Abstract
The essential oils of oregano and thyme are active against a number of food-borne pathogens, such as Escherichia coli O157:H7. Carvacrol is one of the major antibacterial components of these oils, and p-cymene is thought to be its precursor in the plant. The effects of carvacrol and p-cymene on protein synthesis in E. coli O157:H7 ATCC 43895 cells were investigated. Bacteria were grown overnight in Mueller-Hinton broth with a sublethal concentration of carvacrol or p-cymene, and their protein compositions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and confirmed by Western blotting. The presence of 1 mM carvacrol during overnight incubation caused E. coli O157:H7 to produce significant amounts of heat shock protein 60 (HSP60) (GroEL) (P < 0.05) and inhibited the synthesis of flagellin highly significantly (P < 0.001), causing cells to be aflagellate and therefore nonmotile. The amounts of HSP70 (DnaK) were not significantly affected. p-Cymene at 1 mM or 10 mM did not induce HSP60 or HSP70 in significant amounts and did not have a significant effect on flagellar synthesis. Neither carvacrol (0.3, 0.5, 0.8, or 1 mM) nor p-cymene (0.3, 0.5, or 0.8 mM) treatment of cells in the mid-exponential growth phase induced significant amounts of HSP60 or HSP70 within 3 h, although numerical increases of HSP60 were observed. Motility decreased with increasing concentrations of both compounds, but existing flagella were not shed. This study is the first to demonstrate that essential oil components induce HSP60 in bacteria and that overnight incubation with carvacrol prevents the development of flagella in E. coli O157:H7.
Escherichia coli O157:H7 is a pathogen that causes serious food-borne infections and can lead to hemolytic uremic syndrome, particularly in children (4). The chief source is bovine feces, and a variety of foods of both plant and animal origin, such as meat and dairy products, vegetables, and fruit, can become contaminated (4, 27, 40).
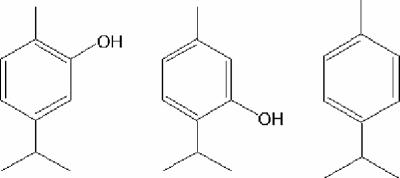
Studies have shown that the essential oils of the herbs oregano and thyme are effective against strains of E. coli (12, 33). The major antibacterial components of these oils are carvacrol and its isomer thymol (6). Both are approved food flavorings in the United States and Europe (8, 10) and have potential as antibacterial additives in food and feed (5, 32). A number of feed additives and food preservatives containing essential oils or carvacrol are already commercially available (21, 28, 38). p-Cymene is also a constituent of oregano and thyme oils but is less effective against food-related pathogens (6, 12, 37) and is thought to be a precursor to carvacrol and thymol in the plant (35). The chemical structures of these compounds are shown in Fig. 1.
FIG. 1.
Structural formulae of the essential oil components (left to right) carvacrol, thymol, and p-cymene.
The precise target(s) of the antibacterial action of essential oils and their components has not yet been fully established. Changes in the fatty acid composition of bacterial cell membranes (an increase in unsaturated fatty acids) have been observed when cells are exposed to sublethal concentrations of essential oil components (11). Carvacrol and thymol damage the outer membrane of gram-negative bacteria and increase the general permeability of the cytoplasmic membrane, leading to leakage of ATP (14, 20). Carvacrol possesses ATPase-inhibiting activity (14, 15); in any case, it appears to dissipate the proton motive force (35). There are also indications for the inhibition of other enzymes (39). p-Cymene has been shown to have lipolytic properties (9).
When bacteria are subjected to stress in the form of toxic substances, they generally increase their synthesis of stress proteins or heat shock proteins (HSPs). The HSP60 and HSP70 families of proteins are molecular chaperones and play a part in the assembly of newly synthesized polypeptides into their native conformation and in the folding and repair of cytosolic proteins (26). HSP60 (GroEL) and its cofactor GroES provide a compartment inside which proteins can fold while being protected from the cytosol. HSP70 (DnaK) holds nascent and newly synthesized polypeptides stable on the ribosomes (18, 26). The induction of HSPs has been observed for E. coli cells subjected to stress in the forms of ethanol, high osmotic stress, high temperature, and the presence of phenol (24, 25). As yet, increased HSP production has not been reported for bacteria treated with carvacrol, thymol, p-cymene, or other essential oil components.
The purpose of this study was to determine which changes in protein synthesis could be detected when E. coli O157:H7 cells were grown at sublethal concentrations of the essential oil components carvacrol and p-cymene.
MATERIALS AND METHODS
Essential oil components.
Carvacrol and p-cymene were obtained from Sigma Aldrich Chemicals, Zwijndrecht, The Netherlands. Stock solutions in 99.8% ethanol were made on the day of use. The final concentration of ethanol in the broth was never more than 1% vol/vol and in pilot experiments was shown to have no influence on the synthesis of HSPs or flagella or the motility of cells (data not shown).
Bacterial strains and growth conditions.
Escherichia coli O157:H7 ATCC 43895 cells were tested for their reactions to the presence of essential oil components in two ways. First, overnight (16 h) cultures were grown in Mueller-Hinton broth (MHB; Oxoid, Basingstoke, United Kingdom) at 37°C, with or without the addition of 1.0 mM carvacrol or p-cymene. Second, exponential-phase cells for testing for a concentration-dependent effect of essential oil components were produced according to a growth curve previously carried out by growing cells in 100 ml MHB at 37°C with shaking to an optical density (OD) at 620 nm of 0.5 and a cell density of approximately 1 × 106 CFU/ml. Aliquots of 10 ml were centrifuged at 2,000 × g for 5 min at room temperature, and the cells were resuspended in MHB with 0 mM, 0.3 mM, 0.5 mM, 0.8 mM, or 1.0 mM of the relevant essential oil component. Incubation continued at 37°C for 3 h, after which the cells were harvested for protein analysis.
Protein extraction.
Whole-cell protein extractions were made by separating E. coli cells from the suspension by centrifugation in an Eppendorf 5415 C tube at 2,000 × g for 5 min at room temperature, two washes in phosphate-buffered saline (Cambrex Bioscience, Verviers, Belgium), and resuspension in sterile distilled water. Portions were kept apart for protein assay. The suspensions were mixed 1:1 with Laemmli buffer, heated at 95°C for 10 min, and cooled on ice.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and protein identification.
Proteins were analyzed by electrophoresis on Tris-HCl ready gels with 10% cross polymer in a Protean III electrophoresis system (Bio-Rad, Hercules, CA) with the prestained marker SeeBlue Plus2 (Invitrogen, Carlsbad, CA) and the benchmark protein ladder (Invitrogen) molecular standard. The protein concentrations were determined by using protein assay dye reagent concentrate (Bio-Rad, Munich, Germany), and the samples were normalized for equal amounts of protein (approximately 2 μg/lane). The protein bands were made visible by staining with Coomassie blue R250, and bands of interest were identified by amino acid sequencing. Some were transferred to polyvinylidene fluoride membrane by electroblotting, and N-terminal sequence analysis was performed by using an Applied Biosystems-Perkin Elmer sequencer model 476A at the Sequence Centre, Institute of Biomembranes, Utrecht University, The Netherlands. The sequences were screened for similarity to proteins in the NCBI database. Other bands of interest were excised and analyzed by trypsin digestion followed by matrix-assisted laser desorption ionization-time of flight (mass spectrometry) by Gentaur, Brussels, Belgium. The peptide sequences in combination with the determined masses of the proteins were used in a search of the NCBI database.
Western blotting.
After electrophoresis, the proteins were electroblotted at 100 V onto nitrocellulose membranes (0.2 μm) (Bio-Rad) and the membranes were blocked with 0.5% blocking reagent (Roche Diagnostics, Mannheim, Germany). The membranes were then incubated for 1 h with mouse antibodies against GroEL (monoclonal antibody [MAb] LK2) (3), DnaK (MAb 7D9 prepared against mycobacterial HSP70 and cross-reactive with E. coli DnaK), or flagellin (MAb 15D8; Bioveris, Oxford, United Kingdom). After incubation with goat anti-mouse immunoglobulin G conjugated with horseradish peroxidase (Invitrogen), the proteins were made visible by staining with a solution of dioctyl sodium sulfosuccinate and 3,3′,5,5′-tetra methyl benzidine (Merck, Darmstadt, Germany). The positive controls for GroEL and DnaK were recombinant preparations of mycobacterial HSP60 and HSP70, respectively. Salmonella enteritidis flagellin provided by Alphons van Asten was used as the positive control. No blotting was carried out against the cofactor for GroEL (GroES). The blots were scanned with a GS-700 imaging densitometer (Bio-Rad, Veenendaal, The Netherlands), and the appropriate bands were quantified by using Quantity One software (Bio-Rad). The ratios of test to control band densities were calculated and are presented (means ± standard deviations [SD]) in bar charts in the figures.
Motility tests.
The motilities of the cells were determined by the hanging-drop technique as follows: a droplet of the culture was suspended from a glass coverslip over a microscope slide with a central concavity and observed under a light microscope. The bacterial cells were observed at a magnification of ×1,000 for 5 min and classified by a method established by Gill and Holley (14). Suspensions in which the majority of cells were actively moving and tumbling were classed as motile; suspensions in which a minority of cells were either moving or tumbling were classed as having reduced motility; and suspensions showing only Brownian movement were classed as nonmotile.
Visualization of flagella.
A 3-μl portion of the bacterial suspension was placed on a microscope slide and covered with a glass coverslip. One drop of flagella stain (Becton Dickinson, Sparks, MD) was applied to the edge of the coverslip and allowed to diffuse through the suspension by capillary action. The slides were observed under differential interference contrast by using a Leica DMRE light microscope with a 630× oil immersion objective and photographed with a Photometrics CoolSnap FX camera using IPLab software.
Determination of viable cell counts.
Viable cell counts were carried out by serial 10-fold dilution of samples in sterile physiological saline solution (0.85% NaCl) and plating out of appropriate dilutions in duplicate on MH agar (Oxoid). The plates were incubated at 37°C for 24 h.
Statistical analysis.
The densitometric data for the blots against HSPs and flagellin after overnight culture with carvacrol or p-cymene and after carvacrol treatment in the exponential phase were compared by a one-way analysis of variance and the Bonferroni post hoc test of significant difference. These data are based on three independent experiments.
RESULTS
Overnight culture with carvacrol or p-cymene.
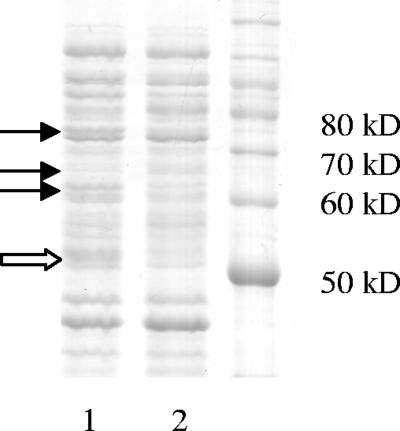
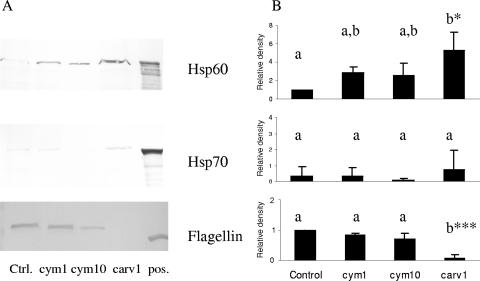
When E. coli O157:H7 cells were grown overnight in MHB in the presence of 1 mM carvacrol, several protein bands with higher or lower expression levels than the control sample were detected on an SDS-PAGE gel (Fig. 2). Two proteins of approximately 60 and 70 kDa (indicated by black arrows in Fig. 2) were identified by N-terminal amino acid sequencing as HSP60 and HSP70. A protein band of approximately 65 kDa that was down regulated in cells grown with carvacrol (also indicated with a black arrow) was identified as flagellin. The mass was determined as 59,918 Da, and flagellin was identified as the source protein for the peptide sequences with a probability-based molecular-weight search score of 294 (P < 0.05). In some experiments, an additional carvacrol-affected protein band was observed at 50 kDa (Fig. 2), but this was not consistent. Western blot analysis was used to confirm these findings, and representative blots are shown in Fig. 3. The carvacrol-induced increase in the amount of HSP60 was significant (P < 0.05), but there was no significant change for HSP70. The reduction in the amount of flagellin formed after overnight culture with 1 mM carvacrol was very highly significant (P < 0.001) (Fig. 3).
FIG. 2.
SDS-PAGE gel stained with Coomassie blue showing changes in protein synthesis by E. coli O157:H7 strain ATCC 43895 cells when grown overnight in MHB with 1.0 mM carvacrol (lane 1) and in MHB only (lane 2). A molecular marker is shown in lane 3. The black arrows indicate increased amounts of 60-kDa and 70-kDa proteins and a decrease in 65-kDa protein. The white arrow indicates an increase in a 50-kDa protein, which did not appear consistently.
FIG. 3.
Effects on proteins in E. coli O157:H7 ATCC 43895 cells after overnight culture in presence of carvacrol or p-cymene. (A) Representative Western blot of HSP60, HSP70, and flagellin after 16 h of culture in (from left to right) MHB (Ctrl.), MHB with 1.0 mM p-cymene (cym1), MHB with 10.0 mM p-cymene (cym10), and MHB with 1.0 mM carvacrol (carv1). The right-hand lane shows the positive control (pos.) containing purified preparations of the protein antigens. (B) Relative density results from three independent experiments, quantified by densitometric analysis of the Western blots represented in panel A. The bars represent the means ± SD of the results. Treatment results that are significantly different from each other are indicated with different letters (*, P < 0.05; ***, P < 0.001).
Western blot analysis of proteins after overnight culture in the presence of the less-antimicrobially active substance, p-cymene, was also carried out; the effects of 1 mM and 10 mM p-cymene on protein levels are also shown in Fig. 3. p-Cymene at 1 mM produced no significant changes in the amounts of HSP60, HSP70, or flagellin. Even when the concentration of p-cymene was increased to 10 mM, no significant changes in the amounts of HSPs or flagellin were detected (Fig. 3).
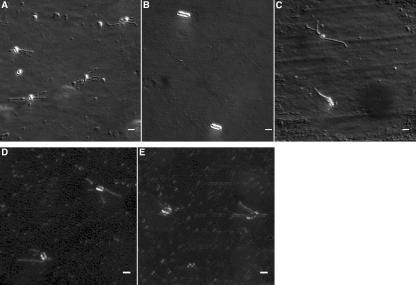
Cells incubated with carvacrol were nonmotile as determined by the hanging-drop technique, while cells grown in broth containing p-cymene were motile or had reduced motility, depending on the concentration present (Table 1). The lack of flagella after growth in carvacrol was also visualized by using differential interference contrast microscopy. The representative pictures in Fig. 4 show normal, flagellated cells grown in control broth (Fig. 4A) and cells lacking flagella after growth in broth containing 1 mM carvacrol (Fig. 4B). Cells grown in carvacrol appeared longer and smoother than normal cells. Apparently normally flagellated cells grown in broth containing 1 mM p-cymene are shown in Fig. 4C.
TABLE 1.
Effect of carvacrol and p-cymene on the motility of E. coli O157:H7 ATCC 43895 cellsa
| Treatment during: | Essential oil component added | Concn of essential oil component (mM) | Motility classification
|
||
|---|---|---|---|---|---|
| Motile | Reduced motility | Nonmotile | |||
| Overnight incubation | Control | 0 | X | ||
| Carvacrol | 1 | X | |||
| p-Cymene | 1 | X | |||
| 10 | X | ||||
| Mid-exponential growth phase | Control | 0 | X | ||
| Carvacrol | 0.3 | X | |||
| 0.5 | X | ||||
| 0.8 | X | ||||
| 1.0 | X | ||||
| p-Cymene | 0.3 | X | |||
| 0.5 | X | ||||
| 0.8 | X | ||||
Cells were grown overnight in MHB in the presence of the substance or were treated in the exponential growth phase.
FIG. 4.
Differential interference contrast images of E. coli O157:H7 ATCC 43895 cells after overnight incubation in MHB showing (A) normal cells with flagella; (B) aflagellate cells grown in MHB containing 1 mM carvacrol; and (C) flagellate cells grown in MHB containing 1 mM p-cymene. Cells retained their flagella when treated in the exponential phase with (D) 1 mM carvacrol or (E) 1 mM p-cymene. The bars represent 2 μm.
Carvacrol treatment in exponential phase.
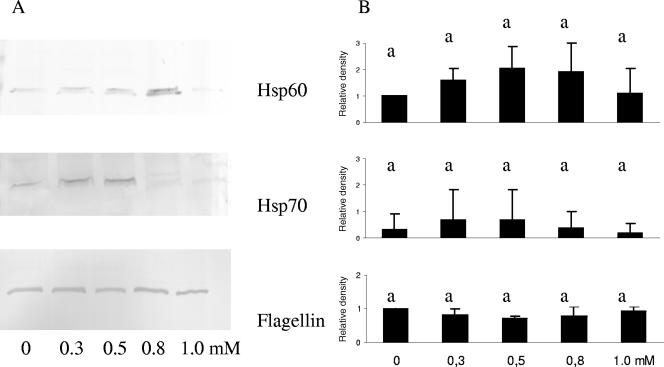
Western blot analysis revealed that when cells in the exponential phase of growth were treated with increasing concentrations of carvacrol of 0, 0.3, 0.5, 0.8, and 1.0 mM for a period of 3 h, no significant changes in HSP60, HSP70, or flagellin levels were detected (Fig. 5). There was, however, a numerical increase in the amounts of HSP60 produced with increasing concentrations of carvacrol from 0 to 0.3 to 0.5 mM that leveled off at 0.8 mM and decreased again at 1.0 mM (Fig. 5).
FIG. 5.
Effects on proteins in E. coli O157:H7 ATCC 43895 cells after addition of carvacrol to cells in exponential phase. (A) Representative Western blots of HSP60 (GroEL), HSP70 (DnaK), and flagellin after treatment with increasing concentrations of carvacrol. Cells were grown in MHB, resuspended in MHB containing carvacrol, and incubated for a further 3 h. (B) Relative density results from three independent experiments, quantified by densitometric analysis of the Western blots represented in panel A. The bars represent the means ± SD of the results. Treatment results that are significantly different from each other are indicated with different letters (P < 0.05).
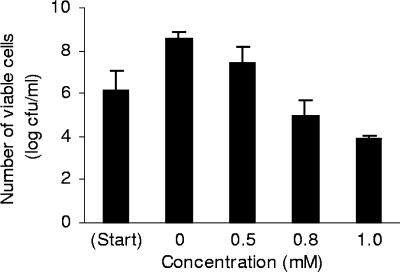
Colony counts carried out before treatment of the cells and at the moment of cell harvesting revealed that the numbers of viable cells increased during the 3-h incubation period in the control and 0.5 mM carvacrol treatments and decreased with the 0.8 mM and 1.0 mM carvacrol treatments (Fig. 6). Adding carvacrol at 0.8 or 1.0 mM to cells in the mid-exponential growth phase apparently reduced the numbers of viable cells and would therefore have reduced the number of cells available to make HSPs, which would account for the apparent leveling off in HSP60 amounts from 0.8 mM carvacrol.
FIG. 6.
Effect of carvacrol on viability of E. coli O157:H7 ATCC 43895 cells in the exponential growth phase. Cells grown in MHB to an OD of 0.5 were centrifuged and resuspended in broth containing 0, 0.5, 0.8, or 1.0 mM carvacrol for 3 h. The bars show the means ± SD of the results of two experiments.
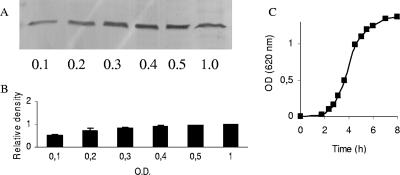
The decrease in the amount of flagellin due to carvacrol treatment in the exponential phase (Fig. 5) was not significant. This indicates that cells at this point in the growth curve already possessed flagella and that the flagella were not shed, as confirmed by the photographs in Fig. 4D. A Western blot against flagellin using cells harvested throughout the exponential growth phase in normal broth confirmed that the amount of flagellin is at a high level before the culture reaches mid-exponential phase (OD = 0.5), which was the point at which carvacrol was added (Fig. 7). The motility of cells in the exponential phase of growth decreased with increasing concentrations of carvacrol (Table 1).
FIG. 7.
Amounts of flagellin protein in E. coli O157:H7 ATCC 43895 cells during exponential growth phase. (A) Representative Western blot of flagellin during growth in MHB. The OD of the bacterial culture is indicated. (B) Relative density results from two independent experiments, quantified by densitometric analysis of the Western blots represented in panel A. The bars represent the means ± SD of the results. (C) Growth curve for the strain in MHB. The data points are the means of two experiments.
p-Cymene treatment in exponential phase.
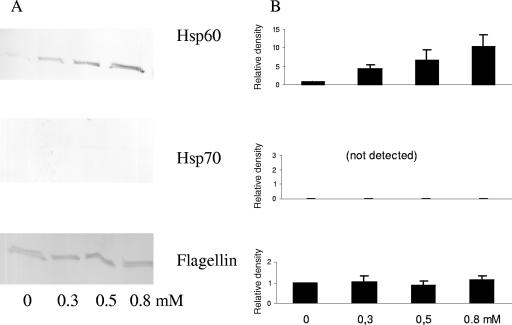
Western blot analysis showed that cells in the exponential phase treated with increasing concentrations of p-cymene of 0, 0.3, 0.5, and 0.8 mM for a period of 3 h exhibited no significant changes in HSP60, HSP70, or flagellin levels (Fig. 8). There was, however, a numerical increase in the amounts of HSP60 produced with increasing concentrations of p-cymene (Fig. 8). Flagella were not shed from existing cells (Fig. 4E). Cell motility decreased with increasing concentrations of p-cymene (Table 1).
FIG. 8.
Effects on proteins in E. coli O157:H7 ATCC 43895 cells after addition of p-cymene to cells in exponential phase. (A) Representative Western blot of HSP60 (GroEL), HSP70 (DnaK), and flagellin synthesis after treatment with increasing concentrations of p-cymene. The cells were grown in MHB, resuspended in MHB containing p-cymene, and incubated for a further 3 h. (B) Relative density results from two independent experiments, quantified by densitometric analysis of the Western blots represented in panel A. The bars represent the means ± SD of the results.
DISCUSSION
In this report, some effects of carvacrol and p-cymene on protein synthesis in E. coli O157:H7 ATCC 43895 cells are described. Although it has been shown that treatment with phenol caused E. coli to synthesize the HSP HtpG (25), to our knowledge, this is the first study to show that the essential oil component carvacrol, a methylated phenol, induces HSP60 in bacteria.
When cells were cultured overnight in the presence of 1.0 mM carvacrol, almost no flagellin was synthesized and the cells were therefore aflagellate and nonmotile. Flagellin can make up as much as 8% of total cell protein in E. coli, and the synthesis of flagella requires a considerable amount of energy (24, 29). Being able to cease flagellin production when conditions are unfavorable (for instance, when phenolic compounds are present in toxic amounts) and to conserve energy for other cell functions may therefore be a survival tactic (24). Salicylate, whose chemical structure also includes an aromatic ring, has also been shown to inhibit the motility of E. coli in a concentration-dependent manner and to block the synthesis of flagellin (23).
The greater surface smoothness of cells grown overnight with carvacrol observed in this study (Fig. 4B) has previously been reported in a study of the effects of oregano oil (of which carvacrol is one of the major components) on E. coli O157:H7 and may be due to modifications in the composition and relative percentages of peptides in the peptidoglycans in the cell wall (7).
That p-cymene had less effect than carvacrol on the induction of HSPs and on the inhibition of flagellin synthesis is consistent with it being far less toxic to E. coli (6, 12, 22). In overnight culture, p-cymene did not have a significant influence even when added at a 10-fold-greater concentration than carvacrol (Fig. 3).
Under normal broth culture conditions, this E. coli strain produced flagellin from early in the exponential phase of growth and production was at a very high level before an OD of 0.5 was reached (Fig. 7). The introduction of carvacrol in mid-exponential growth phase was therefore unlikely to cause a decrease in the total amount of flagellin, particularly since the presence of the higher concentrations of carvacrol reduced the ability of cells to replicate and reduced the numbers of viable bacteria (Fig. 6). From the data provided by blots and photographs (Fig. 4, 5, and 8), it is also apparent that adding carvacrol or p-cymene in the exponential growth phase did not cause bacteria to shed flagella. It appears that carvacrol must be present while cells are dividing in order to have an inhibitory effect on flagellin.
In this study, carvacrol and p-cymene added to exponentially growing cells reduced motility in a concentration-dependent manner (Table 1). An earlier study also reported that the motility of exponential-phase E. coli O157:H7 cells decreased with increasing concentrations of carvacrol (14). In that report, 1 mM carvacrol in broth was not enough to arrest motility, possibly because no ethanol was used to aid solution of the carvacrol. However, carvacrol at 5 mM caused an immediate reduction in motility and at 10 mM it caused an immediate cessation of motility and cell death (14). It was recently demonstrated that the speed of the flagellar rotary motor in E. coli is directly proportional to the proton motive force (13). These observations on motility appear to back up the proposal that carvacrol in some way causes dissipation of the proton motive force (36).
The fact that E. coli cells grown in the presence of carvacrol have no flagella could have implications for the use of carvacrol as an antibacterial additive for foodstuffs or animal feeds. Bacterial flagella, specifically, flagellin, activate the host immune response during infection (19, 30), and bacteria often repress the production of flagella after colonization (31). If the use of carvacrol as a food additive were to render any bacteria in the food aflagellate, these bacteria could more easily remain undetected by the immune systems of people or animals that consumed the food, which would be undesirable. On the other hand, there could be advantages to rendering bacteria aflagellate because, under certain circumstances, cells without flagella have been shown to be significantly less able to adhere to epithelial cells and to be less invasive than flagellated cells. For example, the flagella of enteropathogenic E. coli have been shown to be directly involved in adherence to epithelial cells in vitro (16). Flagella were found to be necessary to enable long-term infection and colonization by E. coli O157:H7 in poultry (2), although this was not the case in pigs or in ruminant models (1). The net effect of carvacrol may, therefore, be advantageous; aflagellate cells may be less able to invade the host, and therefore, detection by the host's immune system may not be so critical.
The maximum concentration of carvacrol used in this study (1 mM, corresponding to approximately 0.015% [vol/vol]) was chosen because, being sublethal, it would enable observations of the physiological changes in growing cells. Concentrations of essential oil components in foods and feed that have shown antimicrobial effects are as follows: 0.1 to 0.25% essential oil components in soft cheese (28); 0.7 to 2.1% (vol/wt) oregano oil (approximately 0.3 to 0.8% (vol/wt) carvacrol equivalent) in eggplant salad (34); 500 ppm oregano oil (approximately 0.02% carvacrol equivalent) in pig feed (38); and 0.1 to 1.0% rubbed thyme leaves (approximately 0.001 to 0.01% thymol equivalent) in feed for weanling piglets (17). The concentration of carvacrol used in this study is therefore lower than or in the same range as antimicrobially active concentrations of essential oil components which have been achieved in foodstuffs and animal feed. The concentrations used here are therefore physiologically relevant. The question is whether carvacrol has the same effects on HSP levels and flagella when used in practice. This topic has significant implications for the practical application of carvacrol and merits further study.
In conclusion, this study shows that the presence of the antibacterial essential oil component carvacrol (1 mM) during overnight incubation stimulates E. coli O157:H7 to produce significant amounts of HSP60 (GroEL), but not HSP70 (DnaK), and prevents the synthesis of flagellin, causing cells to be aflagellate and therefore nonmotile. The less-active antimicrobial component p-cymene does not induce HSP60 or HSP70 or prevent the synthesis of flagellin, even when added at a 10-fold-higher concentration. During a 3-h treatment of exponentially growing cells, neither carvacrol nor p-cymene induced significant amounts of HSP60 or HSP70, although numerical increases of HSP60 levels were observed. Both compounds reduce bacterial motility to an extent dependent on the concentration added to the growth medium.
Acknowledgments
We thank Alphons van Asten for supplying the flagellin control and Betty Jongerius and Elsbeth van Liere for their assistance in this study.
Footnotes
Published ahead of print on 25 May 2007.
REFERENCES
- 1.Best, A., R. M. La Ragione, D. Clifford, W. A. Cooley, A. R. Sayers, and M. J. Woodward. 2006. A comparison of Shiga-toxin negative Escherichia coli O157 aflagellate and intimin deficient mutants in porcine in vitro and in vivo models of infection. Vet. Microbiol. 113:63-72. [DOI] [PubMed] [Google Scholar]
- 2.Best, A., R. M. La Ragione, A. R. Sayers, and M. J. Woodward. 2005. Role for flagella but not intimin in the persistent infection of the gastrointestinal tissues of specific-pathogen-free chicks by Shiga toxin-negative Escherichia coli O157:H7. Infect. Immun. 73:1836-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boog, C. J., E. R. De Graeff-Meeder, M. A. Lucassen, R. Van der Zee, M. M. Voorhorst-Ogink, P. J. Van Kooten, H. J. Geuze, and W. Van Eden. 1992. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J. Exp. Med. 175:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan, R. L., and M. P. Doyle. 1997. Foodborne disease significance of Escherichia coli O157:H7 and other enterohemorrhagic E. coli. Food Technol. 51:69-76. [Google Scholar]
- 5.Burt, S. A. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 94:223-253. [DOI] [PubMed] [Google Scholar]
- 6.Burt, S., R. Vlielander, H. P. Haagsman, and E. J. A. Veldhuizen. 2005. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J. Food Prot. 68:919-926. [DOI] [PubMed] [Google Scholar]
- 7.Caillet, S., F. Shareck, and M. Lacroix. 2005. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Escherichia coli O157:H7. J. Food Prot. 68:2571-2579. [DOI] [PubMed] [Google Scholar]
- 8.Center for Food Safety and Applied Nutrition. 2006. EAFUS: a food additive database. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Washington, DC. http://www.cfsan.fda.gov/∼dms/eafus.html. Consulted 22 May 2006.
- 9.Choi, H.-S. 2006. Lipolytic effects of citrus peel oils and their components. J. Agric. Food Chem. 54:3254-3258. [DOI] [PubMed] [Google Scholar]
- 10.Commission of the European Communities. 1999. Commission decision of 23 February 1999 adopting a register of flavouring substances used in or on foodstuffs drawn up in application of regulation (EC) no. 2232/96 of the European Parliament and of the Council of 28 October 1996 (notified under number C(1999) 399) (text with EEA relevance) (1999/217/EC), p. 1-137. Official Journal of the European Communities L084, 27/03/1999. Commission of the European Communities, Brussels, Belgium.
- 11.Di Pasqua, R., N. Hoskins, G. Betts, and G. Mauriello. 2006. Changes in membrane fatty acids composition of microbial cells induced by addition of thymol, carvacrol, limonene, cinnamaldehyde and eugenol in the growing media. J. Agric. Food Chem. 54:2745-2749. [DOI] [PubMed] [Google Scholar]
- 12.Dorman, H. J. D., and S. G. Deans. 2000. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 88:308-316. [DOI] [PubMed] [Google Scholar]
- 13.Gabel, C. V., and H. C. Berg. 2003. The speed of the flagellar rotary motor of Escherichia coli varies linearly with proton motive force. Proc. Natl. Acad. Sci. USA 100:8748-8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill, A. O., and R. A. Holley. 2006. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant essential oils aromatics. Int. J. Food Microbiol. 108:1-9. [DOI] [PubMed] [Google Scholar]
- 15.Gill, A. O., and R. A. Holley. 2006. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 111:170-174. [DOI] [PubMed] [Google Scholar]
- 16.Girón, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 17.Hagmuller, W., M. Jugl-Chizzola, K. Zitterl-Eglseer, C. Gabler, J. Spergser, R. Chizzola, and C. Franz. 2006. The use of Thymi herba as feed additive (0.1%, 0.5%, 1.0%) in weanling piglets with assessment of the shedding of haemolysing E. coli and the detection of thymol in the blood plasma. Berl. Muench. Tieraerztl. Wochenschr. 119:50-54. [PubMed] [Google Scholar]
- 18.Hartl, F.-U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 20.Helander, I. M., H.-L. Alakomi, K. Latva-Kala, T. Mattila-Sandholm, I. Pol, E. J. Smid, L. G. M. Gorris, and A. Von Wright. 1998. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 46:3590-3595. [Google Scholar]
- 21.Jamroz, D., T. Wertecki, M. Houszka, and C. Kamel. 2006. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Physiol. Anim. Nutr. 90:255-268. [DOI] [PubMed] [Google Scholar]
- 22.Juliano, C., A. Mattana, and M. Usai. 2000. Composition and in vitro antimicrobial activity of the essential oil of Thymus herba-barona Loisel growing wild in Sardinia. J. Essential Oil Res. 12:516-522. [Google Scholar]
- 23.Kunin, C. M., H. H. Tong, and L. O. Bakaletz. 1995. Effect of salicylate on expression of flagella by Escherichia coli and Proteus, Providencia, and Pseudomonas spp. Infect. Immun. 63:1796-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, C., C. Louise, W. Shi, and J. Adler. 1993. Adverse conditions which cause lack of flagella in Escherichia coli. J. Bacteriol. 175:2229-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason, C. A., J. Dünner, P. Indra, and T. Colangelo. 1999. Heat-induced expression and chemically induced expression of the Escherichia coli stress protein HtpG are affected by the growth environment. Appl. Environ. Microbiol. 65:3433-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayhew, M., and F.-U. Hartl. 1996. Molecular chaperone proteins, p. 922-937. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. I. ASM Press, Washington, DC. [Google Scholar]
- 27.Mead, P. S., and P. M. Griffin. 1998. Escherichia coli O157:H7. Lancet 352:1207-1212. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza-Yepes, M. J., L. E. Sanchez-Hidalgo, G. Maertens, and F. Marin-Iniesta. 1997. Inhibition of Listeria monocytogenes and other bacteria by a plant essential oil (DMC) in Spanish soft cheese. J. Food Saf. 17:47-55. [Google Scholar]
- 29.Neidhardt, F. C. 1987. Chemical composition of Escherichia coli, p. 3-6. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, DC. [Google Scholar]
- 30.Ren, T., D. S. Zamboni, C. R. Roy, W. F. Dietrich, and R. E. Vance. 2006. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathogens 2:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen, A., and D. E. Higgins. 2006. The MogR transcriptional repressor regulates nonhierarchical expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathogens 2:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Si, W., J. Gong, R. Tsao, T. Zhou, H. Yu, C. Poppe, R. Johnson, and Z. Du. 2006. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 100:296-305. [DOI] [PubMed] [Google Scholar]
- 33.Skandamis, P., K. Koutsoumanis, K. Fasseas, and G.-J. E. Nychas. 2001. Inhibition of oregano essential oil and EDTA on Escherichia coli O157:H7. Ital. J. Food Sci. 13:65-75. [Google Scholar]
- 34.Skandamis, P. N., and G.-J. E. Nychas. 2000. Development and evaluation of a model predicting the survival of Escherichia coli O157:H7 NCTC 12900 in homemade eggplant salad at various temperatures, pHs, and oregano essential oil concentrations. Appl. Environ. Microbiol. 66:1646-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ultee, A., M. H. J. Bennink, and R. Moezelaar. 2002. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 68:1561-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ultee, A., E. P. W. Kets, and E. J. Smid. 1999. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 65:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ultee, A., R. A. Slump, G. Steging, and E. J. Smid. 2000. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J. Food Prot. 63:620-624. [DOI] [PubMed] [Google Scholar]
- 38.Van Krimpen, M. M., and G. P. Binnendijk. 2001. Ropadiar as alternative for antimicrobial growth promoter in diets of weanling pigs. Report 205. Praktijkonderzoek Veehouderij, Lelystad, The Netherlands.
- 39.Wendakoon, C. N., and M. Sakaguchi. 1995. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J. Food Prot. 58:280-283. [DOI] [PubMed] [Google Scholar]
- 40.WHO. 2002. Food safety and foodborne illness. World Health Organization fact sheet 237, revised January 2002. World Health Organization, Geneva, Switzerland.