Abstract
Prior exposure of a vaccinee to certain species of environmental mycobacteria can prime the immune system against common mycobacterial antigens, which can in turn reduce the subsequent efficacy of live attenuated mycobacterial vaccines (such as Mycobacterium bovis BCG), in both human and livestock vaccination programs. In this study, two strains of Mycobacterium avium, both isolated from New Zealand livestock, were investigated to determine their growth characteristics and effects on the immune system in murine models. Markedly different effects on the immune system were observed; an IS901-negative strain (WAg 207) induced significant up-regulation of cell surface activation markers (major histocompatibility complex II, CD80, and CD86) on in vitro-derived dendritic cells and induced the release of proinflammatory monokines (interleukin-1β [IL-1β], IL-6, and tumor necrosis factor alpha) in dendritic cell-macrophage cocultures following direct in vitro contact of cells with bacteria. In contrast, an IS901-positive strain (WAg 206) had none of these effects. When mice were exposed to M. avium via oral infection prior to BCG parenteral immunization, both strains were shown to be capable of decreasing subsequent antigen-stimulated gamma interferon secretion by splenic lymphocytes, although this effect was more significant for strain WAg 206. Both strains also induced a mycobacterial antigen-specific serological response in M. avium-sensitized and BCG-immunized mice; this response was greater in WAg 206-sensitized mice, and there was a predominance of immunoglobulin G1 antibody. The down-regulation of IFN-γ responses and the up-regulation of antibody responses are characteristic of a switch to a type 2 immune response. The different results may be linked to the inherent growth characteristics of the two strains, since WAg 206 was shown to grow slowly in murine macrophages in vitro and to cause a persistent systemic infection following infection in vivo, while WAg 207 grew fast and did not persist in mice. The implications of these findings for BCG vaccination protocols are discussed.
Members of the Mycobacterium avium-Mycobacterium intracellulare complex are ubiquitous in the environment, and they are a major confounding influence on the specificity of intradermal skin tests used to detect infection with Mycobacterium tuberculosis in humans or infection with Mycobacterium bovis in farmed livestock. Crude antigens, in the form of purified protein derivative (PPD) from cultures of M. tuberculosis or M. bovis, are used as restimulation antigens in in vivo intradermal skin tests, as well as in ex vivo blood-based diagnostic assays, to identify tuberculosis infections. False-positive results are common, and it is believed that these results are due to cross-reactive immune responses induced by transient exposure to, or infection by, members of the M. avium-M. intracellulare complex (1, 21).
In 2002 Buddle et al. reported that presensitization of cattle with M. avium was associated with poor vaccine responses to M. bovis bacillus Calmette-Guerin (BCG) (4). After this, an experimental study investigating the potentially deleterious effects of different field isolates of M. avium on the protective efficacy of BCG vaccination was performed using a guinea pig model (6). In this study the workers identified one strain of M. avium (designated WAg 206) which could suppress the protective effect of BCG vaccination against a virulent M. bovis challenge infection (6). The immune mechanisms which underlie these findings have not been investigated yet. Neither cattle nor guinea pigs lend themselves well to in-depth studies of the immune response. In order to elucidate the mechanisms involved, we used a mouse model to study the effects of two M. avium strains (WAg 206 and WAg 207) on immune functions both in vitro and in vivo (6).
The cells of the mononuclear phagocyte (MNP) system (comprising monocyte-derived macrophages and dendritic cells [DC]) are the first point of contact between the immune system and M. avium-M. intracellulare complex species. Mycobacteria are phagocytosed and placed into a phagolysosome, where they can be destroyed if the MNP system is properly activated, or they may persist within the host cell for prolonged periods depending on the virulence of the strain. A number of mycobacterial cell wall components have been identified as components that are important in the induction of inflammatory signals from MNP (20). Signaling through Toll-like-receptor 2 appears to be essential for induction of costimulatory molecules and for secretion of cytokines which are essential for the MNP to function as antigen-presenting cells (15, 20). Appropriate activation of antigen-presenting cells, accompanied by the processing and presentation of mycobacterial antigen on major histocompatibility complex II (MHC II) molecules, results in generation of a protective, type 1 immune response. This is not always the case, however, and inappropriate responses driven by genetic factors and environmental influences can compromise the response to BCG vaccination and thereby the protection that it is able to provide against tuberculosis.
It is therefore conceivable that prior exposure to or infection with M. avium-M. intracellulare complex species could influence subsequent immune responses to vaccines against tuberculosis, such as BCG. One mechanism that has been proposed is that this prior sensitization to mycobacterial antigens may lead to rapid elimination of BCG before it can generate a protective immune response (3). Another possibility is that early exposure to cross-reactive mycobacteria may imprint an inappropriate, type 2 response on the immune system which negatively influences subsequent responses to BCG vaccination. Data suggesting that the early expression of interleukin-4 (IL-4) in response to BCG vaccination is associated with poor protective efficacy (11, 16) support this hypothesis.
In the present study, we examined the response following direct in vitro exposure of components of the immune system to different strains of M. avium, focusing on defined MNP cell types that are likely to first encounter mycobacteria during infection (namely, DC and macrophages). In addition, we investigated patterns of antigen-specific antimycobacterial immune reactivity in BCG-immunized mice in vivo and tried to determine how these responses may be modulated by prior exposure of the mice to purported cross-sensitizing strains of M. avium.
MATERIALS AND METHODS
Sources, preparation, and use of M. bovis BCG and M. avium WAg 206 and WAg 207.
M. avium strains WAg 206 (an IS901-positive strain) and WAg 207 (IS901 negative) were grown in Middlebrook 7H9 broth supplemented with 1% glycerol and 10% oleic acid-albumin-dextrose-catalase. The strains were grown until the mid-log phase and then harvested and frozen in 1-ml aliquots. Numbers of CFU were determined by plating aliquots onto Middlebrook 7H11 agar plates and counting the colonies after 4 weeks. M. bovis BCG (Pasteur strain 1172) was cultured in Middlebrook 7H9 broth, and organisms were allowed to grow to the mid-log phase before they were harvested and subsequently used for mouse immunization.
Mice, infection with M. avium, and immunization with BCG.
Specific-pathogen-free BALB/c mice that were 6 to 10 weeks old were obtained from the Department of Laboratory Animal Sciences, University of Otago, Otago, New Zealand.
For infection with M. avium, groups of six mice were inoculated orally with 108 or 106 CFU of either WAg 206 or WAg 207 using a dropper pipette containing bacteria in broth medium. In order to determine the persistence of M. avium in mice at the systemic level, splenic homogenates were prepared from euthanized animals 8 weeks postinfection and plated onto Middlebrook 7H11 agar for enumeration of bacteria.
In some experiments, groups of six mice were immunized parenterally with M. bovis BCG at various times following M. avium oral infection to investigate the effect of M. avium presensitization on the subsequent pattern of immunological reactivity in vivo. In this analysis, the mice received 106 CFU of BCG via subcutaneous injection into the nape of the neck 8, 16, or 24 weeks following the initial M. avium infection.
Preparation of cells for in vitro study. (i) Generation of bone marrow-derived dendritic cells (BMDC) and isolation of peritoneal macrophages.
Bone marrow was obtained from the hind legs of BALB/c mice. Red blood cells were removed by ammonium chloride lysis, and the remaining cells were cultured in Dulbecco modified Eagle medium (DMEM) (Invitrogen Ltd., Auckland, New Zealand) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Invitrogen Ltd.) and 20 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (obtained from Escherichia coli using the pET32a expression system [Otago University, Otago, New Zealand]). On day 4, cells were fed with fresh DMEM supplemented with 10% FCS and 20 ng/ml recombinant granulocyte-macrophage colony-stimulating factor. DC were harvested on day 6 and resuspended at a concentration of 106 cells/ml (final purity of DC, >85% CD11c positive, as determined by flow cytometric analysis). To isolate macrophages, mice were inoculated with 1 ml of proteose peptone broth (Difco Ltd., Fort Richards, MD) intraperitoneally. Three days later mice were sacrificed, and the peritoneum was washed twice with 3 ml of sterile phosphate-buffered saline. Peritoneal cells were harvested by centrifugation (300 × g for 5 min) and were resuspended in DMEM supplemented with 2% FCS. Cells were plated in 24-well plates, and macrophages were allowed to adhere for 2 h. Nonadherent cells were washed off with medium, and the remaining macrophages were cultured in DMEM supplemented with 5% FCS.
(ii) Preparation of splenocytes.
Single-cell splenocyte suspensions were prepared in DMEM supplemented with 5% heat-inactivated FCS and 50 μM β-mercaptoethanol. Splenocytes were washed by centrifugation in DMEM, lymphocytes were counted, the concentration of lymphocytes was adjusted to 2 × 106 cells/ml, and then the cells were plated in 24-well plates (Nunc, Roskilde, Denmark). Samples were cultured in DMEM (Gibco BRL, United States) as a control, with 50 μg/ml of M. bovis PPD (PPD-B) (CSL, Parkville, Australia), or with 12 μg/ml concanavalin A (Sigma, St. Louis, MO) as a positive control for 72 h.
In vitro coculture experiments. (i) BMDC cultures.
BMDC were pulsed for 24 h with the two strains of M. avium. In brief, 106 DC were plated in 24-well plates in 1 ml of DMEM containing 10% FCS. Live bacteria were added at a multiplicity of infection (MOI) of 5 bacteria/DC; 24 h later, DC were harvested, and surface marker expression was assessed by flow cytometric analysis. The positive control for this experiment comprised cells stimulated by addition of 1 μg/ml bacterial lipopolysaccharide (LPS) (Sigma).
(ii) Macrophage-DC cocultures and macrophage infection experiments.
Our previous research showed that macrophages are efficient processing cells for whole mycobacteria (10) and can induce potent cytokine production if macrophage-processed mycobacterial components are subsequently transferred to DC. To prepare this coculture system, peritoneal macrophages were first isolated as described above and pulsed for 24 h with the two strains of M. avium (at an MOI of 5 bacteria/macrophage); 24 h later, 106 DC were added, and the coculture was incubated for a further 24 h. Supernatants were subsequently removed for cytokine detection. The positive control for this experiment comprised cells stimulated by addition of 1 μg/ml LPS plus 5 ng/ml recombinant murine gamma interferon (IFN-γ) (R&D Systems, Pharmaco, Auckland, New Zealand).
To measure the intracellular survival and growth of M. avium, 5 × 104 macrophages were seeded into wells of 96-well tissue culture plates in DMEM containing 10% FCS, allowed to adhere, and then infected with live M. avium WAg 206 or WAg 207 at an MOI of 1 bacterial cell/macrophage. Extracellular bacteria were removed by washing the cells three times in medium 2 h after bacteria were pulsed onto the macrophages. The viability of the macrophages was assessed throughout the culture period by trypan blue exclusion (macrophages exhibited good viability for up to 4 to 7 days postinfection, but the viability declined during further culture and cells died between days 7 and 10; for this reason only periods that were up to 4 days long were analyzed in this experiment).
After various times (up to 4 days), intracellular bacterial growth was assessed by a uracil incorporation assay and/or by enumerating viable bacilli via plating. For uracil incorporation, macrophage cultures were pulsed for the final 24 h of the designated culture period with 50 μl of [5,6-3H]uracil (5 μCi; Amersham Bioscience); the cells were subsequently lysed in situ by addition of 0.8% saponin in water, the lysates were harvested, and the uracil incorporation was assessed by measuring beta emission (expressed in cpm). For plate counting, cells were lysed in situ by addition of 0.8% saponin 48 h after infection, and the lysates were plated onto Middlebrook agar for enumeration of the surviving, intracellular bacteria.
Measurement of immunological responses. (i) Cytokine detection and flow cytometric analysis.
Cell-free tissue culture supernatants were assessed to determine the presence of the inflammatory cytokines IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) (macrophage-DC cocultures) or IFN-γ (PPD-B-stimulated splenocyte cultures) by using a multiplex bead-based assay (Bio-Rad, California). The assays were performed according to the manufacturer's instructions. In brief, bead sets containing different amounts of a fluorescent dye were added to 96-well flat-bottomed filter plates. Each bead set had antibodies specific for different cytokines on the surface. Plates were washed, and standards and supernatants were added. The samples were then incubated for 30 min and washed, and a secondary antibody cocktail was added to the plates, which were incubated for a further 30 min. The plates were washed, and a 1/100 dilution of streptavidin/phycoerythrin (Bio-Rad) was added to each well. The plates were incubated for a further 10 min at room temperature before they were washed again and 100 μl/well of an assay buffer was added. Each plate was placed in a BioPlex reader (Bio-Rad, Australia) that determined the fluorescence produced by each bead. The fluorescence was directly proportional to the amount of cytokine bound to a bead. The amount of each cytokine in the supernatant was extrapolated by using standard curves obtained by using serial dilutions of known amounts of recombinant murine cytokines run at the same time as the samples. The concentrations of the standards ranged from 50,000 to 2 pg/ml.
For flow cytometric analysis of cell surface activation marker expression on DC, harvested DC were washed by centrifugation, and a cell pellet was resuspended and incubated for 30 min with 1 μg of one of the following anti-mouse monoclonal antibodies: MHC II, CD80, and CD86 (BD Pharmingen). All antibodies were directly labeled with phycoerythrin. Cells were washed, and the surface staining was analyzed using a FACSCalibur flow cytometer (BD Biosciences).
(ii) Serum antibody detection.
Serum was collected from the various groups of mice (at necropsy). Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with 50 μg/ml of PPD-B (CSL, Parkville, Australia). The plates were washed and blocked, and serial dilutions of serum samples and standards were added. The plates were then incubated overnight at 4°C. Goat anti-mouse immunoglobulin G1 (IgG1) and IgG2a antibodies (Sigma, St. Louis, MO) were added at recommended dilutions, and the plates were incubated for 1 h at room temperature. Peroxidase-labeled anti-goat monoclonal antibody (Serotec, Oxford, United Kingdom) was added to each well, and the plates were incubated for a further 45 min before addition of the 3,3′,5,5-tetramethylbenzidine substrate (Bio-Rad, United States). The reaction was stopped by addition of 1 N H2SO4, and the optical density at 450 nm was determined. The standards for this assay comprised serial dilutions of known amounts of myeloma-expressed murine IgG1 or IgG2a antibodies that were applied directly to ELISA plates (in the absence of PPD-B) and probed directly with goat antibodies.
Statistical analysis.
Immune response data for treatment groups were compared by one-way analysis of variance, employing Tukey's posthoc tests to identify significance when there were three or more group treatment variables. In vivo infection and bacterial persistence data for WAg 206- and WAg 207-infected mice were compared using Fisher's exact chi-square test to compare the proportions of animals in which either strain persisted and using a Mann-Whitney U test to compare the median splenic bacterial burdens in the two groups.
RESULTS
Modulation of accessory cell function in vitro by M. avium.
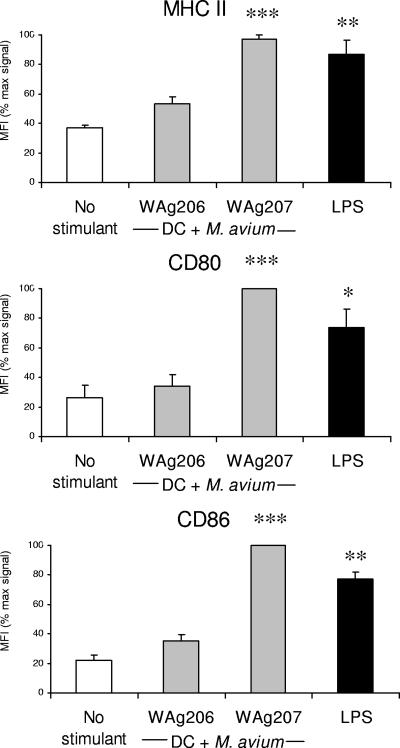
The potential of M. avium strains WAg 206 and WAg 207 to modulate murine accessory cell function was investigated by coculturing live bacilli in the presence of single or dual cell populations. Cell surface expression of antigen-presenting complex (MHC II) or costimulation (CD80, CD86) molecules on BMDC was not affected by the presence of M. avium strain WAg 206 (Fig. 1). In contrast, the mean levels of expression of these markers increased significantly in response to coculture with WAg 207 (Fig. 1), and the level was at least equal to the level observed when a standard DC-activating stimulus (LPS) was used.
FIG. 1.
Modulation of cell surface activation marker expression on BMDC following exposure to different strains of M. avium. BMDC were pulsed for 24 h with M. avium WAg 206 or WAg 207 prior to assessment of surface activation marker expression by flow cytometry. The bars indicate the average mean fluorescence intensity (MFI) for each marker, and the error bars indicate the standard errors of the means for three replicate experiments (data were normalized as percentages of the maximal mean fluorescence intensity signal in each case). The asterisks indicate that there was significant activation compared to the results obtained with nonstimulated (control) DC, as follows: two asterisks, P < 0.01; and three asterisks, P < 0.001. DC activated by 1 μg/ml LPS were used as positive controls.
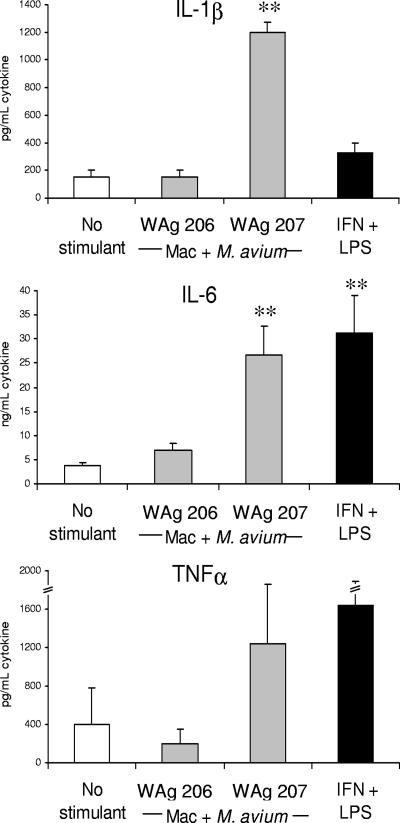
M. avium strain WAg 207 was also shown to significantly enhance monokine production following coculture with murine macrophages and DC in dual-cell systems (Fig. 2). Significant increases in IL-1β and IL-6 levels were observed after exposure of macrophage-DC cocultures to WAg 207, and the levels were equal to or greater than the levels observed following exposure of macrophage-DC cocultures to a standard monokine-inducing stimulus (LPS plus IFN-γ). The levels of TNF-α were also increased in cocultures exposed to WAg 207, although the effect was not statistically significant (Fig. 2). Exposure of macrophage-DC cocultures to WAg 206 did not up-regulate proinflammatory monokine production (Fig. 2).
FIG. 2.
Modulation of cytokine production by murine macrophage-DC cocultures following exposure to different strains of M. avium. Elicited peritoneal macrophages were infected with M. avium WAg 206 or WAg 207 and maintained in culture for 24 h. BMDC were then added and cocultured for a further 24 h prior to collection of cell-free supernatants for cytokine analysis. The bars indicate the mean levels of each cytokine, and the error bars indicate the standard errors of the means for three replicate experiments. The asterisks indicate that there was significant production of cytokine by cells compared to the production in nonstimulated controls, as follows: one asterisk, P < 0.05; two asterisks, P < 0.01. Macrophages activated by 1 μg/ml LPS plus 5 ng/ml IFN-γ were used as positive controls. Mac, macrophages.
Ability of M. avium strains to modulate mycobacterial antigen-specific immune responses in BCG-immunized mice following oral presensitization with M. avium.
In order to determine whether in vivo presensitization of mice to different M. avium strains could modulate the subsequent patterns of immune reactivity to mycobacterial antigens, mice were infected via the oral route with WAg 206 or WAg 207 and maintained for 8, 16, or 24 weeks before they were immunized parenterally with 106 CFU BCG. The subsequent specific immune reactivity in these mice was assessed ex vivo by monitoring the serological reactivity to mycobacterial antigens and by determining the ability of PPD-stimulated splenocytes to produce IFN-γ. Antigen-specific levels of IL-4 and IL-5 were also assessed, but these molecules were not detectable (data not shown).
As shown in Fig. 3, for mice that were immunized with BCG measurable levels of IFN-γ were observed upon ex vivo stimulation of their splenocytes with PPD-B. Suppression of IFN-γ production was observed for mice that had been presensitized to either WAg 206 or WAg 207 (Fig. 3); the degree of suppression was first apparent in mice that had been presensitized for 16 weeks (prior to BCG immunization). In mice maintained for 24 weeks prior to BCG immunization, the degree of suppression induced by M. avium was statistically significant (Fig. 3); when the IFN-γ responses of the two M. avium-presensitized groups were compared, it was evident that the IFN-γ levels were significantly lower in the mice presensitized to strain WAg 206 than in the mice presensitized to WAg 207 (Fig. 3).
FIG. 3.
Effect of oral presensitization of mice to different strains of M. avium prior to parenteral BCG immunization on the subsequent mycobacterial antigen-specific cellular immune responsiveness. BALB/c mice were presensitized to M. avium strain WAg 206 or WAg 207 by oral inoculation of 106 CFU/mouse; control mice were presensitized with only phosphate-buffered saline (PBS). At 8, 16, or 24 weeks following sensitization, all mice were immunized by subcutaneous injection of 106 CFU of BCG/mouse. Four weeks postimmunization, mice were euthanized, and single-cell splenocyte suspensions were prepared for analysis of in vitro IFN-γ production. The bars indicate the levels of IFN-γ in culture supernatants following in vitro restimulation of splenic lymphocytes with PPD-B, and the error bars indicate the standard errors of the means for three mice/group. Two asterisks indicate that the responses in M. avium-presensitized mice were significantly different than the responses in phosphate-buffered saline-presensitized mice (P < 0.01). In addition, there was a significant difference between WAg206-treated mice and WAg207-treated mice at 24 weeks (P < 0.05). The IFN-γ responses in control mice that did not receive BCG were <40 pg/ml (data not shown).
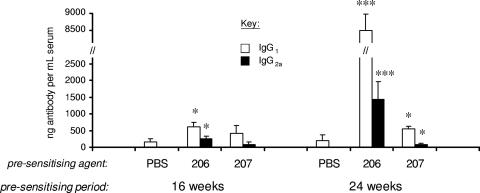
In contrast to IFN-γ production, mice that were immunized with BCG did not produce measurable levels of antigen-specific IgG antibodies, unless they had received some form of presensitization to M. avium (Fig. 4). Compared to control (nonpresensitized mice), mice presensitized to WAg 207 for 24 weeks (prior to BCG immunization) produced significantly elevated levels of PPD-B-specific antibody, while in mice presensitized to WAg 206 a significantly elevated antibody response was evident whether they had been presensitized for 16 or 24 weeks prior to BCG immunization (Fig. 4). When the antibody levels in groups of M. avium-presensitized mice were compared, both the IgG1 and IgG2a levels were significantly higher in WAg 206-presensitized mice than in WAg 207-presensitized mice at 24 weeks (P < 0.001). In all cases, the predominant IgG response comprised antibodies belonging to the IgG1 subclass. For mice that had been presensitized to M. avium (prior to BCG immunization), the mean ratios of IgG1 to IgG2a in WAg 206-presensitized mice were 4.8:1 and 17.5:1 for the 16- and 24-week experiments, respectively; this compares to ratios of IgG1 to IgG2a in WAg 207-presensitized mice of 1.5:1 and 9.5:1, respectively, for the same times. In naïve animals (animals not exposed to Mycobacterium), no Mycobacterium-specific antibodies were detected.
FIG. 4.
Effect of oral presensitization of mice to different strains of M. avium prior to parenteral BCG immunization on the subsequent mycobacterial antigen-specific antibody response. BALB/c mice were presensitized to M. avium strain WAg 206 or WAg 207 by oral inoculation of 106 CFU/mouse; control mice were presensitized with only phosphate-buffered saline (PBS). At 16 or 24 weeks following sensitization, all mice were immunized by subcutaneous injection of 106 CFU of BCG/mouse. Four weeks postimmunization, mice were euthanized, and sera were obtained for an antibody ELISA. The bars indicate the mean levels of PPD-B-specific IgG1 and IgG2a antibodies, and the error bars indicate the standard errors of the means for seven mice/group. The asterisks indicate that the responses in M. avium-presensitized mice were significantly different than the response in the control phosphate-buffered saline-presensitized mice, as follows: one asterisk, P < 0.05; three asterisks, P < 0.001. The antibody responses in control mice that did not receive BCG were below the level of detection (data not shown).
In vitro and in vivo M. avium persistence and growth characteristics.
After 24 to 48 h of infection of macrophages in vitro, the growth rates of intracellular M. avium strains WAg 206 and WAg 207 were similar (Fig. 5a). However, from day 3 to day 4 postinfection, WAg 207 bacteria grew rapidly, whereas WAg 206 bacteria replicated more steadily over the same period (the fact that intracellular WAg 206 was replicating, albeit slowly, was indicated by the finding that the mean level of uracil incorporation was significantly higher at 96 h than at 24 h [P = 0.001]). When the growth characteristics of the two strains were compared statistically after 48 h, it was evident that the intracellular numbers of M. avium strain WAg 207 cells were significantly greater than those of WAg 206 cells (Fig. 5b), although live bacilli of both strains were present.
FIG. 5.
Intracellular growth characteristics of M. avium strains WAg 206 and WAg 207. Murine peritoneal macrophages were seeded into tissue culture plates (5 × 104 cells/well) and infected with M. avium strain WAg 206 or WAg 207 at an MOI of 1:1. The intracellular growth of M. avium was determined by a uracil incorporation assay or by counting mycobacterial colonies on Middlebrook 7H11 agar. The graphs show differences between the growth characteristics of the two strains. (a) Growth kinetics for 4 days of culture (mean cpm following uracil incorporation). The error bars indicate the standard errors of the means for three replicate experiments per time. (b) Comparison of the numbers of CFU per culture sample at 2 days. The bars indicate the means, and the error bars indicate the standard errors of the means for six replicates per time. One asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001.
In vivo oral route infection studies showed that M. avium WAg 206 persisted in the spleens of 7 of 10 mice at 8 weeks after infection, compared to the persistence of M. avium WAg 207 in 1 of 10 mice (Table 1). Both the proportion of mice in which WAg 206 was persistent and the median splenic bacterial burden were significantly higher in WAg 206-infected mice than in WAg 207-infected animals (Table 1).
TABLE 1.
Summary of incidence and burden of M. avium systemic infections in orally infected micea
| Strain | Dose | No. of mice culture positive/no. tested | Median M. avium burden (CFU) (range) |
|---|---|---|---|
| WAg 206 | 106 | 3/5 | 8 × 104 (0-240,000) |
| 108 | 4/5 | 2.4 × 105 (0-3,520,000) | |
| Combined | 7/10b | 2 × 104c | |
| WAg 207 | 106 | 0/5 | 0 |
| 108 | 1/5 | 0 (0-1,360,000) | |
| Combined | 1/10 | 0 |
Groups of 10 mice were infected orally with M. avium strain WAg 206 or WAg 207 using an inoculum containing 106 CFU (five mice/strain) or 108 CFU (five mice/strain).
There was a significantly higher proportion of culture-positive spleens in WAg 206-infected mice than in WAg 207-infected mice (P < 0.05).
There was a significantly higher median M. avium burden in the spleens of WAg 206-infected mice than in the spleens of WAg 207-infected mice (P < 0.05).
DISCUSSION
The presence of an IS901 insertion element is considered to be a potential virulence marker in M. avium (12, 13), although this may be true only for infection in avian species (17). In the current study, both test strains of M. avium (WAg 206, an IS901-positive isolate, and WAg 207, an IS901-negative isolate) were isolated from New Zealand farmed livestock (6), and hence both strains have the capacity to initiate infection in ruminants. Furthermore, in oral route infection studies with guinea pigs, both of these strains have been shown to persist in gastrointestinal tract lymphatic tissues, while only WAg 206 was able to disseminate to somatic lymphatic tissues (6), suggesting that strain WAg 206 is more virulent than strain WAg 207. Here, we showed that the ways in which these two strains interact with mononuclear leukocytes differ markedly, which may provide some insight into processes underlying differences in bacterial persistence. Strain WAg 207 induced activation of murine DC (as shown by significant up-regulation of cell surface accessory molecules) and also stimulated monokine secretion in DC-macrophage cocultures; in contrast, WAg 206 had none of these effects. In previous work workers have found that M. avium activates mononuclear cells via ligation of Toll-like-receptor 2 (19), and the resulting cytokine patterns have been reported to be different depending on the bacterial strain (2). Our data added to this paradigm by showing that both cell surface activation marker expression and cytokine secretion are under strain-dependent regulatory control and that different effects of different strains of M. avium may initiate different patterns of immune reactivity in a host in vivo, which in turn influence the outcome of an infection process.
Coculture experiments in which macrophages and DC were combined were performed in the present study, based on previous observations in our laboratory that while macrophages are the primary targets of infection, it is the DC which initiate the subsequent T-cell responses (requiring that there is an interaction between these two types of cells and that antigen is passed from infected macrophages to DC [10]). In this regard, and since MNP are the components of the immune surveillance system most likely to first encounter infecting mycobacteria, the immunological results obtained here provide some clues regarding the different patterns of bacterial persistence observed in rodents. In the present study, WAg 207 was shown to replicate rapidly within murine macrophages in vitro, whereas strain WAg 206 was shown to replicate more slowly in the intracellular environment. Of the monokines activated by WAg 207 (but not by WAg 206), TNF-α in particular is known to be induced strongly in MNP following contact with avirulent nonpersistent mycobacteria but to be produced only at a low level by cells contacting virulent persistent mycobacteria (8). Since the production of autocrine- and paracrine-signaling monokines is one means by which phagocytes initiate an inflammatory response to control intracellular mycobacterial infection (9), the inability of WAg 206 to induce cytokine production may explain its persistence in vitro in the intracellular environment. Furthermore, in in vivo studies, strain WAg 206 was shown to persist in the reticuloendothelial tissues of mice following oral infection, while strain WAg 207 did not persist (except in 1 of 10 mice exposed to a very high dose, 108 CFU). These data extend observations made in a previous study of mice (3), which showed that certain M. avium isolates (of several strains of environmental mycobacteria tested) were able to persist in vivo following experimental parenteral injection (oral administration of M. avium was not reported in this previous study). Thus, in the present study, combined in vitro and in vivo data indicate that the IS901-negative strain WAg 207 initially infects MNP but in doing so activates host cellular reactivity, which probably contributes to its limited persistence in vivo; in contrast, the IS901-positive strain WAg 206 replicates more slowly following intracellular infection but does not activate sentinel cells, which in turn probably contributes to its dissemination to (and persistence in) the systemic lymphatic system in vivo.
Previous work in our laboratory has shown that oral exposure of mice to killed M. avium WAg 206 or WAg 207 does not induce a significant in vitro or in vivo immune response (data not shown). Therefore, it is unlikely that nonviable M. avium affects BCG vaccination, and for this reason in our studies we focused on testing live M. avium bacilli. However, despite the apparent ability of live strain WAg 206 to evade immune surveillance sufficiently to persist in the murine host in vivo, it is evident that this strain does invoke a degree of immune recognition, as shown by the development of a pronounced ability to modulate immune reactivity in response to BCG vaccination. This confirms observations made in a previous study, in which we found that live WAg 206 (but not WAg 207) could compromise BCG vaccine-mediated protection against virulent M. bovis aerosol challenge in guinea pigs (6). Both M. avium strains were capable of suppressing antigen-stimulated IFN-γ production in BCG-immunized mice and of inducing a de novo IgG antibody response to M. bovis antigens, although these responses were more pronounced in mice presensitized to WAg 206. Since antigen-induced production of IFN-γ following immunization is considered an essential component of BCG vaccine-mediated protection (14), the implication is that the M. avium strains tested (particularly strain WAg 206) are able to suppress vaccine-mediated protection by compromising the magnitude of the effector immune response. Previous studies (3, 7) have shown that several strains of environmental mycobacteria are capable of cross-sensitizing mice sufficiently to diminish the subsequent IFN-γ response to BCG immunization, and this correlates with diminished protective capacity of the vaccine against aerosol challenge with M. tuberculosis. However, in those studies the workers used mixtures of organisms and, unlike us, did not identify which individual strains had this potential.
The development of serological reactivity in hosts exposed to mycobacteria is usually an indication of progression of the immune response away from protective Th1-dominated cell-mediated immunity toward a Th2-dominated humoral response (18, 22). In the present study we did not detect any PPD-specific IL-4 or IL-5 protein; however, it was noteworthy that while M. avium strains WAg 206 and WAg 207 both promoted IgG antibody reactivity in BCG-immunized mice, the response in WAg 206-presensitized mice was significantly more biased toward a Th2-type pattern, dominated by IgG1 subclass reactivity. This reinforces the notion that WAg 206 in particular skews the mycobacterial antigen-specific immune response in BCG-immunized mice toward a Th2-dominated phenotype, which further supports previous data showing that this strain is capable of compromising BCG vaccine-mediated protective efficacy. Further studies are required to determine whether the predominance of Th2 reactivity in WAg 206-infected mice also correlates with disease progression and increased pathology, as has been observed in nonrodent animal studies (11).
The data presented here increase our understanding of how certain strains of environmental mycobacteria, in this case M. avium, might initiate infection in a host following oral exposure. They also indicate how these strains may interact with the immune system in vivo and how they may cross-sensitize a host sufficiently to compromise the efficacy of vaccination programs in which other species of live, attenuated mycobacteria are employed. It has been suggested that the failure of some BCG vaccination programs for humans (5) and livestock (4) may be attributable to prior cross-sensitization to environmental mycobacteria. Our results confirm this suggestion, and while they do not totally eliminate the possibility that a memory response prematurely eliminates BCG (3), we believe that our data demonstrate that this phenomenon could also be due to inappropriate imprinting of the immune system, promoting a nonprotective Th2-dominated phenotype. Additionally, we found that this effect is not likely to be found with all strains of M. avium but may instead be linked to differences in the abilities of the organisms to persist in the host, which are in turn related to their abilities to invoke or evade the host's immune surveillance system at the accessory cell level. The practical conclusion from this study is that the longer a given strain of environmental M. avium is able to persist in a host, the greater its potential to interfere with the protective immune responses that are desired when live attenuated mycobacterial vaccines are used.
Acknowledgments
This research was funded by the Foundation for Research Science and Technology (New Zealand) and Otago University. Sarah Young was supported by an HRC Health Sciences Fellowship (New Zealand).
We thank Frank Cross (Otago University) for his help with preparation of the manuscript.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Black, G. F., H. M. Dockrell, A. C. Crampin, S. Floyd, R. E. Weir, L. Bliss, L. Sichali, L. Mwaungulu, H. Kanyongoloka, B. Ngwira, D. K. Warndorff, and P. E. Fine. 2001. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J. Infect. Dis. 184:322-329. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal, A., J. Lauber, R. Hoffmann, M. Ernst, C. Keller, J. Buer, S. Ehlers, and N. Reiling. 2005. Common and unique gene expression signatures of human macrophages in response to four strains of Mycobacterium avium that differ in their growth and persistence characteristics. Infect. Immun. 73:3330-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, L., J. Feino Cunha, J. Weinreich, A. Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buddle, B. M., B. J. Wards, F. E. Aldwell, D. M. Collins, and G. W. de Lisle. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20:1126-1133. [DOI] [PubMed] [Google Scholar]
- 5.Chihota, V. N., N. Z. Nyazema, S. Mashingaidze, and B. Mutandiro. 1998. TB infection: an exploratory study of BCG protective properties and the possible role of environmental mycobacteria. Cent. Afr. J. Med. 44:145-148. [PubMed] [Google Scholar]
- 6.de Lisle, G. W., B. J. Wards, B. M. Buddle, and D. M. Collins. 2005. The efficacy of live tuberculosis vaccines after presensitization with Mycobacterium avium. Tuberculosis 85:73-79. [DOI] [PubMed] [Google Scholar]
- 7.Demangel, C., T. Garnier, I. Rosenkrands, and S. T. Cole. 2005. Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect. Immun. 73:2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falcone, V., E. B. Bassey, A. Toniolo, P. G. Conaldi, and F. M. Collins. 1994. Differential release of tumor necrosis factor-alpha from murine peritoneal macrophages stimulated with virulent and avirulent species of mycobacteria. FEMS Immunol. Med. Microbiol. 8:225-232. [DOI] [PubMed] [Google Scholar]
- 9.Flesch, I. E., and S. H. Kaufmann. 1993. Role of cytokines in tuberculosis. Immunobiology 189:316-339. [DOI] [PubMed] [Google Scholar]
- 10.Girvan, A., F. E. Aldwell, G. S. Buchan, L. Faulkner, and M. A. Baird. 2003. Transfer of macrophage-derived mycobacterial antigens to dendritic cells can induce naive T-cell activation. Scand. J. Immunol. 57:107-114. [DOI] [PubMed] [Google Scholar]
- 11.Hook, S., F. Griffin, C. Mackintosh, and G. Buchan. 1996. Activation of an interleukin-4 mRNA-producing population of peripheral blood mononuclear cells after infection with Mycobacterium bovis or vaccination with killed, but not live, BCG. Immunology 88:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunze, Z. M., S. Wall, R. Appelberg, M. T. Silva, F. Portaels, and J. J. McFadden. 1991. IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Mol. Microbiol. 5:2265-2272. [DOI] [PubMed] [Google Scholar]
- 13.Kunze, Z. M., F. Portaels, and J. J. McFadden. 1992. Biologically distinct subtypes of Mycobacterium avium differ in possession of insertion sequence IS901. J. Clin. Microbiol. 30:2366-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray, D. N. 2001. Determinants of vaccine-induced resistance in animal models of pulmonary tuberculosis. Scand. J. Infect. Dis. 33:175-178. [DOI] [PubMed] [Google Scholar]
- 15.Quesniaux, V. J., D. M. Nicolle, D. Torres, L. Kremer, Y. Guerardel, J. Nigou, G. Puzo, F. Erard, and B. Ryffel. 2004. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172:4425-4434. [DOI] [PubMed] [Google Scholar]
- 16.Rook, G. A., R. Hernandez-Pando, K. Dheda, and G. Teng Seah. 2004. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 25:483-488. [DOI] [PubMed] [Google Scholar]
- 17.Semret, M., C. Y. Turenne, P. de Haas, D. M. Collins, and M. A. Behr. 2006. Differentiating host-associated variants of Mycobacterium avium by PCR for detection of large sequence polymorphisms. J. Clin. Microbiol. 2006. 44:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa, A. O., S. Henry, F. M. Maroja, F. K. Lee, L. Brum, M. Singh, P. H. Lagrange, and P. Aucouturierm. 1998. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin. Exp. Immunol. 111:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweet, L., and J. S. Schoreym. 2006. Glycopeptidolipids from Mycobacterium avium promote macrophage activation in a TLR2- and MyD88-dependent manner. J. Leukoc. Biol. 80:415-423. [DOI] [PubMed] [Google Scholar]
- 20.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Reyn, C. F., T. W. Barber, R. D. Arbeit, C. H. Sox, G. T. O'Connor, R. J. Brindle, C. F. Gilks, K. Hakkarainen, A. Ranki, and C. Bartholomew. 1993. Evidence of previous infection with Mycobacterium avium-Mycobacterium intracellulare complex among healthy subjects: an international study of dominant mycobacterial skin test reactions. J. Infect. Dis. 168:1553-1558. [DOI] [PubMed] [Google Scholar]
- 22.Welsh, M. D., R. T. Cunningham, D. M. Corbett, R. M. Girvin, J. McNair, R. A. Skuce, D. G. Bryson, and J. M. Pollock. 2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 114:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]