Abstract
The question of whether Campylobacter jejuni produces a cholera toxin-like toxin (CTLT) has been controversial. The objective of this study was to identify the factor that cross-reacts with CT from C. jejuni. Filtrates of C. jejuni grown in four different liquid media reported to promote CTLT production were tested by Chinese hamster ovary (CHO) cell elongation assay and for reactivity with CT antibody using GM1 ganglioside enzyme-linked immunosorbent assay (ELISA) and immunoblotting. Protein sequence was determined by matrix-assisted laser desorption ionization-time of flight (MALDI TOF-TOF). Filtrates from seven reference strains reported to produce CTLT and from 80 clinical strains were negative in the CHO cell assay, but those from three reference strains and 16 clinical strains were positive by GM1 ELISA. All strains tested, including C. jejuni NCTC 11168, which does not contain a CT gene homologue, possessed a 53-kDa protein which reacted with CT antibody by immunoblotting. This band was identified as the major outer membrane protein, PorA, of C. jejuni. CT antibody reacted by immunoblotting with a recombinant PorA, but antibody to the recombinant PorA did not react with CT. Our results indicate that C. jejuni does not produce a functional CTLT, but the reactivity of PorA with CT antibody would lead to the erroneous conclusion that C. jejuni produces a functional CTLT.
Campylobacter jejuni is a major food-borne pathogen (3). It also causes the major neurological sequela Guillain-Barré syndrome (21). A study in 1997 estimated that C. jejuni infections would cost the U.S. economy 8 billion dollars annually (2). The predominant diarrheal syndrome caused by C. jejuni in developed countries is inflammatory diarrhea and in developing countries is watery diarrhea (28, 29). The putative virulence factors of C. jejuni include the ability to adhere to and invade epithelial cells, iron acquisition systems (9), cytotoxins, cytolethal distending toxin, and an enterotoxin that resembles cholera toxin (CT) and the heat-labile enterotoxin (LT) of Escherichia coli (31). We refer to this enterotoxin as cholera toxin-like toxin (CTLT). It is believed that CTLT might contribute to watery diarrhea (31). However, there is controversy as to the existence of CTLT. Many groups have reported the production of CTLT by C. jejuni strains (1, 6, 8, 12, 16, 18, 26), while others have failed to do so (14, 24, 25, 30). Moreover, attempts to demonstrate genetic sequences homologous to the genes encoding CT and LT have failed (25, 22). This gave rise to the speculation that there is some material in the culture medium that cross-reacts with CT (14). Thus, the issue of production of CTLT by C. jejuni has confused and frustrated investigators for the past quarter of a century (26). In this report, we present the identity of the protein of C. jejuni that cross-reacts with CT.
MATERIALS AND METHODS
Bacteria.
The following strains of C. jejuni were studied: enterotoxin-positive strains CCUG 8731, CCUG 6951, CCUG 6968, and CCUG 8680 (obtained from the culture collection center, University of Goteborg, Goteborg, Sweden); enterotoxin-positive strains 180 ip and 189 ip (provided by G. Ruiz-Palacios, National Institute of Medical Science and Nutrition, Mexico Districto Federal, Mexico); enterotoxin-positive strain CJ0094400 (provided by A. Lee, University of New South Wales, Sydney, Australia); CT gene-negative, fully sequenced C. jejuni strain NCTC 11168 (23) (obtained from B. W. Wren, London School of Hygiene and Tropical Medicine, London, United Kingdom); 10 clinical strains from the International Centre for Diarrheal Diseases Research, Bangladesh, Dhaka, Bangladesh (provided by M. Rahman); and 70 clinical strains obtained from patients treated at Mubarak Al-Kabeer Hospital, Jabriya, Kuwait, during 2000 to 2004. Most of the clinical isolates were from patients with noninflammatory diarrhea. All the strains were confirmed as C. jejuni by standard bacteriological tests (20). An enterotoxigenic Escherichia coli (ETEC) strain, H10407, producing LT served as a positive control for enterotoxin production.
Production of CTLT.
All C. jejuni cultures were incubated at 42°C in a microaerophilic atmosphere generated with BBL Campy GasPak (BBL, Becton Dickinson, Sparks, MD). Initially, all strains were screened for CTLT production in Casamino Acids-yeast extract broth supplemented with 1.0 μg/ml of ferric chloride (CAYEF medium) which was incubated in a shaker incubator for 24 h (18). Subsequently, selected strains were screened for CTLT production in three other media as follows. Cultures in brucella broth (BBL) supplemented with 0.25% each of l-asparagine, l-serine, and l-glutamic acid (Sigma, St. Louis, MO) (BASG broth) were incubated for 72 h (26). Cultures in G-C medium supplemented with 0.1% IsoVitaleX (BBL) (GCIV medium) were incubated in a shaker incubator for 48 h (11). The effect of adding polymyxin B (2 mg/ml; Sigma) to release the toxin from the periplasmic space was studied in GCIV medium (11). BASG broth supplemented with 0.05% l-cystine, 0.1% corn starch, 0.5% yeast extract, and 0.48% dextrose (all from Sigma; pH 7.1) was incubated for 24 h (8). Culture supernatants were filtered through 0.22-μm cellulose acetate filters (Sartorius, Goettingen, Germany), and serial doubling dilutions (starting with a 1:2 dilution) of the filtrates were tested immediately for toxin.
Serial doubling dilutions of 50×-concentrated filtrate (suspended in phosphate-buffered saline [PBS] [pH 7.2] to 1/50 the original volume after lyophilization) from CAYEF broth culture were also tested. The concentrated filtrate was dialyzed against PBS (pH 7.2) using Spectrapor molecular porous membrane tubing (Spectrum Medical Industries, Los Angeles, CA) at 4°C for 20 h and filtered through a 0.45-μm-pore-size membrane filter (TPP) before testing.
Chinese hamster ovary (CHO) cell assay for toxins.
CHO cells distributed in 100-μl amounts (in Ham's F-12 medium with 1% fetal bovine serum; Gibco, Paisley, United Kingdom) in flat-bottomed wells of 96-well microtiter plates (Nunc, Rochester, NY) were inoculated with serial doubling dilutions of 100 μl of toxin preparation in F-12 medium and incubated at 37°C in a 5% CO2 atmosphere (10). After 24 h, the monolayer was fixed in methanol and stained with Giemsa before the cells were counted. A positive toxin effect was scored when ≥50% of the monolayer was affected at a filtrate dilution of 1:4 or more.
Ganglioside GM1 ELISA for detection of CTLT.
Flat-bottomed wells of Linbro/Titertek microtiter plates (Flow Laboratories, McLean, VA) were coated with 100 μl (containing 0.15 μg) of ganglioside GM1 (Sigma) in PBS (pH 7.2). After 20 h of incubation at 37°C, the wells were blocked with 100 μl of 1% bovine serum albumin (Sigma) in PBS-Tween 20 at 37°C for 30 min. Various dilutions of the culture filtrate in PBS-Tween 20 were added in 100-μl quantities and incubated overnight at 37°C. Then, 100 μl of rabbit polyclonal antiserum to CT (Sigma) (1:1,000 dilution in PBS-Tween 20) was added and incubated at 37°C for 2 h. This was followed by the addition of mouse monoclonal anti-rabbit antibody conjugated to peroxidase (Sigma) (1:1,000 dilution in PBS-Tween 20) and incubation at 37°C for 1 h. After addition of substrate solution (2,2′-azino-bis[ethylbenzthiazocine-6-sulfonic acid]) (Sigma) and incubation at 37°C for 30 min, the optical density (OD) was measured at 410 nm. We established a cutoff OD value of 0.402 as positive. This was the mean OD plus 2 standard deviations of ODs obtained with the medium control (CAYEF medium) tested six times by enzyme-linked immunosorbent assay (ELISA) (24).
Direct ELISA for detection of CTLT.
For direct ELISA, the GM1 ELISA was modified in that the wells were not precoated with ganglioside. C. jejuni strains were grown on sheep blood agar microaerophilically for 48 h. Cells were suspended in PBS (pH 7.2) to McFarland's opacity of 10 (3 × 109 cells per ml) and sonicated (10). One hundred microliters of the sonicate was used to coat the wells overnight at 4°C. The remainder of the steps were as for GM1 ELISA. However, we established a cutoff OD value of 0.12 as positive. This was the mean OD plus 2 standard deviations obtained with the PBS control tested six times by ELISA.
Passage of C. jejuni through a rat ileal loop.
Adult Sprague-Dawley rats that had fasted for 24 h (according to institutional policies) were anesthetized using xylazine (100 mg/ml; Phoenix Scientific, St. Joseph, MO) and ketamine (100 mg/ml; Apex Laboratories, Somersby, New South Wales, Australia). Loops were constructed in the small intestine. A volume of 0.5 ml of an overnight culture of C. jejuni grown in brucella broth (BBL) was inoculated into the loop. After 20 h, the rats were killed by ether anesthesia. The ratio of fluid accumulated (ml) to the length of the loop (cm) was calculated. C. jejuni was recovered from the fluid by culturing on CAMP-BAP medium (Oxoid, Basingstoke, Hampshire, United Kingdom). C. jejuni colonies from this medium were inoculated into brucella broth (BBL), and the culture was passaged through the ileal loops a number of times until the C. jejuni strain(s) induced significant fluid accumulation. The secreted fluid was sterilized using 0.45-μm-pore-size membranes (TPP), and the filtrate was tested in the CHO cell assay.
SDS-PAGE and immunoblotting.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (0.1% SDS and 12% polyacrylamide separating gel) by the method of Laemmli (15) and stained with Coomassie blue. For immunoblotting, the separated proteins were transferred electrophoretically to nitrocellulose (Bio-Rad, Hercules, CA) and then blocked with 5% skim milk in PBS (pH 7.2). When the antigen was CT, the rabbit CT antibody and normal rabbit serum were used at 1:3,000 and rabbit GST-PorA fusion antibody (see below) was used at 1:500 to 1:20,000. When the antigen was GST-PorA fusion protein, the rabbit CT antibody and normal rabbit serum were used at 1:1,000, while rabbit GST-PorA fusion antibody was diluted 1:20,000. When the antigen was extract of C. jejuni, rabbit CT antibody was used at a dilution of 1:1,000. The secondary antibody (peroxidase-conjugated, affinity-purified goat anti-rabbit immunoglobulin G [Fc fragment specific], at a 1:1,000 dilution) (Jackson Immunoresearch Laboratories, West Grove, PA) was added, followed by development with enhanced chemiluminescence Western blotting detection reagents according to the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, NJ).
GST-PorA fusion protein of C. jejuni.
A recombinant clone, pGSTP7RIL, containing the porA gene, which encodes the major outer membrane protein (OMP), PorA, from C. jejuni strain C31, was used. This clone expressed PorA as a fusion with glutathione S-transferase (GST). The GST-PorA fusion protein was prepared as described previously (10). Polyclonal antiserum to this fusion protein raised in rabbits was used in some immunoblot studies (10).
Identification of protein bands.
After SDS-PAGE analysis, the desired band was excised, and the gel piece was washed with 50% methanol/5% acetic acid and destained with 50% acetonitrile/50 mM ammonium bicarbonate. Sequential washes of the gel piece were carried out alternating with 20 mM ammonium bicarbonate and acetonitrile. The gel pieces were dehydrated with acetonitrile and then rehydrated with 0.5 μg trypsin solution, 20 mM ammonium bicarbonate, and CaCl2 added to a final concentration of 1 mM. After overnight incubation, the gel pieces were sonicated, and 0.5 μl was added to 0.5 μl α-hydroxycinnamic acid and spotted onto a matrix-assisted laser desorption ionization (MALDI) target plate. Both mass spectrometry (MS) and tandem time-of-flight (TOF-TOF) spectra were acquired and processed on an Applied Biosystems 4700 MALDI TOF-TOF using 4000 series Explorer software (Applied Biosystems, Foster City, CA). The data were searched against the National Center for Biotechnology Information database using Applied Biosystems GPS Explorer software with MASCOT.
RESULTS AND DISCUSSION
CHO cell assay for enterotoxin.
None of the CAYEF culture filtrates of reportedly CTLT-positive reference strains from Mexico, Australia, or Sweden or the 80 clinical strains from Kuwait and Bangladesh caused elongation of ≥50% of CHO cells at a dilution of 1:4. The filtrate from the negative control strain, NCTC 11168, was negative as expected. Culture filtrate of ETEC strain H10407 caused elongation of the entire monolayer. Twelve strains were screened for elongation of CHO cells using filtrates from three additional media (see Materials and Methods). These strains were the four Swedish strains, the two Mexican strains, the Australian strain, and five Kuwaiti clinical strains (strains 85, 89, 106, 111, and 112). The rat ileal loop-passaged Australian strain and one Kuwaiti strain, 85 (after four ileal loop passages when fluid secretion was positive) (see below), were also tested. None of the strains caused elongation of the cells with the filtrates from these media. Serial doubling dilutions (1:2 to 1:128) in PBS (pH 7.2) of 50×-concentrated filtrates from CAYEF cultures of CCUG 6968 and CJ0094400 were negative for elongation of CHO cells. Thus, in spite of screening numerous freshly isolated local clinical strains and also control strains from various laboratories and using several media that were reported to promote enterotoxin production by C. jejuni, none of the strains produced any enterotoxin in our hands. This failure to demonstrate enterotoxin production is consistent with the findings of others (14, 19, 24, 25, 30).
Rat ileal loop passage.
The two Mexican strains, the Australian strain, and five selected ELISA-positive Kuwaiti strains (no. 85, 89, 106, 111, and 112) were passaged four times through rat ileal loops. At the end of the passage, Australian strain CJ0094400 and Kuwaiti strain 85 each gave a fluid-to-loop-length ratio of 0.3. The control ETEC strain H10407 gave a ratio of 0.8 without the need for prior passage.
Other workers have also reported induction of fluid secretion by repeated passage of C. jejuni strains through rat ileal loops (13, 26, 27). However, the fluids induced by the two C. jejuni strains were negative for CHO cell elongation, while that induced by the control ETEC strain H10407 was positive. This finding is similar to the observation from another study that C. jejuni-induced fluid in the rat ileal loop assay did not cause elongation of CHO cells (7). This is consistent with the notion that fluid secretion was not due to CTLT production but was due to another mechanism(s) (5).
GM1 ganglioside ELISA.
The CAYEF culture filtrates of the positive reference cultures of C. jejuni, filtrate from the negative reference strain NCTC 11168, and those of all Kuwaiti and Bangladeshi strains were tested by ELISA for material cross-reacting with CT. The Swedish strains CCUG 8680 and CCUG 6968, the Australian strain CJ0094400, and 16 of 70 Kuwaiti strains gave OD readings of ≥0.402, indicating a positive test. The other strains were negative. The positive control ETEC strain H10407 gave an OD reading of 0.701. This identification of material cross-reacting with CT antibody in GM1 ganglioside ELISA from some C. jejuni cultures agrees with the findings from other studies (12, 16, 17, 18).
SDS-PAGE and immunoblotting.
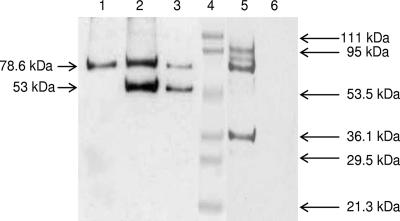
Culture supernatants of numerous C. jejuni strains (both GM1 ELISA-positive and -negative strains) yielded two prominent bands of 78.6 kDa and 53 kDa reacting with CT antiserum. When blots were probed with normal rabbit serum, only the 78.6-kDa band from all strains reacted (Fig. 1). ETEC H10407 yielded a different profile (data not shown).
FIG. 1.
Immunoblot of proteins from bacterial culture supernatants. SDS-PAGE-separated proteins transferred to nitrocellulose membrane were probed with antibodies. Lane 1, C. jejuni NCTC 11168 (GM1 ELISA negative); lane 2, C. jejuni NCTC 11168; lane 3, C. jejuni CCUG 6968 (GM1 ELISA positive); lane 4, molecular mass markers; lane 5, ETEC H10407; lane 6, medium control. Lane 1 was probed with normal rabbit serum, and all other lanes except lane 4 were probed with rabbit CT antibody. The positions of standard molecular mass markers are shown on the right and those of C. jejuni proteins on the left.
Amino acid sequencing of the specific CT antibody-reactive protein band.
Tryptic digests of the 53-kDa CT antibody-reactive band were analyzed by MALDI TOF-TOF, which identified the protein as the major OMP PorA of C. jejuni (23) (Fig. 2). The 53-kDa reactive band was seen even with NCTC 11168, which does not possess a CT gene homologue.
FIG. 2.
Protein match with 37% sequence coverage of the CT antibody-reactive 53-kDa protein of C. jejuni with the major OMP PorA. Peptides identified by MALDI TOF-TOF are shown in bold, while underlined peptides indicate that at least 50% of potential b and y ions matched.
Cross-reactivity of GST-PorA and CT.
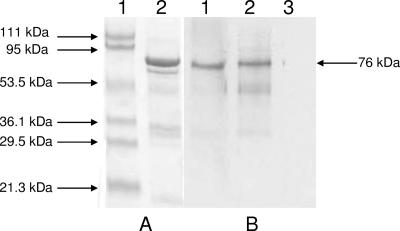
Rabbit CT antibody reacted with GST-PorA fusion protein by Western blotting. There was no reaction with normal rabbit serum (Fig. 3). However, antibody produced against GST-PorA did not react with CT (data not shown). A similar one-way reaction has been observed in other bacterial infections (4). The mechanism behind this one-way reaction is not clear, but it may depend upon the differential accessibility of a common epitope(s) in the two different antigens. Although PorA shares 4% and 12% similarity with CtxB from El Tor and classical biotypes of Vibrio cholerae O1, respectively, we could not identify any potential common epitopes between PorA and CtxB. Extensive epitope mapping of the proteins would be required to identify the precise antibody recognition regions.
FIG. 3.
SDS-PAGE and immunoblot analyses of GST-PorA fusion protein. SDS-PAGE-separated fusion protein (A) was transferred to nitrocellulose and probed with antibodies (B). (A) Lane 1, molecular mass markers; lane 2, GST-PorA fusion stained with Coomassie blue. (B) GST-PorA fusion probed with rabbit CT antibody (lane 1), rabbit homologous antibody (lane 2), and normal rabbit serum (lane 3). The positions of molecular mass standards are shown on the left and that of the GST-PorA fusion is shown on the right.
Direct ELISA.
All seven reference strains from Sweden, Mexico, and Australia, the enterotoxin-negative strain NCTC 11168, five Kuwaiti strains (no. 85, 89, 106, 111, and 112) from among the 16 GM1 ELISA-positive strains, and five selected Kuwaiti strains negative by GM1 ELISA were tested by direct ELISA. All were positive, with OD values of ≥0.2. Since the CT cross-reactive material PorA is an OMP, most of it remains bound to the bacterial cells, with only small amounts shed into the medium. This explains why some strains were positive only by GM1 ELISA, while all tested strains were positive by both direct ELISA and immunoblotting.
In our immunoblot studies with CT antibody, two bands of 78.6 kDa and 53 kDa were visible, but the larger band was due to a nonspecific reaction with normal rabbit serum. One of the factors that contributed to the conclusion that C. jejuni strains produce an enterotoxin resembling CT was the reactivity of culture filtrates with CT antibody in GM1 ELISA. Our results show unequivocally that C. jejuni strains possess an antigen that reacts with CT antibody. That antigen is the major OMP, PorA. If a porA mutant could be constructed, we hypothesize that it would no longer bind CT antibody. However, PorA performs important bacterial functions such as regulating the flow of solutes across the cell membrane. Because of this essential housekeeping function, a porA mutation may well be lethal or highly unstable. Indeed, there are no reports of successful construction of a porA mutant in C. jejuni; moreover, other species of Campylobacter, including C. coli, C. lari, and C. fetus, possess a porA gene that can be amplified with the primers used for C. jejuni (10). Assuming that the PorA proteins produced by different species of Campylobacter are antigenically similar, all of them should bind CT antibody.
Acknowledgments
This study was supported by a Kuwait University research grant (number MI02/01) and by an Australian Research Council grant (CEO562063).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Bok, H. E., A. S. Greeff, and H. H. Crewe-Brown. 1991. Incidence of toxigenic Campylobacter strains in South Africa. J. Clin. Microbiol. 29:1262-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buzby, J. C., B. M. Allos, and T. Roberts. 1997. The economic burden of Campylobacter-associated Guillain-Barre syndrome. J. Infect. Dis. 176(Suppl. 2):S192-S197. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2006. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, United States, 2005. Morb. Mortal. Wkly. Rep. 55:392-395. [PubMed] [Google Scholar]
- 4.Chart, H., T. Cheasty, D. Cope, R. J. Gross, and B. Rowe. 1991. The serological relationship between Yersinia enterocolitica O9 and Escherichia coli O157 using sera from patients with yersiniosis and haemolytic uraemic syndrome. Epidemiol. Infect. 107:349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everest, P. H., A. T. Cole, C. J. Hawkey, S. Knutton, H. Goossens, J.-P. Butzler, J. M. Ketley, and P. H. Williams. 1993. Roles of leukotriene B4, prostaglandin E2, and cyclic AMP in Campylobacter jejuni-induced intestinal fluid secretion. Infect. Immun. 61:4885-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everest, P. H., H. Goossens, J.-P. Butzler, D. Lloyd, S. Knutton, J. M. Ketley, and P. H. Williams. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319-325. [DOI] [PubMed] [Google Scholar]
- 7.Everest, P. H., H. Goossens, P. Sibbons, D. R. Lloyd, S. Knutton, R. Leece, J. M. Ketley, and P. H. Williams. 1993. Pathological changes in the rabbit ileal loop model caused by Campylobacter jejuni from human colitis. J. Med. Microbiol. 38:316-321. [DOI] [PubMed] [Google Scholar]
- 8.Goossens, H., J.-P. Butzler, and Y. Takeda. 1985. Demonstration of cholera-like enterotoxin production by Campylobacter jejuni. FEMS Microbiol. Lett. 29:73-76. [Google Scholar]
- 9.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5-21. [DOI] [PubMed] [Google Scholar]
- 10.Khan, I., B. Adler, S. Haridas, and M. J. Albert. 2005. PorA protein of Campylobacter jejuni is not a cytotoxin mediating inflammatory diarrhoea. Microb. Infect. 7:853-859. [DOI] [PubMed] [Google Scholar]
- 11.Klipstein, F. A., and R. F. Engert. 1984. Properties of crude Campylobacter jejuni heat-labile enterotoxin. Infect. Immun. 45:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klipstein, F. A., and R. F. Engert. 1985. Immunological relationship of the B subunits of Campylobacter jejuni and Escherichia coli heat-labile enterotoxins. Infect. Immun. 48:629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klipstein, F. A., R. F. Engert, and H. Short. 1986. Enzyme-linked immunosorbent assays for virulence properties of Campylobacter jejuni clinical isolates. J. Clin. Microbiol. 23:1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konkel, M. E., Y. Lobet, and W. Cieplak. 1992. Examination of multiple isolates of Campylobacter jejuni for evidence of cholera toxin-like activity, p. 193-198. In I. Nachamkin, M. J. Blaser, and L. S. Tomkins (ed.), Campylobacter jejuni: current status and future trends. ASM Press, Washington, DC.
- 15.Laemmli. U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Lindblom, G.-B., M. Johny, K. Khalil, K. Mazhar, G. M. Ruiz-Palacios, and B. Kaijser. 1990. Enterotoxigenicity and frequency of Campylobacter jejuni, C. coli and C. laridis in human and animal stool isolates from different countries. FEMS Microbiol. Lett. 54:163-167. [DOI] [PubMed] [Google Scholar]
- 17.Mathan, V. I., D. P. Rajan, F. A. Klipstein, and R. F. Engert. 1984. Enterotoxigenic Campylobacter jejuni among children in South India. Lancet ii:981. [DOI] [PubMed] [Google Scholar]
- 18.McCardell, B. A., J. M. Madden, and J. T. Stanfield. 1986. Effect of iron concentration on toxin production in Campylobacter jejuni and C. coli. Can. J. Microbiol. 32:395-401. [DOI] [PubMed] [Google Scholar]
- 19.McFarland, B. A., and S. D. Neill. 1992. Profiles of toxin production by thermophilic Campylobacter of animal origin. Vet. Microbiol. 30:257-266. [DOI] [PubMed] [Google Scholar]
- 20.Nachamkin, I. 1999. Campylobacter and Arcobacter, p. 716-726. In P. R. Murray, E. J. Barron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 21.Nachamkin, I., B. M. Allos, and T. W. Ho. 1998. Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 11:555-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsvik, Ø., K. Wachsmuth, G. Morris, and J. C. Feeley. 1984. Genetic probing of Campylobacter jejuni for cholera toxin and E. coli heat-labile enterotoxin. Lancet i:449. [DOI] [PubMed] [Google Scholar]
- 23.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, D. Clingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, R. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Perez, G. I., D. L. Cohn, R. L. Guerrant, C. M. Patton, L. B. Reller, and M. J. Blaser. 1989. Clinical and immunological significance of cholera-like toxin and cytotoxin production by Campylobacter species in patients with acute inflammatory diarrhea in the USA J. Infect. Dis. 160:460-468. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Perez, G. I., D. N. Taylor, P. D. Echeverria, and M. J. Blaser. 1992. Lack of evidence of enterotoxin involvement in pathogenesis of Campylobacter diarrhea, p. 184-192. In I. Nachamkin, M. J. Blaser, and L. S. Tomkins (ed.), Campylobacter jejuni: current status and future trends. ASM Press, Washington, DC.
- 26.Ruiz-Palacios, G. M., N. I. Torres, B. R. Ruiz-Palacios, J. Torres, E. Escamilla, and J. Tamayo. 1983. Cholera-like enterotoxin produced by Campylobacter jejuni: characterization and clinical significance. Lancet 30:250-253. [DOI] [PubMed] [Google Scholar]
- 27.Saha, S. K., N. P. Singh, and S. C. Sanyal. 1988. Enterotoxigenicity of chicken isolates of Campylobacter jejuni in ligated ileal loops of rats. J. Med. Microbiol. 26:87-91. [DOI] [PubMed] [Google Scholar]
- 28.Skirrow, M. B., and M. J. Blaser. 1995. Campylobacter jejuni, p. 825-848. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, NY.
- 29.Taylor, D. N. 1992. Campylobacter infections in developing countries, p. 20-30. In I. Nachamkin, M. J. Blaser, and L. S. Tomkins (ed.), Campylobacter jejuni: current status and future trends. ASM Press, Washington, DC.
- 30.Wadstrom, T. S., S. B. Baloda, K. Krovacek, A. Faris, S. Bengtson, and M. Walder. 1983. Swedish isolates of Campylobacter jejuni/coli do not produce cytotonic or cytotoxic enterotoxins. Lancet ii:911. [DOI] [PubMed] [Google Scholar]
- 31.Wassenaar, T. M. 1997. Toxin production by Campylobacter spp. Clin. Microbiol. Rev. 10:466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]