Abstract
The gene encoding the α-agarase from “Alteromonas agarilytica” (proposed name) has been cloned and sequenced. The gene product (154 kDa) is unrelated to β-agarases and instead belongs to a new family of glycoside hydrolases (GH96). The α-agarase also displays a complex modularity, with the presence of five thrombospondin type 3 repeats and three carbohydrate-binding modules.
Agars are the main cell wall components of numerous red macroalgae. These polymers consist of 3,6-anhydro-l-galactoses and d-galactoses alternately linked by α-(1,3) and β-(1,4) linkages (10). Agars constitute a crucial carbon source for a number of marine bacteria which secrete agarolytic enzymes, mainly β-agarases (EC 3.2.1.81), which hydrolyze the β-(1,4) linkages (for a review, see reference 12). They are found in three distinct families of glycoside hydrolases (GH), families GH16, GH50, and GH86 (CAZY database [6]). Structural data are available only for the GH16 β-agarases from Zobellia galactanivorans, AgaAZg and AgaBZg (1, 2, 9). In contrast, “Alteromonas agarilytica” (proposed name) secretes an α- agarase (EC 3.2.1.158) which randomly hydrolyzes the α-(1,3) linkages in agars, releasing agarotetraose as its main end product (13, 15, 18). In this context, we sought to determine whether the agarases of the α type share any structural relationships with β-agarases. We report here the cloning of the α-agarase gene agaA from “A. agarilytica,” and we demonstrate that α-agarases define a new GH family.
Cloning of the agaA gene.
The α-agarase from “A. agarilytica” (AgaAAa) was purified as previously described (13), and N-terminal and internal peptide sequences were determined (Pasteur Institute, France) as follows: (i) ETLELQAESFANSGGFF and (ii) QPRVYNPNEHIVAEIQGPAT, respectively. With degenerated oligonucleotides derived from these microsequences (CARGCIGARTCITTYGCIAA and TAYAAYCCNAAYGARCAYAT, respectively), a 2.5-kb DNA fragment was amplified by PCR using “A. agarilytica” genomic DNA and labeled with [α-32P]dCTP. This radiolabeled probe was used to screen an “A. agarilytica” genomic library, prepared as previously described (5). Among the ∼5,000 recombinant clones, two positive clones (pAA1 and pAA2) were identified, with inserts of 7.4 kb and 17.9 kb in length, respectively. Southern blotting analysis and plasmid mapping indicated that both inserts encompassed the same gene, which is present in only one copy in the genome. Plasmid pAA1 was sequenced on both strands over 4,651 bp, and a single open reading frame was identified and referred to as agaA (4,287 bp). Potential −35 and −10 promoter regions (TTGATC and TACACA, respectively) and a Shine-Dalgarno sequence (GGAG) were identified upstream of the start codon. A possible transcription termination codon was found downstream of the TAA stop codon. The deduced gene product is a preprotein of 1,429 residues (154 kDa) which includes the peptides A and B. A signal peptide cleaved between A26 and E27 is predicted by SIGNALP (4), consistent with the N-terminal sequencing of the purified extracellular α-agarase.
Several attempts were made to overexpress AgaAAa and its isolated modules with Escherichia coli, with pET or pGEX vectors, under various conditions. Unfortunately, the constructs always yielded inclusion bodies. However, within 1 week of culture at 22°C on Zd agar broth (3), the E. coli clones harboring the plasmid pAA1 dug a hole in the substratum, indicating agar degradation. Therefore, under the control of its own promoter, agaA was successfully translated into an active, recombinant enzyme, confirming that this gene indeed encodes the α-agarase.
AgaAAa is a complex, modular protein with a catalytic domain that defines a new GH family.
Only the N-terminal region of AgaAAa displays significant sequence similarity with proteins in the UniProt database. Based on InterProScan (14) analysis, eight distinct modules were identified in this region (Fig. 1A), including five thrombospondin type 3 (TSP3) repeats (TSP3-1 [amino acids D171 to G203], TSP3-2 [D360 to L392], TSP3-3 [D393 to A425], TSP3-4 [D426 to L458], and TSP3-5 [D459 to G491]) and three carbohydrate-binding modules from family 6 (CBM6-1 [amino acids E27 to R159], CBM6-2 [E209 to T343], and CBM6-3 [S659 to L792]).
FIG. 1.
(A) Modular architecture of the α-agarase AgaAAa. CBM6 and GH96 refer to carbohydrate-binding modules of family 6 and the glycoside hydrolase module of family 96, respectively. Gray boxes correspond to the TSP3 repeats. (B) Structure-based alignment of the TSP3 repeats of the human thrombospondin (PDB code 1UX6), of the α-agarase AgaAAa, and of the cellulase CelG from Pseudoalteromonas haloplanktis (trEMBL code O86099). (C) Structure-based alignment of the three CBM6s from the α-agarase AgaAAa and of the agar-specific CBM6s tethered to the β-agarases Aga16BSd (GenPept no., ABD80437) and Aga86ESd (GenPept no., ABD81915) from Saccharophagus degradans. These modules are compared to the secondary structures of the CBM6 appended to Aga16BSd (PDB code 2CDO). Alpha helices and beta strands are represented as helices and arrows, respectively, and beta turns are marked with TT. The black triangles mark the residues involved in the recognition of the nonreducing end of agarose chains. Figure 1B and C were prepared using ESPrit software (7) and use the same shading codes.
The characterized protein that most closely matches the TSP3 repeats of AgaAAa is the cellulase CelG from Pseudoalteromonas haloplanktis. In CelG, the TSP3 repeats constitute an extended linker connecting the GH5 catalytic module and a C-terminal CBM5 (17). The TSP3 repeats of AgaAAa present about 50% sequence identity with their counterparts in CelG. They also display ∼30% sequence identity with the “true” type 3 repeats found in human thrombospondin, whose crystal structure has been solved (11). These modules lack secondary structures and are organized around a core of calcium ions coordinated by conserved aspartates [DXDXDGXX(D/N)XXDXC motif]. The conserved cysteine is involved in a disulfide bridge linking adjacent TSP3 repeats, strengthening their stability (11). This motif is strictly conserved in each of the TSP3 repeats of AgaAAa (Fig. 1B), indicating that these modules adopt a similar structure and likely bind calcium ions. This is consistent with the observation that α-agarase activity is stabilized by the presence of calcium ions (13, 18).
In BLASTp searches with the three CBM6 modules from AgaAAa, the highest E values are always obtained for the CBM6 sequences tethered to β-agarases, while they decrease significantly with CBM6 linked to nonagarolytic enzymes. Only the CBM6s attached to the β-agarases from Saccharophagus degradans, Aga16BSd and Aga86ESd, were shown to actually bind agarose (8). A pair-wise comparison indicates a strong sequence identity (51%) between the CBM6-1 and the CBM6 from Aga16BSd (Fig. 1C), while those of CBM6-2 and CBM6-3 are more divergent (28% and 26%, respectively). The crystal structure of the CBM6 from Aga16BSd revealed that five residues are critical for the recognition of the nonreducing end of the agarose chain, Asn39, Tyr40, Trp97, Trp127, and Asn130 (8). Four of these residues are strictly conserved in CBM6-1, while Tyr40 is replaced by a similar aromatic amino acid, Phe64 (Fig. 1C). Together, these results strongly suggest that CBM6-1 is an agar-binding module and likely displays selectivity toward the nonreducing termini of agarose chains. In contrast, the five critical residues are only partially conserved in CBM6-2 and CBM6-3. Therefore, the specificities of these CBM6 sequences are less certain.
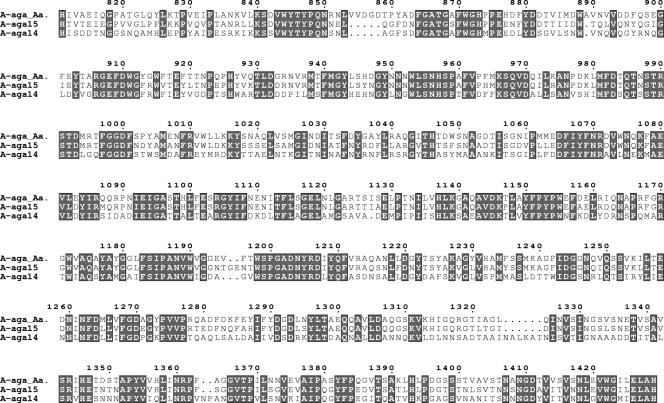
Finally, a BLASTp search of the patent data bank at NCBI identified two proteins from a marine bacterium with a strong sequence identity with the C-terminal region of AgaAAa (49% and 77%; Fig. 2). These proteins, also described as α-agarases (16), encompass three modules, two N-terminal CBM6s and the C-terminal module conserved with AgaAAa. Since the N-terminal region of AgaAAa encompasses only additional, noncatalytic modules, its conserved C-terminal region (Asn809 to His1429) likely contains its active site. Together, these three catalytic modules (∼620 residues) constitute a new family of glycoside hydrolases, referred to as family GH96 (CAZY database). Therefore, α-agarases are structurally unrelated to the β-agarases from the families GH16, GH50, and GH86.
FIG. 2.
Sequence alignment of the catalytic module of AgaA from A. agarilytica and of Aga14 and Aga15 (16). Shaded boxes enclose conserved positions. Figure 2 was prepared using ESPrit software and the same shading codes as seen in Fig. 1B and C.
Accession numbers.
The nucleotide sequence of the α-agarase gene from “Alteromonas agarilytica” has been deposited in GenBank with the accession number AF121273. Its amino acid sequence is available in Swiss-Prot under the accession number Q9LAP7.
Acknowledgments
We gratefully acknowledge the help of the Conseil Régional de Bretagne, which supported this research through a Ph.D. fellowship to D.F. and a research grant (PRIR number 98C389).
Footnotes
Published ahead of print on 18 May 2007.
REFERENCES
- 1.Allouch, J., W. Helbert, B. Henrissat, and M. Czjzek. 2004. Parallel substrate binding sites in a beta-agarase suggest a novel mode of action on double-helical agarose. Structure 12:623-632. [DOI] [PubMed] [Google Scholar]
- 2.Allouch, J., M. Jam, W. Helbert, T. Barbeyron, B. Kloareg, B. Henrissat, and M. Czjzek. 2003. The three-dimensional structures of two beta-agarases. J. Biol. Chem. 278:47171-47180. [DOI] [PubMed] [Google Scholar]
- 3.Barbeyron, T., A. Gerard, P. Potin, B. Henrissat, and B. Kloareg. 1998. The kappa-carrageenase of the marine bacterium Cytophaga drobachiensis. Structural and phylogenetic relationships within family 16 glycoside hydrolases. Mol. Biol. Evol. 15:528-537. [DOI] [PubMed] [Google Scholar]
- 4.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 5.Colin, S., E. Deniaud, M. Jam, V. Descamps, Y. Chevolot, N. Kervarec, J. C. Yvin, T. Barbeyron, G. Michel, and B. Kloareg. 2006. Cloning and biochemical characterization of the fucanase FcnA: definition of a novel glycoside hydrolase family specific for sulfated fucans. Glycobiology 16:1021-1032. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho, P. M., and B. Henrissat. 1999. Carbohydrate-active enzymes: an integrated database approach, p. 3-12. In H. J. Gilbert, G. Davies, B. Henrissat, and B. Svensson (ed.), Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 7.Gouet, P., X. Robert, and E. Courcelle. 2003. ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 31:3320-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henshaw, J., A. Horne-Bitschy, A. L. van Bueren, V. A. Money, D. N. Bolam, M. Czjzek, N. A. Ekborg, R. M. Weiner, S. W. Hutcheson, G. J. Davies, A. B. Boraston, and H. J. Gilbert. 2006. Family 6 carbohydrate binding modules in beta-agarases display exquisite selectivity for the non-reducing termini of agarose chains. J. Biol. Chem. 281:17099-17107. [DOI] [PubMed] [Google Scholar]
- 9.Jam, M., D. Flament, J. Allouch, P. Potin, L. Thion, B. Kloareg, M. Czjzek, W. Helbert, G. Michel, and T. Barbeyron. 2005. The endo-beta-agarases AgaA and AgaB from the marine bacterium Zobellia galactanivorans: two paralogue enzymes with different molecular organizations and catalytic behaviours. Biochem. J. 385:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kloareg, B., and R. Quatrano. 1988. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev. 26:259-315. [Google Scholar]
- 11.Kvansakul, M., J. C. Adams, and E. Hohenester. 2004. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. EMBO J. 23:1223-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel, G., P. Nyval-Collen, T. Barbeyron, M. Czjzek, and W. Helbert. 2006. Bioconversion of red seaweed galactans: a focus on bacterial agarases and carrageenases. Appl. Microbiol. Biotechnol. 71:23-33. [DOI] [PubMed] [Google Scholar]
- 13.Potin, P., C. Richard, C. Rochas, and B. Kloareg. 1993. Purification and characterization of the alpha-agarase from Alteromonas agarlyticus (Cataldi) comb. nov., strain GJ1B. Eur. J. Biochem. 214:599-607. [DOI] [PubMed] [Google Scholar]
- 14.Quevillon, E., V. Silventoinen, S. Pillai, N. Harte, N. Mulder, R. Apweiler, and R. Lopez. 2005. InterProScan: protein domains identifier. Nucleic Acids Res. 33:116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochas, C., P. Potin, and B. Kloareg. 1994. NMR spectroscopic investigation of agarose oligomers produced by an alpha-agarase. Carbohydr. Res. 253:69-77. [DOI] [PubMed] [Google Scholar]
- 16.Tomono, J., Y. Nomura, H. Sagawa, T. Sakai, and I. Kato. July 2003. Alpha-agarase and process for producing the same. U.S. patent 6,599,729.
- 17.Violot, S., N. Aghajari, M. Czjzek, G. Feller, G. K. Sonan, P. Gouet, C. Gerday, R. Haser, and V. Receveur-Brechot. 2005. Structure of a full-length psychrophilic cellulase from Pseudoalteromonas haloplanktis revealed by X-ray diffraction and small angle X-ray scattering. J. Mol. Biol. 348:1211-1224. [DOI] [PubMed] [Google Scholar]
- 18.Young, K. S., S. S. Bhattacharjee, and W. Yaphe. 1978. Enzymic cleavage of the alpha-linkages in agarose to yield agarooligosaccharides. Carbohydr. Res. 66:207-212. [Google Scholar]