Abstract
Human granulocytic anaplasmosis (HGA) is caused by the obligate intracellular bacterium Anaplasma phagocytophilum. The critical role of gamma interferon (IFN-γ) for induction of severe inflammatory histopathology, even in the absence of a significant bacterial load, was previously demonstrated in a murine model of HGA. We hypothesized that NK, NKT, and possibly CD8+ cytotoxic T cells participate in the development of histopathologic lesions with A. phagocytophilum infection. Mice were mock infected or infected with low- or high-passage A. phagocytophilum and assayed for hepatic histopathology and splenocyte immunophenotype during the first 21 days after infection. Compared to high-passage A. phagocytophilum-infected mice, low-passage A. phagocytophilum-infected mice had more severe hepatic lesions and increased apoptosis. The hepatic histopathology severity in low-passage A. phagocytophilum-infected mice peaked on day 2 at the time of peak plasma IFN-γ levels and gradually decreased through day 21. Low-passage A. phagocytophilum-infected mice also showed significantly increased levels of lymphocyte NK1.1/FasL expression on days 4 to 7 corresponding to early, severe hepatic inflammation, whereas the levels of NKT cells were substantially lower on day 4, suggesting that there was NKT cell involvement. This result supports the concept that NK1.1+ cells, including NK and NKT cells, are major components in the early pathogenesis of A. phagocytophilum infection.
Human granulocytic anaplasmosis (HGA) is an emerging, tick-borne disease that ranges in severity from mild to fatal. Its causative agent, Anaplasma phagocytophilum, is an obligate intracellular bacterium that propagates within neutrophil vacuoles (13). Fever, malaise, headache, myalgia, thrombocytopenia, and leukopenia are common during symptomatic infections in humans. Horses, dogs, and other animals that develop febrile disease and acute infection have clinical signs similar to those observed in humans (13, 17, 22, 26). Several models of HGA exist, including infection of mice, which do not have clinical signs but develop histopathology characteristic of human and equine infections, and infection of horses, which nearly precisely mimics human infection, including the spectrum of clinical severity (7, 22, 23). In vitro passage alters the clinical severity in horses such that worse clinical signs and laboratory features are observed in animals inoculated with low-passage A. phagocytophilum than in animals inoculated with high-passage A. phagocytophilum (26).
The mechanisms of pancytopenia with HGA are unclear, but some evidence points to pathogen activation of macrophages by proinflammatory cytokines, such as gamma interferon (IFN-γ) (1, 24). Despite its ability to avoid killing by innate immunity (31), A. phagocytophilum paradoxically induces some innate immune responses that contribute to tissue injury and probably disease (29). Previous studies in our laboratory examined host immune responses in a murine model and demonstrated the critical role of IFN-γ in induction of severe inflammatory histopathology, even in the absence of significant bacterial loads (23, 24, 28). Since innate immune responses and early IFN-γ production are important in the development of histopathologic lesions in mice (24, 29), we hypothesized that NK cells and possibly CD8+ cytotoxic T cells could be activated and participate in the development of histopathologic lesions with A. phagocytophilum infection. Moreover, as observed with horses (26), we hypothesized that these cellular and histopathologic responses vary depending upon the length of time that the inoculum was propagated in vitro. To better understand the events during the initial phases of infection with A. phagocytophilum, we examined the immunophenotype and activation status of splenocytes, their relationship to proinflammatory histopathology, and the production of important cytokines in a murine model of HGA.
MATERIALS AND METHODS
Experimental animals, A. phagocytophilum culture, and infection of mice.
Female B6 mice that were 6 weeks old were purchased from The Jackson Laboratories (Bar Harbor, ME). All animals were maintained and used in strict accordance with the guidelines issued by the Johns Hopkins University School of Medicine for animal care.
A. phagocytophilum strain WebsterT was maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS) and 2 mM l-glutamine until >90% of the cells contained morulae. Cultures were coordinated so that low- and high-passage cultures were available for simultaneous inoculation; the difference between the low- and high-passage cultures was approximately 3.5 months of continuous in vitro growth. On the day of inoculation, passage 8 (low-passage) and passage 22 (high-passage) A. phagocytophilum-infected and uninfected HL-60 cells were centrifuged (200 × g, 10 min) to concentrate them, and then each cell pellet was resuspended in serum-free RPMI 1640 medium for inoculation.
Twenty-eight mice were assigned to each of the low-passage and high-passage A. phagocytophilum-infected and mock-infected HL-60 cell groups (total, 84 mice). Challenge was performed by intraperitoneal injection of 1 ml (106 cells/ml) containing either heavily infected (>90% of cells infected) or uninfected (mock-infected) HL-60 cells.
Necropsy.
Four mice in each group (low-passage and high-passage A. phagocytophilum infected and mock infected) were necropsied 1 h after inoculation and on days 2, 4, 7, 10, 14, and 21. The mice were sedated with CO2 gas and then exsanguinated by cardiac puncture. The spleen and liver were sterilely harvested. Part of the spleen was placed in RPMI 1640 medium containing 1× penicillin/streptomycin (Invitrogen Life Technologies, Carlsbad, CA) and 10% FBS for immunophenotyping. The liver was fixed in a zinc fixation solution (BD Pharmingen, San Diego, CA) and embedded in paraffin for hematoxylin and eosin staining and histopathologic assessment. Since histopathologic lesions may not be normally distributed among infected and uninfected mice, hepatic histopathologic changes in infected and mock-infected mice were continuously ranked for severity, focusing on the size, density, and cellularity of inflammatory lesions, the degree of necrosis and/or apoptosis, and the number of inflammatory foci. All evaluations were performed by investigators blinded to the type of mouse treatment and were conducted by two microscopists to ensure the fidelity of ranking. When two groups were compared, the groups were reranked to ensure that there were the continuous variables required for nonparametric analysis. Statistical analysis was performed using one-sided nonparametric statistical tests (Mann-Whitney and Kruskall-Wallis tests) to compare median ranks of groups; P values of <0.05 were considered significant, and variability was displayed by providing maximum and minimum ranks for each group considered. Although inoculation of uninfected HL-60 cells resulted in lower degrees of hepatic histopathologic severity, mock infection caused some histopathologic alterations similar to those observed in infected mice. To normalize for pathology in mock-infected mice (mice inoculated with uninfected HL-60 cells), the median rank for the mock-infected group at each time was subtracted from the rank of each individual infected mouse. Normalized results were reranked to establish continuous variables and to determine significant differences in hepatic histopathology between the low-passage A. phagocytophilum-infected mice and the high-passage A. phagocytophilum-infected mice.
Flow cytometry analysis (immunophenotyping).
Spleens from individual mice (12 mice at each time) were minced and dispersed to obtain single-cell suspensions. Cells were washed and lysed in a hypotonic salt solution to remove erythrocytes, and then the remaining cells were washed and resuspended in RPMI 1640 medium with 10% FBS and 1× penicillin/streptomycin. The single-cell suspensions were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD19 (B cells; BD Pharmingen), FITC-conjugated anti-CD4 (L3T4) (BD Pharmingen), FITC-conjugated FasL (activated cells; BD Pharmingen), R-phycoerythrin (R-PE)-conjugated anti-NK1.1 (NKR-P1B and NKR-P1C) (NK cells; BD Pharmingen), R-PE-conjugated anti-CD8a (Ly-2) (BD Pharmingen), and isotype-matched control antibodies (BD Pharmingen) for 20 min on ice. The stained cells were washed twice with phosphate-buffered saline containing 0.5% bovine serum albumin (Sigma, St. Louis, MO) and 0.02% NaN3. Stained cells were initially gated to identify lymphocyte populations and then identified and quantitated by flow cytometry. All flow cytometry data were normalized to expression in mock-infected mice by subtracting the mean proportion of stained cells in mock-infected mice at each time from the individual values for the proportion of expressing cells in infected animals at the same time. Normalized values for each group at each time were then used to calculate a normalized mean expression ± standard deviation. Normalized mean values were compared using paired, one-way Student's t tests, and P values of <0.05 were considered significant.
To determine whether NKT cells could play a role in the inflammatory process, spleens from A. phagocytophilum-infected (passage 15) and mock-infected mice were harvested on day 4, when NK cell populations were peaking. Splenocytes were prepared as described above and stained with R-PE-conjugated anti-NK1.1 and FITC-conjugated T-cell receptor (TCR) monoclonal antibodies.
Cytokine assays.
Levels of IFN-γ in the plasma were determined using a mouse IFN-γ Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) according to the manufacturer's recommendations. Briefly, standard or plasma samples were added to duplicate antibody capture wells and incubated for 2 h at room temperature. Plates were washed and reacted with biotinylated anti-IFN-γ at room temperature for 2 h. After washing, captured IFN-γ was detected by addition of the substrate solution, and the optical density at 540 nm was determined using a microplate reader. Statistical significance was determined by application of one-sided Student's t tests and Kolmogorov-Smirnov two-sample tests, and P values of <0.05 were considered significant.
RESULTS
A. phagocytophilum infection-induced immunopathology.
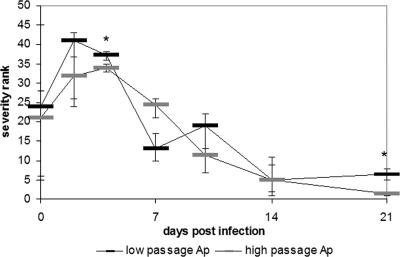
A. phagocytophilum hepatic histopathology was evaluated over a 21-day period. As expected, none of the mice exhibited clinical signs of illness with infection. Infected mice often contained small localized accumulations of lymphocytes, macrophages, and occasionally neutrophils, with or without apoptotic cells. These lesions were most frequently observed in the hepatic lobules, but some were also observed in periportal regions or adjacent to portal veins. Most infected animals also had a mild to moderate degree of hepatitis, with lymphohistiocytic infiltrates in hepatic lobules (Fig. 1). Low-passage A. phagocytophilum-infected mice had more severe hepatic lesions and a larger number of apoptotic cells than either high-passage A. phagocytophilum-infected mice or mock-infected mice up to day 7 postinfection (P = 0.03, as determined by a Mann-Whitney U test). The severity of hepatic histopathology peaked on day 2 and gradually decreased throughout the 21 days of the experiment, returning to near the baseline at day 21. High-passage A. phagocytophilum-infected mice also had hepatic inflammatory lesions and rank scores greater than those of mock-infected mice on days 2 through 14, although the severity ranks were less than those of low-passage A. phagocytophilum-infected mice (Fig. 2).
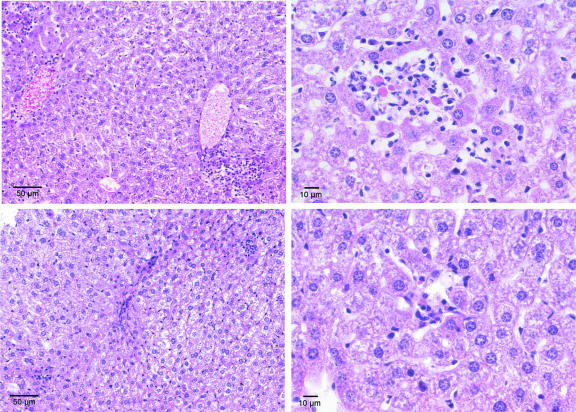
FIG. 1.
Hepatic histopathology in low-passage A. phagocytophilum-infected mice (top panels) and high-passage A. phagocytophilum-infected mice (bottom panels). Note the larger inflammatory lesions (top left panel) and lobular infiltrates of predominantly lymphocytes and macrophages with edema, focal necrosis, and apoptotic cells (top right panel). The bottom panels show similar anatomic compartments with smaller inflammatory lesions and infiltrates and less hepatocyte injury. The preparations were stained with hematoxylin and eosin.
FIG. 2.
Median hepatic histopathology ranks for mock-infected versus low- or high-passage A. phagocytophilum-infected mice at the time of infection through 21 days postinfection. Higher ranks correspond to higher degrees of severity. The error bars indicate the maximum and minimum ranks for replicate mice in each group at each time. An asterisk indicates that the P value is <0.05, as determined by a Wilcoxon signed-rank test comparing median ranks after reranking at each time. Ap, A. phagocytophilum.
NK1.1/FasL, CD8, and CD4 expression on splenic lymphocytes following infection with A. phagocytophilum.
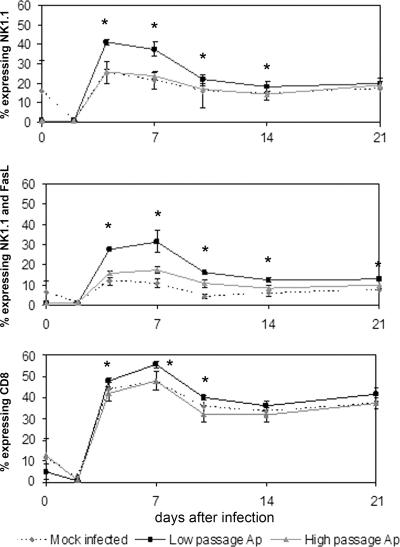
The relative proportions of NK1.1- and NK1.1/FasL (double-positive)-expressing splenic lymphocytes were significantly different in different groups (Fig. 3). Compared to both mock-infected and high-passage A. phagocytophilum-infected mice, animals infected with low-passage A. phagocytophilum had significantly increased levels of NK1.1 expression in splenic lymphocytes on days 4 to 7 that corresponded with early and more severe hepatic inflammation (days 2 to 4). Many of the NK1.1+ cells also expressed FasL, and NK1.1/FasL-expressing cells in low-passage A. phagocytophilum-infected mice were significantly more abundant at every time after day 2 (compared to mock-infected mice) or day 4 (compared to high-passage A. phagocytophilum-infected mice) (Fig. 3). Likewise, compared to the spleens from mock-infected animals, NK1.1/FasL-expressing cells were more abundant in spleens from high-passage A. phagocytophilum-infected mice, but not to the degree observed with low-passage A. phagocytophilum infections. Total FasL expression in splenocytes was significantly higher in infected animals than in mock-infected animals at every time from day 4 to 21 and was higher in low-passage A. phagocytophilum-infected mice than in high-passage A. phagocytophilum-infected mice (not shown).
FIG. 3.
Total NK1.1, NK1.1/FasL, and CD8 expression in splenic lymphocytes was significantly higher in low-passage A. phagocytophilum-infected mice than in high-passage A. phagocytophilum-infected or mock-infected mice. Splenic lymphocytes were analyzed by flow cytometry using monoclonal antibodies to NK1.1 alone (top panel), to NK1.1 and FasL (middle panel), and to CD8 (bottom panel). Staining was performed individually, as described in Materials and Methods. The data are expressed as the proportion of splenic lymphocytes expressing the specific immunophenotype and are means ± standard deviations. An asterisk indicates that the P value is <0.05 for a comparison of low-passage A. phagocytophilum-infected mice and high-passage A. phagocytophilum-infected mice. Ap, A. phagocytophilum-infected mice.
Although to a lesser degree than NK1.1+ cells, CD8+ lymphocyte populations also expanded more in spleens of low-passage A. phagocytophilum-infected animals (Fig. 3), peaking on days 4 to 10; the CD8+ lymphocyte population densities were not different in mock-infected and high-passage A. phagocytophilum-infected mice. The CD4 lymphocyte proportions increased in both infected and mock-infected animals, ranging from 20 to 41%, and, with the exception of day 10, did not vary in mock-infected and infected animals (not shown). FasL expression was not examined for either CD4 or CD8+ lymphocytes.
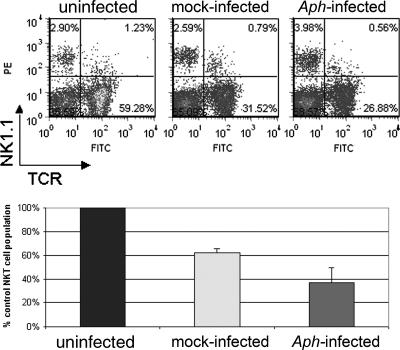
These data show that A. phagocytophilum infection drives FasL expression on NK1.1-expressing cells, which expand rapidly after infection, particularly in low-passage A. phagocytophilum-infected mice. Moreover, low-passage bacteria stimulated total NK (NK and NKT) and CD8+ lymphocyte responses not observed with either high-passage A. phagocytophilum-infected or mock-infected animals. NKT cells, as defined by NK1.1/TCR expression in splenocytes, were examined only on day 4 in infected and mock-infected animals and in a single uninfected animal. At this time, the number of NKT cells in infected animals was 37 to 74% of the number in mock-infected mice and 23 to 46% of the number detected in uninfected mice (Fig. 4).
FIG. 4.
NK1.1/TCR expression in splenic lymphocytes of mice infected with A. phagocytophilum or mock infected at 4 days. The top panel shows the two-color flow cytometric distribution of NK1.1- and TCR-expressing cells. The bottom panel shows the reductions in NKT cell populations in mock-infected and A. phagocytophilum-infected mice relative to uninfected mice; the error bars indicate standard deviations. The results shown are representative of three replicates. Aph, A. phagocytophilum.
IFN-γ production.
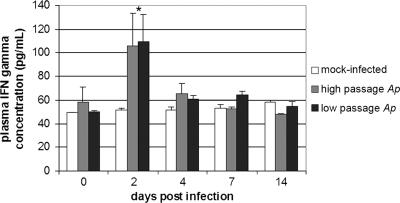
IFN-γ was analyzed in plasma samples through day 14; the average level was 52.7 ± 3.3 pg/ml in plasma from mock-infected mice, and the level did not increase to more than 58 pg/ml through day 14 (P ≥ 0.068). The plasma IFN-γ concentrations were not different in low- and high-passage A. phagocytophilum-infected animals and were different from the concentrations in mock-infected animals only on day 2, when the concentrations doubled (105 and 109 pg/ml for high- and-low passage A. phagocytophilum-infected animals, respectively), although the P values did not reach levels of significance (P = 0.099 and 0.064, respectively, as determined by Student's t tests) (Fig. 5). However, the results of IFN-γ analyses were not normally distributed, and when the nonparametric Kolmogorov-Smirnov two-sample test was used, the levels induced by low-passage A. phagocytophilum infection but not the levels induced by high-passage A. phagocytophilum infection differed from the levels for mock-infected animals through day 14 (P < 0.024 versus P ≥ 0.222).
FIG. 5.
Plasma IFN-γ concentrations after infection with low- or high-passage A. phagocytophilum or after mock infection. At day 2, the concentrations in A. phagocytophilum-infected mice were higher than the concentrations in mock-infected mice. An asterisk indicates that the P value is <0.05 for a comparison with mock-infected mice. The error bars indicate standard errors of the means. Ap, A. phagocytophilum.
DISCUSSION
The mechanisms of tissue injury with A. phagocytophilum infection are poorly understood. Certain aspects of the clinical course, including toxic shock-like manifestations and acute respiratory distress syndrome (4, 13, 22, 36), and pathological findings, such as hepatocyte apoptosis, suggest that host immune factors play a role in the pathogenesis of HGA. The niche occupied by obligate intracellular bacteria also presents a significant challenge to host immunity. Typically, infections with these organisms elicit a cell-mediated immune response that results in the production of IFN-γ and up-regulation of other proinflammatory and cytotoxic responses effective at killing intracellular pathogens (24, 35). Owing to the intracellular niche of A. phagocytophilum, many suspect that T-cell immunity is important for control of this organism, and in fact, humans with HGA develop very high levels of the Th1 cytokine IFN-γ during active infection (14). Mice also exhibit a substantial increase in the plasma IFN-γ level with infection (1, 23), and the lack of A. phagocytophilum growth restriction in SCID and other mouse strains is consistent with an important role for acquired immunity in controlling bacterial burdens and complete bacterial killing (7, 19, 32). In addition to control of A. phagocytophilum infection, IFN-γ production in the murine HGA model is a major contributor to inflammatory tissue injury. This is known since histopathologic lesions in IFN-γ knockout mice are completely abrogated despite a marked increase in the pathogen load, implying a role for immune-mediated pathology (24).
An alternate model for HGA is the horse, which develops a febrile illness very similar to that observed in humans (26), and the histopathologic findings for humans, horses, mice, and other animals that develop acute infections are also strikingly similar (22). In addition, horses experimentally infected with A. phagocytophilum propagated in vitro for various intervals develop modified disease manifestations, and in vitro culture intervals greater than approximately 2 months (18 or more passages) result in a phenotype with reduced disease severity (26). The mechanisms that account for differential clinical severity in horses are unclear but appear to be unrelated to the continually changing expression of major surface protein 2 (Msp2), the immunodominant surface protein of A. phagocytophilum (10). To better understand the mechanisms that drive the altered disease severity, we sought to reproduce differential histopathologic findings observed with experimental equine infection in the murine model after infection with isogenic low- and high-passage in vitro-propagated A. phagocytophilum. In addition, we sought to demonstrate whether histopathologic phenotypes are associated with immunophenotypic changes in potential immune effectors, focusing on probable sources of IFN-γ.
Our findings confirm previous studies showing the likely critical role of innate immune induction as a mechanism for tissue injury and inflammatory histopathology in the HGA mouse model (29). Similar to other observations with the HGA mouse model, histopathologic lesions that likely represent anatomic correlates of disease peak earlier and are larger in mice infected by low-passage A. phagocytophilum than in mice infected by high-passage A. phagocytophilum, and the peak occurs at days 0 through 4, when adaptive immunity is not yet established or is just beginning. In agreement with this observation is the detection of higher levels of IFN-γ in the plasma of infected mice than in the plasma of mock-infected mice peaking on day 2, at the peak of histopathologic injury with low-passage A. phagocytophilum. Although only demonstrated by nonparametric statistical means, the greater levels of IFN-γ induced by low-passage A. phagocytophilum infection are consistent with the early and greater histopathologic severity in these mice. This early peak for both IFN-γ, which has been demonstrated to be a critical component of histopathologic injury and disease in HGA (24), and histopathologic inflammatory hepatic injury in infected mice strongly supports a role for innate immune-mediated injury. Although mock infection also results in some hepatic injury, the severity is less than the severity with an infection, and it is unlikely that the host HL-60 cells survive long or that chemokines produced by these cells have substantial biological activity in mice.
Since NK1.1-expressing cells, either NK or NKT, are innate immune cells that can produce abundant IFN-γ, we examined their role and their activation in this process (6, 21, 27). In agreement with the differential histopathologic severity in low-passage A. phagocytophilum-infected mice, all NK1.1+ and activated (FasL-expressing) NK1.1+ cells from these animals comprised a significantly greater proportion of the splenocytes on days 4 and 7 than the cells in either high-passage A. phagocytophilum-infected or mock-infected mice comprised. Similarly, CD8+ lymphocytes also expanded on days 4 through 10 postinfection in mice infected with low-passage A. phagocytophilum compared with the levels in high-passage A. phagocytophilum- and mock-infected animals. Although CD8+ T cells can also express NK1.1 with activation (3), they are unlikely to have comprised an important population at early times in naïve mice. The differential expansion of NK and activated NK cells, as well as CD8+ cells, suggests that these cells may play an important role in the differential histopathologic inflammatory severity. However, the peak hepatic inflammation occurred marginally earlier than the expansion of both activated NK1.1+ and CD8+ lymphocytes, and both types of cells comprised an exceedingly small proportion of splenocytes on day 2, at the time of peak inflammation and plasma IFN-γ levels. Whether the infiltrating cells in liver lesions include a large population of NKT or NK cells is not known since specific immunohistologic studies were not conducted. However, it is well recognized that up to 30% of the lymphocytes in the liver are NKT cells, and these cells are important mediators that link innate immunity and adaptive immunity (15, 16).
Another potential significant source of IFN-γ during early phases of infection includes NKT cells, which are implicated in infections by the related organism Ehrlichia muris (25). Given the discrepancy in the kinetics of NK and CD8+ lymphocyte expansion at day 4 and later versus peak plasma IFN-γ and hepatic inflammation at day 2, a role for NKT cells was suggested. This suggestion was also supported by (i) significantly reduced proportions of NK1.1+ cells (including both NK and NKT cells) at day 2 in splenocytes from infected animals, suggesting the potential for activation-induced loss of NKT cells populations (12, 18, 34); and (ii) the known disproportionate distribution of NKT cells to the liver, where activation could have more dramatic local effects, such as inflammatory histopathology (16). A role for NKT cells is further supported by the demonstration that the NK1.1/TCR-expressing cells in infected animals are 37 to 74% of the cells in mock-infected mice and less than 23 to 46% of the cells observed in uninfected mice. Although there is debate about the precise nature of the apparent early loss of NKT cells with some infections (activation-induced cell death [15] versus down-regulation of TCR expression [18]), the reduced detection of these cells is correlated with IFN-γ production and the induction of tissue histopathology in models of salmonellosis and listeriosis (2, 5, 33). Moreover, these data are consistent with the concept of cross talk between NKT and NK cells that are the active early components that bridge innate immunity and adaptive immunity (8).
Several recent observations need to be considered regarding the immunopathogenesis of A. phagocytophilum infections. First, we recently demonstrated that the highly variable Msp2s, the dominant immunoreactive surface proteins of A. phagocytophilum, are poor stimuli for T-cell responses. Second, we showed that induction of innate immunity by A. phagocytophilum, while unable to restrict bacterial propagation in vivo, contributes significantly to inflammatory histopathology (29). The data here supplement these observations by reinforcing the role of innate immunity, chiefly focusing on NK and NKT cells and to a lesser extent on CD8+ lymphocytes of the adaptive immune response. Since Msp2 seems to be not involved (10), the nature of the immunostimulatory ligands of A. phagocytophilum becomes an important question. We previously demonstrated macrophage activation via Toll-like receptor 2 when animals were exposed to A. phagocytophilum (11), and NK and NKT cells are well known to become activated via this pathogen-associated molecular pattern receptor (9, 30). The likely involvement of NKT cells as important sources of IFN-γ that drive the proinflammatory phenotype in both E. muris (25) and A. phagocytophilum infections raises important questions. Do these bacteria contain glycolipid ligands (20, 25) that could be the stimulus for many of the inflammatory histopathology and disease manifestations observed in animal models of HGA and in human disease, and does their expression vary with in vitro propagation or under natural circumstances? Further analysis of the roles of cytotoxic cells such as NK, NKT, and CD8+ lymphocytes and the bacterial ligands that trigger their effector responses may provide important new clues to the immunopathogenesis of HGA.
Acknowledgments
This work was supported by grant R01 AI41213 to J.S.D. from the National Institute of Allergy and Infectious Diseases.
We thank Nicole Barat for excellent technical support.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 2 April 2007.
REFERENCES
- 1.Akkoyunlu, M., and E. Fikrig. 2000. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect. Immun. 68:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrunategui-Correa, V., L. Lenz, and H. S. Kim. 2004. CD1d-independent regulation of NKT cell migration and cytokine production upon Listeria monocytogenes infection. Cell. Immunol. 232:38-48. [DOI] [PubMed] [Google Scholar]
- 3.Assarsson, E., T. Kambayashi, J. K. Sandberg, S. Hong, M. Taniguchi, L. Van Kaer, H.-G. Ljunggren, and B. J. Chambers. 2000. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J. Immunol. 165:3673-3679. [DOI] [PubMed] [Google Scholar]
- 4.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199-205. [PubMed] [Google Scholar]
- 5.Berntman, E., J. Rolf, C. Johansson, P. Anderson, and S. L. Cardell. 2005. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur. J. Immunol. 35:2100-2109. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A. 1997. Natural killer cell regulation during viral infection. Biochem. Soc. Trans. 25:687-690. [DOI] [PubMed] [Google Scholar]
- 7.Bunnell, J. E., E. R. Trigiani, S. R. Srinivas, and J. S. Dumler. 1999. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J. Infect. Dis. 180:546-550. [DOI] [PubMed] [Google Scholar]
- 8.Carnaud, C., D. Lee, O. Donnars, S.-H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647-4650. [PubMed] [Google Scholar]
- 9.Chalifour, A., P. Jeannin, J. F. Gauchat, A. Blaecke, M. Malissard, T. N′Guyen, N. Thieblemont, and Y. Delneste. 2004. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 104:1778-1783. [DOI] [PubMed] [Google Scholar]
- 10.Choi, K. S., D. G. Scorpio, N. C. Barat, and J. S. Dumler. 2007. Msp2 variation in Anaplasma phagocytophilum in vivo does not stimulate T cell immune responses or interferon-gamma production. FEMS Immunol. Med. Microbiol. 49:374-386. [DOI] [PubMed] [Google Scholar]
- 11.Choi, K. S., D. G. Scorpio, and J. S. Dumler. 2004. Anaplasma phagocytophilum ligation to Toll-like receptor (TLR) 2, but not to TLR4, activates macrophages for nuclear factor-kappa B nuclear translocation. J. Infect. Dis. 189:1921-1925. [DOI] [PubMed] [Google Scholar]
- 12.Crowe, N. Y., A. P. Uldrich, K. Kyparissoudis, K. J. Hammond, Y. Hayakawa, S. Sidobre, R. Keating, M. Kronenberg, M. J. Smyth, and D. I. Godfrey. 2003. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J. Immunol. 171:4020-4027. [DOI] [PubMed] [Google Scholar]
- 13.Dumler, J. S., K. S. Choi, J. C. Garcia-Garcia, N. S. Barat, D. G. Scorpio, J. W. Garyu, D. J. Grab, and J. S. Bakken. 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11:1828-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumler, J. S., E. R. Trigiani, J. S. Bakken, M. E. Aguero-Rosenfeld, and G. P. Wormser. 2000. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin. Diagn. Lab Immunol. 7:6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberl, G., and H. R. MacDonald. 1998. Rapid death and regeneration of NKT cells in anti-CD3epsilon- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity 9:345-353. [DOI] [PubMed] [Google Scholar]
- 16.Emoto, M., and S. H. Kaufmann. 2003. Liver NKT cells: an account of heterogeneity. Trends Immunol. 24:364-369. [DOI] [PubMed] [Google Scholar]
- 17.Greig, B., K. M. Asanovich, P. J. Armstrong, and J. S. Dumler. 1996. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J. Clin. Microbiol. 34:44-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada, M., K. Seino, H. Wakao, S. Sakata, Y. Ishizuka, T. Ito, S. Kojo, T. Nakayama, and M. Taniguchi. 2004. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int. Immunol. 16:241-247. [DOI] [PubMed] [Google Scholar]
- 19.Hodzic, E., J. W. Ijdo, S. Feng, P. Katavolos, W. Sun, C. H. Maretzki, D. Fish, E. Fikrig, S. R. Telford III, and S. W. Barthold. 1998. Granulocytic ehrlichiosis in the laboratory mouse. J. Infect. Dis. 177:737-745. [DOI] [PubMed] [Google Scholar]
- 20.Kinjo, Y., E. Tupin, D. Wu, M. Fujio, R. Garcia-Navarro, M. R. Benhnia, D. M. Zajonc, G. Ben-Menachem, G. D. Ainge, G. F. Painter, A. Khurana, K. Hoebe, S. M. Behar, B. Beutler, I. A. Wilson, M. Tsuji, T. J. Sellati, C. H. Wong, and M. Kronenberg. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7:978-986. [DOI] [PubMed] [Google Scholar]
- 21.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225-274. [DOI] [PubMed] [Google Scholar]
- 22.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29-37. [DOI] [PubMed] [Google Scholar]
- 23.Martin, M. E., J. E. Bunnell, and J. S. Dumler. 2000. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J. Infect. Dis. 181:374-378. [DOI] [PubMed] [Google Scholar]
- 24.Martin, M. E., K. Caspersen, and J. S. Dumler. 2001. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 158:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattner, J., K. L. Debord, N. Ismail, R. D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, D. Walker, B. Beutler, L. Teyton, P. B. Savage, and A. Bendelac. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525-529. [DOI] [PubMed] [Google Scholar]
- 26.Pusterla, N., J. E. Madigan, K. M. Asanovich, J. S. Chae, E. Derock, C. M. Leutenegger, J. B. Pusterla, H. Lutz, and J. S. Dumler. 2000. Experimental inoculation with human granulocytic Ehrlichia agent derived from high- and low-passage cell culture in horses. J. Clin. Microbiol. 38:1276-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharton, T. M., and P. Scott. 1993. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scorpio, D. G., M. Akkoyunlu, E. Fikrig, and J. S. Dumler. 2004. CXCR2 blockade influences Anaplasma phagocytophilum propagation but not histopathology in the mouse model of human granulocytic anaplasmosis. Clin. Diagn. Lab Immunol. 11:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scorpio, D. G., F. D. von Loewenich, H. Gobel, C. Bogdan, and J. S. Dumler. 2006. Innate immune response to Anaplasma phagocytophilum contributes to hepatic injury. Clin. Vaccine Immunol. 13:806-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu, H., T. Matsuguchi, Y. Fukuda, I. Nakano, T. Hayakawa, O. Takeuchi, S. Akira, M. Umemura, T. Suda, and Y. Yoshikai. 2002. Toll-like receptor 2 contributes to liver injury by Salmonella infection through Fas ligand expression on NKT cells in mice. Gastroenterology 123:1265-1277. [DOI] [PubMed] [Google Scholar]
- 31.von Loewenich, F. D., D. G. Scorpio, U. Reischl, J. S. Dumler, and C. Bogdan. 2004. Frontline: control of Anaplasma phagocytophilum, an obligate intracellular pathogen, in the absence of inducible nitric oxide synthase, phagocyte NADPH oxidase, tumor necrosis factor, Toll-like receptor (TLR) 2 and TLR4, or the TLR adaptor molecule MyD88. Eur. J. Immunol. 34:1789-1797. [DOI] [PubMed] [Google Scholar]
- 32.Wang, T., M. Akkoyunlu, R. Banerjee, and E. Fikrig. 2004. Interferon-gamma deficiency reveals that 129Sv mice are inherently more susceptible to Anaplasma phagocytophilum than C57BL/6 mice. FEMS Immunol. Med. Microbiol. 42:299-305. [DOI] [PubMed] [Google Scholar]
- 33.Way, S. S., T. R. Kollmann, A. M. Hajjar, and C. B. Wilson. 2003. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J. Immunol. 171:533-537. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, M. T., C. Johansson, D. Olivares-Villagomez, A. K. Singh, A. K. Stanic, C. R. Wang, S. Joyce, M. J. Wick, and L. Van Kaer. 2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. USA 100:10913-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winslow, G. M., and C. Bitsaktsis. 2005. Immunity to the ehrlichiae: new tools and recent developments. Curr. Opin. Infect. Dis. 18:217-221. [DOI] [PubMed] [Google Scholar]
- 36.Wong, S., and L. J. Grady. 1996. Ehrlichia infection as a cause of severe respiratory distress. N. Engl. J. Med. 334:273. [DOI] [PubMed] [Google Scholar]