Abstract
Noroviruses (NVs) are the most frequent cause of outbreaks of gastroenteritis in common settings, with surface-mediated transfer via contact with fecally contaminated surfaces implicated in exposure. NVs are environmentally stable and persistent and have a low infectious dose. Several disinfectants have been evaluated for efficacy to control viruses on surfaces, but the toxicity and potential damage to treated materials limits their applicability. Sterilox hypochlorous acid (HOCl) solution (HAS) has shown broad-spectrum antimicrobial activity while being suitable for general use. The objectives of this study were to evaluate the efficacy of HAS to reduce NV both in aqueous suspensions and on inanimate carriers. HOCl was further tested as a fog to decontaminate large spaces. HOCl effectiveness was evaluated using nonculturable human NV measured by reverse transcriptase PCR (RT-PCR) and two surrogate viruses, coliphage MS2 and murine NV, that were detected by both infectivity and RT-PCR. Exposing virus-contaminated carriers of ceramic tile (porous) and stainless steel (nonporous) to 20 to 200 ppm of HOCl solution resulted in ≥99.9% (≥3 log10) reductions of both infectivity and RNA titers of tested viruses within 10 min of exposure time. HOCl fogged in a confined space reduced the infectivity and RNA titers of NV, murine NV, and MS2 on these carriers by at least 99.9% (3 log10), regardless of carrier location and orientation. We conclude that HOCl solution as a liquid or fog is likely to be effective in disinfecting common settings to reduce NV exposures and thereby control virus spread via fomites.
Noroviruses (NVs) are the leading cause of nonbacterial gastroenteritis worldwide and are estimated to be responsible for 80 to 90% of reported outbreaks, particularly in public settings such as cruise ships, hotels, and health care facilities (19, 25). NV outbreaks in these common settings have economic impacts resulting from the costs to disinfect affected areas to prevent the spread and recurrence of NV infection, the likely shutdown of workplaces, and the hospitalizations of affected persons. The main modes of transmission are via the fecal-oral route by consumption of contaminated foods or water (4, 5, 26, 27), by direct or indirect person-to-person transmission via hands or fomite transfer (11, 12, 14, 17, 23, 35), or by airborne contamination. NV outbreaks are difficult to control due to the viruses' having high attack rates, environmental stability, and a low human infectious dose estimated to be between 10 and 100 virions per 50% human infectious dose (10).
Chemical disinfection is an effective measure to interrupt the environmental spread of infectious viruses. However, the effectiveness of antimicrobial agents is highly influenced by the type of virus, by exposure conditions such as organic loading and contact time, and by biocidal activity and concentration. Generally, glutaraldehyde and sodium hypochlorite are known to be effective against nonenveloped RNA viruses, such as NVs, whereas detergents and most lipophilic disinfectants (e.g., quaternary ammonium compounds) have been reported to have weaker virucidal activity (2, 16). Aside from efficacy, the use of these biocides in practical situations and settings must satisfy other requirements such as low toxicity to personnel and low potential to damage treated materials (29).
Sterilox systems (PuriCore Inc.) electrochemically generate hypochlorous acid (HOCl) solutions (HAS) at a range of concentrations to meet the requirements of different applications. These HAS exhibit broad-spectrum antimicrobial activity against a range of organisms including spore-forming bacteria and nonenveloped viruses (PuriCore Inc., personal communication). HOCl is also involved in a number of biological interactions with mammalian cells and tissues (28). A recent study has shown that fogged Sterilox HOCl was able to decontaminate environmental surfaces carrying antibiotic-resistant Staphylococcus aureus (methicillin resistant) and Acinetobacter (14). Despite the widespread use and proven effectiveness of HOCl against other viral pathogens, it has not been studied for its ability to disinfect surfaces contaminated with NV or other human caliciviruses.
Therefore, the objectives of this study were to determine the effectiveness of HAS against NV in aqueous suspension and dried onto porous and nonporous hard surfaces using a human NV isolate and two surrogate organisms, a murine NV and coliphage MS2. A method of environmentally decontaminating large, confined spaces carrying NV by using an HOCl fogging system was also evaluated.
MATERIALS AND METHODS
Viruses.
Human NV used in this study was obtained from cases of an outbreak of gastroenteritis at the University of North Carolina campus in 2004 and characterized as genotype II.4 (GII.4). A frozen stool sample obtained from a patient was aliquoted into several 100-μl volumes of 10% stool suspensions and stored at −80°C. An aliquot was thawed and made into 1% stool suspensions (wt/vol) in phosphate-buffered saline (PBS; pH 7.5) on the day of an experiment. Frozen NV aliquots were not frozen and thawed more than twice in order to maintain virion stability.
Bacteriophage MS2.
Bacteriophage MS2 (ATCC 15597-B1) was cultivated using Escherichia coli Famp (ATCC 700891), as previously described (34). MS2 was partially purified from infected cell lysate by recovery of the resulting supernatant after moderate-speed centrifugation (3,000 × g, 20 min, 4°C). The titer of the partially purified MS2 stock was approximately 1010 PFU per ml.
MNV-1 stock and culture.
A murine NV (MNV-1), obtained from the laboratory of Herbert W. “Skip” Virgin, Washington University in St. Louis, was grown and assayed for infectivity in RAW 264.7 cells (ATCC TIB-71). Cells were grown in Eagle's minimum essential medium (MEM) supplemented with 10% low-endotoxin fetal bovine serum (SH30070.03; HyClone, Logan, UT), kanamycin (250 μg/ml), 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 0.1 mM Eagle's nonessential amino acids, and 13 mM sodium bicarbonate. After 2 days of incubation in 150-ml flasks to obtain >90% confluent cell layers, cells were infected with MNV-1 at a multiplicity of infection of 0.1 to 0.05 PFU/cell for 5 to 6 days at 37°C. The viruses in infected cell lysates were partially purified by three cycles of freezing and thawing, followed by removal of cell debris by centrifugation (3,000 × g, 20 min, 4°C). The titer of MNV-1 was approximately 108 PFU per ml.
The infectivity of MNV-1 was assayed by a plaque technique in RAW 264.7 cells that had been seeded into 60-mm plates at a density of 106 viable cells per plate and then incubated at 37°C in 5% CO2. After 2 days, spent medium was decanted, and cells were inoculated with 100 to 200 μl of virus suspensions plus 0.5 ml of medium having the same composition as complete MEM except for containing only 3% fetal bovine serum. After a 1-h incubation, plates were aspirated of excess liquid and overlaid with 0.5% agarose in complete MEM. After 48 h, cells received a second overlay with 0.5% agarose in complete MEM containing 0.5% neutral red (3.3 g/liter in Dulbecco's PBS; Sigma-Aldrich). Plates having 5 to 50 plaques were counted, and the virus titer was expressed as PFU per ml.
RNA extraction.
Viral RNA was extracted from sample suspensions using the QIAamp viral mini kit according to the manufacturer's instructions (QIAGEN Inc., Valencia, CA). Briefly, 100 to 200 μl of virus suspension was mixed with an equal volume of guanidinium thiocyanate solution, followed by neutralization with an equal volume of 100% ethanol. Viral RNA was further purified by the use of a QIAamp mini column (QIAGEN viral RNA kit). Purified RNA samples were stored at −80°C until reverse transcriptase PCR (RT-PCR) assay.
Virus primer design.
To construct the GII.4-specific primer set, we sequenced a 343-bp amplicon of the G2SKF and G2FKR primer pair of Kojima et al. (18), targeting the highly conserved capsid region of NV GII groups, and the resulting sequence information was used to design a primer pair specific for GII.4, designated NoroG2SKF/NoroG2SKR. This primer set generates a 212-bp PCR product, corresponding to nucleotides 5153 to 5364 in the reference strain of Lordsdale virus (X86557). For MS2 and MNV-1, primer sets targeting the RNA polymerase region of each virus were designed to generate amplicons of 234 and 231 bp, respectively. Detailed information on primer sets for each virus is shown in Table 1.
TABLE 1.
Primers used for this study
| Strain | Primer | Sequence (5′→3′) | Polaritya | Location |
|---|---|---|---|---|
| Human NV | G2SKF | CNTGGGAGGGCGATCGCAA | + | 5046b |
| G2FKR | CCRCCNGCATRHCCRTTRTACAT | − | 5352b | |
| NoroF | CCCAGAGGTCAACAATGAGG | + | 5153b | |
| NoroR | AAAGGTAGGGATTCAGATCAGG | − | 5364b | |
| MNV-1 | JV91 | CAGCAGTCTTTGTGAATGAGG | + | 5041c |
| JV92 | CGAGGCGAAATGGAAAAC | − | 5271c | |
| MS2 | MS2REPS1 | TAAGCTACGGGAGCGGAATG | + | 1982d |
| MS2REPA1 | GCTTGTTCAGCGAACTTCTTG | − | 2215d |
+, forward primer; −, reverse primer.
Location of the 5′ end of the primer in the nucleotide sequence of Lordsdale virus (X86557) (19).
Location of the 5′ end of the primer in the nucleotide sequence of MNV-1 (DQ285629).
Location of the 5′ end of the primer in the nucleotide sequence of coliphage MS2 (NC 001417.1) (34).
RT-PCR assay.
Each reaction tube contained 5 μl 5× buffer, 1 μl of dNTP (deoxynucleoside triphosphate) mix (containing 10 mM of each dNTP) to a final concentration of 400 mM each, 0.5 μl of each primer to a final concentration of 50 pmol/liter, 1 μl QIAGEN OneStep RT-PCR enzyme mix, and RNase-free water to a total volume of 22 μl. A 2.5-μl volume of extracted sample RNA was added, and tubes were placed into the thermocycler (PTC-200; MJ Research, Waltham, MA). Cycling conditions for NV were as follows: for the broadly reactive primer set G2SKF and G2FKR, 42°C for 60 min, 95°C for 15 min, and 40 cycles consisting of 30 s, 1 min, and 1 min, respectively, for denaturing (94°C), annealing (50°C), and extension (72°C), with a 10-min final extension step (72°C); for primer set NoroF/NoroR specific for NV GII.4, 45°C for 30 min, 95°C for 15 min, and 40 cycles consisting of 30 s, 1 min, and 1 min, respectively, for denaturing (94°C), annealing (58°C), and extension (72°C), with a 10-min final extension step (72°C); for MS2, 45°C for 30 min, 95°C for 15 min, and 40 cycles consisting of 30 s for denaturing (94°C), 1 min for annealing (48°C), and 1 min for extension (72°C), with a 10-min final extension step (72°C); for MNV-1, 45°C for 30 min, 94°C for 15 min, and 40 cycles consisting of 30 s for denaturing (94°C), 1 min for annealing (50°C), and 30 s for extension (72°C), with a 10-minute final extension step (72°C).
The RNA titer of each virus was calculated by end point titration, which determines the highest dilution of virus stock that is positive by RT-PCR amplification. This method was used to quantify the initial RNA titer or the remaining RNA titer after treatments. Four to six carriers per treatment were used to determine disinfection efficiency. Amplification reaction products (15 μl) were resolved by electrophoresis (1 h, 120 V) on 2% agarose gels, stained with ethidium bromide, and visualized by UV transillumination.
Surface disinfection.
No. 4 finish-polished stainless steel and ceramic tiles were used as representative nonporous and porous surfaces, respectively. Prior to use, carriers 1.0 by 1.0 cm in size were pretreated with 0.1% Tween 80, rinsed in sterile, distilled water and then in 70% ethanol, air dried, wrapped in aluminum foil, and then autoclaved for 15 min at 121°C.
A pooled virus suspension in 1% stool was prepared by adding 100-μl volumes of undiluted MS2, MNV-1 stocks, and 10% human NV stool suspension into 700 μl of PBS to make a 1% stool suspension volume of 1 ml. A 25-μl volume of this 1% stool virus suspension was air dried onto the center of each carrier surface for triplicate carriers. As a negative control, 25 μl of sterile PBS (pH 7.5) was pipetted onto a carrier surface. The stability of the viruses dried onto carriers was determined for time periods of up to 2 days at room temperature.
HAS at a pH of 5.5 to 6.2 was generated electrolytically from a dilute NaCl solution in a Sterilox Technologies model 2100 system according to the manufacturer's instructions. The stability of test viruses in HAS undiluted (about 200 ppm free chlorine) and diluted fivefold (about 40 mg/liter) and 10-fold (about 20 ppm free chlorine) in chlorine demand-free water was determined. Volumes of 25 μl of 1% NV stool suspension and diluted MS2 stock were combined with 1.2 ml of HAS, and after specified contact times, 25 μl of 6% sodium thiosulfate was added to neutralize residual free chlorine.
For surface disinfection experiments, virus suspensions dried on each carrier for 2 to 3 h were treated with 1.2 ml of HAS of 50 to 190 ppm. After specified contact times, 0.275 ml of 16% beef extract (pH 8.0) and 0.025 ml of 6% sodium thiosulfate were added into the 1.2-ml chlorine solution to elute viruses and neutralize the HOCl, respectively. Plates were mixed on a rotary shaker for 20 min to facilitate virus elution and neutralization of chlorine. Eluents were transferred into sterile 1.5-ml microcentrifuge tubes and were stored at −80°C until virus assay.
HAS fogging experiment.
Following a protocol of PuriCore Inc., fogging disinfection experiments were carried out in a 9-by-9-by-9-ft room in which the air ventilation had been shut down to better control airflow during the experiment. The fogger (Curtis Dyna-Fog, Westfield, IN) was loaded with HAS at 180 to 200 ppm available free chlorine (AFC) and used to generate fogs at a 0.4 liter/min fogging rate. The size range of generated droplets was between 20 and 50 μm. Prior to the fogging treatment, contaminated carriers were laid out in the following pattern: five horizontally placed (upward-facing) contaminated surface carriers were evenly located within a distance of 60 cm, and five vertically placed surface carriers were parallel to the horizontal carriers but at a height of one foot above the horizontal carriers. The freshly prepared HAS fog was generated 1.5 m away from the carriers, at an angle of 30° toward the horizontal surface carrier designated H_C for 10 min, and then was fogged individually toward all five vertically and horizontally laid tiles within a 1-min period. After 1 h to allow for the settling of fog within the room, each carrier was placed into a well of a 24-well plate, each well containing a solution of 1.5 ml of 3% beef extract-0.05% sodium thiosulfate. The plate was mechanically rotated for 15 min, after which the solution was transferred into sterile, 1.5-ml microcentrifuge tubes and stored at −80°C until assay. Duplicate fogging experiments were performed.
Data analysis.
Antimicrobial activity of HAS for suspension, carrier, and fogging experiments was determined by calculating log10 (Nd/N0), where N0 is the titer of detectable viruses (by infectivity or viral RNA amplification) recovered from untreated samples and Nd is the titer of viable viruses (by infectivity or viral RNA amplification) from treated samples. Data obtained from at least two independent experiments were averaged, and statistical analysis of groups was performed using either the Wilcoxon matched-pairs signed-rank test or the Kruskal-Wallis test to identify significant differences in virus concentrations or log10 virus reductions for treatments or viruses (3).
RESULTS
Stability of RNA viruses on test environmental surfaces.
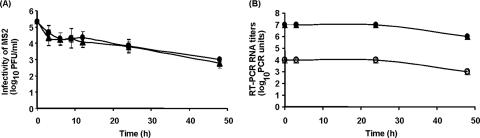
Initial log10 titers (± standard deviations) of MNV-1, MS2, and human NV in 25 μl of 1% stool samples were determined to be 6.3 ± 0.1 log10 PFU/ml (MNV-1), 8 to 9 log10 PFU/ml (MS2), and 5 to 6 log10 RT-PCR units/ml (NV) (Fig. 1). Following 3 h of air drying of virus suspensions on both carrier materials (ceramic tile and stainless steel), MS2 infectivity on ceramic tile and stainless steel carriers decreased to 4.4 ± 0.50 and 4.7 ± 0.45 log10 PFU/ml, which corresponded to 1.1 and 2.5% of initial titers, respectively (Fig. 1A). After 2 days, MS2 infectivity levels on both carriers further declined by approximately 1.5 log10 PFU/ml from their levels after initial drying. For the same conditions, the detectable RNA levels of both NV and MS2 eluted from both carriers remained unchanged from initial levels after 24 h of air drying, but they decreased by approximately 1 log10 after 2 days of drying (Fig. 1B).
FIG. 1.
The persistence of viruses on environmental surfaces. (A) Recovery rate of MS2 (infectivity) and (B) RT-PCR RNA titers of MS2 (• and ▴) and NV (○ and ▵) on two kinds of dried carriers at an ambient temperature of 25°C. • and ○, stainless steel; ▴ and ▵, ceramic tile.
Efficacy of (Sterilox) liquid HAS against viruses in suspension and dried on surfaces.
As shown in Table 2, exposure of test viruses to HAS at AFC doses ranging from about 20 to 200 ppm consistently achieved greater than a 3 log10 (>99.9%) reduction of infectious MS2 within a contact time of 20 s in aqueous suspension tests at room temperature. Results summarize the outcomes of eight replicate experiments with four replicate samples per HAS dose in each experiment. For the same exposure conditions, reductions in detectable RNA titers of MS2 also were at least 3 log10 at 20 s for HAS doses of 38 or 18.8 ppm. Likewise, exposure to undiluted HAS or diluted solutions of 38 and 18.8 ppm also reduced RNA titers of human NV by at least 3 log10 (99.9%) in a contact time of 20 seconds.
TABLE 2.
Inactivation of viruses in aqueous suspension and dried on carrier surfaces by Sterilox HASa
| Assay type and HOCl concn (ppm AFC) | Minimum contact time to achieve at least 3 log10 (99.9%) virus reduction
|
||
|---|---|---|---|
| Suspension test (s) | Carrier test (min)
|
||
| Ceramic tile | Stainless steel | ||
| Infectivity (MS2) | |||
| 188 | 20 | 1 | 1 |
| 38 | 20 | 5 | 5 |
| 18.8 | 20 | 5 | 5 |
| RT-PCR titer (MS2) | |||
| 188 | 20 | 1 | 1 |
| 38 | 20 | 5 | 5 |
| 18.8 | 20 | 5 | 5 |
| RT-PCR titer (human NV) | |||
| 188 | 20 | 1 | 1 |
| 38 | 20 | 5 | 10 |
| 18.8 | 20 | 10 | 5 |
Results of 12 replicate suspension experiments (6 replicate suspensions per HAS concentration per experiment) and 12 replicate carrier tests (6 replicate carriers per HAS concentration per test). The exposure time for viruses in all replicate samples per test condition to be reduced by >3 log10 or >99.9%, to below the virus detection limit, is given. Exposure times in suspension tests were 20, 40, and 60 seconds, and in carrier tests they were 0.67, 1, 2, 5, and 10 min.
In the carrier tests, MS2 infectivity on both ceramic tile and stainless steel carriers was reduced by >3 log10 (>99.9%) after 1 min of contact time with undiluted HAS (Table 2). On exposure to five- and 10-fold-diluted HAS (38 and 18.8 ppm, respectively), the minimum contact time to achieve at least 3 log10 MS2 infectivity reductions on both carriers was 5 min. Undiluted HAS also achieved at least 3 log10 reductions of viral RNA for both MS2 and human NV in one minute of contact time. Using five- and 10-fold-diluted HAS, contact times of at least 10 min were necessary to obtain at least 3 log10 viral RNA reductions.
Stability of Sterilox HAS AFC concentration and pH during fogging.
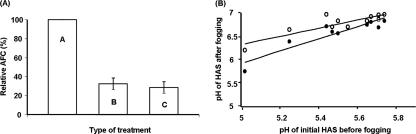
The initial concentration of AFC in freshly generated HAS was measured at 203 ± 12 mg/liter. The decrease in average AFC of HAS immediately after fogging, based on recovery of the liquid that accumulated on exposed carrier surfaces, was about 140 mg/liter, corresponding to an approximately 70% loss of initial AFC (Fig. 2A). AFC concentrations of HAS in liquid collected after 1 h of settling of fogs further decreased only slightly.
FIG. 2.
Effect of fogging on (A) pH levels and (B) AFC concentration of Sterilox HAS. A, initial HAS before fogging; B, HAS after 10-min fogging; C, HAS after 10-min fogging and 1-h settling. •, HAS after 10-min fogging; ○, HAS after 10-min fogging and 1-h settling.
When HAS in which pH conditions were adjusted to between 5.0 and 5.8 were subjected to fogging, appreciable pH elevations were always observed in the recovered solution, regardless of the initial pH condition (Fig. 2B). The degree of pH elevation appeared to be relatively constant, with only narrow variations. When HOCl fogs were further monitored for pH after additional time periods beyond 1 h of settling on surfaces, pH changes were negligible compared to the pH elevations of HOCl after initial fogging and settling.
Overall, when HAS at an initial pH of 5.0 to 5.2 and an initial AFC concentration of approximately 190 ppm was used for fogging experiments, pH remained below 6.2, and AFC was >50 ppm throughout the cycle of fogging treatment.
Virucidal activity of fogged Sterilox HOCl.
The results of duplicate fogging experiments with five ceramic tile carriers contaminated with 20 μl of pooled viruses in 1% stool and placed either horizontally or vertically in an area of 50 cm by 30 cm are summarized in Table 3. In these experiments, initial infectious titers of MNV-1 and MS2 were 4.3 and 4.7 log10 PFU/ml, respectively, in experiment 1 and 4.7 and 6.3 log10, respectively, in experiment 2, with lower detection limits of 1 log10 PFU/ml. After fogging treatment, no infectious viruses were ever detected on all 10 carriers in both experiments. Corresponding RNA titer reductions of all three viruses tested were always at least 3 log10 PCR units, and median reductions were >6, >5, and 5 log10 for human NV, MNV-1, and MS2, respectively. When RNA reductions of the three different viruses by HOCl fogging were analyzed by the Kruskal-Wallis test (3), they were not significantly different (P > 0.05). However, there were small but detectable spatial variations in the effectiveness of fogging (Table 3). Viral RNA titer reductions on horizontal carriers ranged from 5 to 7 log10 PCR units, whereas greater variations in viral reductions were observed on vertical carriers, ranging from 3 to 8 log10 PCR units. When these data for horizontal and vertical orientations were subjected to statistical analysis for each virus, they were not significantly different for human NV or MS2 (P > 0.05), but they were significant for MNV-1 (P = 0.03) by the Wilcoxon matched-pairs signed-rank test (3). Despite these possible spatial differences in effectiveness, HAS fogging consistently reduced viruses on carriers by at least 3 log10, with the majority of tiles showing greater than 5 log10 reductions in viral RNA titer.
TABLE 3.
Reduction of viruses dried on ceramic tile surfaces by fogging of Sterilox HOCl
| Carrierb | Virus RNA reduction (log10 PCR units)a
|
Virus infectivity reduction (log10 PFU)a
|
|||
|---|---|---|---|---|---|
| Human NV | MNV-1 | MS2 | MNV-1 | MS2 | |
| H_L2 | >6 | >5.5 | 5.5 | >3.5 | >4.0 |
| H_L1 | >6 | >5.5 | 6 | >3.5 | >4.0 |
| H_C | >6 | >5.5 | 5.5 | >3.5 | >4.0 |
| H_R1 | >6 | >5.5 | 6 | >3.5 | >4.0 |
| H_R2 | >5.5 | >5.5 | 5.5 | >3.5 | >4.0 |
| V_L2 | >5.5 | >4.5 | 5.5 | >3.5 | >4.0 |
| V_L1 | >4.5 | >5 | 5 | >3.5 | >4.0 |
| V_C | >6 | >5 | 6.5 | >3.5 | >4.0 |
| V_R1 | 4.5 | 4.5 | 6 | >3.5 | >4.0 |
| V_R2 | >4.5 | >4.5 | 4.5 | >3.5 | >4.0 |
Values are average data from two independent experiments. > indicates that virus in treated sample was below assay detection limits. Virus RNA reduction values were determined by RT-PCR assay. Virus infectivity reduction values were determined by infectivity assay.
Vertical and horizontal carriers were designated V and H, respectively. L1 (R1) and L2 (R2) indicate direction of carriers (left and right side from center-placed [C] carriers, respectively, relative to the position of the fogger) and the distance of the carrier from the center, with numbers 1 and 2 indicating distances of 12.5 cm and 25 cm from the center, respectively.
DISCUSSION
Due to the absence of a culture assay for human NVs, reliable surrogate viruses are needed to estimate the effects of various environmental exposure conditions and antiviral treatments on the infectivity of these viruses. Bacteriophage MS2, belonging to serotype (genogroup) I of the RNA coliphages within the family Leviviridae, resembles NV because it is a plus-sense, single-stranded RNA virus with icosahedral symmetry and is of similar virion size (8). MS2 has been widely recognized as an indicator organism to measure fecal contamination of water, oysters, and other environmental media; to predict the persistence of enteric viruses in soils and other environmental media; and to determine enteric virus response to disinfectants (15, 22, 31).
In this study, MS2 remained stable, with fewer than 3 log10 reductions on stainless steel and ceramic tiles after 7 days, a sufficient period of time for viruses to spread via fomites to hands or vice versa (Fig. 1). Similar results were previously demonstrated for other enteric viruses (hepatitis A virus, astrovirus, and rotavirus), which are often implicated in outbreaks in defined settings (1, 30). Although the persistence of human NVs in the environment is uncertain, due to the lack of infectivity assays, epidemiological evidence suggests long-term NV persistence as being responsible for persistent and recurrent outbreaks (12, 13). Given the long persistence of MS2, the similar elution patterns of MS2 and human NV from surfaces, and their similar detection by RT-PCR, MS2 may resemble human NV in persistence of infectivity, elution from surfaces, and detectability.
HAS at concentrations between about 20 and 200 ppm AFC produced rapid and extensive inactivation of NVs and bacteriophage MS2 in aqueous suspensions and when dried on ceramic tile or stainless steel carriers. Minimum contact times to achieve at least 3 log10 reductions of viruses were three- to 15-fold longer in carrier tests than in suspension tests when exposure to HAS ranged from 18 to 188 ppm. The observed difference in virucidal activity of HOCl between suspension and carrier tests has been previously documented in other disinfection studies (36). The reasons for this difference remain uncertain, but it is assumed that there is less exposure to biocides when viruses are present on surfaces, perhaps due to reduced accessibility of the entire virus to the disinfectant chemical (29). Based on the observed greater virus resistance on surfaces than in suspension, carrier tests are considered a more suitable and realistic model system for assessing the efficiencies of chemical disinfectants against viruses on environmental (fomitic) surfaces than are aqueous suspension tests.
The results of this study indicate that carrier types (a porous versus a nonporous carrier) did not show appreciable differences in virus persistence or in the virucidal efficacy of HAS for the exposure conditions and viruses tested. The effect of the specific carrier type on the virucidal activity of disinfectants has been examined only rarely and remains uncertain (1). Abad et al. observed that hepatitis A virus and rotavirus were highly persistent on both nonporous carriers and porous carriers in a comparison of four different viruses (hepatitis A virus, rotavirus, poliovirus, and NV) (1). In our study, the detectability of infectious MS2 recovered from a porous carrier (ceramic tile) after drying was slightly lower than that from a nonporous carrier (stainless steel). However, reductions of viral RNA recovered from the two carrier types were similar. There were no appreciable differences between human NV and MS2 based on recovery from both carriers following HOCl disinfection.
Simultaneous assays for viral RNA and infectivity showed that RNA reduction appeared to be smaller than or slower than infectivity reduction. It is well recognized that the RT-PCR assay is likely to overestimate virus infectivity and underestimate virus reductions caused by various treatments (32). It is possible that the remaining viral RNA of inactivated viruses might still be infectious. However, from a practical, human health risk standpoint, this persistent viral RNA is likely to be of little consequence if loss of infectivity has resulted in alterations causing loss of virion integrity (36). Nevertheless, the possibility that conventional cell culture assays based on detection of viral cytopathogenic effects are potentially unable to detect fastidious but infectious viruses that no longer produce cytopathogenic effects may need further consideration for the exposure conditions investigated in this study (6).
On the basis of both infectivity and RNA assays, it is likely that HAS used at a concentration of 200 ppm AFC is effective at decontaminating inert environmental surfaces carrying NVs and probably other enteric viruses in a 1-min contact time. When diluted 10-fold, HAS at an AFC concentration of about 20 ppm is still effective in decontaminating environmental surfaces carrying NVs in a 10-min contact time.
To evaluate the virucidal efficacy of HOCl fogs against human NVs, we employed a murine NV (MNV-1) as well as coliphage MS2. MNV-1 is genetically similar to human NV and is capable of surviving extreme acid conditions, which is a typical feature of enteric viruses, including human NV (9). Furthermore, a comparison of inactivation kinetics of MNV-1 to that of feline calicivirus, which is generally used as an animal virus model for human NV, showed that MNV-1 was more resistant to free chlorine and UV disinfection than feline calicivirus (G. W. Park, J. Vinjé, and M. D. Sobsey, presented at the 106th General Meeting of the American Society for Microbiology, Orlando, FL, 21 to 25 May 2006).
Fogs of HAS were effective against viruses on surfaces. Human NV, MNV-1, and coliphage MS2 on ceramic tiles were reduced by a minimum of 3 log10 PFU and mostly >5 log10, regardless of the location and orientation of carriers (either vertical or horizontal). HAS fogs were highly effective in the microbial disinfection of environmental surfaces, even though the fogging process changed the physical and chemical properties of the disinfectant. Fogging reduced the AFC concentration by approximately 70% and increased the pH by about 1.3 ± 0.11 pH units. Len et al. reported appreciable losses of chlorine in electrolyzed oxidizing water similar to those in HAS in an open or agitated system and assumed the loss of chlorine resulted from evaporation of chlorine gas (20). Fogging is a mechanical action that creates small particles that can accelerate the interfacial mass transfer of chlorine gas (24), thereby resulting in appreciable chlorine loss. Because the changes in the properties of HAS after fogging were predictable, preadjustment of the AFC and pH of the solution prior to fogging made it possible to control the levels of these parameters within the desirable range to appreciably inactivate microbes after fogging.
Variations of viral RNA titer reductions were occasionally observed with the orientations of the carriers exposed to fogging. Burfoot and colleagues also reported differences in the efficacy of fogs by orientation of carriers, with the ratio of reduction of microorganisms on horizontal surfaces being 20-fold greater than on vertical surfaces. This observed variation of the effectiveness of fogging was attributed to possible differences in the amount of deposition of the disinfectants (7). The difference sometimes observed in viral RNA titer reductions between vertical and horizontal carriers in this study also could be due to a difference in disinfectant deposition. Nevertheless, fog disinfection with HAS always achieved greater than 3 log10 reductions and mostly >5 log10 reductions in both the infectivity and RNA titers of all tested viruses on both vertical and horizontal surfaces, suggesting that it is an effective approach to reduce NVs on surfaces.
Several authors have proposed that a disinfectant may be recognized as effective in decontaminating environmental surfaces carrying viruses if it causes a 3 log10 reduction in virus titer, taking into consideration the titer of viruses shed into the environment (21, 33). We examined the efficacy of HOCl use as a fog, based on a disinfection protocol by PuriCore Inc. PuriCore guidelines recommend cleaning prior to fogging disinfection of environmental surfaces. In this study, a 1% stool suspension of viruses on environmental carriers was selected as a reasonable model to examine the effects of viral load on disinfection efficacy, based on the organic loads likely to be present on precleaned porous and nonporous surfaces. HAS at concentrations ranging from 20 to 200 ppm, representing the likely chlorine concentration range achieved during fogging, always gave at least 3 log10 reductions of tested viruses both by viral infectivity and RNA assays. These findings indicate that HAS applied as either a liquid or a fog at 20 to 200 ppm of AFC was effective to decontaminate the inert environmental surfaces tested when they carried NVs and other viruses.
Continued NV-associated outbreaks in common settings highlight the need for effective disinfection. We conclude that dilute solutions of HOCl, containing as little as 20 to 200 mg/liter free chorine, are effective for disinfection of surfaces contaminated with NVs. Furthermore, we conclude that the use of fogs of Sterilox HOCl is likely to be effective in disinfecting large areas to control NV presence and thereby prevent the spread as well as the recurrence of human NV infection from environmental surface exposures.
Acknowledgments
This research was supported by PuriCore Inc.
We are grateful for the technical assistance of Douglas Wait and Dorothy Thompson of the University of North Carolina laboratory.
Footnotes
Published ahead of print on 4 May 2007.
REFERENCES
- 1.Abad, F. X., R. M. Pintó, and A. Bosch. 1994. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 60:3704-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abad, F. X., R. M. Pintó, and A. Bosch. 1997. Detection of human enteric viruses on fomites. FEMS Microbiol. Lett. 156:107-111. [DOI] [PubMed] [Google Scholar]
- 3.Altman, D. G. 1991. Practical statistics for medical research, 1st ed. Chapman & Hall, London, United Kingdom.
- 4.Anderson, A. D., V. D. Garrett, J. Sobel, S. S. Monroe, R. L. Fankhauser, K. J. Schwab, J. S. Bresee, P. S. Mead, C. Higgins, J. Campana, and R. I. Glass. 2001. Multistate outbreak of Norwalk-like virus gastroenteritis associated with a common caterer. Am. J. Epidemiol. 154:1013-1019. [DOI] [PubMed] [Google Scholar]
- 5.Berg, D. E., M. A. Kohn, T. A. Farley, and L. M. McFarland. 2000. Multi-state outbreaks of acute gastroenteritis traced to fecal-contaminated oysters harvested from Louisiana. J. Infect. Dis. 181:S381-S386. [DOI] [PubMed] [Google Scholar]
- 6.Blackmer, F., K. A. Reynolds, C. P. Gerba, and I. L. Pepper. 2000. Use of integrated cell culture-PCR to evaluate the effectiveness of poliovirus inactivation by chlorine. Appl. Environ. Microbiol. 66:2267-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burfoot, D., K. Hall, K. Brown, and Y. Xu. 1999. Fogging for the disinfection of food processing factories and equipment. Trends Food Sci. Technol. 10:205-210. [Google Scholar]
- 8.Calender, R. 1988. The bacteriophages, vol. 1. Oxford University Press, New York, NY.
- 9.Cannon, J. L., E. Papafragkou, G. W. Park, J. Osborne, L.-A. Jaykus, and J. Vinjé. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J. Food Prot. 69:2761-2765. [DOI] [PubMed] [Google Scholar]
- 10.Caul, E. O. 1994. Small structured viruses: airborne transmission and hospital control. Lancet 343:1240-1242. [DOI] [PubMed] [Google Scholar]
- 11.Chadwick, P. R., and R. McCann. 1994. Transmission of a small round structure virus by vomiting during a hospital outbreak of gastroenteritis. J. Hosp. Infect. 26:251-259. [DOI] [PubMed] [Google Scholar]
- 12.Cheesbrough, J. S., L. Barkiss-Jones, and D. W. G. Brown. 1997. Possible prolonged environmental survival of small round structured viruses. J. Hosp. Infect. 35:325-326. [DOI] [PubMed] [Google Scholar]
- 13.Cheesbrough, J. S., J. Green, C. I. Gallimore, P. A. Wright, and D. W. G. Brown. 2000. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol. Infect. 125:93-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark, J., S. P. Barrett, M. Rogers, and R. Stapleton. 2006. Efficacy of super-oxidized water fogging in environmental decontamination. J. Hosp. Infect. 64:386-390. [DOI] [PubMed] [Google Scholar]
- 15.Dore, W. J., K. Henshilwood, and D. N. Lees. 2000. Evaluation of F-specific RNA bacteriophage as a candidate human enteric virus indicator for bivalve molluscan shellfish. Appl. Environ. Microbiol. 66:1280-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 41:51-57. [DOI] [PubMed] [Google Scholar]
- 17.Green, J., P. A. Wright, C. I. Gallimore, O. Mitchell, P. Morgan-Capner, and D. W. G. Brown. 1998. The role of environmental contamination with small round structured viruses in a hospital outbreak investigated by reverse-transcriptase polymerase chain reaction assay. J. Hosp. Infect. 39:39-45. [DOI] [PubMed] [Google Scholar]
- 18.Kojima, S., T. Kageyama, S. Fukushi, F. B. Hoshino, M. Shinohara, K. Uchida, K. Natori, N. Takeda, and K. Katayama. 2002. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J. Virol. Methods 100:107-114. [DOI] [PubMed] [Google Scholar]
- 19.Koopmans, M., C.-H. von Bonsdorff, J. Vinje, D. de Medici, and S. Monroe. 2002. Foodborne viruses. FEMS Microbiol. Rev. 26:187-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Len, S. V., Y. C. Hung, D. Chung, J. L. Anderson, M. C. Erickson, and K. Morita. 2002. Effects of storage conditions and pH on chlorine loss in electrolyzed oxidizing (EO) water. J. Agric. Food Chem. 50:209-212. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Evans, N., V. S. Springthorpe, and S. A. Sattar. 1986. Chemical disinfection of human rotavirus-contaminated inanimate surfaces. J. Hyg. 97:163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maillard, J. Y., T. S. Beggs, M. J. Day, R. A. Hudson, and A. D. Russell. 1994. Effect of biocides on MS2 and K coliphages. Appl. Environ. Microbiol. 60:2205-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks, P. J., I. B. Vipond, D. Carlisle, D. Deakin, R. E. Fey, and E. O. Caul. 2000. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol. Infect. 124:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McRay, R. J., P. Dineen, and E. D. Kitzke. 1964. Disinfectant fogging techniques. Soap Chem. Spec. 40:112-114. [Google Scholar]
- 25.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parashar, U. D., L. Dow, R. L. Fankhauser, C. D. Humphrey, I. J. Miller, T. Ando, K. S. Williams, C. R. Eddy, J. S. Noel, T. Ingram, J. S. Bresee, S. S. Monroe, and R. I. Glass. 1998. An outbreak of viral gastroenteritis associated with consumption of sandwiches: implications for the control of transmission by food handlers. Epidemiol. Infect. 121:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponka, A., L. Maunula, C.-H. von Bonsdorff, and O. Lyytikainen. 1999. An outbreak of calicivirus associated with consumption of frozen raspberries. Epidemiol. Infect. 123:469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pullar, J. M., M. C. M. Vissers, and C. C. Winterbourn. 2000. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life 50:259-266. [DOI] [PubMed] [Google Scholar]
- 29.Russell, A. D., W. B. Hugo, and G. A. J. Ayliffe. 1999. Disinfection, preservation and sterilization, 3rd ed. Blackwell Science, Osney Mead, Oxford, United Kingdom.
- 30.Rzezutka, A., and N. Cook. 2004. Survival of human enteric viruses in the environment and food. FEMS Microbiol. Rev. 28:441-453. [DOI] [PubMed] [Google Scholar]
- 31.Shin, G. A., and M. D. Sobsey. 2003. Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Appl. Environ. Microbiol. 69:3975-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobsey, M. D., D. A. Battigelli, G.-A. Shin, and S. Newland. 1988. RT-PCR amplification detects inactivated viruses in water and wastewater. Water Sci. Technol. 38:91-94. [Google Scholar]
- 33.Springthorpe, V. S., J. L. Grenier, N. Lloyd-Evans, and S. A. Sattar. 1986. Chemical disinfection of human rotaviruses: efficacy of commercially-available products in suspension tests. J. Hyg. 97:139-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Environmental Protection Agency. 2001. Method 1602: male-specific (F+) and somatic coliphage in water by single agar layer (SAL) procedure. Office of Water Analytical Methods, U.S. Environmental Protection Agency, Washington, DC.
- 35.Vipond, I. B. 2001. The role of viruses in gastrointestinal disease in the home. J. Infect. 43:38-41. [DOI] [PubMed] [Google Scholar]
- 36.Young, D. C., and D. G. Sharp. 1979. Partial reactivation of chlorine-treated echovirus. Appl. Environ. Microbiol. 37:766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]