Abstract
The effect of the glutathione reductase (GshR) activity of Lactobacillus sanfranciscensis DSM20451T on the thiol levels in fermented sourdoughs was determined, and the oxygen tolerance of the strain was also determined. The gshR gene coding for a putative GshR was sequenced and inactivated by single-crossover integration to yield strain L. sanfranciscensis DSM20451TΔgshR. The gene disruption was verified by sequencing the truncated gshR and surrounding regions on the chromosome. The gshR activity of L. sanfranciscensis DSM20451TΔgshR was strongly reduced compared to that of the wild-type strain, demonstrating that gshR indeed encodes an active GshR enzyme. The thiol levels in wheat doughs fermented with L. sanfranciscensis DSM20451 increased from 9 μM to 10.5 μM sulfhydryl/g of dough during a 24-h sourdough fermentation, but in sourdoughs fermented with L. sanfranciscensis DSM20451TΔgshR and in chemically acidified doughs, the thiol levels decreased to 6.5 to 6.8 μM sulfhydryl/g of dough. Remarkably, the GshR-negative strains Lactobacillus pontis LTH2587 and Lactobacillus reuteri BR11 exerted effects on thiol levels in dough comparable to those of L. sanfranciscensis. In addition to the effect on thiol levels in sourdough, the loss of GshR activity in L. sanfranciscensis DSM20451TΔgshR resulted in a loss of oxygen tolerance. The gshR mutant strain exhibited a strongly decreased aerobic growth rate on modified MRS medium compared to either the growth rate under anaerobic conditions or that of the wild-type strain, and aerobic growth was restored by the addition of cysteine. Moreover, the gshR mutant strain was more sensitive to the superoxide-generating agent paraquat.
Reduced glutathione (γ-GluCysGly [GSH]) and oxidized glutathione (GSSG) are both naturally occurring in wheat flour (11, 17). These sulfhydryl compounds are capable of undergoing a disulfide-sulfhydryl interchange with other low-molecular-weight thiol compounds, as well as gluten proteins, resulting in the cleavage or reformation of disulfide bonds in wheat dough (10). The formation of the glutenin macropolymer in wheat doughs, which determines dough rheology and gas retention and, thus, bread volume and texture, is dependent on intermolecular disulfide bonds between glutenin proteins (10, 47). Oxidizing or reducing agents that influence the thiol exchange reactions between GSH and gluten proteins are therefore important components of baking improvers to standardize and to control dough rheology and bread texture in wheat baking.
It was recently shown that Lactobacillus sanfranciscensis increases the levels of low-molecular-weight thiol components, as well as thiol levels, in gluten proteins during sourdough fermentation (43). This reduction of disulfide bonds by L. sanfranciscensis, in addition to the pH-dependent activities of cereal proteases, may determine the gluten quality. The effect of L. sanfranciscensis on disulfide exchange reactions in wheat doughs was attributed to the glutathione reductase (GshR) activity of this organism (43).
GshR is a member of the family of flavoprotein disulfide oxidoreductases. The enzyme catalyzes the NADPH-dependent reduction of glutathione disulfide. In addition to its technological relevance in wheat doughs, glutathione has an important function as a redox buffer in bacterial cells. Glutathione is the major nonprotein thiol compound in living cells, and it was found to be involved in the resistance to osmotic stress (33), toxic electrophiles (8), and oxidative stress (4, 32). Glutathione also acts as an electron donor for both the scavenging of reactive oxygen, e.g., from respiration, and metabolic reactions, such as the reduction of hydroperoxides and lipid peroxides (24). GshR plays an essential role in cell defense against oxygen stress by maintaining a high intracellular GSH/GSSG status (46a). GshR has been purified and characterized from several bacteria, e.g., Cyanobacterium anabaena PCC7120 (15) and Pseudomonas aeruginosa (27). In Escherichia coli, glutathione-based reduction systems contribute to protection against oxidative stress (4); however, the GshR from E. coli seems to play a minor role compared to those of the thioredoxin reductases (32).
Lactic acid bacteria are known for their ability to accumulate GSH (48). Streptococcus mutans possesses a sulfhydryl uptake system (37). GshRs were characterized from Streptococcus thermophilus CNRZ368 (26) and Enterococcus faecalis (25). In Lactococcus lactis, the increased accumulation of GSH under aerobic conditions was interpreted as a regulatory mechanism that protects L. lactis cells against oxidative stress (19). To date, a functional characterization of GshR in lactobacilli and its contribution to the oxygen tolerance of these organisms has not been reported.
It was the aim of this study to verify the hypothesis that the effects of L. sanfranciscensis on thiol levels in wheat doughs are attributable to the glutathione activity of this organism. Other lactobacilli of relevance in sourdough fermentation were screened for GshR activity, and their effects on thiol levels were analyzed. Furthermore, the contribution of GshR to the oxygen tolerance of L. sanfranciscensis DSM20451T was examined, and its oxygen tolerance was compared to that of a CyuC-defective mutant of Lactobacillus reuteri. Deletion of the cell wall-bound cystine binding protein CyuC in L. reuteri decreases the oxygen tolerance of the mutant strains (28). The gene for CyuC of L. reuteri (previously MAP, mucus adhesion protein, and BspA, basic surface protein) is part of an operon consisting of genes for a cystathionine-γ-lyase, an ATP binding protein, a hydrophobic membrane protein, and a surface-bound cystine binding protein (28, 39, 40). Cystathionine-γ-lyase accepts cysteine, cystine, and methionine as substrates for conversion to low-molecular-weight thiol compounds (31). It was suggested that four proteins encoded by the operon act in concert by extracellular binding, ATP-dependent transport, and the conversion of cystathionine-γ-lyase.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Lactobacilli were cultivated in modified MRS medium (mMRS) (34) containing 10 g liter−1 maltose and 5 g liter−1 fructose. To maintain plasmids in the cells, 10 μg liter−1 erythromycin was added where indicated. L. sanfranciscensis DSM20451T was cultivated anaerobically at 30°C, and L. reuteri BR11 (40) and Lactobacillus pontis LTH2587 at 37°C. Escherichia coli DH5α was cultivated aerobically in Luria-Bertani medium at 37°C. To maintain plasmids in the cells, 100 μg liter−1 ampicillin was added.
Plasmids.
E. coli DH5α was transformed with the plasmid pME-1 (38), which was used for the construction of the integration vector pME-1ΔgshR as described below. L. reuteri BR11 (formerly L. fermentum BR11) was transformed with the plasmid PNG201 according to the method of Turner et al. (40) to obtain an L. reuteri BR11ΔcyuC strain defective in the l-cystine binding protein CyuC.
General molecular techniques.
General techniques regarding cloning, DNA manipulations, and agarose gel electrophoresis were performed as described by Sambrook et al. (29). Chromosomal DNA of L. sanfranciscensis was isolated according to the method of Lewington et al. (18), and E. coli plasmid DNA was isolated with a Wizard Plus SV Minipreps DNA purification system from Promega (Madison, WI). Restriction endonuclease digestions and ligations with T4-DNA ligase were performed by following the recommendations of the supplier (Fermentas, St. Leon-Rot, Germany). PCR was carried out in thermocyclers (Applied Biosystems, Foster City, CA) by using Taq polymerase and deoxynucleoside triphosphates from Invitrogen (Burlington, Canada). PCR products were purified using the QIAquick PCR purification kit (QIAGEN, Mississauga, Canada). Sequencing was carried out by the dideoxy method using a GenomeLab DTCS quick-start kit (Beckman Coulter, Fullerton, CA) in combination with an Applied Biosystems model 377A automated sequencing system. Nucleotide and amino acid sequence analysis was carried out by using DNASTAR for Windows software (DNASTAR, Madison, WI). Transformations were performed with a Bio-Rad gene pulser apparatus (Bio-Rad Laboratories, Hercules, CA) in 0.2-mm cuvettes (Bio-Rad Laboratories, Hercules, CA) at 2.5 kV, 25 μF, and 200 Ω for E. coli and at 1.2 kV, 25 μF, and 1,000 Ω for lactobacilli.
Southern hybridization.
Genomic DNA was digested with EcoRV, HindIII, and NcoI, separated on a 0.7% agarose gel, and then transferred to nylon membranes (Amersham Biosciences). A 921-bp fragment of L. sanfranciscensis DSM20451T obtained with primers GTDHV1 and GTDHR2 (Table 1) was labeled with digoxigenin DNA-labeling mixture (Roche Diagnostics). Hybridization and washing were performed according to the manufacturer's instructions.
TABLE 1.
Primers used for genetic manipulations
| Primer | Sequence (5′ to 3′)a | Use |
|---|---|---|
| gshknockV | TAT ATG GAT CCA ACA TGA TGT TAA GGA AT | PCR and cloning |
| gshknockR | TAT ATG GAT CCA TTC GAA AAT GGC AGT TG | PCR and cloning |
| GTDHV | TAT ATT TGG GGA GTG GAC | PCR |
| GTDHR | ATT CGA AAA TGG CAG TTG | PCR |
| GTDHV1 | GGG AGT GGA CAT GGA ACG | PCR and Southern blotting |
| GTDHR2 | ATT CGA AAA TGG CAG TTG | PCR and Southern blotting |
| eryV | GAC TCA AAA CTT TAT TAC TTC | PCR |
| T7 | GTA ATA CGA CTC ACT ATA GGG C | PCR |
| Deg-gshRV | GGY GGH ACT TGY CCW AAY | PCR |
| Deg-gshRR | ATH CCS ACT TGM GCW A | PCR |
| gshRV1 | GTG ATC AGG CAG AAG ATT C | Inverse PCR |
| gshRR1 | GCA ATC ACA ATT TTA TCT GC | Inverse PCR |
| gshRV2 | AGA TTC AAT TAG TAC GAT TCT | Inverse PCR |
| gshRR2 | CAA TTA ATC TCT GGA ATT CCA | Inverse PCR |
| cyuC-for3 | GCT CCT TAT GCT TAT C | PCR |
| cyuC-rev3 | CGT GCA TCA AAT CTT TG | PCR |
BamHI restriction sites are underlined.
Sequencing and insertional inactivation of the GshR gene by single-crossover integration.
Based on a 771-bp fragment of the GshR gene from L. sanfranciscensis DSM20451T (43), primers gshRV1/V2 and gshRR1/R2 were designed for inverse PCR (Table 1). Chromosomal DNA of L. sanfranciscensis was digested with PstI, religated, and used as a template for inverse PCR to yield a product with a size of about 2,500 bp. For insertional inactivation of the GshR gene, a 765-bp fragment of the GshR gene was obtained with PCR using primers gshknockV and gshknockR (Table 1), carrying BamHI restriction sites. Digestion and ligation into the BamHI restriction site of plasmid pME-1 resulted in the nonreplicating integration vector pME-1ΔgshR, which was cloned in E. coli DH5α and isolated with the Wizard Plus SV Minipreps DNA purification system.
For the preparation of electrocompetent cells of L. sanfranciscensis, the strain was grown on mMRS medium supplemented with 1% (wt/vol) glycine to an optical density at 590 nm (OD590) of 0.7. The cells were harvested by centrifugation at 4°C (4,000 × g, 15 min) and washed four times with 50 ml of 10 mM MgCl2 solution, once with glycerol (10%, vol/vol), and once with glycerol-sucrose solution (10%, vol/vol; 0.5 M). The cells were resuspended in glycerol-sucrose solution and stored at −80°C in 100-μl aliquots. All washing and storage solutions were cooled on ice. After electroporation, the cells were incubated in mMRS at 30°C for 3 h prior to plating on mMRS with 10 ppm erythromycin. To verify the insertion of plasmid pME-1ΔgshR into the GshR gene in cells from erythromycin-resistant colonies, PCR was carried out with primers targeting the regions upstream and downstream of the GshR gene (GTDHV and GTDHR, respectively) and the plasmid-borne regions from pME-1 (eryV and T7) (Table 1). The PCR products obtained with primers T7/GTDHV and GTDHR/eryV were sequenced.
Preparation of doughs and determination of pH and cell counts in sourdough.
Wheat flour was obtained at a local supermarket (ash content of 0.4 to 0.5 g/100 g). Sourdoughs were prepared with 22.5 g flour and 22.5 g sterile tap water, inoculated with cells from a 15-ml overnight culture that had been washed twice with tap water, and incubated at 30°C. Chemically acidified doughs were prepared by adding 10 μl of a mixture of acetic acid and lactic acid (1:4, vol/vol) to match the pH of sourdoughs. Dough pH and cell counts were determined as described previously (35).
Measurement of free thiol groups in SDS-soluble protein fractions.
Dough extraction was performed with 50 mM sodium phosphate buffer (pH 6.9) containing 1.5% sodium dodecyl sulfate (SDS) at a 1:10 (wt/vol) extraction ratio (35), and the concentrations of free thiol groups in dough were determined with DTNB (5,5-dithiobis-2-nitrobenzoic acid) (1). The SDS-soluble extract (225 μl) was mixed with 450 μl reagent A (50% n-propanol in 50 mM sodium phosphate buffer, pH 8.0, saturated with nitrogen gas) and 22.5 μl reagent B (39.6 mg DTNB in 10 ml 0.5 M sodium phosphate buffer, pH 7.0). After incubation for 30 min in the dark, the absorbance at 405 nm was measured. GSH solutions with concentrations ranging from 0 to 0.42 mM were used for calibration. Experiments were performed at least in triplicate, and statistical significance was assessed at the 95% confidence level using Student's t test.
GshR and cystathionine-γ-lyase activities of L. sanfranciscensis, L. reuteri, and L. pontis.
The GshR activities in the extracts were measured at 25°C by monitoring the oxidation of NADPH in the reaction mixture (1 ml) at 340 nm. The reaction mixture contained 640 μl sodium phosphate buffer (1:1) plus 5 mM EDTA, 120 μl GSSG (10 mM), 100 μl NADPH (1 mM), and 100 μl extracts. The reduced GSH content was measured with DTNB by adding 20 μl of reagent B as described above. The GshR activity was calculated with reference to controls that were incubated without the addition of crude cell extract. The levels of cystathionine-γ-lyase activity were determined according to the method of Smacchi and Gobbetti (31).
Activity staining of GshR on SDS-polyacrylamide gel electrophoresis (PAGE) gels.
Activity staining of GshR was performed after the separation of crude cellular extracts on 12% SDS-polyacrylamide gels (12). The gel was immersed and shaken twice for 10 min in 25% (vol/vol) isopropanol in 10 mM Tris-HCl buffer (pH 7.9) to remove the SDS and then finally equilibrated for renaturation in 50 mM Tris-HCl buffer (pH 7.9) for 15 min. The gel was soaked in the substrate solution (25 ml 50 mM Tris-HCl buffer, pH 7.9, containing 4.0 mM GSSG, 1.5 mM β-NADPH, and 2 mM DTNB) with gentle shaking for 20 min. After a brief rinse with 50 mM Tris-HCl buffer (pH 7.9), the GshR activity was detected by negative staining in darkness with 50 ml 1.2 mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and 1.6 mM phenazine methosulfate for 10 min at room temperature. A clear zone against the blue background indicated GshR activity.
Determination of the effect of paraquat on growth rates.
According to the method of Turner et al. (40), 500 μl of log-phase cells were added to 4.5 ml of mMRS containing either 500 μl of sterile double-distilled water (ddH2O) (0 mM paraquat), 166 μl of 1 M paraquat (methyl viologen; Sigma) and 333 μl of sterile ddH2O (30 mM paraquat), or 270 μl of 1 M paraquat and 230 μl of sterile ddH2O (49 mM paraquat). The cultures were incubated at 30°C without shaking. Growth was monitored by measuring the OD600.
Determination of intracellular and extracellular sulfhydryl levels.
Cysteine transport by L. sanfranciscensis was determined according to the method of Turner et al. (40). Cells grown aerobically to the mid-exponential phase were harvested by centrifugation, washed twice in KPM solution (0.1 M K2HPO4 adjusted to pH 6.5 with H3PO4 and containing 10 mM MgSO4 · 7H2O), and suspended in KPM to an OD of 0.5. Portions (0.5 ml each) of this suspension were supplemented with 10 μl of 10 μM l-cysteine and 10 μl of 1 M d-glucose, and the suspension was then incubated at 30°C for 1 h. The cells were then pelleted, and the supernatant was removed and put on ice. Fifty microliters of a 10 mM solution of DTNB in KPM was added to the supernatant, and the absorbance at 412 nm was measured. The pelleted cells were washed twice with 1 ml of KPM and resuspended in a solution containing 200 μl of water, 4 μl of 0.5 M EDTA, 10 μl of 1 M Tris-HCl (pH 8), 20 μl of 10 mM DTNB, and 100 μl of 10% SDS, added successively. This mixture was incubated at 30°C for 1 h, cellular debris was removed by centrifugation, and the absorbance of the supernatant was measured at 412 nm. Assay mixtures containing no bacterial cells or no l-cysteine served as controls. The sulfhydryl concentrations were calculated based on the absorbance at 412 nm and the molar extinction coefficient of 5-thio-2-nitrobenzoic acid of 13.6 liters (mol cm)−1 and were corrected to an optical density of 1.0. The intracellular accumulation of thiols during incubation was calculated as follows: ([thiol](cells in KPM-cystine) [thiol](cells in KPM without cysteine)) × (cell density)−1. The decrease in extracellular thiols during incubation was calculated as follows: [thiol](KPM-cysteine) − [thiol](cells in KPM-cysteine). The results are reported as the means ± standard deviations of five independent determinations.
Sequence and expression of a gene coding for a CyuC-like protein in L. sanfranciscensis DSM20451T.
A gene coding for a CyuC-like protein was sequenced based on several rounds of PCR with primers derived from the cyuC of L. reuteri BR11. RNA was isolated from cells of L. sanfranciscensis that were grown aerobically (shaking at 220 rpm) in 50 ml of MRS broth to an OD595 of 0.5. Bacterial cells in the supernatant were harvested by centrifugation (15 min, 4,500 relative centrifugal force) and resuspended in 3 ml Tris-HCl buffer (50 mM, pH 7.0) with 3 ml RNAprotect (QIAGEN, Hilden, Germany). This cell suspension was used for RNA isolation with the QIAGEN RNeasy mini kit. DNA was removed by incubation with RQ1 RNase-free DNase (Promega, Mannheim, Germany). Reverse transcription was performed by incubating RNA with random hexamer primers (random hexadeoxynucleotides; Promega) at 70°C for 10 min. After cooling on ice, 1 μl deoxynucleoside triphosphates (25 mM), 1 μl reverse transcriptase (200 U μl−1; Moloney murine leukemia virus reverse transcriptase, RNase H minus; Promega), 5 μl reaction buffer (supplied with reverse transcriptase), and 5 μl RNase-free water were added. The sample was incubated at 25°C for 10 min and subsequently at 42°C for 110 min, and the reaction was stopped by heating the sample at 72°C for 15 min. A fragment of the CyuC-like protein of L. sanfranciscensis was amplified using Taq polymerase and primers cyuC-for3 and cyuC-rev3 and cDNA as a template (Table 1). All PCRs were also carried out with DNase-digested RNA preparations to verify the absence of chromosomal DNA.
Nucleotide sequence accession numbers.
The nucleotide sequences of the L. sanfranciscensis DSM20451T GshR (gshR) and CyuC-like-protein genes have been assigned the GenBank accession numbers DQ866807 and EF422159, respectively.
RESULTS
Nucleotide and amino acid sequences of L. sanfranciscensis DSM20451T gshR and its product.
The sequence of a complete open reading frame termed gshR encoding a putative GshR was obtained by inverse PCR. Sequence analysis indicated the presence of an imperfect Shine-Dalgarno sequence (AAGGAG), putative −10 and −35 sequences corresponding to consensus sequences proposed for lactobacilli (23), and a palindromic sequence (TAAAAACATGTTTTTA) downstream from the termination codon, indicating that the GshR gene is expressed as monocistronic mRNA. Southern hybridization of genomic DNA from L. sanfranciscensis DSM20451 with a probe targeting gshR demonstrated that its chromosome harbors a single copy of the gene (data not shown).
gshR codes for a 446-amino-acid protein (GshR) with a predicted relative molecular weight of 48,614 and a predicted pI of 4.79. BLAST searches showed high similarities to bacterial GshRs that were previously characterized (Cyanobacterium anabaena EMBL X89712, 31% identity and 58% similarity over 427 amino acids; Pseudomonas aeruginosa EMBL X54201, 31% identity and 50% similarity over 444 amino acids; and Enterococcus faecalis EMBL AE016830, 29% identity and 55% similarity over 436 amino acids). The GshR of L. sanfranciscensis contains two dinucleotide binding motifs and a GG doublet that are highly conserved in different GshRs (41). Most GshRs contain the highly conserved NAD(P)H binding site sequence GXGYIAX18RX5R (21); however, in L. sanfranciscensis, the first arginine residue in the Rx5R motif is replaced by histidine. The ATG and GD motifs that are present in most flavoproteins with two dinucleotide binding domains (41) are also present in the GshR of L. sanfranciscensis.
Insertional inactivation of the GshR gene and GshR activity of the mutant strain.
L. sanfranciscensis DSM20451T was transformed with the nonreplicating plasmid pME-1ΔgshR, yielding strain L. sanfranciscensis DSM20451ΔgshR. Sequencing of the disrupted GshR gene ensured that a single-crossover integration of pME-1ΔgshR into the chromosomal gshR gene of DSM20451ΔgshR had taken place (data not shown). Crude cellular extracts of the ΔgshR mutant exhibited a GshR activity of 14 nmol (min mg)−1, which in comparison to an activity of 45 nmol (min mg)−1 in the wild-type strain indicates that gshR encodes an active GshR.
Thiol levels in sourdoughs fermented with L. sanfranciscensis DSM20451T and L. sanfransiscensis DSM20451TΔgshR and chemically acidified doughs.
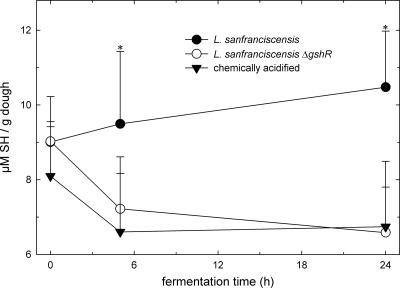
To determine whether GshR is involved in the reduction of thiol groups in wheat sourdoughs, the thiol levels were quantified in SDS extracts of wheat sourdoughs fermented with L. sanfranciscensis DSM20451T and DSM20451TΔgsh. Samples were taken from unfermented doughs after 5 h of incubation, corresponding to exponentially growing cells in sourdough, and after 24 h of incubation, corresponding to stationary cells. Chemically acidified dough was used as a control. Both strains grew to high cell counts (7.0 × 108 ± 0.5 × 108 CFU/g) after 24 h of fermentation. The pH of doughs fermented with L. sanfranciscensis DSM 20451T was 4.38 ± 0.05 after 5 h and 3.45 ± 0.05 after 24 h. Fermentation with L. sanfranciscensis increased the thiol levels in dough (Fig. 1). In chemically acidified doughs, the thiol levels decreased during fermentation. During fermentation with the gshR mutant strain, the pH decreased to 4.32 ± 0.05 after 5 h of fermentation and to 3.47 ± 0.05 after 24 h. The thiol contents of sourdoughs fermented with the gshR mutant were comparable to those of chemically acidified doughs.
FIG. 1.
Thiol levels of SDS extracts from chemically acidified wheat doughs and doughs fermented with L. sanfranciscensis DSM20451T and L. sanfranciscensis DSM20451TΔgshR. Shown are the means ± standard deviations of the results from five independent experiments. Data differing significantly (P < 0.05) from those for chemically acidified doughs are marked with asterisks.
Glutathione activities of other sourdough lactobacilli.
Previous studies indicated that the obligate heterofermentative lactobacilli L. reuteri and L. pontis exert effects on thiol levels in wheat doughs comparable to the effect of L. sanfranciscensis (N. Vermeulen, J. Kretzer, H. Machalitza, R. F. Vogel, and M. G. Gänzle, unpublished data). Strains of these species and other lactobacilli were screened on biochemical and genetic levels for GshR activity to establish whether their effects on thiol exchange reactions are also attributable to GshR activities. Moreover, L. reuteri BR11 and the cognate mutant strain L. reuteri BR11ΔcyuC, which is deficient in a cystine uptake system (13, 40), were included in the analysis. By use of the degenerate primers deg/gshRV and deg/gshRR, L. brevis TMW 1.57, L. plantarum TMW 1.460, L. johnsonii TMW 1.192, L. frumenti TMW 1.635, L. acidophilus TMW 1.18, L. hilgardii TMW 1.45, and L. pentosus TMW 1.10 were found to harbor GshR genes related to the gshR of L. sanfranciscensis, but no amplification product was obtained with L. reuteri TMW 1.106, L. pontis LTH2587, or L. reuteri BR11 (data not shown and Table 2). The absence of GshR activity in L. pontis and L. reuteri was verified by determination of the levels of activity in crude cellular extracts after separation on SDS-PAGE gels (Fig. 2). Strains without GshR activity exhibited cystathionine-γ-lyase activity (Table 2).
TABLE 2.
Presence and activities of GshR, gshR, and cysteine-γ-lyase in sourdough lactobacilli
| Strain | GshR activity (nmol min−1 mg−1)a | Presence of gshRb | Presence of cystathionine-γ-lyase activity | Presence of cystathionine-γ-lyasec |
|---|---|---|---|---|
| L. sanfranciscensis DSM20451 | 45 | + | − | −d |
| L. sanfranciscensis DSM20451ΔgshR | 14 | − | − | ND |
| L. reuteri BR11 | − | − | + | +e |
| L. reuteri BR11ΔcyuC | − | − | + | ND |
| L. pontis LTH2587 | − | − | + | +d |
GshR activity in crude cellular extract. −, absence of GshR activity in crude cellular extracts after separation by SDS-PAGE.
Detection of gshR was performed by using the degenerate primers deg/gshRV and deg/gshRR.
Detection of cystathionine-γ-lyase was performed by PCR.
See reference 45.
See reference 40.
FIG. 2.
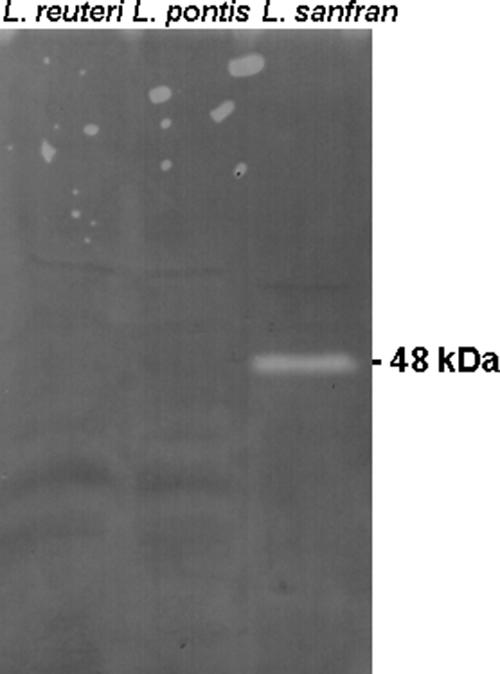
Detection of GshR acivity in crude cellular extracts of L. sanfranciscensis DSM20451T, L. pontis LTH2587, and L. reuteri BR11 after separation of crude cellular extracts by SDS-PAGE. The location of GshR, with a predicted relative molecular mass of 48,614 kDa, is indicated.
Thiol levels in sourdough fermented with L. reuteri BR11, L. reuteri BR11ΔcyuC, and L. pontis LTH2587.
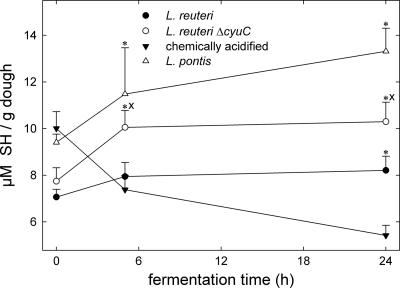
The effects of GshR-negative, heterofermentative lactobacilli on thiol levels in dough were determined with L. pontis strain LTH 2587, as well as L. reuteri BR11 and BR11ΔcyuC. All strains acidified wheat doughs to pHs ranging from 3.20 to 3.32 after 24 h of incubation. Remarkably, the effects of the GshR-negative strains on thiol levels in dough were qualitatively comparable to that of L. sanfranciscensis (Fig. 3). The thiol levels in doughs fermented with L. reuteri BR11ΔcyuC were consistently higher than the levels in doughs fermented with the corresponding wild-type strain.
FIG. 3.
Thiol levels in sourdoughs fermented with L. reuteri BR11, L. reuteri BR11ΔcyuC, and L. pontis LTH2587 and chemically acidified doughs. Shown are the means ± standard deviations of the results from three independent experiments. Data differing significantly (P < 0.05) from those for chemically acidified doughs are marked with asterisks, and significant differences (P < 0.05) between doughs fermented with L. reuteri BR11 and BR11ΔcyuC are marked with an “x.” SH, sulfhydril.
Contribution of GshR to the oxygen tolerance of L. sanfranciscensis.
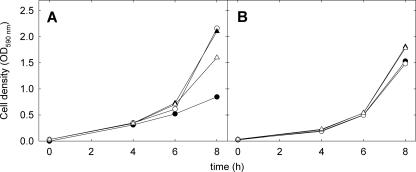
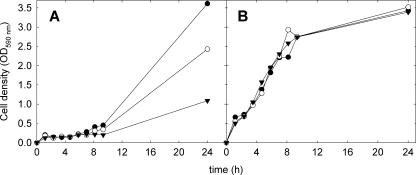
The tolerance of L. sanfranciscensis DSM20451Tand its ΔgshR mutant towards oxygen and superoxide radicals was determined in mMRS medium which was supplemented with 0.5 g liter−1 cysteine and in mMRS without cysteine. The wild-type strain tolerated aerobic conditions in either medium, whereas growth of the ΔgshR mutant was inhibited in the presence of oxygen (Fig. 4). This difference was not observed in the absence of oxygen or in the presence of 0.5 g liter−1 cysteine (Fig. 4). To determine the sensitivity of L. sanfranciscensis DSM20451TΔgshR to the superoxide radicals, the growth rates of this strain and L. sanfransicensis DSM20451T were compared in medium supplemented with 0, 30, or 49 mmol of the superoxide-generating agent paraquat. The growth of L. sanfranciscensis DSM20451T was unaffected by paraquat, but paraquat strongly inhibited the growth of L. sanfranciscensis DSM20451TΔgshR in the absence of cysteine (Fig. 5). When cysteine was added to the medium, paraquat did not affect the growth of either strain (data not shown).
FIG. 4.
Growth rates of L. sanfranciscensis DSM20451T (triangles) and DSM20451TΔgshR (circles) under aerobic conditions (black symbols) and anaerobic conditions (open symbols). Experiments were carried out in mMRS without cysteine (A) and in mMRS containing 0.5 g liter−1 cysteine (B). The results shown are representative of three independent experiments.
FIG. 5.
Growth rates of L. sanfranciscensis DSM20451TΔgshR (A) and L. sanfranciscensis DSM20451T (B) in mMRS without the addition of cysteine. Zero (•), 30 (○), or 49 (▾) mM paraquat was added to the media. The results shown are representative of two independent experiments.
Cysteine and cystine transport by L. sanfranciscensis.
To affirm that cysteine transport complements the protective effect of GshR during aerobic growth, the levels of cysteine transport by aerobically grown cells of L. sanfranciscensis DSM20451T and DSM20451TΔgshR were estimated by the determination of intra- and extracellular thiol levels after incubation in buffer with cysteine. The intracellular sulfhydryl levels prior to cysteine supplementation were 3.9 ± 1.0 and 3.6 ± 1.2 nmol/unit of cell density for the wild-type and mutant strains, respectively, and the intracellular thiol levels increased by 37 ± 7 and 32 ± 8 nmol/unit of cell density, respectively, upon the addition of cysteine to the cellular suspensions. A corresponding decrease of thiol levels in the buffer, of 110 ± 7 and 103 ± 6 nmol/liter, respectively, was observed.
L. reuteri BR11 harbors separate transport systems for cystine and cysteine. Cystine transport is mediated by CyuC and cognate ATP binding and membrane-spanning proteins (13, 14). L. sanfranciscensis DSM20451T harbors an open reading frame coding for a 264-amino-acid protein. The predicted gene product is 46% identical and 63% similar to CyuC of L. reuteri BR11. The expression of cyuC in L. sanfranciscensis was verified by the amplification of a 637-bp fragment of cyuC from a cDNA library obtained from exponentially growing cells of L. sanfranciscensis DSM20451T.
DISCUSSION
Effect of GshR on thiol exchange reactions in wheat sourdoughs.
In this study, the functional characterization of a GshR in lactobacilli was carried out by using a GshR-deficient mutant of L. sanfranciscensis DMS20451T. Although the enzyme is located in the cytoplasm, the accumulation of thiols in the extracellular medium was attributable to GshR activity. The import of glutathione was previously demonstrated in S. mutans (30); however, glutathione in wheat doughs undergoes thiol exchange reactions with cyst(e)ine and other thiols (10). Thus, the transport of reduced or oxidized thiol compounds other than glutathione across the cytoplasmic membrane may account for the effects of metabolism on extracellular thiol levels. Previously, the intracellular conversion of cystine or cysteine in L. reuteri was shown to increase extracellular thiol levels (22).
L. sanfranciscensis increased thiol levels in wheat doughs, whereas a decrease of thiols was observed in wheat doughs fermented with L. sanfranciscensis DSM20451TΔgshR. The extracellular accumulation of thiols is particularly relevant in wheat doughs. The quality and quantity of gluten proteins in wheat flours is of paramount importance for wheat bread quality, and the intermolecular disulfide cross-links of glutenin subunits to form the glutenin macropolymer are dependent on the presence or absence of low-molecular-weight sulfhydryl compounds (10). The elasticity and viscosity of wheat sourdoughs decreases during fermentation because of altered protein net charge, disruption of thiol cross-linking of gluten protein, and proteolytic degradation of glutenin subunits (2, 5, 35, 43). The disruption of disulfide cross-links in the gluten macropolymer occurs early during fermentation and is dependent on the presence of heterofermentative lactic acid bacteria in the dough (43). In comparison, proteolytic degradation of gluten proteins occurs only after extended fermentation times corresponding to the production of pHs of less than 4.5, and a comparable extent of gluten proteolysis occurs in aseptic and fermented doughs (35, 36). In keeping with the different time scales of proteolysis and thiol exchange, a comparison of the fundamental rheological properties of aseptic acidified wheat doughs and sourdoughs with the same pH revealed significant differences between fermented and unfermented doughs after 6 h of fermentation, but not after 24 h of fermentation (5).
Thiol exchange reactions in wheat doughs are furthermore relevant in applications targeting the complete proteolytic degradation of gluten proteins. In wheat doughs, proteolysis is limited by the activities of proteolytic enzymes, but the substrate solubility becomes the limiting factor of protein degradation upon the addition of external proteases (9, 35, 44). The disruption of disulfide cross-links between gluten proteins by chemical reducing agents or heterofermentative lactobacilli is required to achieve a virtually quantitative hydrolytic degradation of gluten proteins in wheat doughs (35, 44).
The effects of L. reuteri and L. pontis on the thiol levels in sourdough were comparable to the effect of L. sanfranciscensis, but these strains did not exhibit GshR activity. Remarkably, L. reuteri BR11ΔcyuC, which has a phenotype comparable to that of the gshR-deficient L. sanfranciscensis with respect to its tolerance to oxygen (40), increased the thiol levels in dough compared to the levels found with the cognate wild-type strain, indicating a role for cystine metabolism via cystathionine-γ-lyase for thiol exchange reactions in wheat doughs. The effect of CyuC deletion in L. reuteri was less pronounced than that of the GshR deletion in L. sanfranciscensis. It is counterintuitive that the loss of cystine transport increased extracellular thiol levels; however, the L. reuteri CyuC mutant strain remains capable of extracellular accumulation of thiols from substrates other than cystine (14).
Influence of GshR during aerobic life of L. sanfranciscensis DSM20451T.
This study additionally considered a potential role of GshR in the oxygen tolerance of L. sanfranciscensis. Generally, aerobic growth of lactic acid bacteria requires the presence of catalase and/or NADH oxidases to remove hydrogen peroxide (6, 22). Several thiol-active enzyme systems additionally contribute to the tolerance of lactic acid bacteria to oxygen, including the thioredoxin-thioredoxin reductase couple (16, 42, 46), cyst(e)ine uptake and metabolism (40), and the glutathione-GshR system.
Streptococci harbor GshRs that enable the cells to create a reducing environment and which are overexpressed during aerobic growth (26, 37, 49). Some strains of L. lactis accumulate glutathione in response to aerobic conditions (19). This study demonstrated that gshR-deficient mutants of L. sanfranciscensis DSM20451T exhibited a decreased tolerance to oxygen and superoxide. Moreover, the insertional deletion of gshR reduced the aerobic growth rate of L. sanfranciscensis but did not fully eliminate oxygen tolerance in this strain. Putative thioredoxin, glutaredoxin, and thioredoxin reductase genes may serve as additional pathways to maintain intracellular redox homeostasis in the absence of an active GshR.
Oxygen tolerance in L. sanfranciscensis DSM20451TΔgshR could be restored by the addition of cysteine to the medium, indicating that the gshR mutant strain is more sensitive to oxidative stress because it is unable to maintain high intracellular levels of thiols. Little is known about cysteine transport systems in lactic acid bacteria; in Saccharomyces cerevisiae, several permeases with broad specificities contribute to cysteine transport (7). Bacterial cystine transport systems exhibit a high specificity for cystine (3, 13, 14). In agreement with data reported for cysteine transport in L. reuteri (40), L. sanfranciscensis internalized cysteine to increase intracellular thiol levels. Moreover, the strain expressed a gene product with high homology to the CyuC of L. reuteri. Because L. sanfranciscensis does not exhibit cystathionine-γ-lyase activity to liberate thiols from cystine or cysteine, it remains unclear whether cystine transport contributes to intracellular thiol homeostasis or serves nutritional requirements.
In conclusion, this study demonstrated that the GshR of L. sanfranciscensis plays an important role in disulfide exchange reactions in wheat doughs, indicating that the GshR activity of L. sanfranciscensis may contribute to the beneficial effects of sourdough fermentation on bread texture. Furthermore, a contribution of GshR to the oxygen tolerance of L. sanfranciscensis was shown. It is remarkable that gshR homologues were furthermore detected in several other species of lactobacilli, whereas L. pontis and L. reuteri, species that are not capable of growth at aerobic conditions (34), harbored no gshR homologue and exhibited no GshR activity. Thus, lactobacilli differ in their use of thiol-dependent redox systems, and the contributions of the various thiol compounds to the oxygen tolerance and aerobic growth of lactobacilli may prove a relevant area of future research.
Acknowledgments
We thank Phillip Giffard, Queensland University of Technology, Brisbane, Australia, for providing L. reuteri BR11 and the cognate bspA-cyuC deletion mutant. Christine Ippy is acknowledged for excellent technical support.
The Canada Research Chairs program and Ernst Böcker GmbH and Co. KG are acknowledged for financial support. Part of this study was supported by the German Federal Ministry of Economics and Technology (BMWi) via the AiF-German Federation of Industrial Research Associations Otto von Guericke (project no. AiF-FV 14492 N) and the Research Association of the German Food Industry (FEI).
Footnotes
Published ahead of print on 11 May 2007.
REFERENCES
- 1.Antes, S., and H. Wieser. 2000. Quantitative determination and localization of thiol groups in wheat flour, p. 211-214. In P. R. Shewry and A. S. Tatham (ed.), Wheat gluten. Proceedings of the 7th International Workshop. Royal Society of Chemistry, Cambridge, United Kingdom.
- 2.Arendt, E. K., L. A. M. Ryan, and F. Dal Bello. 2007. Impact of sourdough on the texture of bread. Food Microbiol. 24:165-174. [DOI] [PubMed] [Google Scholar]
- 3.Burguière, P., S. Auger, M.-F. Hullo, A. Danchin, and I. Martin-Verstraete. 2004. Three different systems participate in l-cystine uptake in Bacillus subtilis. J. Bacteriol. 186:4875-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmel-Harel, O., and G. Storz. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439-461. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, C. I., T. J. Schober, D. Dockery, K. O'Sullivan, and E. K. Arendt. 2004. Wheat sourdough fermentation: effects of time and acidification on fundamental rheological properties. Cereal Chem. 81:409-417. [Google Scholar]
- 6.De Angelis, M., and M. Gobbetti. 2004. Environmental stress responses in Lactobacillus: a review. Proteomics 4:106-122. [DOI] [PubMed] [Google Scholar]
- 7.During-Olsen, L., B. Regenberg, C. Gjermansen, M. C. Kielland-Brandt, and J. Hansen. 1999. Cysteine uptake by Saccharomyces cerevisiae is accomplished by multiple permeases. Curr. Genet. 35:609-617. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson, G. P. 1999. Protective mechanisms against toxic electrophiles in Escherichia coli. Trends Microbiol. 7:242-247. [DOI] [PubMed] [Google Scholar]
- 9.Gobbetti, M., C. G. Rizzello, R. Di Cagno, and M. De Angelis. 2007. Sourdough lactobacilli and celiac disease. Food Microbiol. 24:187-196. [DOI] [PubMed] [Google Scholar]
- 10.Grosch, W., and H. Wieser. 1999. Redox reactions in wheat dough as affected by ascorbic acid. J. Cereal Sci. 29:1-16. [Google Scholar]
- 11.Hird, F. J. R., I. W. D. Crocker, and W. L. Jones. 1968. Low molecular weight thiols and disulfides in flour. J. Sci. Food Agric. 19:602-604. [Google Scholar]
- 12.Hou, W.-C., H.-J. Liang, C.-C. Wang, and D.-Z. Liu. 2004. Detection of glutathione reductase after electrophoresis on native or sodium dodecyl sulfate polyacrylamide gels. Electrophoresis 25:2926-2931. [DOI] [PubMed] [Google Scholar]
- 13.Hung, J., M. S. Turner, T. Walsh, and P. M. Giffard. 2005. BspA (CyuC) in Lactobacillus fermentum BR11 is a highly expressed high-affinity l-cystine-binding protein. Curr. Microbiol. 50:33-37. [DOI] [PubMed] [Google Scholar]
- 14.Hung, J., D. Cooper, M. S. Turner, T. Walsh, and P. M. Giffard. 2003. Cystine uptake prevents production of hydrogen peroxide by Lactobacillus fermentum BR11. FEMS Microbiol. Lett. 227:93-99. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, F., and B. Mannervik. 1999. Optimized heterologous expression of glutathione reductase from Cyanobacterium anabaena PCC 7120 and characterization of the recombinant protein. Protein Expr. Purif. 15:92-98. [DOI] [PubMed] [Google Scholar]
- 16.Jobin, M. P., D. Garmyn, C. Divies, and J. Guzzo. 1999. Expression of the Oenococcus oeni trxA gene is induced by hydrogen peroxide and heat shock. Microbiology 145:1245-1251. [DOI] [PubMed] [Google Scholar]
- 17.Kuninori, T., and H. Matsumoto. 1964. Dehydro-l-ascorbic acid reducing system in flour. Cereal Chem. 41:39-46. [Google Scholar]
- 18.Lewington, J., S. D. Greenaway, and B. J. Spillane. 1987. Rapid small scale preparations of bacterial genomic DNA suitable for cloning and hybridization analysis. Lett. Appl. Microbiol. 5:51-53. [Google Scholar]
- 19.Li, Y., J. Hugenholtz, T. Abee, and D. Molenaar. 2003. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 69:5739-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Loprasert, S., W. Whangsuk, R. Sallabhan, R., and S. Mongkolsuk. 2005. The unique glutathione reductase from Xanthomonas campestris: gene expression and enzyme characterization. Biochem. Biophys. Res. Commun. 331:1324-1330. [DOI] [PubMed] [Google Scholar]
- 22.Lountos, G. T., R. Jiang, W. B. Wellborn, T. L. Thaler, A. S. Bommarius, and A. M. Orville. 2006. The crystal structure of NAD(P)H oxidase from Lactobacillus sanfranciscensis: insights into the conversion of O2 into two water molecules by the flavoenzyme. Biochemistry 45:9648-9659. [DOI] [PubMed] [Google Scholar]
- 23.McCracken, A., M. S. Turner, P. Giffard, L. M. Hafner, and P. Timms. 2000. Analysis of promoter sequences from Lactobacillus and Lactococcus and their activity in several Lactobacillus species. Arch. Microbiol. 173:383-389. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, A. J., and R. Hell. 2005. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth. Res. 86:435-457. [DOI] [PubMed] [Google Scholar]
- 25.Patel, M. P., J. Marcinkeviciene, and J. S. Blanchard. 1998. Enterococcus faecalis glutathione reductase: purification, characterization and expression under normal and hyperbaric O2 conditions. FEMS Microbiol. Lett. 166:155-163. [DOI] [PubMed] [Google Scholar]
- 26.Pébay, M., A. C. Holl, J. M. Simonet, and B. Decaris. 1995. Characterization of the gor gene of the lactic acid bacterium Streptococcus thermophilus CNRZ368. Res. Microbiol. 146:371-383. [DOI] [PubMed] [Google Scholar]
- 27.Perry, A. C., N. Ni Bhriain, N. L. Brown, and D. A. Rouch. 1991. Molecular characterization of the gor gene encoding glutathione reductase from Pseudomonas aeruginosa: determinants of substrate specificity among pyridine nucleotide-disulphide oxidoreductases. Mol. Microbiol. 5:163-171. [PubMed] [Google Scholar]
- 28.Roos, S., P. Aleljung, N. Robert, B. Lee, T. Wadstrom, M. Lindberg, and H. Jonsson. 1996. A collagen binding protein from Lactobacillus reuteri is part of an ABC transporter system? FEMS Microbiol. Lett. 144:33-38. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Sherrill, C., and R. C. Fahey. 1998. Import and metabolism of glutathione by Streptococcus mutans. J. Bacteriol. 180:1454-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smacchi, E., and M. Gobbetti. 1998. Purification and characterization of cystathionine gamma-lyase from Lactobacillus fermentum DT41. FEMS Microbiol. Lett. 166:197-202. [DOI] [PubMed] [Google Scholar]
- 32.Smirnova, G. V., N. G. Muzyka, M. N. Glukhovchenko, T. A. Krasnykh, and O. N. Oktyabrsky. 1999. Oxidative stress resistance of Escherichia coli strains deficient in glutathione biosynthesis. Biochemistry (Moscow) 64:1111-1116. [PubMed] [Google Scholar]
- 33.Smirnova, G. V., T. A. Krasnykh, and O. N. Oktyabrsky. 2001. Role of glutathione in the response of Escherichia coli to osmotic stress. Biochemistry (Moscow) 66:973-978. [DOI] [PubMed] [Google Scholar]
- 34.Stolz, P., G. Böcker, W. P. Hammes, and R. F. Vogel. 1995. Utilization of electron acceptors by lactobacilli isolated from sourdough. II. Lactobacillus pontis, L. reuteri, L. amulovorus, and L. fermentum. Z. Lebensm. Unters. Forsch. 201:402-410. [Google Scholar]
- 35.Thiele, C., M. G. Gänzle, and R. F. Vogel. 2003. Fluorescence labeling of wheat proteins for determination of gluten hydrolysis and depolymerization during dough processing and sourdough fermentation. J. Agric. Food Chem. 51:2745-2752. [DOI] [PubMed] [Google Scholar]
- 36.Thiele, C., S. Grassl, and M. G. Gänzle. 2004. Gluten hydrolysis and depolymerization during sourdough fermentation. J. Agric. Food Chem. 52:1307-1314. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, E. L. 1984. Disulfide reduction and sulfhydryl uptake by Streptococcus mutans. J. Bacteriol. 157:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tieking, M., M. A. Ehrmann, R. F. Vogel, and M. G. Gänzle. 2005. Molecular and functional characterization of a levansucrase from Lactobacillus sanfranciscensis. Appl. Microbiol. Biotechnol. 66:655-663. [DOI] [PubMed] [Google Scholar]
- 39.Turner, M. S., P. Timms, L. M. Hafner, and P. M. Giffard. 1997. Identification and characterization of a basic cell surface-located protein from Lactobacillus fermentum BR11. J. Bacteriol. 179:3310-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner, M. S., T. Woodberry, L. M. Hafner, and P. M. Giffard. 1999. The bspA locus of Lactobacillus fermentum BR11 encodes an l-cystine uptake system. J. Bacteriol. 181:2192-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallon, O. 2000. New sequence motifs in flavoproteins: evidence for common ancestry and tools to predict structure. Proteins Struct. Funct. Genet. 38:95-114. [DOI] [PubMed] [Google Scholar]
- 42.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. D. Ehrlich, and E. Maguin. 2002. Stress responses in lactic acid bacteria. Antonie Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]
- 43.Vermeulen, N., J. Kretzer, H. Machalitza, R. F. Vogel, and M. G. Gänzle. 2006. Influence of redox reactions catalysed by homo- and hetero-fermentative lactobacilli on gluten in wheat sourdoughs. J. Cereal Sci. 43:137-143. [Google Scholar]
- 44.Vermeulen, N. 2006. Aroma relevant metabolic activities of lactobacilli during wheat sourdough fermentation. Doctoral thesis. TU München, Faculty Center of Life Sciences, Weihenstephan, Germany.
- 45.Vermeulen, N. C., C. Thiele, M. G. Gänzle, and R. F. Vogel. 2003. Abstr. 2nd Int. Symp. Sourdough, Brussels, Belgium, p. 49. IMDO, Brussels, Belgium.
- 46.Vido, K., H. Diemer, A. van Dorsselaer, E. Leize, V. Juillard, A. Gruss, and P. Gaudu. 2005. Roles of thioredoxin reductase during the aerobic life of Lactococcus lactis. J. Bacteriol. 187:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Viña, J. (ed.). 1990. Glutathione: metabolism and physiological functions. CRC Press, Boca Raton, FL.
- 47.Weegels, P. L., R. J. Hamer, and J. D. Schofield. 1996. Functional properties of wheat glutenin. J. Cereal Sci. 23:1-17. [Google Scholar]
- 48.Wiederholt, K. M., and J. L. Steele. 1994. Glutathione accumulation in lactococci. J. Dairy Sci. 77:1183-1188. [Google Scholar]
- 49.Yamamoto, Y., Y. Kamio, and M. Higuchi. 1999. Cloning, nucleotide sequence, and disruption of Streptococcus mutans glutathione reductase gene (gor). Biosci. Biotechnol. Biochem. 63:1056-1062. [DOI] [PubMed] [Google Scholar]