and S2E12 (lane 2) fermented for 48 h at 37°C. After one-dimensional SDS-PAGE, proteins were electrotransferred for R5-based Western blot analysis. S2E12 ingredients are described in Materials and Methods.
2DE.
2DE analysis of the CAD resolved 218 albumin-globulin polypeptides with pIs of 4.15 to 9.7 and molecular masses of 6.0 to 74.5 kDa. No albumin-globulin polypeptides in S2E12 were detectable. Seventy-six gliadin polypeptides in the CAD were detected by 2DE analysis. Polypeptides had pIs of 6.5 to 9.9 and molecular masses of 9.2 to 54.1 kDa. No gliadin polypeptides in S2E12 were detectable. 2DE analysis of the CAD resolved 193 glutenin polypeptides with pIs of 4.10 to 9.45 and molecular masses of 11.05 to 81.8 kDa (Fig. 2A). Although marked hydrolysis was found, some glutenin polypeptides persisted in S2E12 (Fig. 2B). Of the 193 spots identified for the CAD, 160 showed hydrolysis factors which ranged from 95 to 100% and 24 and 9 demonstrated hydrolysis of 50 to 70% and 30 to 50%, respectively.
FIG. 2.
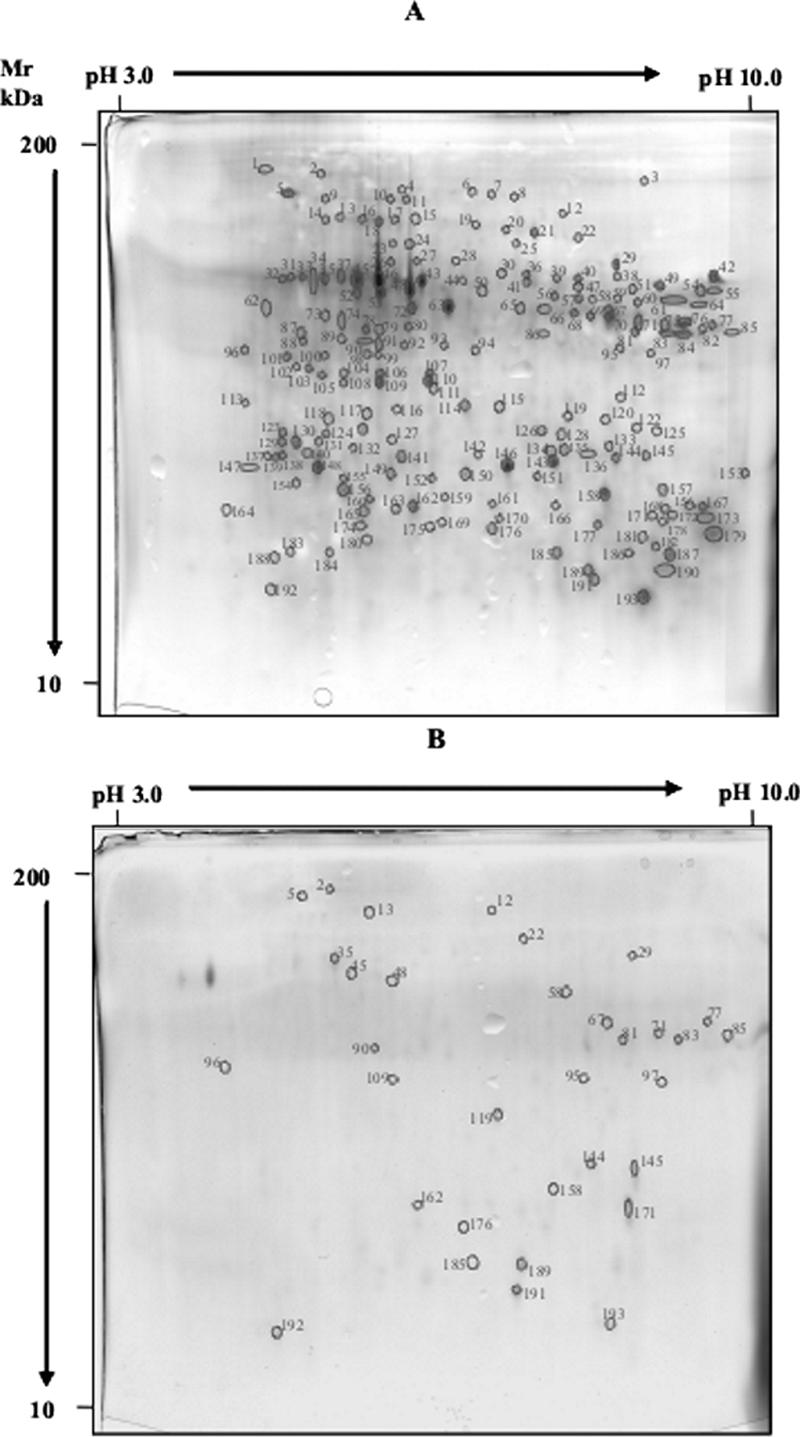
2DE analysis of glutenin polypeptides of CAD (A) and S2E12 (B) fermented for 48 h at 37°C. S2E12 ingredients are described in Materials and Methods. Mr, molecular mass.
MALDI-TOF MS.
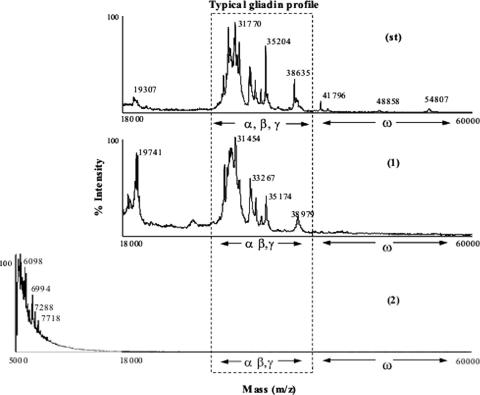
CAD and S2E12 were subjected to extraction by 60% ethanol and analyzed by MALDI-TOF MS (Fig. 3). Gliadin peaks corresponding to the European gliadin standard and the CAD completely disappeared for S2E12. To look for small polypeptides, the m/z range used to analyze S2E12 was extended. A few peaks corresponding to molecular masses of less than ca. 8 kDa were found.
FIG. 3.
MALDI-TOF mass spectra of the European gliadin reference (st), CAD (1), and S2E12 (2) fermented for 48 h at 37°C. S2E12 ingredients are described in Materials and Methods.
Protein and peptide concentrations.
Spray-dried flour from CAD contained 0.29% water/salt-soluble proteins (mainly albumins and globulins; 16.3% of the total organic nitrogen content), 0.66% gliadins (37.1% of the total organic nitrogen content), and 0.83% glutenins (46.6% of the total organic nitrogen content), with a total organic nitrogen content of 1.78% that approximately corresponded to the protein concentration (the organic nitrogen content multiplied by 5.70 is 10.15%, versus a protein concentration of 10.3%) of the wheat flour used. The spray-dried flour from S2E12 showed a marked increase in the water/salt-soluble fraction to 1.66% (90.7% of the total organic nitrogen content), which corresponded to the accumulation of water/salt-soluble low-molecular-mass peptides and amino acids derived from the hydrolysis of all wheat proteins. Indeed, no organic nitrogen was detectable in the gliadin fraction, and the level of glutenins decreased to 0.17% (9.3%). The concentrations of free amino acids confirmed this difference: the CAD had ca. 1,050 mg/kg, while S2E12 contained ca. 14,622 mg/kg. Leu, Val, Glu, Ile, and Pro were present at the highest concentrations.
The Tricine-SDS-PAGE of the 60% ethanol-soluble fraction of S2E12 confirmed the presence of a few polypeptides with molecular masses of less than ca. 8 kDa. No immunogenic polypeptides were detected by the R5 monoclonal antibody in Tricine-SDS-PAGE. Both the 60% ethanol-soluble and the water/salt-soluble fractions of S2E12 were further analyzed using SCX-LC/CapLC followed by ESI-Q-TOF MS. Fractions were collected from the SCX-LC eluate whenever a peak with significant absorbance was observed; each of the fractions was then subjected to CapLC-ESI-Q-TOF MS analysis. Mass spectra related to each peak detected in the relevant total ion current traces were elaborated in order to search for m/z of [M + nH]n+ (where n is a variable) ions arising from gliadin peptides. No peak could be related to such species. One of the SCX-LC fractions from the water/salt-soluble fraction was spiked with 1 ppm of the synthetic epitope spanning residues 62 to 75 of α-gliadin (1) and analyzed by CapLC-ESI-Q-TOF MS. A very intense peak was found, and the corresponding mass spectra confirmed the attribution of the epitope to the fragment comprising residues 62 to 75. Therefore, if present, gliadin peptides with molecular masses lower than 3 kDa (the detectable molecular mass limit for the ESI-Q-TOF MS analysis) had concentrations well below 1 ppm.
Kinetics of hydrolysis of the 33-mer.
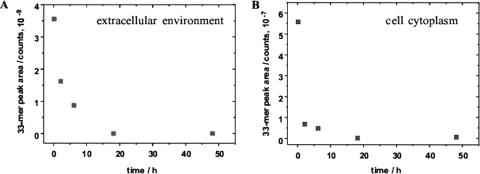
To elucidate the mechanism of the hydrolysis of gliadin epitopes, lactobacillus pools 1 and 2 were incubated in a buffer system with 2 mM 33-mer. The concentrations of the 33-mer peptide both in the extracellular environment and the cell cytoplasm were monitored for 48 h using HPLC-ESI-ion trap MS. The 33-mer was found in the cell cytoplasm as soon as the incubation started, although its concentration could be estimated to be 100-fold lower than the one in the extracellular environment (Fig. 4). Remarkable decreases in the peptide concentrations in both media were also observed, although the decrease in the cytoplasm was sharper. About 70% of the 33-mer was degraded in 6 h, and its hydrolysis was completed after 18 h. No traces of the 33-mer were detectable in the cell cytoplasm. Potential 33-mer hydrolysis products were searched for in both samples, after each of the incubation periods. No evidence of their presence, at least at the minimum detectable concentration (20 ppm), was found. This minimum concentration was estimated by injecting each sample with the synthetic analogue of peptide QLQPFPQPQLPY, a potential hydrolytic product of the 33-mer.
FIG. 4.
Time dependence of the peak areas obtained for the 33-mer in the selected ion monitoring mode in the HPLC-ESI-ion trap MS analysis of the extracellular environment (A) and lactobacillus cell cytoplasm (B).
PBMC proliferation.
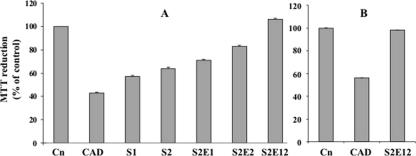
Polypeptides soluble in 60% ethanol and glutenins were extracted from CAD and the sourdoughs, subjected to PT hydrolysis, and used to treat PBMCs. The MTT assay was used to asses the lymphocyte proliferation, which decreased in proportion to the activation of apoptosis in the presence of the antigen. The proliferation rate of PBMCs subjected to PT digests of polypeptides soluble in 60% ethanol increased according to the intensity of gluten hydrolysis in the sourdoughs (Fig. 5A and Table 1). The rate of proliferation in the presence of the PT digest from S2E12 was the same as that in the negative control. The same result was found for the PT digest of glutenins from S2E12 (Fig. 5B).
FIG. 5.
Effects of the PT digests of polypeptides soluble in 60% ethanol (A) and glutenins (B) extracted from CAD and sourdoughs S1, S2, S2E1, S2E2, and S2E12 on PBMC proliferation. Cn, negative control. The proliferative response in the presence of the culture medium alone (negative control) was taken as 100%, and the percentage of inhibition in response to each PT digest was calculated. Sourdough ingredients are described in Materials and Methods. Data are the means ± standard deviations from three independent fermentations each analyzed twice.
IFN-γ production by PBMCs and iTCLs.
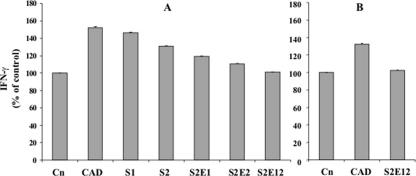
The exposure of lymphocytes to PT digests from different sourdoughs resulted in a progressive decrease in the cytokine production (Fig. 6A). The release of IFN-γ by PBMCs treated with the PT digest from S2E12 was the same as that in the negative control. No differences in glutenins between the negative control and PBMCs treated with the PT digest from S2E12 were found (Fig. 6B).
FIG. 6.
Effects of the PT digests of polypeptides soluble in 60% ethanol (A) and glutenins (B) extracted from CAD and sourdoughs S1, S2, S2E1, S2E2, and S2E12 on IFN-γ production by PBMCs. Cn, negative control. The IFN-γ production in the presence of the culture medium alone was taken as 100% and used as a standard to assess the responses of PBMCs treated with PT digests. Sourdough ingredients are described in Materials and Methods. Data are the means ± standard deviations from three independent fermentations each analyzed twice.
IFN-γ production in the culture supernatants was measured after 48 h of gliadin stimulation of long-term iTCLs established from intestinal mucosae of CD patients and highly responsive to tTG-PT-gliadin. iTCLs produced significant levels of IFN-γ in response to gliadins from cv. Sagittario and albumin-globulin, gliadin, and glutenin fractions from the CAD (Fig. 7). The IFN-γ level induced by the PT digest of CAD-derived gliadins was comparable to that in the positive control (cv. Sagittario). PT-glutenin stimulation resulted in lower levels of IFN-γ production than stimulation with albumin-globulin and gliadin fractions from CAD. On the contrary, no IFN-γ production was found following stimulation with any of the PT digests corresponding to S2E12.
FIG. 7.
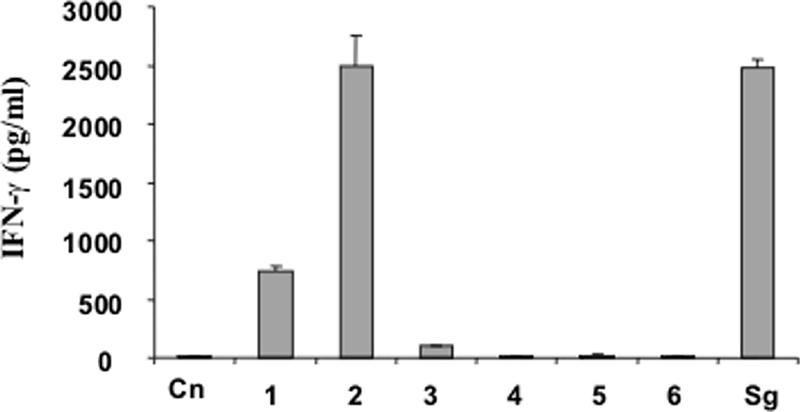
Effect of tTG-deamidated PT digests of proteins extracted from CAD (columns 1, 2, and 3) and S2E12 (columns 4, 5, and 6) on IFN-γ production by iTCLs. Columns: Cn, negative control (medium alone); 1 and 4, albumins and globulins; 2 and 5, gliadins; 3 and 6, glutenins; Sg, PT digest of gliadins from cv. Sagittario. The IFN-γ level in 48-h cell culture supernatant was measured by a sandwich ELISA. S2E12 ingredients are described in Materials and Methods. Data are the means ± standard deviations from two independent fermentations each analyzed twice.
Bread making.
After the fermentation of S2E12, the water was removed and the pretreated wheat flour was used for bread making by using baker's yeast and structuring agents. This bread (sourdough bread) was compared to baker's yeast bread made with untreated flour and without structuring agents. The specific loaf volume (vol/wt) of the sourdough bread was similar to that of the baker's yeast bread (1.9 ± 0.06 cm3/g versus 2.06 ± 0.06 cm3/g, respectively).
DISCUSSION
Although new and attractive molecular treatments are under investigation (21, 25, 32, 37), presently, a GFD remains the only effective therapy for CD. Nevertheless, a GFD also has some drawbacks. It is expensive, gluten-free products have poor sensory and shelf life properties, and the diet is hard to follow and needs continuous monitoring by dieticians, also due to occasional malnutrition problems (18, 41). On the other hand, the development of wheat varieties that are free of toxic polypeptide sequences is considered unlikely because of the high degree of sequence homology existing among members of the cereal protein family and because cereals like wheat are hexaploid (18).
Novel proteomic technologies to eliminate toxic epitopes during food processing were pursued in this study. Food-grade sourdough lactobacilli, as cell factories for multiple and complementary proteolytic enzymes, supplemented with fungal proteases were used. This approach conceptually resembles that of other studies (24, 29, 34, 35), which used microbial PEPs but as an oral supplement to the diet. Flavobacterium meningosepticum PEP-catalyzed cleavage activity was shown to be complementary to intestinal brush border enzymes. The pretreatment of gluten with PEP of F. meningosepticum prevented the development of fat or carbohydrate malabsorption in the majority of those patients who were subjected to a 2-week gluten challenge (30). A newly identified PEP from A. niger efficiently degrades T-cell-stimulatory peptides as well as a PT digest of gluten and intact gluten (39). In our study, the addition of fungal proteases routinely used in bread making was useful to start the primary proteolysis of wheat proteins. After primary hydrolysis, medium-sized polypeptides, including the 33-mer and analogue epitopes, are efficiently transported into lactobacillus cytoplasm and subjected to peptidase activities (17).
Previous in vivo, ex vivo, and in vitro assays showed that a few of the sourdough lactobacilli (pool 1) considered in this study, although markedly decreasing the toxicity of gluten epitopes (6, 7, 10, 11), were helpful only in eliminating the risk of gluten cross contamination. Aiming at manufacturing a bread made of wheat flour alone that could be tolerated by CD patients, further efforts were made in this study to increase the hydrolyzing capacity during sourdough fermentation (7, 10, 11). Besides adding fungal proteases, we employed a new pool (pool 2) of selected L. sanfranciscensis strains (8). These strains are characterized by marked peptidase activity towards Pro-rich peptides. As required by the Codex Alimentarius Commission, the hydrolyzed wheat flour contained <20 ppm of gluten. This threshold was also confirmed by the R5-based competitive ELISA (15) that allows the quantification of hydrolyzed gliadins, the level of which may be underestimated in some cases by the R5-based sandwich ELISA, in foods that have been processed by enzymatic procedures. The gluten concentration was also consistent with the recommendation of the Prolamins Working Group, which suggested a lower limit than the previous threshold of 200 ppm (40). After hydrolysis, S2E12 spray-dried flour was a mixture of mainly water/salt-soluble low-molecular-mass peptides and amino acids. By using several complementary immunological, electrophoresis, and MS techniques, it was shown that gliadin and albumin-globulin were completely degraded, as were the glutenins for the most part. As determined by SCX-LC/CapLC-ESI-Q-TOF MS and R5-based Western blot analyses, no epitopes with molecular masses of ca. <8 kDa were detectable in the 60% ethanol-soluble and water/salt-soluble fractions. About 20% of the glutenin fraction persisted in the sourdough S2E12. Glutenins may contain sequences (e.g., glt04 residues 707 to 742) that activate T cells from the small intestine and result in the secretion of large amounts of IFN-γ (38, 44). High-molecular-mass glutenin subunits stimulate T-cell lines from some CD patients and exacerbate CD in vivo (9). For these reasons, the present diagnostic value of the R5 antibody for detecting toxic epitopes may be in part limited, and in vitro assays (e.g., those involving iTCLs and PBMCs) are imposed to confirm the absence of toxicity (20).
Although peripheral lymphocytes cannot reflect the cytokine profile of the tissue under analysis, some studies (1, 2) have shown the suitability of PBMCs as a model system. Besides, the in vitro incubation of PBMCs from CD patients with PT digests provides a noninvasive system to screen the toxicity of sourdough samples. As expected, the PT digests from the CAD caused a marked decrease in PBMC proliferation due to the related activation of apoptosis that occurs when highly activated lymphocytes again encounter the antigen (47). Responses to PT digests from various sourdoughs differed and culminated with the response to the PT digest from S2E12, which activated PBMCs to the level in the negative control. IFN-γ production is a Th1-specific event (2), whereas proliferation is an index of T-cell activation. Indeed, the proliferation of PBMCs of CD patients is inhibited by anti-DR antibody, indicating that class II molecules are involved in the presentation of gliadin peptides to peripheral T cells (28). IFN-γ coordinates many aspects of the innate and adaptive immune responses, and it is the principal cytokine produced by αβ CD4+ reactive T cells upon gluten activation (26, 27, 32, 45). The PT digests of 60% ethanol-soluble polypeptides and glutenins from sourdough S2E12 had the same capacity to induce IFN-γ as the negative control. To exclude immunoreactivity, the immune responses of iTCLs to the PT digest of S2E12 was also assayed (3, 13, 20, 36, 42). Six gluten-specific iTCLs obtained from jejunal explants increased IFN-γ production when stimulated by protein fractions from the CAD. None of the iTCLs used in this study demonstrated immunoreactivity when treated with the PT digest of S2E12.
Technologically, an expected perplexity concerns the suitability of such wheat flour to ensure bread quality given the complete degradation of gluten. Spray-dried flour from S2E12 was used as an ingredient in bread made by conventional short-time fermentation with baker's yeast and structuring agents. The specific loaf volume of this bread was just slightly lower than that of baker's yeast bread, and the bread had the typical flavor of sourdough wheat bread as judged by an internal panel test. Given the encouraging results of this study, research including a long-term in vivo challenge of CD patients is in progress.
Acknowledgments
This work was supported by the Italian Ministry of University and Research, project no. 12819, D.D. 1801 (31 December 2004).
Footnotes
Published ahead of print on 18 May 2007.
REFERENCES
- 1.Anderson, R. P., P. Degano, J. Godkin, D. P. Jewell, and A. V. S. Hill. 2000. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6:337-342. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, R. P., D. A. van Heel, J. A. Tye-Din, D. P. Jewell, and A. V. S. Hill. 2006. Antagonists and non toxic variants of the dominant wheat gliadin T cell epitope in coeliac disease. Gut 55:485-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arentz-Hansen, H., R. Korner, O. Molberg, H. Quarsten, W. Vader, Y. M. Kooy, K. E. A. Lundin, F. Koning, P. Roepstorff, L. M. Sollid, and S. N. McAdam. 2000. The intestinal T cell response to alpha-gliadin in adult coeliac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 191:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bini, L., B. Magi, B. Marzocchi, F. Arcuri, S. Tripodi, M. Cintorino, J. C. Sanchez, S. Frutiger, and D. Hochstrasser. 1997. Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis 18:2832-2841. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.De Angelis, M., C. G. Rizzello, A. Fasano, M. G. Clemente, C. De Simone, M. Silano, M. De Vincenzi, I. Losito, and M. Gobbetti. 2006. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue. Biochim. Biophys. Acta 1762:80-93. [DOI] [PubMed] [Google Scholar]
- 7.De Angelis, M., R. Coda, M. Silano, F. Minervini, C. G. Rizzello, R. Di Cagno, O. Vicentini, M. De Vincenzi, and M. Gobbetti. 2006. Fermentation by selected sourdough lactic acid bacteria to decrease the intolerance to rye and barley flours. J. Cereal Sci. 43:301-314. [Google Scholar]
- 8.De Angelis, M., R. Di Cagno, G. Gallo, M. Curci, S. Siragusa, C. Crecchio, E. Parente, and M. Gobbetti. 2007. Molecular and functional characterization of Lactobacillus sanfranciscensis strains isolated from sourdoughs. Int. J. Food Microbiol. 114:69-82. [DOI] [PubMed] [Google Scholar]
- 9.Dewar, D. H., M. Amato, H. J. Ellis, E. L. Pollock, N. Gonzalez-Cinca, H. Wieser, and P. J. Ciclitira. 2006. The toxicity of high molecular weight glutenin subunits of wheat to patients with coeliac disease. Eur. J. Gastroenterol. Hepatol. 18:483-491. [DOI] [PubMed] [Google Scholar]
- 10.Di Cagno, R., M. De Angelis, S. Auricchio, L. Greco, C. Clarke, M. De Vincenzi, C. Giovannini, M. D'Archivio, F. Landolfo, and M. Gobbetti. 2004. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 70:1088-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.di Cagno, R., M. De Angelis, G. Alfonsi, M. De Vincenzi, M. Silano, O. Vincentini, and M. Gobbetti. 2005. Pasta made from durum wheat semolina fermented with selected lactobacilli as a tool for a potential decrease of the gluten intolerance. J. Agric. Food Chem. 53:4393-4402. [DOI] [PubMed] [Google Scholar]
- 12.Diosdado, B., E. van Oort, and C. Wijmenga. 2005. “Coelionomics”: towards understanding the molecular pathology of coeliac disease. Clin. Chem. Lab. Med. 43:685-695. [DOI] [PubMed] [Google Scholar]
- 13.Ellis, H. J., E. L. Pollock, W. Engel, J. S. Fraser, S. Rosen-Bronson, H. Wieser, and P. J. Ciclitira. 2003. Investigation of the putative immunodominant T cell epitopes in coeliac disease. Gut 52:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Society of Paediatric Gastroenterology and Nutrition. 1990. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch. Dis. Child. 65:909-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferre, S., E. Garcìa, and E. Mendez. 2004. Measurement of hydrolysed gliadins by a competitive ELISA based on monoclonal antibody R5: analysis of syrups and beers. In M. Stern (ed.), Proceedings of the 18th Meeting of the Working Group on Prolamin Analysis and Toxicity. Verlag Wissenschaftliche Scripten, Zwickau, Germany.
- 16.Gallagher, E., T. R. Gormley, and E. K. Arendt. 2004. Recent advances in the formulation of gluten-free cereal-based products. Trends Food Sci. Technol. 15:143-152. [Google Scholar]
- 17.Gobbetti, M. 1998. The sourdough microflora: interactions between lactic acid bacteria and yeasts. Trends Food Sci. Technol. 9:267-274. [Google Scholar]
- 18.Hamer, R. J. 2005. Coeliac disease: background and biochemical aspects. Biotechnol. Adv. 23:401-408. [DOI] [PubMed] [Google Scholar]
- 19.Hernando, A., I. Valdes, and E. Mendez. 2003. New strategy for the determination of gliadins in maize- or rice-based foods matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: fractionation of gliadins from maize or rice prolamins by acidic treatment. J. Mass Spectrom. 38:862-871. [DOI] [PubMed] [Google Scholar]
- 20.Kilmartin, C., H. Wieser, M. Abuzakouk, J. Kelly, J. Jackson, and C. Feighery. 2006. Intestinal T cell responses to cereal proteins in celiac disease. Dig. Dis. Sci. 51:202-209. [DOI] [PubMed] [Google Scholar]
- 21.Kim, C. Y., H. Quarsten, E. Bergseng, C. Khosla, and L. M. Sollid. 2004. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc. Natl. Acad. Sci. USA 101:4175-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontakou, M., R. T. Przemioslo, and R. P. Sturgess. 1995. Cytokine mRNA expression in the mucosa of treated coeliac patients after wheat peptide challenge. Gut 37:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundin, K. E., H. Scott, T. Hansen, G. Paulsen, T. S. Halstensen, O. Fausa, E. Thorsby, and L. M. Sollid. 1993. Gliadin-specific, HLA-DQ (alpha 1*0501, beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178:187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marti, T., O. Molberg, Q. Li, G. M. Gray, C. Khosla, and L. M. Sollid. 2005. Prolyl endopeptidase-mediated destruction of T cell epitopes in whole gluten: chemical and immunological characterization. J. Pharmacol. Exp. Ther. 312:19-26. [DOI] [PubMed] [Google Scholar]
- 25.Molberg, O., S. McAdam, K. E. A. Lundin, C. Kristiansen, H. Arentz-Hansen, K. Kett, and L. M. Sollid. 2001. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur. J. Immunol. 31:1317-1323. [DOI] [PubMed] [Google Scholar]
- 26.Monteleone, I., G. Monteleone, G. Del Vecchio Blanco, P. Vavassori, S. Cucchiara, T. T. MacDonald, and F. Pallone. 2004. Regulation of the T helper cell type 1 transcription factor T-bet in coeliac disease mucosa. Gut 53:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsen, E. M., F. L. Jahnsen, K. E. Lundin, F. E. Johansen, O. Fausa, L. M. Sollid, J. Jahnsen, H. Scott, and P. Brandtzaeg. 1998. Gluten induces an intestinal cytokine response strongly dominated by interferon γ in patients with celiac disease. Gastroenterology 115:551-563. [DOI] [PubMed] [Google Scholar]
- 28.O'Keeffe, J., K. Mills, J. Jackson, and Y. Feighery. 1999. T cell proliferation, MHC class II restriction and cytokine products of gliadin-stimulated peripheral blood mononuclear cells (PBMC). Clin. Exp. Immunol. 117:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piper, J. L., G. M. Gray, and C. Khosla. 2004. Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo. J. Pharmacol. Exp. Ther. 311:213-219. [DOI] [PubMed] [Google Scholar]
- 30.Pyle, G. G., B. Paaso, B. E. Anderson, D. A. Allen, T. Marti, Q. Li, M. Siegel, C. Khosla, and G. M. Gray. 2005. Effect of pretreatment of food gluten with prolyl endopeptidase on gluten-induced malabsorption in celiac sprue. Clin. Gastroenterol. Hepatol. 3:687-694. [DOI] [PubMed] [Google Scholar]
- 31.Rewers, M. 2005. Epidemiology of celiac disease: what are the prevalence, incidence, and progression of celiac disease? Gastroenterology 128:47-51. [DOI] [PubMed] [Google Scholar]
- 32.Salvati, V. M., G. Mazzarella, C. Gianfrani, M. K. Levings, R. Stefanile, and B. De Giulio. 2005. Recombinant human IL-10 suppresses gliadin dependent T cell activation in ex vivo cultured celiac intestinal mucosa. Gut 54:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 34.Shan, L., O. Molberg, I. Parrot, F. Hausch, F. Filiz, G. M. Gray, L. M. Sollid, and C. Koshla. 2002. Structural basis for gluten intolerance in coeliac sprue. Science 297:2275-2279. [DOI] [PubMed] [Google Scholar]
- 35.Shan, L., T. Marti, L. M. Sollid, G. M. Gray, and C. Khosla. 2004. Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for celiac sprue. Biochem. J. 383:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sollid, L. M. 2002. Coeliac disease: dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2:647-655. [DOI] [PubMed] [Google Scholar]
- 37.Sollid, L. M., and C. Khosla. 2005. Future therapeutic options for celiac disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2:140-147. [DOI] [PubMed] [Google Scholar]
- 38.Stepniak, D., L. W. Vader, Y. Kooy, P. A. van Veelen, A. Moustakas, N. A. Papandreou, E. Eliopoulos, J. W. Drijfhout, G. K. Papadopoulus, and F. Koning. 2005. T-cell recognition of HLA-DQ2-bound gluten peptides can be influenced by an N-terminal proline at p-1. Immunogenetics 57:8-15. [DOI] [PubMed] [Google Scholar]
- 39.Stepniak, D., L. Spaenij-Dekking, C. Mitea, M. Moester, A. de Ru, R. Bak-Pablo, P. van Veelen, L. Edens, and F. Koning. 11 May 2006, posting date. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am. J. Physiol. Gastrointest. Liver Physiol. 291:G621-G629. doi: 10.1152/ajpgi.00034.2006. [DOI] [PubMed] [Google Scholar]
- 40.Stern, M., P. J. Ciclitira, R. van Eckert, C. Feighery, F. W. Janssen, E. Mendez, T. Mothes, R. Troncone, and H. Wieser. 2001. Analysis and clinical effects of gluten in coeliac disease. Eur. J. Gastroenterol. Hepatol. 13:741-747. [DOI] [PubMed] [Google Scholar]
- 41.Thompson, T., M. Dennis, L. A. Higgins, A. R. Lee, and M. K. Sharret. 2005. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 18:163-169. [DOI] [PubMed] [Google Scholar]
- 42.Troncone, R., G. Mazzarella, N. Leone, M. Mayer, M. De Vincenzi, L. Greco, and S. Auricchio. 1998. Gliadin activates mucosal cell mediated immunity in cultured rectal mucosa from coeliac patients and a subset of their siblings. Gut 43:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdés, I., E. Garcia, M. Lorente, and E. Méndez. 2003. Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur. J. Gastroenterol. Hepatol. 15:465-474. [DOI] [PubMed] [Google Scholar]
- 44.van de Wal, Y., M. C. Kooy, P. van Veelen, W. Vader, S. A. August, J. W. Drijfhout, S. A. Peña, and F. Koning. 1999. Glutenin is involved in the gluten-driven mucosal T cell response. Eur. J. Immunol. 29:3133-3139. [DOI] [PubMed] [Google Scholar]
- 45.Wapenaar, M. C., M. J. van Belzen, J. H. Fransen, A. F. Sarasqueta, R. H. Houwen, J. W. Meijer, C. J. Mulder, and C. Wijmenga. 2004. The interferon γ gene in celiac disease: augmented expression correlates with tissue damage but no evidence for genetic susceptibility. J. Autoimmun. 23:183-190. [DOI] [PubMed] [Google Scholar]
- 46.Weiss, W., C. Volgelmeier, and A. Gorg. 1993. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers’ asthma. Electrophoresis 14:805-816. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, J., X. Xu, and Y. Liu. 2004. Activation-induced cell death in T cells and autoimmunity. Cell. Mol. Immunol. 1:186-192. [PubMed] [Google Scholar]