Abstract
Host defense mechanisms against Pneumocystis carinii are not fully understood. Previous work in the murine model has shown that host defense against infection is critically dependent upon host CD4+ T cells. The recently described Th17 immune response is predominantly a function of effector CD4+ T cells stimulated by interleukin-23 (IL-23), but whether these cells are required for defense against P. carinii infection is unknown. We tested the hypothesis that P. carinii stimulates the early release of IL-23, leading to increases in IL-17 production and lung effector CD4+ T-cell population that mediate clearance of infection. In vitro, stimulation of alveolar macrophages with P. carinii induced IL-23, and IL-23p19 mRNA was expressed in lungs of mice infected with this pathogen. To address the role of IL-23 in resistance to P. carinii, IL-23p19−/− and wild-type control C57BL/6 mice were infected and their fungal burdens and cytokine/chemokine responses were compared. IL-23p19−/− mice displayed transient but impaired clearance of infection, which was most apparent 2 weeks after inoculation. In confirmatory studies, the administration of either anti-IL-23p19 or anti-IL-17 neutralizing antibody to wild-type mice infected with P. carinii also caused increases in fungal burdens. IL-17 and the lymphocyte chemokines IP-10, MIG, MIP-1α, MIP-1β, and RANTES were decreased in the lungs of infected IL-23p19−/− mice in comparison to their levels in the lungs of wild-type mice. In IL-23p19−/− mice infected with P. carinii, there were fewer effector CD4+ T cells in the lung tissue. Collectively, these studies indicate that the IL-23-IL-17 axis participates in host defense against P. carinii.
Pneumonia caused by Pneumocystis carinii remains an important cause of morbidity and mortality in the immunocompromised host (26, 27, 30). Host defense mechanisms against P. carinii are poorly understood, but CD4+ T cells have a pivotal role in combating P. carinii infection, as evidenced by its association with HIV infection (12, 34, 36). In murine models, Scid mice, which lack CD4+ T cells, develop progressive P. carinii infection which eventually causes death (40). However, if CD4+ cells from immunocompetent mice are adoptively transferred into Scid mice, recipient mice will clear P. carinii from the lung (12). The importance of the CD4+ T lymphocytes in host defense against P. carinii is further supported by animal work from our laboratory that shows that normal mice inoculated with P. carinii can resolve the infection without treatment, while mice that are depleted of CD4+ lymphocytes with a monoclonal antibody (Ab) are susceptible to P. carinii infection (3, 35). When CD4+ depletion is stopped, CD4+ T cells are recruited to lung tissue and the infection resolves (35). Collectively, these studies support a key role for the CD4+ T cells in host defense against P. carinii. The signals responsible for recruitment and activation of these cells during infection remain incompletely understood.
Interleukin-23 (IL-23), a member of the IL-6 family of cytokines, is a heterodimer with stimulating activity for memory CD4+ T cells (1, 14, 28, 29). IL-23 is composed of the IL-12p40 subunit and a unique p19 subunit which bears homology to IL-12p35. Like IL-12, IL-23 is produced by activated myeloid antigen-presenting cells such as dendritic cells and macrophages (18, 28, 31, 33). Given its structural similarity to IL-12, as well as its ability to stimulate gamma interferon (IFN-γ) production by human T cells (28), IL-23 was initially believed to induce the Th1 response, with the important distinction that its actions are restricted to memory CD4+ T cells. However, studies have since suggested that IL-23 likely functions to expand committed Th17 effectors to maintain and extend their function (22, 24, 42). These Th17 cells are distinguished from previously described Th1 and Th2 cells by their expression of IL-17, but not IFN-γ or IL-4, upon stimulation (1, 17). However, whether the IL-23-IL-17 axis is important in host responses to P. carinii is unknown. We investigated the effect of IL-23 deficiency on host responses to this pathogen in a murine model of infection. In addition, we also examined whether IL-23 modulated the host response through the IL-17 pathway.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free BALB/c and C57BL/6 mice were purchased at 4 to 5 weeks of age from Hilltop Lab Animals (Scottsdale, PA). C57BL/6 Scid and Scid/NCr (BALB/c background) mice were purchased at 5 to 6 weeks of age from Jackson Laboratory (Bar Harbor, ME) and NCI/Charles River Breeding Labs (Wilmington, MA), respectively. IL-23p19−/− mice were kindly provided by Nico Ghilardi (Genentech, South San Francisco, CA) (8). Animals were housed in filter-topped cages and fed autoclaved chow and water ad libitum. All caging procedures and surgical manipulations were done under a laminar flow hood. All procedures were approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center.
P. carinii inoculation.
P. carinii for inoculation was prepared, as described earlier, by using lung homogenates from chronically infected Scid mice (35). In brief, Scid mice chronically infected with P. carinii were injected with a lethal dose of pentobarbital and the lungs were removed and frozen in 1 ml of phosphate-buffered saline (PBS) at −70°C. The lungs were homogenized in 10 ml PBS by forcing tissue through a sterile 70-μm nylon strainer (BD Biosciences, Bedford, MA). The homogenates were centrifuged at 500 × g for 10 min at 4°C. The cell pellet was resuspended in PBS, and 1:5 and 1:10 dilutions were stained with modified Giemsa stain (Diff-Quick; Dade Behring, Newark, DE). The number of cysts was quantified microscopically, and the inoculum concentration was adjusted with PBS to 2 × 106 cysts/ml. Recipient mice were anesthetized with intraperitoneal ketamine-xylazine (200 mg per kg/10 mg per kg) and injected intratracheally with 2 × 105 cysts per mouse. C57BL/6 mice received P. carinii inoculum prepared from C57BL/6 Scid mice, while BALB/c mice received lung homogenates from Scid/NCr mice. Mice were sacrificed at serial time intervals after challenge by a lethal dose of pentobarbital and aortic transection. The right lungs were homogenized in 1 ml TRIzol (Invitrogen, Carlsbad, CA) and frozen at −70°C for subsequent RNA isolation, and the left lungs were snap-frozen in an ethyl alcohol-dry ice bath and stored at −70°C for protein analysis.
BAL.
Animals were sacrificed as described above. The trachea was exposed through a midline incision and cannulated with a polyethylene catheter. The lungs were lavaged with 2 ml of sterile Ca2+- and Mg2+-free PBS containing 0.6 mM EDTA. Bronchoalveolar lavage (BAL) cells were collected by centrifugation at 500 × g for 5 min. The cell pellets were resuspended in either PBS with 0.05% sodium azide (for flow cytometry) or 1 ml TRIzol (for RNA assay).
In vitro stimulation of alveolar macrophage line MH-S and BAL cells with P. carinii.
Totals of 1 × 106 MH-S cells (a mouse alveolar macrophage line, ATCC CRL-2019) or BAL cells from uninfected BALB/c mice were incubated in 24-well plates with 0.1 ml of lung homogenate containing 2 × 105 cysts of P. carinii. After serial time intervals, supernatants were assayed for cytokine/chemokine production by using a Bio-Plex system (Bio-Rad, Richmond, CA) and a biological activity assay of IL-23 (induction of splenocyte IL-17) was performed as has been previously described (1, 10). Cells were harvested in 1 ml TRIzol for total RNA extraction. Control cells were incubated with 0.1 ml of lung homogenate from uninfected mice.
IL-23 biological activity assay.
Spleens from normal BALB/c mice were passed through a 40-μm nylon cell strainer (BD Biosciences), and red cells were lysed. Adherent cells were removed, and the remaining splenocytes were cultured at 2 × 106/ml in medium composed of a 1:1 ratio of RPMI 1640 (Invitrogen, Carlsbad, CA) and conditioned supernatants from P. carinii-exposed macrophages. The supernatant IL-17 concentration was determined at 2, 24, and 48 h by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
Real-time RT-PCR.
Following total RNA isolation per the manufacture's protocol (TRIzol; Invitrogen), RNA purity and concentration were determined by spectrophotometric absorbance at 260 and 280 nm. Equal amounts of total RNA were added to each reverse transcription-PCR (RT-PCR) (or one-step RT-PCR) reaction mixture. Real-time PCR was carried out using a two-step TaqMan RT-PCR (Applied Biosystems, Foster City, CA) for P. carinii rRNA (44) and a one-step brilliant quantitative RT-PCR (Stratagene, La Jolla, CA) for IL-23p19 and IL-17 mRNA. All reactions were performed on a Stratagene Mx3000P. Data were converted to transcript copy numbers using standard curves of known copy numbers of P. carinii rRNA, p19, or IL-17 cRNA, as described previously (32). For P. carinii rRNA (44), the primers and probe sequences are 5′-ATG AGG TGA AAA GTC GAA AGG G-3′, 5′-TGA TTG TCT CAG ATG AAA AAC CTC TT-3′, and 5′-6-FAM-AAC AGC CCA GAA TAA TGA ATA AAG TTC CTC AAT TGT TAC-TAMRA-3′. For IL-23p19 mRNA, the primers and probe sequences are 5′-TGG CTG TGC CTA GGA GTA GCA-3′, 5′-TTC ATC CTC TTC TTC TCT TAG TAG ATT CAT A-3′, and 5′-6-FAM-CTC TGC ATG CTA GGC TGG AAC GCA C-3BHQ_1-3′, and for IL-17 mRNA, they are 5′-GCT CCA GAA GGC CCT CAG A-3′, 5′-CTT TCC CTC CGC ATT GAC A-3′, and 5′-6-FAM-ACC TCA ACC GTT CCA CGT CAC CCT G-3BHQ_1-3′.
Flow cytometric analysis of lung lymphocytes.
BAL cells were stained with optimal concentrations of fluorochrome-conjugated Abs specific for murine CD4, CD44, and CD62L (BD Biosciences) for 45 min at 4°C. Isotype control Ab staining was used to assist in gating. After the cells were washed three times with PBS-sodium azide, they were fixed with 0.05% paraformaldehyde in PBS-sodium azide. The surface expression levels of these molecules were determined by using a FACSCalibur cytofluorometer (BD Biosciences).
Preparation of lung homogenates and ELISA and Bio-Plex analysis of cytokine levels.
The left lungs were homogenized (Omni TH homogenizer; Omni International, Warrenton, VA) in 0.5 ml PBS containing 0.5% Triton X-100 and Roche complete protease inhibitor cocktail (Mannheim, Germany). The lung homogenates were cleared of debris and cells by centrifugation at 10,000 × g for 10 min and frozen at −70°C for later ELISA analysis. The IL-17, MIG, IP-10, I-TAC, and MIP-1β concentrations were determined by ELISA (R&D Systems). The MIP-1α and RANTES concentrations were determined by cytokine bead array (Bio-Rad).
IL-17 and IL-23 neutralization.
For IL-17 neutralization experiments, P. carinii-infected C57BL/6 mice were lightly anesthetized with ketamine-xylazine at day 5 after P. carinii inoculation and 1 μg of neutralizing anti-murine IL-17 Ab (R&D Systems) was given to each mouse intranasally. Ab was administered twice a week for up to 4 weeks afterwards. Control mice received 1 μg of goat immunoglobulin gamma (Sigma, St Louis, MO). For IL-23 neutralization experiments, anti-IL-23p19 Ab (R&D Systems) or isotype control was premixed with P. carinii inoculum at 10 μg/ml final concentration just prior to intratracheal inoculation. Mice then received 1 μg of Ab intranasally twice a week, similar to the IL-17 neutralization experiments.
Statistics.
Data are reported as the means ± standard errors of the means (SEMs). Differences in levels of effector CD4+ T cells between experimental groups were tested using a two-way analysis of variance followed by a Holm-Sidak multiple comparison procedure. Other data were analyzed using Student's t test. Statistical significance was accepted when P was less than 0.05.
RESULTS
Alveolar macrophage cytokine/chemokine expression in response to P. carinii in vitro.
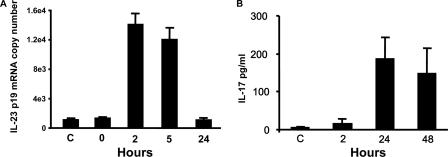
To determine whether P. carinii induces alveolar macrophage IL-23 expression, MH-S cells were incubated with 2 × 105 cysts in vitro. There was significant induction of IL-23p19 mRNA as early as 2 h after exposure (Fig. 1A).
FIG. 1.
IL-23 expression in alveolar macrophages in response to P. carinii infection in vitro. (A) MH-S cell IL-23p19 mRNA expression at indicated times after exposure. (B) Splenocyte IL-17 production induced by conditioned supernatants from P. carinii-exposed MH-S cells. MH-S cells were infected with P. carinii for 24 h prior to supernatant harvest and transfer onto BALB/c splenocytes. At the indicated times postexposure, splenocyte supernatants were collected for IL-17 assay. The data are expressed as the means ± SEMs and are representative of three separate experiments. n = 3 per group. C, control.
To confirm IL-23 biological activity in supernatants from P. carinii-exposed MH-S cell cultures, cell- and cyst-free conditioned supernatants were added to adherent-cell-depleted splenocytes, and IL-17 concentrations were then determined at 2, 24, and 48 h after incubation. IL-17 from splenocytes stimulated with media from P. carinii-exposed MH-S cells was increased at 24 and 48 h (Fig. 1B). Minimal IL-17 (6 pg/ml) was induced by conditioned media from MH-S cells exposed to normal mouse lung homogenate. Thus, P. carinii stimulates both IL-23 mRNA and protein expression in alveolar macrophages in vitro.
To determine whether neutralization of IL-23 in the in vitro macrophage stimulation cultures affects cytokine or chemokine production by macrophages, MH-S cells were incubated with 0.1 ml of lung homogenate containing 2 × 105 cysts of P. carinii with or without the presence of IL-23p19 Ab (2 μg/ml final concentration) for 24 h. For the controls, cells were either untreated or incubated with lung homogenate from normal uninfected mice or anti-IL-23p19 Ab (2 μg/ml). Cell supernatants were assayed for production of IL-10, IL-12p70, IL-1β, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage (GM)-CSF, IL-6, tumor necrosis factor alpha, KC, IP-10, MIG, and I-TAC. MH-S cells incubated with P. carinii produced significant amounts of IL-6, G-CSF, and IL-1β in comparison to untreated and Ab-only controls (Table 1). Neutralization of IL-23 did not significantly change the expression of these cytokines. P. carinii stimulated the release of MIG and I-TAC, but there was no observable suppression after incubation with anti-IL-23p19 Ab. No consistent results were observed for IP-10. Minimal amounts of IL-10, IL-12p70, GM-CSF, tumor necrosis factor alpha, and KC were produced in response to P. carinii.
TABLE 1.
Chemokine and cytokine production in alveolar macrophage MH-S cells in response to P. carinii challenge in vitro
| Cytokine or chemokine | Amt (pg/ml) in:
|
||||
|---|---|---|---|---|---|
| MH-S cells | MH-S cells with IL-23p19 Ab | MH-S cells with lung homogenate from normal mice | MH-S cells with P. carinii | MH-S cells with P. carinii and IL-23p19 Ab | |
| IL-6 | 2,765 ± 156 | 2,683 ± 787 | 2,167 ± 322 | 6,537 ± 690 | 4,950 ± 805 |
| G-CSF | 34 ± 3 | 36 ± 11 | 39 ± 3 | 241 ± 54 | 188 ± 19 |
| IL-1β | 10 ± 0.5 | 12 ± 2 | 8 ± 2 | 106 ± 12 | 109 ± 27 |
BAL cell IL-23 expression in response to in vivo P. carinii infection.
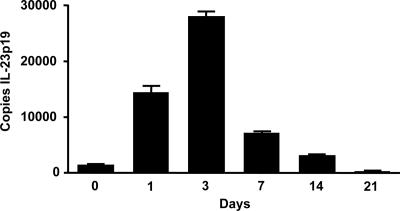
In separate in vivo experiments, the kinetics of BAL cell IL-23 expression in mice intratracheally inoculated with P. carinii was assayed. At 0, 1, 3, and 7 days after inoculation, mice were sacrificed. BAL cell RNA was analyzed for IL-23p19 transcripts by real-time RT-PCR. Mice challenged with P. carinii showed increases in IL-23p19 expression in BAL cells which peaked at 3 days postchallenge (Fig. 2). Thus, IL-23 mRNA is increased in the lungs of P. carinii-infected mice.
FIG. 2.
IL-23p19 mRNA expression in BAL cells following P. carinii challenge. BALB/c mice were inoculated with P. carinii, and BAL cells were harvested at the indicated times postinoculation for mRNA assay. The data are expressed as the means ± SEMs and are representative of two separate experiments. n = 4 per group.
Absence or neutralization of IL-23 and clearance of P. carinii infection.
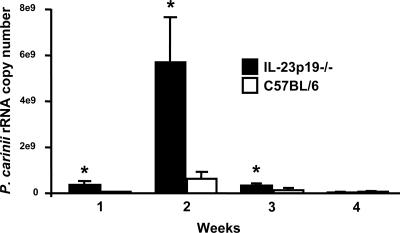
Having established that P. carinii inoculation induces IL-23 expression, experiments were performed to determine whether this cytokine is important to host defense against P. carinii pneumonia. IL-23p19−/− mice and background (C57BL/6) controls were inoculated with P. carinii. The burden of P. carinii in the lungs was determined by real-time RT-PCR for P. carinii rRNA copy number at serial intervals. IL-23p19−/− mice had significantly heavier fungal burdens than wild-type mice at 1, 2, and 3 weeks after inoculation (Fig. 3). However, by week 4, both groups of mice had cleared the infection.
FIG. 3.
Lung P. carinii burden in wild-type and IL-23p19−/− mice. C57BL/6 and IL-23p19−/− mice were inoculated with P. carinii and sacrificed for P. carinii rRNA assay of the right lung at the indicated time points postinoculation. The data are expressed as the means ± SEMs of total rRNA per right lung and are representative of five separate experiments. n = 4 per group. *, P < 0.05 compared with C57BL/6 mice at the same time point.
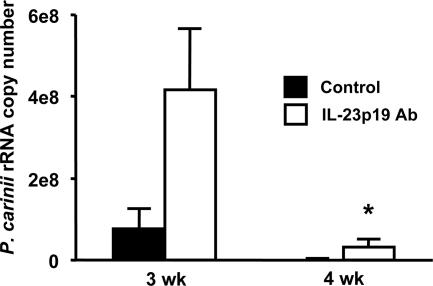
Additional experiments were done to confirm the importance of IL-23 in host defense against P. carinii, using anti-IL-23p19 neutralizing Ab. C57BL/6 mice treated with anti-IL-23 Ab showed higher burdens of infection than control mice at both 3 and 4 weeks (Fig. 4). These differences were significant at 4 weeks, when control mice had a significantly lower fungal burden (3 × 107 versus 1 × 104 copies of P. carinii rRNA; P = 0.024). Similar to the findings in IL-23p19−/− mice, these data showed that the absence of IL-23 compromises host defense against infection.
FIG. 4.
Lung P. carinii burden in C57BL/6 mice treated with anti-IL-23p19 Ab. C57BL/6 mice were inoculated with P. carinii and treated with either anti-IL-23 Ab or isotype control Ab twice a week. At the indicated time points postinoculation, total RNA was isolated from the right lungs for assay of P. carinii rRNA expression levels by real-time RT-PCR. The data are expressed as the means ± SEMs and are representative of two separate experiments. n = 4 per group. *, P < 0.05 compared with control mice at the same time point.
Chemokine responses to P. carinii infection in IL-23p19−/− mice.
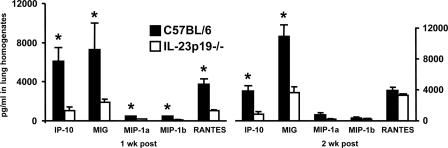
Since host defense against P. carinii depends upon recruitment of T cells into infected lungs, we tested whether IL-23p19−/− mice have impaired or delayed lymphocyte chemokine responses. Lung homogenates from C57BL/6 and IL-23p19−/− mice infected with P. carinii were assayed for expression of IP-10, MIG, MIP-1α, MIP-1β, and RANTES. As anticipated, lungs from wild-type C57BL/6 mice contained significant levels of these chemokines at 1 and 2 weeks postinoculation (Fig. 5). However, chemokine release was significantly suppressed in the lungs of IL-23p19−/− mice. IL-23p19−/− mice had significantly lower amounts of IP-10 and MIG at both 1 and 2 weeks postinoculation than wild-type animals. The amounts of MIP-1α, MIP-1β, and RANTES were also significantly lower in IL-23p19−/− mice at the 1-week time point. Thus, an absence of IL-23 resulted in decreased chemokine synthesis in response to P. carinii. When control C57BL/6 mice are inoculated with uninfected lung tissue, there is no significant release of these chemokines at the 1- and 2-week time points (data not shown).
FIG. 5.
Chemokine expression in wild-type C57BL/6 and IL-23p19−/− mice during P. carinii infection. The mice were inoculated with P. carinii and sacrificed at the indicated time points postinoculation. The expression of chemokines was measured in whole-lung homogenates of the left lung. The data are expressed as the means ± SEMs and are representative of two separate experiments. n = 4 per group. *, P < 0.05 compared with control mice at the same time point.
Lung CD4+ effector T-cell recruitment in response to P. carinii infection in IL-23p19−/− mice.
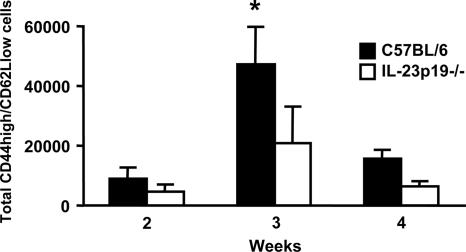
Since IL-23 stimulates effector T cells, and because the absence of IL-23 during infection causes a lymphocyte chemokine defect, we next examined the effector T-cell population in the alveolar space of P. carinii-infected C57BL/6 and IL-23p19−/− mice. Prior to infection, both strains showed essentially no lymphocytes in the BAL cell population (data not shown). At 3 and 4 weeks postinfection, BAL cells were obtained and analyzed by fluorescence-activated cell sorter. As seen in Fig. 6, a significantly greater number of T effector cells (CD4+/CD44high/CD62Llow) were found in the BAL fluid of C57BL/6 mice than in that of IL-23p19−/− mice at 3 weeks postinfection.
FIG. 6.
Lung CD4+ effector T-cell recruitment in wild-type C57BL/6 and IL-23p19−/− mice in response to P. carinii infection. At the indicated times after P. carinii inoculation, BAL cells were collected and stained with fluorochrome-conjugated Abs specific for murine CD4, CD44, and CD62L. The absolute numbers of lymphocytes bearing surface expression of these molecules were determined by using a fluorescence-activated cell sorter. The data are expressed as the means ± SEMs and are representative of two separate experiments. n = 4 per group. *, P < 0.05 compared with wild-type mice at the same time point.
Lung IL-17 expression during P. carinii pneumonia.
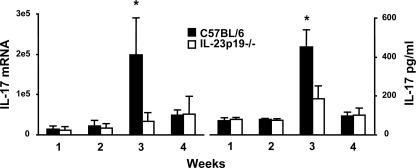
To determine the lung IL-17 response to P. carinii infection and whether IL-23 is requisite for this response, we next examined IL-17 expression in IL-23p19−/− versus C57BL/6 mice. Using real-time RT-PCR, expression of IL-17 mRNA was determined at 1, 2, 3, and 4 weeks postinfection. At the 3-week time point, IL-23p19−/− mice demonstrated significantly lower IL-17 levels than wild-type C57BL/6 mice (Fig. 7, left). Decreased lung homogenate IL-17 content was also confirmed at the protein level (Fig. 7, right). Consistent with the IL-17 mRNA data, there was a significant reduction of IL-17 protein in IL-23p19−/− mice at 3 weeks postinfection compared to that in the C57BL/6 mice.
FIG. 7.
Lung IL-17 production in wild-type and IL-23p19−/− mice in response to P. carinii. C57BL/6 and IL-23p19−/− mice were inoculated with P. carinii, and lungs were harvested at the indicated time points postinoculation. The right lungs were assayed for mRNA content and the left lungs for protein analysis. (Left) Lung IL-17 mRNA expression levels. (Right) Lung IL-17 protein expression levels. The data are expressed as the means ± SEMs and are representative of three separate experiments. n = 4 per group. *, P < 0.05 compared with wild-type mice at the same time point.
Neutralization of IL-17 and clearance of P. carinii infection.
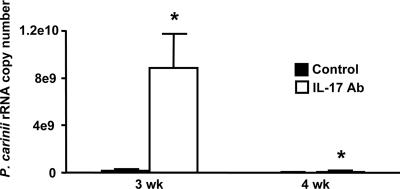
A possible mechanism for the increased intensity of P. carinii infection in IL-23p19−/− mice compared to that in the wild-type mice is defective IL-17 release in response to P. carinii. To test whether IL-17 is important for P. carinii clearance, C57BL/6 mice were administered an anti-IL-17 neutralizing Ab intranasally, and the P. carinii lung burden was determined as previously described. Mice receiving anti-IL-17 Ab showed an approximately 50% reduction in IL-17 protein in lung homogenates at 3 weeks postinfection (data not shown). As shown in Fig. 8, anti-IL-17-treated mice showed a 36-fold increase in P. carinii rRNA at 3 weeks and a 10,000-fold increase at 4 weeks postinfection in comparison to levels in control mice treated with isotype control Ab.
FIG. 8.
Lung P. carinii burden in C57BL/6 mice treated with anti-IL-17 Ab. C57BL/6 mice were inoculated with P. carinii and then treated with anti-IL-17 Ab or isotype control Ab twice a week. At the indicated time points postinoculation, total RNA was isolated from the right lung for assay of P. carinii rRNA expression levels by real-time RT-PCR. The data are expressed as the means ± SEMs and are representative of two separate experiments. n = 4 per group. *, P < 0.05 compared with control mice at the same time points.
DISCUSSION
The current study is the first examination of a role for the IL-23-IL-17 cytokine axis in host defense against P. carinii. IL-23 expression was induced in alveolar macrophages inoculated with P. carinii, and this IL-23 response is necessary for optimal lung IL-17 production during infection. IL-23-deficient mice (IL-23p19−/−) developed a more intense infection than wild-type mice, and Ab neutralization of either IL-23 or IL-17 within the lung significantly increased the susceptibility of wild-type C57BL/6 mice to infection with P. carinii. Together, these findings demonstrate an important role for the IL-23-IL-17 immune pathway in host defense against P. carinii during infection.
Infection models using IL-12p40−/− and IL-12p35−/− mice have shown that there is an IL-12p40-dependent, IL-12p35-independent mechanism of resistance to several microorganisms, including Francisella tularensis (6), Cryptococcus neoformans (5), Salmonella enteritidis (19), Mycobacterium spp. (13), Toxoplasma gondii (20), and murine cytomegalovirus (4). These observed differences in host defense have been attributed to the absence of IL-23, which shares the p40 subunit with IL-12. More recently, the development of IL-23p19−/− mice has allowed investigators to show that IL-23 provides a moderate level of protection against Toxoplasma (20) and Mycobacterium tuberculosis (16) in the absence of IL-12. In this study, we have shown that IL-23 is produced as part of the immune response to P. carinii and that the alveolar macrophage is one of the early cellular sources of this cytokine. In contrast to data observed in studies of Toxoplasma and Mycobacterium tuberculosis infection, our study shows a direct role for IL-23 in controlling pathogen proliferation despite intact IL-12 signaling. It is important to note that IL-23-deficient mice were ultimately capable of clearing P. carinii infection, results similar to those of other published studies of cytokine absence/neutralization in murine models of P. carinii infection (7, 9, 37).
Since neutrophils do not appear to play a central role in host defense against Pneumocystis infection (2, 15, 21, 23), we next examined whether the defects in P. carinii clearance in IL-23p19−/− mice were associated with defects in T-cell recruitment in the lungs in response to the infection. We observed significantly reduced production of the lymphocytic chemokines IP-10, MIG, MIP-1α, MIP-1β, and RANTES in the lungs of IL-23p19−/− mice compared to their production in C57BL/6 mice. Because localized chemokine expression is a prerequisite for infiltration of lymphocytes to the challenge site (41), it appears likely that lower expression of chemokines is in part responsible for the reduced number of effector T cells observed in the lungs of IL-23p19−/− mice. The mechanism through which IL-23 deficiency results in compromised chemokine production has yet to be determined, but a recent study of central nervous system autoimmunity revealed that IL-23 induced elevated expression of chemokine genes, such as CCL7, CCL17, CCL20, CCL22, and CCR1, in cells from draining lymph nodes in vitro (17).
IL-23 does not appear to play a role in stimulating or amplifying the release of macrophage proinflammatory cytokines, at least for MH-S cells stimulated with P. carinii in vitro. MH-S cells cultured with P. carinii showed enhanced release of IL-16, G-CSF, and IL-1β, but this was not altered in the presence of neutralizing anti-IL-23 Ab. These data support a more limited role for IL-23 in the inflammatory response to P. carinii, likely through enhanced chemokine production and/or expansion of T lymphocytes producing IL-17. However, we cannot rule out additional effects of IL-23 in vivo or on lung cells other than alveolar macrophages.
Although transforming growth factor β is a critical cytokine for the commitment of naïve T cells to Th17 development, IL-23 is believed to be important in the expansion and survival of these IL-17-producing cells (22, 38, 39). In addition, an intact IL-23-IL-17 axis seems to be essential for host protection against Citrobacter rodentium, as well as in the pathogenesis of certain autoimmune diseases such as rheumatoid arthritis and experimental autoimmune encephalomyelitis (17, 22, 24, 25). We next investigated whether the IL-23-IL-17 pathway is important during P. carinii challenge. We found that IL-17 production was significantly reduced in IL-23p19−/− mice, showing that IL-23 is indeed important for optimal T-cell production of IL-17. This is consistent with our observations of fewer recruited effector T cells in IL-23p19−/− mice. However, IL-17 production was not completely abrogated in IL-23p19−/− mice. This is in agreement with a recent report that found that IL-23p19−/− mice are able to develop an IL-17 response despite an impaired inflammatory response and deficiencies in bacterial clearance (22). Others have shown that the absence of IL-23 resulted in a profound reduction in the frequency and number of antigen-specific, IL-17-producing CD4+ T cells as well as local IL-17 mRNA production in the lung during M. tuberculosis infection (16). Studies of pulmonary Klebsiella pneumoniae challenge showed that both IL-23 and IL-17 are important in resistance to this pathogen, and IL-23, released from dendritic cells exposed to K. pneumoniae, induced IL-17 production in both CD4+ and CD8+ T cells in vitro (11). Our IL-17 neutralization experiments suggest that the defect in host defense against P. carinii observed in IL-23p19−/− mice may result from defective downstream IL-17 expression. Whether the defect in lung IL-17 expression seen in IL-23p19−/− mice is predominantly due to abrogated recruitment of effector CD4+ T cells or impaired IL-17 expression in a lung-resident lymphocyte population is unknown. The IL-17 neutralization strategy used in our study reduced IL-17 protein in the lung by 50% at 3 weeks post-P. carinii challenge (unpublished data). Because this incomplete IL-17 depletion was nevertheless associated with substantial defects in pathogen control compared to that in control animals, we conclude that the Th17 response is a critical component of the host immune repertoire against P. carinii infection. Since we detected no differences in the levels of lung IFN-γ expression between C57BL/6 and IL-23p19−/− mice infected with P. carinii (unpublished data), we conclude that the pulmonary Th17 response is largely independent of the IFN-γ-dominated Th1 pathway. Indeed, current models regarding the ontogeny of Th17- versus Th1-polarized T-cell responses suggest early separation of common precursor cells during maturation or perhaps distinct origins for these adaptive effector cells (14, 38, 42, 43).
In summary, we found that P. carinii pneumonia induces IL-23 expression and that mice deficient in IL-23 develop more severe infection. Our results indicate that IL-23 plays a role in host defense against P. carinii, but it is not an essential one, in that mice deficient in IL-23 are still able to clear the infection. Given the proven role of IL-23 in several models of autoimmune inflammation (25), substantial interest exists in targeting this cytokine with neutralization immunotherapy. Such therapy will require surveillance for the development of opportunistic infection with pathogens such as P. carinii. Furthermore, our results support the investigation of IL-23 delivery to augment immune function in the immunocompromised host to prevent infection with P. carinii.
Acknowledgments
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grant PO1 HL076100 (J.S.) and National Institute of Allergy, Immunology, and Infectious Diseases grant RO1 AI51677 (J.S.), and by National Center for Research Resources, Department of Health and Human Services, grants 1 P20 RR021970-01 (X.R.) and K08AA015163 (K.H.).
We thank Ping Zhang and Constance Porretta of Immunology Core for help with the flow cytometric analysis.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 2 April 2007.
REFERENCES
- 1.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910-1914. [DOI] [PubMed] [Google Scholar]
- 2.Bang, D., J. Emborg, J. Elkjaer, J. D. Lundgren, and T. L. Benfield. 2001. Independent risk of mechanical ventilation for AIDS-related Pneumocystis carinii pneumonia associated with bronchoalveolar lavage neutrophilia. Respir. Med. 95:661-665. [DOI] [PubMed] [Google Scholar]
- 3.Beck, J., M. Warnock, J. Curtis, M. Sniezek, S. Arrag-Peffer, H. Kaltreider, and J. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5:186-197. [DOI] [PubMed] [Google Scholar]
- 4.Carr, J. A., J. A. Rogerson, M. J. Mulqueen, N. A. Roberts, and A. A. Nash. 1999. The role of endogenous interleukin-12 in resistance to murine cytomegalovirus (MCMV) infection and a novel action for endogenous IL-12 p40. J. Interferon Cytokine Res. 19:1145-1152. [DOI] [PubMed] [Google Scholar]
- 5.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkins, K. L., A. M. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garvy, B., A. Ezekowitz, and A. Harmsen. 1997. Role of gamma interferon in the host immune and inflammatory responses to Pneumocystis carinii infection. Infect. Immun. 65:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghilardi, N., N. Kljavin, Q. Chen, S. Lucas, A. L. Gurney, and F. J. De Sauvage. 2004. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J. Immunol. 172:2827-2833. [DOI] [PubMed] [Google Scholar]
- 9.Hanano, R., K. Reifenberg, and S. Kaufmann. 1998. Activated pulmonary macrophages are insufficient for resistance against Pneumocystis carinii. Infect. Immun. 66:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happel, K. I., P. J. Dubin, M. Zheng, N. Ghilardi, C. Lockhart, L. J. Quinton, A. R. Odden, J. E. Shellito, G. J. Bagby, S. Nelson, and J. K. Kolls. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Happel, K. I., M. Zheng, E. Young, L. J. Quinton, E. Lockhart, A. J. Ramsay, J. E. Shellito, J. R. Schurr, G. J. Bagby, S. Nelson, and J. K. Kolls. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmsen, A., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holscher, C., R. A. Atkinson, B. Arendse, N. Brown, E. Myburgh, G. Alber, and F. Brombacher. 2001. A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 167:6957-6966. [DOI] [PubMed] [Google Scholar]
- 14.Hunter, C. A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5:521-531. [DOI] [PubMed] [Google Scholar]
- 15.Ieki, R., T. Furuta, S. Asano, S. Mori, S. Kudoh, H. Kimura, and F. Takaku. 1989. Effect of recombinant human granulocyte colony-stimulating factor on Pneumocystis carinii infection in nude mice. Jpn. J. Exp. Med. 59:51-58. [PubMed] [Google Scholar]
- 16.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. Desauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J. Immunol. 175:788-795. [DOI] [PubMed] [Google Scholar]
- 17.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langrish, C. L., B. S. McKenzie, N. J. Wilson, R. de Waal Malefyt, R. A. Kastelein, and D. J. Cua. 2004. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 202:96-105. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann, J., S. Bellmann, C. Werner, R. Schroder, N. Schutze, and G. Alber. 2001. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J. Immunol. 167:5304-5315. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman, L. A., F. Cardillo, A. M. Owyang, D. M. Rennick, D. J. Cua, R. A. Kastelein, and C. A. Hunter. 2004. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol. 173:1887-1893. [DOI] [PubMed] [Google Scholar]
- 21.Limper, A. H., K. P. Offord, T. F. Smith, and W. J. Martin II. 1989. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 140:1204-1209. [DOI] [PubMed] [Google Scholar]
- 22.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 23.Mason, G. R., C. H. Hashimoto, P. S. Dickman, L. F. Foutty, and C. J. Cobb. 1989. Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am. Rev. Respir. Dis. 139:1336-1342. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie, B. S., R. A. Kastelein, and D. J. Cua. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17-23. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, C. A., C. L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R. A. Kastelein, J. D. Sedgwick, and D. J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, J., C. Felton, and S. Garay. 1984. Pulmonary complications of the acquired immunodeficiency syndrome: report of a National Heart, Lung and Blood Institute workshop. N. Engl. J. Med. 310:1682-1688. [DOI] [PubMed] [Google Scholar]
- 27.Murray, J., S. Garay, P. Hopewell, J. Mills, G. Snider, and D. Stover. 1987. Pulmonary complications of the acquired immunodeficiency syndrome: an update. Am. Rev. Respir. Dis. 135:504-509. [DOI] [PubMed] [Google Scholar]
- 28.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 29.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K. P. Singh, F. Vega, W. To, J. Wagner, A. M. O'Farrell, T. McClanahan, S. Zurawski, C. Hannum, D. Gorman, D. M. Rennick, R. A. Kastelein, R. de Waal Malefyt, and K. W. Moore. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699-5708. [DOI] [PubMed] [Google Scholar]
- 30.Phair, J., A. Munoz, R. Detels, R. Kaslow, C. Rinaldo, and A. Saah. 1990. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. N. Engl. J. Med. 322:161-165. [DOI] [PubMed] [Google Scholar]
- 31.Pirhonen, J., S. Matikainen, and I. Julkunen. 2002. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J. Immunol. 169:5673-5678. [DOI] [PubMed] [Google Scholar]
- 32.Quinton, L. J., S. Nelson, D. M. Boe, P. Zhang, Q. Zhong, J. K. Kolls, and G. J. Bagby. 2002. The granulocyte colony-stimulating factor response after intrapulmonary and systemic bacterial challenges. J. Infect. Dis. 185:1476-1482. [DOI] [PubMed] [Google Scholar]
- 33.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TRL4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692. [DOI] [PubMed] [Google Scholar]
- 34.Roths, J., and C. Sidman. 1992. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J. Clin. Investig. 90:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shellito, J., V. V. Suzara, W. Blumenfeld, J. M. Beck, H. J. Steger, and T. H. Ermak. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stansell, J. D., D. H. Osmond, E. Charlebois, L. LaVange, J. M. Wallace, B. V. Alexander, J. Glassroth, P. A. Kvale, M. J. Rosen, L. B. Reichman, J. R. Turner, P. C. Hopewell, et al. 1997. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Am. J. Respir. Crit. Care Med. 155:60-66. [DOI] [PubMed] [Google Scholar]
- 37.Steele, C., J. E. Shellito, and J. K. Kolls. 2005. Immunity against the opportunistic fungal pathogen Pneumocystis. Med. Mycol. 43:1-19. [DOI] [PubMed] [Google Scholar]
- 38.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179-189. [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen, M., and B. Stockinger. 2006. TGFbeta1, a ‘Jack of all trades’: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 27:358-361. [DOI] [PubMed] [Google Scholar]
- 40.Walzer, P., C. Kim, M. Linke, C. Pogue, M. Huerkamp, C. Chrisp, A. Lerro, S. Wixson, E. Hall, and L. Shultz. 1989. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect. Immun. 57:62-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward, S. G., and J. Westwick. 1998. Chemokines: understanding their role in T-lymphocyte biology. Biochem. J. 333:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weaver, C. T., L. E. Harrington, P. R. Mangan, M. Gavrieli, and K. M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24:677-688. [DOI] [PubMed] [Google Scholar]
- 43.Wynn, T. A. 2005. T(H)-17: a giant step from T(H)1 and T(H)2. Nat. Immunol. 6:1069-1070. [DOI] [PubMed] [Google Scholar]
- 44.Zheng, M., J. Shellito, L. Marrero, Q. Zhong, J. Stewart, P. Ye, V. Wallace, P. Schwarzenberger, and J. Kolls. 2001. CD4+ T cell independent vaccination against Pneumocystis carinii in mice. J. Clin. Investig. 108:1469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]