Abstract
Enterohepatic Helicobacter species infect the intestinal tracts and biliary trees of various mammals, including mice and humans, and are associated with chronic inflammatory diseases of the intestine, gallstone formation, and malignant transformation. The recent analysis of the whole genome sequence of the mouse enterohepatic species Helicobacter hepaticus allowed us to perform a functional analysis of bacterial factors that may play a role in these diseases. We tested the hypothesis that H. hepaticus suppresses or evades innate immune responses of mouse intestinal epithelial cells, which allows this pathogen to induce or contribute to chronic inflammatory disease. We demonstrated in the present study that the innate immune responses of intestinal epithelial cells to lipopolysaccharide (LPS) via Toll-like receptor 4 (TLR4) and to flagellin-mediated activation via TLR5 are reduced by H. hepaticus infection through soluble bacterial factors. In particular, H. hepaticus lysate and the soluble component LPS antagonized TLR4- and TLR5-mediated immune responses of intestinal epithelial cells. H. hepaticus lysate and LPS inhibited development of endotoxin tolerance to Escherichia coli LPS. Suppression of innate immune responses by H. hepaticus LPS thus may affect intestinal responses to the resident microbial flora, epithelial homeostasis, and intestinal inflammatory conditions.
The enterohepatic helicobacters are a large group of Helicobacter species which colonize the intestinal tracts of various mammals and birds (53, 58). Helicobacter hepaticus, the prototypic enterohepatic helicobacter, was discovered as the cause of liver tumors in a control cohort of mice during an NIH cancer study in 1994 (22). H. hepaticus naturally colonizes the intestinal tract of mice. Several models with immunocompromised mice have been established, and when these mice are infected with H. hepaticus, they develop chronic intestinal inflammatory conditions that closely mimic chronic inflammatory bowel diseases, such as Crohn's disease (14, 33, 38, 58). Further identification and characterization of H. hepaticus infections and other intestinal bacterial infections in mice have heightened the awareness that the composition of the intestinal flora is crucial for the modulation of the immune response and the development of intestinal disorders (14). Although H. hepaticus does not usually colonize humans and seems to be relatively host specific for mice, enterohepatic Helicobacter species other than H. hepaticus have also been identified and cultured from the intestinal tracts of humans, including groups of patients with intestinal diseases such as gallbladder cancer, cholecystitis, pancreatitis, and chronic diarrhea (4, 20, 21, 40, 51). Until now, it has been difficult to determine a direct causal link to specific bacterial virulence factors, but elucidation of the whole genome sequence of H. hepaticus (54) has provided the opportunity to determine the roles of specific bacterial properties in the development of intestinal diseases. Thus far, the only bacterial factor of H. hepaticus that has been studied in some detail is cytolethal distending toxin (CDT) (9, 34, 60). CDT has been associated with increased inflammation or bacterial persistence in experimental infections with H. hepaticus and Campylobacter (23, 24, 60).
The aim of this study was to determine the effects of H. hepaticus and its soluble components on the innate immune responses of intestinal epithelial cells, which interact closely with this bacterium in the in vivo setting. H. hepaticus has been observed previously to colonize bile canaliculi of the liver (22) in mice susceptible to liver disease and to be in close proximity to the intestinal mucosal epithelium in crypts of the mouse colon and cecum (49). Intestinal epithelial cells are sentinels and first-line innate immune defenders against microbial invaders at sites of the intestinal mucosa and, by reacting to microbial components, take part in the maintenance of homeostasis and barrier function in the intestinal tract (47, 48). The interaction of H. hepaticus with intestinal epithelial cells and with specific host cell pattern recognition receptors (PRR), such as Toll-like receptors (TLR), has not been investigated intensively, whereas a role for these receptors in the pathogenesis of chronic inflammatory bowel disease has been firmly established (15, 46). Like other Helicobacter spp., H. hepaticus has been found to be incapable of inducing a TLR5-mediated innate immune reaction by means of its immunoevasive flagellins (3, 36), but potential activation via TLR4 and TLR2 has been suggested (39).
We demonstrate here that H. hepaticus itself is able to evade innate immune responses of mouse intestinal epithelial cells and to suppress the innate response to lipopolysaccharide (LPS) of Escherichia coli (a TLR4 agonist). Furthermore, H. hepaticus LPS is a soluble compound that is able to antagonize TLR4 activation. The ability of H. hepaticus to actively suppress TLR4-mediated innate immune responses to the endogenous microflora may be an important cofactor in the disturbance of intestinal epithelial homeostasis and decreased intestinal immunotolerance and thereby may provide a possible trigger for chronic inflammatory diseases of the intestinal tract.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. hepaticus strains ATCC 51449 (= 3B1), 94-2423, 95-225, 96-284, and 96-1809 (30, 54) were used for lysate, protein, and LPS preparation and cell infection. H. hepaticus was cultured under specific microaerobic conditions (10% CO2, 80% N2, 10% H2) on blood agar plates (Columbia agar base II; Oxoid, Wesel, Germany) supplemented with 10% horse blood and either containing or not containing the following antibiotics: vancomycin (10 mg/liter), polymyxin B (2,500 U/liter), trimethoprim (5 mg/liter), and amphotericin B (4 mg/liter). Unless indicated otherwise, H. hepaticus strains were preincubated on plates for 24 h at 37°C under microaerobic conditions for the infection assays.
Antibodies and reagents.
A polyclonal antiserum against whole H. hepaticus bacteria was raised using a paraformaldehyde-fixed bacterial preparation in rabbits. Highly purified Salmonella enterica serovar Typhimurium FliC flagellin was kindly provided by Shin-Ichi Aizawa. Ultrapure E. coli LPS (K-12 strain D31m4, a rough strain) was manufactured by List Biological Laboratories and was purchased from Axxora Life Sciences Inc. (San Diego, CA). The mitogen phorbol myristate acetate (PMA) was purchased from Sigma, Pam3Cys-SK4 and Pam3Cys-OH were purchased from EMC Microcollections (Tübingen, Germany), all oligonucleotides, including the stabilized oligonucleotides CpG 1668 and GpC 1668, were purchased from MWG Biotech (Ebersberg, Germany), and N-acetyl-d-glucosaminyl-(β-1,4)-N-acetylmuramyl-l-alanyl-d-isoglutamine (GMDP) was purchased from Calbiochem. All reagents that were used were tested to determine their LPS contents either by the manufacturers or in house (Limulus amebocyte assay) and contained less than 1 IU of LPS. All of the TLR agonists mentioned above were tested by the manufacturers for function and were tested by us in well-established human intestinal cell lines and macrophages (Caco-2 cells, transfected HEK293 cells, and the human U937 macrophage line) for function as innate immune activating agents. Anti-mouse MIP-2 and biotinylated anti-mouse MIP-2 antibodies were purchased from Abcam (Cambridge, MA). Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G antibodies and wheat germ agglutinin-Texas Red were purchased from Molecular Probes (Carlsbad, CA).
Cells and cell cultures.
Mouse liver intestinal epithelial cells (clone NCTC 1469, isolated from a C3H/AN mouse) were obtained from the European Collection of Cell Cultures. m-ICcl2 mouse intestinal crypt cells were kindly provided by A. Vandewalle (7, 27). NCTC 1469 cells were cultured in NCTC 135 medium supplemented with 10% fetal bovine serum (FBS). m-ICcl2 cells were cultured in complex medium that contained growth factors and FBS as described by Bens and coworkers (7). Caco-2 human intestinal epithelial cells, originally isolated from a colon adenocarcinoma, were obtained from the ATCC and were cultivated in Dulbecco modified Eagle medium or buffered RPMI 1640 medium supplemented with 10% FBS. The murine monocyte/macrophage cell line J774 was kindly provided by Stefan Odenbreit and was cultivated in Dulbecco modified Eagle medium supplemented with 10% FBS.
Molecular biology methods.
Cellular RNA was prepared from H. hepaticus- or mock-infected cells using a QIAGEN RNeasy kit, with slight modifications (36). Total RNA was repurified on RNeasy columns after DNase I treatment. Reverse transcription of 1 μg of total RNA was performed at 42°C using the oligo(dT)18 primer for 120 min in 20 μl of reaction buffer. The samples were heated for 15 min at 65°C to end the reaction and were stored at −20°C until analysis. For semiquantitative reverse transcriptase PCR (RT-PCR), 1 to 2.5 μl of cDNA was amplified with gene-specific primers for the mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (a housekeeping gene), genes encoding mouse TLRs, MyD88, and Tollip, and various mouse cytokine genes (primer sequences and cycling conditions are available on request). Quantitative real-time PCR was performed with total cDNA and an ABI Prism 7000 Taqman for mouse GAPDH, interleukin-10 (IL-10), tumor necrosis factor alpha (TNF-α), MIP-2, and IL-12p40 genes using the specific QuantiTect primer assays and a Quantitect SYBR green kit (both obtained from QIAGEN, Germany). Reactions (volume of the reaction mixture, 20 μl) were performed as recommended by the manufacturer, and the results were normalized to the GAPDH transcript values for the samples.
Preparation of H. hepaticus lysates.
Bacteria were harvested from plates that had been incubated for 20 to 48 h by resuspending them in phosphate-buffered saline (PBS) (pH 7.4). The suspensions were sonicated for 3 min at a power setting of 7 (Branson Sonifier) to lyse the bacteria. The protein concentrations of the lysates were determined by a bicinchoninic acid test (Pierce) performed according to the manufacturer's instructions. We determined by counting CFU that 1 μg of lysate corresponded to 1 × 106 bacteria.
Cell infections.
Cells were seeded in 24- or 6-well tissue culture plates. Cell infections and coincubations were performed using exponentially growing cell layers (NCTC liver epithelial cell lines, 50% confluence) and either exponential m-ICcl2 cells (3 days) or confluent m-ICcl2 cells (5 days), which did not differ in terms of activation. Cells were washed three times and were preincubated prior to infection for 30 min in fresh medium with or without serum as indicated below and without any antibiotics. At the time of infection, bacteria, which had been washed and resuspended in fresh cell culture medium, were added at different multiplicities of infection (MOI), as indicated below. When different strains of live bacteria were used, the incubation plates were centrifuged at 500 × g for 3 min to synchronize the infection. After coincubation, the supernatants were removed, cleared by centrifugation, and stored at −20°C. For RNA preparation, cells were scraped from the incubation plates into an appropriate amount of medium and pelleted by centrifugation at 10,000 × g for 1 min. The supernatants were removed, and the cell pellets were flash-frozen in liquid nitrogen before storage at −80°C.
Assays of innate immune activation through PRRs.
The competence of mouse cells for innate immune stimulation was examined by coincubating the cells with various well-defined PRR ligands, including pure bacterial flagellin (S. enterica FliC, a TLR5 ligand), the lipopeptide structural analog Pam3Cys-SK4 (a TLR2 ligand) and the corresponding negative control peptide PAM-3Cys-OH, CpG oligonucleotide 1668 (a mouse-specific TLR9 ligand [26]), ultrapure E. coli LPS (a TLR4 ligand), synthetic NOD2 ligand GMDP (55), and PMA as a nonspecific activating agent (control). The concentrations used are indicated below. For innate immune activation assays, cells were seeded and washed as described above for cell infections. After this, the stimuli were added at the concentrations indicated and for the times indicated in the figure legends. After coincubation, supernatants and cells were harvested as described above for cell infections.
Inhibition studies with murine cells.
J774 and NCTC 1469 cells were seeded as described above for cell infections. m-ICcl2 cells were seeded in 24-well plates precoated with 3 μg/cm2 rat tail collagen at a concentration of 1 × 105 cells per well and incubated for 4 to 6 days. The medium was changed every 2 or 3 days. On the day of the experiment, the cells were washed three times with medium and incubated for 30 min. After this, a 30-min preincubation step with viable H. hepaticus bacteria, lysates, or H. hepaticus LPS was performed. After 30 min, the activating agent, ultrapure E. coli LPS unless indicated otherwise, was added; this agent was present throughout the incubation period. Control wells were maintained without E. coli LPS throughout the incubation period. The plates were centrifuged at the start of the coincubation at 500 × g for 5 min if live bacteria were present and then incubated at 37°C for the time desired. After this, supernatants were harvested as described above for cell infections. All experiments were performed in triplicate. For each experimental condition, at least two independent experiments were performed on different days.
Preparation of H. hepaticus LPS.
Bacteria from plates supplemented or not supplemented with polymyxin B, which had been grown for 24 h, were harvested by resuspension in PBS and centrifugation for 5 min at 15,000 × g. The supernatant was discarded, and the bacteria were dissolved in pyrogen-free sterile water. LPS was purified from H. hepaticus lysates by using the hot phenol method combined with several additional purification steps as previously described (29, 44) in order to remove all of the residual proteins and nucleic acids. Briefly, the bacterial lysate was extracted twice with hot phenol at 68°C for 15 min. The two water phases were combined, dialyzed extensively against pyrogen-free water, and dried. The pellet was resuspended in 10 mM Tris HCl (pH 7.5), 100 μg/ml DNase I, 100 μg/ml RNase I and incubated at 37°C for 3 h. Then 10 μg/ml proteinase K was added, and the mixture was incubated at 65°C for 3 h. After this, the LPS was dried and purified further by cetavlon extraction in 0.2 M NaCl and subsequent ethanol precipitation. The pellet was resuspended in pyrogen-free water and dialyzed once against 0.2 M NaCl and twice against pyrogen-free water. The purified LPS was characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining and compared with controls consisting of ultrapure E. coli LPS. To address the concern that the biosynthesis and physicochemical properties of H. hepaticus LPS may change in the presence of the cationic antimicrobial peptide polymyxin B, we compared the activating potential with NCTC clone 1469 hepatocytes and the inhibitory properties for TLR4 activation in m-ICcl2 cells of LPS preparations obtained from bacteria grown in the presence and in the absence of polymyxin B. All LPS preparations, regardless of the culture conditions, exhibited low activating potential with mouse hepatocytes. Also, the capacity of LPS from bacteria grown on polymyxin-free medium to inhibit TLR4 responses was similar to the capacity of polymyxin-exposed bacteria to inhibit TLR4 responses. On average, the capacity of H. hepaticus lysates to inhibit TLR4 responses was reduced 50% when the organism was grown on polymyxin B-free plates. Purified H. hepaticus LPS was shown by mass spectrometry not to contain polymyxin B. Below, only data obtained with bacteria cultured in the presence of antibiotics, including polymyxin B, are shown.
ELISA to determine cytokine release from cells. (i) IL-8.
An IL-8 enzyme-linked immunosorbent assay (ELISA) was performed by using an IL-8 mouse OptEIA ELISA set (555244; BD Bioscience) according to the manufacturer's instructions. Samples were diluted appropriately. (ii) MIP-2. A MIP-2 ELISA was performed like the IL-8 ELISA, with the following reagents and modifications: the primary antibody was 100 μl of a solution containing 250 ng/ml anti-mouse MIP-2 antibody (PP1082P2; Acris monoclonal antibody), the secondary antibody was 100 μl of a solution containing 100 ng/ml biotinylated anti-mouse MIP-2 antibody (PP1082B2; Acris), and 100 μl of avidin horseradish peroxidase (18-4100-51; Ebioscience) diluted 1:1,000 was used for the enzymatic reaction. Incubation with the secondary antibody and with the avidin horseradish peroxidase was performed in two steps; both steps included 1 h of incubation, and there were five washes between the steps. Means and standard deviations for triplicate experiments and duplicate measurements are shown below.
Immunofluorescence assays.
m-ICcl2 cells were seeded at a concentration of 6 × 104 cells per well in 24-well plates on round 12-mm coverslips precoated with 3 μg/cm2 rat tail collagen, incubated for 3 and 8 days, and then infected with live H. hepaticus at an MOI of 50. After coincubation, nonadherent bacteria were removed, and cells were fixed for 2 h with 2% paraformaldehyde in PBS. Adherent bacteria were detected using anti-H. hepaticus antiserum (1:1,000 in blocking buffer) and a goat anti-rabbit immunoglobulin G antiserum coupled to Alexa Fluor 488 (1:5,000 in blocking buffer). The cells were counterstained for actin using Texas Red-X-phalloidin (1:1,000 in blocking buffer; Molecular Probes) and for DNA using 4′,6′-diamidino-2-phenylindole (DAPI) (1:5,000 in PBS) and were mounted on microscope slides. The specimens were examined with a Leica tcs SP2 confocal microscope.
Statistical analysis.
The significance of the results was calculated by using a paired one-sided t test with different variances, and the results are indicated below. A P value of <0.05 was considered significant.
RESULTS
m-ICcl2 mouse intestinal crypt epithelial cells react only weakly with H. hepaticus and its soluble components, while mouse macrophages and liver epithelial cells have strong proinflammatory responses.
The ecological niche of H. hepaticus in vivo is the cecal and colonic epithelia of mice. Our goal was to simulate the in vivo environment of H. hepaticus and obtain insight into the mechanisms of local immune activation or evasion. Therefore, we established a cell culture model using a recently described immortalized mouse intestinal crypt epithelial cell line from 20-day-old fetuses of L-PK/Tag1 transgenic mice, m-ICcl2 (7). This cell line, directly derived from primary fetal epithelial cells, maintains an intestinal crypt cell phenotype in vitro, including enzyme production, similar to that of primary cells and does not have mutations except for the insertion of the artificially engineered simian virus 40 large and small T-antigen oncogene under the control of a tissue-specific promoter, which drives the proliferation of the cells.
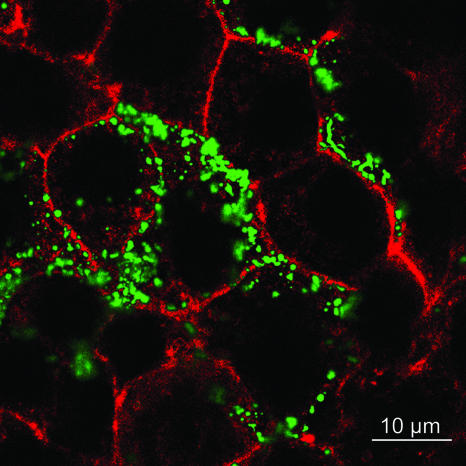
Viable H. hepaticus bacteria exhibited strong adherence to these cells (Fig. 1), suggesting that the cells provide a suitable model system for studying bacterium-cell interactions. When cell-adherent bacteria were counted by microscopic examination after infection at an MOI of 100 bacteria per cell, an average of five adherent bacteria per cell were observed after 1 h of coincubation (50 cells were counted). This low average number did not reflect the real pattern of adherence, since the numbers of adherent bacteria were different for heterogeneous subclusters of cells; some subclusters of cells had a very high bacterial load (approximately 30 bacteria per cell [Fig. 1]), whereas other cells in different morphological clusters had no adherent bacteria. The reason for this cellular heterogeneity in the m-ICcl2 cells is not known, but these cells were originally reported to be morphologically heterogeneous, as demonstrated by a pattern of dome formation in a confluent cell layer (7). Our initial observation with this model was that upon coincubation of live H. hepaticus with these cells for up to 24 h, despite close adherence, there were no detectable cytotoxic effects (data not shown). Nor did bacterial adherence lead to marked activation of the cells, as measured by secretion of the proinflammatory cytokine MIP-2 (Fig. 2A). Lysates of H. hepaticus prepared by ultrasonication likewise activated m-ICcl2 cells only to a very low degree (Fig. 2A), suggesting that all soluble components of H. hepaticus may have a low capacity to activate these cells. However, we observed a strong MIP-2 response to E. coli LPS. We hypothesized that H. hepaticus evades innate immune responses by m-ICcl2 cells or actively suppresses them, since this property may enable the bacteria to persist for a long time in close apposition to the cells without perturbing them. In contrast, viable H. hepaticus and H. hepaticus lysates strongly activated mouse macrophages (J774) and NCTC 1469 hepatocytes to secrete MIP-2 (Fig. 2C). This is consistent with the capacity of this bacterium to elicit innate immune activation and corroborated a recent study in which TLR4- and TLR2-dependent responses to Helicobacter sp. were proposed (39).
FIG. 1.
Adherence of H. hepaticus to intestinal epithelial cells. Murine intestinal cell line m-ICcl2 was coincubated with H. hepaticus at an MOI of 50 for 4 h, after which nonadherent bacteria were removed. Cell membranes labeled with wheat germ agglutinin coupled to Texas Red are red, and bacteria labeled with H. hepaticus-specific antiserum (see Materials and Methods) are green. A representative confocal plane of the specimen is shown as an overlay of green and red fluorescence.
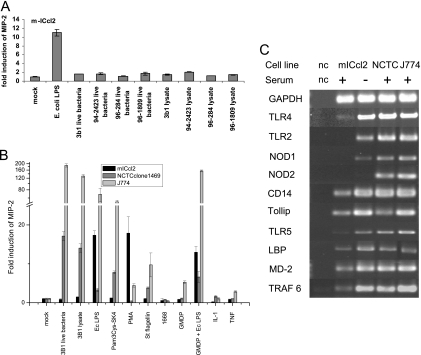
FIG. 2.
Reactivity of murine cell lines with H. hepaticus and different PRR ligands. (A) Cell line m-ICcl2 was coincubated for 4 h with live cells of H. hepaticus strains 3B1, 94-2423, 96-284, and 96-1809 (MOI, 50 bacteria per cell), with H. hepaticus bacterial lysates (25 μg/ml), and with E. coli LPS (Ec LPS) (control; 50 ng/ml). (B) Cell lines m-ICcl2, NCTC 1469, and J774 were coincubated with the following substances: E. coli LPS (10 ng/ml for m-ICcl2 and J774 and 2 ng/ml for NCTC 1469), Pam3Cys-SK4 (2 ng/ml for NCTC and 10 ng/ml for J774 and m-ICcl2), S. enterica serovar Typhimurium FliC flagellin (St flagellin) (100 ng/ml for m-ICcl2 and 200 ng/ml for NCTC 1469 and J774), 2 μM CpG oligonucleotide 1668, GMDP alone (50 μg/ml for m-ICcl2 and 10 μg/ml for NCTC 1469 and J774) or combined with E. coli LPS (10 ng/ml for m-ICcl2 and J774 and 2 ng/ml for NCTC 1469), 20 ng/ml PMA, and the cytokines TNF-α and IL-1β (20 ng/ml for m-ICcl2 and 10 ng/ml for NCTC 1469 and J774). In panels A and B, MIP-2 release into the cell supernatant (see Materials and Methods) is expressed as the fold induction compared to the induction in the mock-infected control. (C) Transcripts of different PRRs and genes involved in TLR signaling in murine cell lines were detected by semiquantitative RT-PCR (see Materials and Methods). Transcripts of m-ICcl2 cells were determined in the presence and in the absence of serum as indicated at the top. nc, negative control.
Intestinal epithelial crypt cells express PRRs and are differentially stimulated by PRR ligands compared to other mouse cells.
The capacities for innate immune responses of mouse intestinal epithelial cell line m-ICcl2 have not been fully characterized. The reaction of these cells to E. coli LPS has been described previously (27), and in another study the workers observed activation by live Toxoplasma gondii (43). Since we observed variable stimulation of three different mouse cell types, m-ICcl2 cells, hepatocytes, and J774 macrophages, with live cells and soluble components of H. hepaticus (Fig. 2A and B), we characterized in a more detailed fashion the responses of these cells to various bacterial components. We used molecules designated microbe-associated molecular patterns in order to determine the roles of specific innate PRRs, including the TLR family, in cell activation (see Materials and Methods). Stimulation of the three mouse cell lines by TLR and NOD2 agonists (see above and Materials and Methods) in the presence of serum (FBS) yielded the following results (Fig. 2B). The intestinal crypt cell line m-ICcl2 did not respond to CpG oligonucleotides (a TLR9 ligand), Pam3Cys-SK4 (a TLR2 ligand), and S. enterica FliC (a TLR5 ligand). The m-ICcl2 cells could be activated only by the TLR4 agonist E. coli LPS, as previously reported (7, 27), and by the mitogen PMA (Fig. 2B). In contrast, NCTC 1469 hepatocytes and J774 macrophages, which were used as controls, were strongly activated by Pam3Cys-SK4, E. coli LPS, and S. enterica FliC (Fig. 2C). All three cell lines exhibited almost no response to the synthetic NOD2 ligand GMDP (Fig. 2B). However, when GMDP was combined with E. coli LPS, a synergistic effect, which led to an approximately twofold increase, was detected with hepatocytes and macrophages. In contrast, signaling in the m-ICcl2 cecal cells was reduced when E. coli LPS was combined with GMDP compared to the signaling in the cells incubated with E. coli LPS alone (Fig. 2B).
In order to match the functional assays with the availability of receptors in the mouse cells, we analyzed the transcripts of the genes encoding TLR PRRs and NOD1/NOD2 and of other essential genes encoding adaptor and signaling proteins in the innate activation pathways. The transcript abundance of these genes was determined by semiquantitative cDNA analysis (RT-PCR) in the absence of activating stimuli (Fig. 2C). In untreated m-ICcl2 cells in the presence of serum, TLR4 and TLR5 mRNAs were expressed, while no transcript was detected for TLR2 (Fig. 2C), which supported the finding that these cells did not respond to the TLR2 agonist in the presence of FBS. In contrast, TLR2 mRNA was detected in m-ICcl2 cells in serum-free medium. The TLR4 coreceptors CD14, MD-2, and LPS-binding protein (LBP) were expressed both in the presence of serum and in the absence of serum. Both the NCTC 1469 hepatocytes and the J774 macrophages expressed mRNAs of TLR2, TLR4, TLR5, and the TLR4 coreceptor CD14. We detected transcription of the negative regulator of TLR signaling Tollip and the downstream mediator of TLR-induced signaling TRAF6 in all three cell lines tested. No transcripts of the peptidoglycan recognition receptors NOD1 and NOD2 were detected in m-ICcl2 cells grown in serum-containing medium, although we detected a small amount of the NOD1 transcript in m-ICcl2 cells when they were cultivated in serum-free medium. The other two cell lines produced both NOD1 and NOD2 receptor mRNAs. This result was consistent with the observed synergistic effect of GMDP with E. coli LPS in the macrophages and the hepatocytes, while the functional synergistic effect was not observed in m-ICcl2 cells.
Effect of isolated H. hepaticus LPS on mouse intestinal crypt epithelial cells.
At this point, we concluded that m-ICcl2 intestinal crypt epithelial cells, which allowed intimate bacterial adherence, seemed almost anergic to H. hepaticus live cells and lysates of these cells and therefore apparently to all soluble factors of the bacteria. This raised the question of why the m-ICcl2 cells were not activated by H. hepaticus soluble factors, although H. hepaticus LPS had been determined previously to be a possible cell-activating factor in vitro (29, 39) and these cells are responsive to the TLR4 ligand (see above) (27). To answer this question, we isolated H. hepaticus LPS from strains 3B1 and 95-225 and determined its activation potential, as measured by MIP-2 secretion, in m-ICcl2 cells and in the other murine cell lines as controls (Fig. 3). Like the bacterial components used in our activation assays, isolated H. hepaticus LPS activated m-ICcl2 cells only weakly. However, it activated the liver epithelial cells more strongly and, to a lesser extent, also the mouse macrophages (Fig. 3).
FIG. 3.
Induction of an innate immune response by purified H. hepaticus LPS in different mouse cell lines. m-ICcl2, NCTC 1469, and J774 cells were coincubated with H. hepaticus strain 3B1 LPS (Hh LPS) (0.6 μg/ml) for 6 h in medium either with or without FBS. 3B1 lysate (25 μg/ml) and E. coli LPS (Ec LPS) (10 ng/ml for m-ICcl2 and J774 and 2 ng/ml for NCTC 1469) were included as controls. The concentration of MIP-2 in the supernatant is expressed as the fold induction compared to the induction in the mock-infected control.
Inhibitory effect of live H. hepaticus and H. hepaticus whole-cell lysates on innate immune stimulation via TLR4.
We next investigated whether H. hepaticus components, which showed low activating potential, may have an inhibitory effect on innate immune activation of intestinal epithelial cells. We first coincubated m-ICcl2 cells with live H. hepaticus or lysates and, after a 30-min preincubation step, added a TLR4-activating ligand, ultrapure E. coli LPS. Since we and other researchers (27) observed maximal activation (MIP-2 secretion) of the cells by E. coli LPS at 4 to 6 h (data not shown), we used these times to assess inhibition. Live bacteria and H. hepaticus lysates of different strains inhibited TLR4-mediated responses by m-ICcl2 cells to similar extents (as determined by MIP-2 release) (Fig. 4A). In general, MIP-2 secretion was reduced by 30 to 70% in the cells coincubated with both E. coli LPS and live H. hepaticus or lysate compared to the secretion in cells incubated with E. coli LPS alone, when 10 ng E. coli LPS and 25 μg H. hepaticus lysate were used (Fig. 4A). This inhibitory effect was highly reproducible. This result suggested that in addition to live bacteria, soluble components of H. hepaticus may inhibit innate immune responses of mouse intestinal epithelial cells. As a control, m-ICcl2 cells were coincubated with PMA after preincubation with live H. hepaticus or lysate. This combination did not result in inhibition by H. hepaticus molecules, but there was a slight increase in MIP-2 secretion (data not shown). Sustained activation of the cells by PMA in the presence of lysed or live H. hepaticus, in addition to confirming the low cytoxicity of H. hepaticus bacteria or lysates (compared to control levels) (data not shown), suggested that the viability or signaling capacities of the m-ICcl2 cells in general were not negatively affected by coincubation with H. hepaticus bacteria and lysates. The level of inhibition of MIP-2 secretion by both live H. hepaticus and bacterial lysates after E. coli LPS stimulation (TLR4-mediated activation) was dependent on the ratio of H. hepaticus live bacteria or lysate to the activating agent E. coli LPS (Fig. 4A). In both J774 and NCTC 1469 cells, which were used as controls, no inhibition resulting from preincubation with H. hepaticus whole bacteria and lysates was observed after E. coli LPS stimulation (not shown), and both these cell lines were highly activated by H. hepaticus live bacteria and lysates alone (Fig. 2B).
FIG. 4.
Inhibitory effects of H. hepaticus live bacteria, lysates, and LPS on E. coli LPS-induced stimulation of m-ICcl2 cells. (A) m-ICcl2 cells were coincubated for 4 h with different amounts of E. coli LPS and antagonized with 5, 25, or 100 μg/ml 3B1 lysate or with live bacteria at an MOI of 25 or 100 bacteria per cell. (B) m-ICcl2 cells were stimulated with either 10 ng/ml of E. coli LPS (Ec LPS) alone or 10 ng/ml of E. coli LPS combined with 25 μg/ml H. hepaticus 3B1 lysate (Hh lysate), 0.6 μg/ml H. hepaticus 3B1 LPS, or 0.15 μg/ml H. hepaticus 95-225 LPS for 6 h in medium with or without 2% FBS. The concentration of MIP-2 in the supernatant is expressed as the fold induction compared to the induction in the mock-infected control. An asterisk indicates that the P value is <0.01, and a number sign indicates that the P value is <0.05.
H. hepaticus LPS is one soluble component responsible for inhibition of TLR4-mediated responses of mouse intestinal epithelial cells.
m-ICcl2 mouse intestinal epithelial cells are not responsive to agonists of TLR2, TLR5, NOD2, and TLR9. They were activated only slightly by H. hepaticus live bacteria, heat-killed bacteria, H. hepaticus bacterial lysates, or H. hepaticus LPS. However, live H. hepaticus or lysates inhibited innate immune responses elicited in m-ICcl2 cells by the TLR4 agonist E. coli LPS. These inhibition results suggested that H. hepaticus LPS and/or other soluble H. hepaticus components are able to suppress TLR4-mediated responses of mouse intestinal epithelial cells.
Therefore, we repeated the experiments in which activation by E. coli LPS was inhibited, and we replaced the preincubation with live bacteria and lysates by preincubation with purified H. hepaticus LPS preparations (approximately 15- to 100-fold excess) before coincubation with E. coli LPS. After both 6 and 24 h of coincubation in the presence or absence of serum (2% FBS), reduced MIP-2 secretion in m-ICcl2 cells was observed when the activating agent E. coli LPS was combined with H. hepaticus LPS prepared from two different strains (preincubation and coincubation) compared to the secretion observed for cells coincubated with E. coli LPS alone (Fig. 4B). This result was reproduced in more than three independent experiments. The inhibitory effect of H. hepaticus LPS on E. coli LPS activation of m-ICcl2 cells was concentration dependent (not shown). We also observed inhibition by H. hepaticus lysates and LPS of m-ICcl2 activation after the cells were challenged with heat-inactivated E. coli K-12 whole bacteria (data not shown).
In contrast, in several independent experiments with J774 macrophages, we observed no inhibitory effect of H. hepaticus LPS after stimulation by E. coli LPS in the presence or absence of fetal calf serum (data not shown). In NCTC 1469 cells, a slight inhibitory effect of H. hepaticus LPS was observed, which was more pronounced in the absence of serum (not shown).
H. hepaticus lysate inhibits development of endotoxin tolerance and promotes increased susceptibility to E. coli LPS in intestinal epithelial cells.
As described previously, coincubation of m-ICcl2 cells with E. coli LPS leads to reduced susceptibility to a second challenge with E. coli LPS (28). Therefore, we examined whether components of H. hepaticus inhibit the development of tolerance to LPS in this cell line. We confirmed that an initial challenge with E. coli LPS led to reduced MIP-2 secretion when a second challenge with E. coli LPS was applied (Fig. 5). However, when the cells were first challenged with H. hepaticus lysate combined with E. coli LPS, the susceptibility to a second challenge with E. coli LPS was not reduced. In contrast, coincubation with H. hepaticus lysate alone or combined with E. coli LPS resulted in increased susceptibility to a second challenge with E. coli LPS (Fig. 5). An initial application of purified H. hepaticus LPS alone also did not promote development of endotoxin tolerance to a subsequent challenge with E. coli LPS (Fig. 5).
FIG. 5.
Inhibition of the development of endotoxin tolerance in m-ICcl2 cells by H. hepaticus. m-ICcl2 cells were coincubated for 6 h with the following stimuli alone or in combination: mock infection, E. coli LPS (Ec LPS) (10 ng/ml), H. hepaticus LPS (Hh LPS) (0.6 μg/ml), and H. hepaticus lysate (Hh lysate) (25 μg/ml). Then the supernatants were harvested (first coincubation), and the cells were washed three times and incubated for 16 h in serum-containing medium. After this, the cells were coincubated for another 6 h with 10 ng/ml E. coli LPS, and the supernatants were collected (second coincubation). The bars indicate the mean MIP-2 concentrations in the supernatants after the first and second coincubations for triplicate experiments, and the error bars indicate the standard deviations. An asterisk indicates coincubation conditions that resulted in a significant change (P < 0.01, as determined by an unpaired, one-sided t test) in MIP-2 induction after the second E. coli LPS stimulation compared to cells that were mock infected during the first coincubation.
Determination of amounts of cytokine transcripts in m-ICcl2 cells after coincubation with H. hepaticus soluble factors alone or with E. coli LPS.
We next examined whether the results obtained by measuring MIP-2 protein secretion in m-ICcl2 cells could be supported by determining the amounts of transcripts of cytokines (IL-10, TNF-α, MIP-2, and IL-12p40) by real-time PCR after coincubation of m-ICcl2 cells under serum-free conditions with combinations of E. coli LPS and H. hepaticus preparations (Fig. 6).
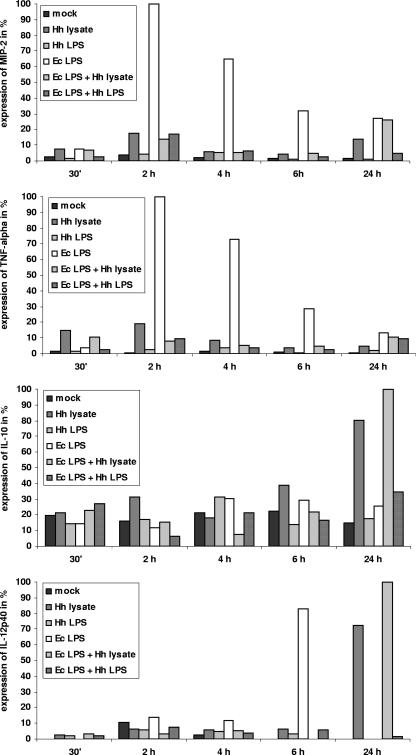
FIG. 6.
Amounts of transcripts (as determined by real-time PCR) of marker cytokine genes (MIP-2, TNF-α, IL-10, IL-12p40) after coincubation with E. coli LPS alone or in combination with H. hepaticus lysates or LPS in m-ICcl2 cells. Real-time PCRs were performed (see Materials and Methods) with cDNA of m-ICcl2 cells coincubated under different conditions for 30 min or 2, 4, 6, or 24 h. Coincubations were performed with 25 μg/ml 3B1 lysate (Hh lysate), 0.75 μg/ml 3B1 LPS (Hh LPS), 50 ng/ml E. coli LPS (Ec LPS), 50 ng/ml E. coli LPS and 25 μg/ml 3B1 lysate, or 50 ng/ml E. coli LPS and 0.75 μg/ml 3B1 LPS. The amounts of transcripts are expressed as percentages relative to the highest induction value in each panel, which was defined as 100%.
The expression of MIP-2 mRNA was time dependent and at 2 to 6 h was highest in cells coincubated with E. coli LPS alone (Fig. 6). The level of the MIP-2 transcript was much lower in m-ICcl2 cells cocultured with H. hepaticus lysate or LPS alone than in m-ICcl2 cells incubated with E. coli LPS. Similar to the protein results, the level of the MIP-2 transcript was lower in cells coincubated with E. coli LPS along with H. hepaticus lysate or LPS (reductions of 83, 90, and 92% at 2, 4, and 6 h, respectively, for H. hepaticus LPS) than in cells incubated with E. coli LPS alone (Fig. 6). For the TNF-α transcript, weak expression was detected when cells were coincubated with E. coli LPS alone (Fig. 6), although the amount of TNF-α protein secreted was below the detection limit of our ELISA system (not shown). The levels of TNF-α transcripts were reduced like the levels of MIP-2 transcripts when E. coli LPS was combined with H. hepaticus lysate (92% reduction at 2 h) or H. hepaticus LPS (90% reduction at 2 h) (Fig. 6). The amounts of the transcripts of the TLR4 and TLR5 receptor genes, the Tollip and TRAF6 TLR downstream adaptor genes, and the MD-2, CD14, and LBP cofactor genes were not regulated under any of these conditions (not shown). The transcript profiles of IL-10 and IL-12p40 (a subunit of both IL-12 and IL-23) were distinct at later times. The IL-10 transcript was not significantly upregulated except at 24 h when E. coli LPS was combined with H. hepaticus lysate or when H. hepaticus lysate alone was used (Fig. 6). The IL-12p40 transcript was almost not regulated up to 6 h for all cell incubation conditions except E. coli LPS alone. While the IL-12p40 transcript was undetectable in the presence of E. coli LPS at 24 h, addition of H. hepaticus lysate alone or combined with E. coli LPS led to sustained upregulation of IL-12p40 at 24 h.
Inhibition of TLR5-induced proinflammatory responses in human intestinal epithelial cell lines by H. hepaticus.
Our next goal was to determine if the inhibitory activity exhibited by H. hepaticus was specific for TLR4 activation. Since m-ICcl2 cells were activated only via TLR4 and NCTC 1469 and J774 cells were strongly activated by multiple PRR ligands and by H. hepaticus (live, lysed, or isolated LPS), all of these cells were not suitable for defining more specifically the cellular target(s) or pathway of the inhibitory activity of H. hepaticus LPS. Furthermore, IL-1β or TNF-α did not activate m-ICcl2 cells sufficiently (Fig. 2B); thus, these conditions also could not be used to determine the specificity of signaling inhibition by H. hepaticus and its components for TLR4 or the TLR-IL-1 receptor pathway. In order to analyze the specificity of the inhibitory effects of H. hepaticus LPS, we used Caco-2 human colon epithelial cells. In contrast to m-ICcl2 cells, this cell line can be stimulated to secrete chemokines, such as IL-8, via flagellin/TLR5 stimulation but, like most other human intestinal epithelial cells, is not activated via TLR4 or TLR2 (36; unpublished results). In contrast to H. hepaticus lysates, which activated Caco-2 cells approximately 40-fold (not shown), isolated H. hepaticus LPS did not induce IL-8 chemokine secretion (used as an activation marker) in these cells (Fig. 7). Inhibition of Caco-2 cell activation by H. hepaticus LPS after flagellin (S. enterica serovar Typhimurium FliC, a TLR5 agonist) stimulation did not occur in the presence of serum (10% FBS), which suggested that the inhibitory effect of H. hepaticus LPS may be specific for TLR4 activation. However, when we coincubated FliC flagellin with Caco-2 cells in combination with H. hepaticus LPS under serum-free conditions, induction of IL-8 secretion via TLR5 was almost completely abolished (Fig. 7).
FIG. 7.
Inhibition of flagellin-induced innate immune response by H. hepaticus LPS in human Caco-2 cells. Caco-2 cells were coincubated with either 10 ng/ml S. enterica serovar Typhimurium flagellin or 50 ng/ml PMA alone or combined with 0.6 μg/ml 3B1 LPS (Hh LPS) for 6 h. As controls, the cells were also coincubated with 25 μg/ml 3B1 lysate or 0.6 μg/ml 3B1 LPS alone. The level of IL-8 in the supernatant is expressed as the fold induction compared to the induction in the mock-infected control. The asterisk indicates that the P value is <0.01.
DISCUSSION
In the present study, we established an in vitro epithelial cell culture model to mimic natural H. hepaticus infection in mice, using m-ICcl2 immortalized primary mouse intestinal epithelial cells that have a crypt phenotype (7). H. hepaticus exhibited very good adherence to this cell line, which is consistent with the natural habitat of this bacterium, which is found in high numbers close to the epithelia of intestinal crypts in the mouse cecum. Interestingly, these mouse intestinal epithelial cells exhibited very weak innate immune reactions to H. hepaticus infection, which stimulated us to study the potential immunomodulatory or immunoevasive activities of H. hepaticus with these intestinal epithelial cells further. It has been established previously by us and other workers that Helicobacter species and related species have specific and general mechanisms for innate immune evasion and suppression, including evasion of TLR5 (3, 10, 11, 59). H. hepaticus is able to activate via TLR2, which appears to be the most important PRR for live Helicobacter species in vivo (39). TLR2 and TLR4 PRRs generally exhibit limited activity in intestinal epithelial cells from adults (6, 42, 45) but are quite active in immune cells (e.g., macrophages).
The m-ICcl2 intestinal epithelial cells did not exhibit activation via TLR2, but they exhibited a response to the TLR4 ligand. These cells exhibited very weak activation by live intact H. hepaticus cells, bacterial lysates, or H. hepaticus LPS of different strains, indicating that the TLR4-stimulating activity of H. hepaticus and its LPS is weak, which is consistent with previous reports which showed that the activity of Helicobacter LPS was low compared to the activities of other types of bacterial LPS (10, 11). The structures of H. hepaticus LPS and lipid A, which are not known in detail, seem to be different from the structures of Helicobacter pylori LPS and lipid A in terms of the lipid content (shorter-chain fatty acids) (29). Among the LPS from the helicobacters, LPS from H. hepaticus and other enterohepatic helicobacters exhibited the lowest activity in a Limulus amebocyte assay (29). H. hepaticus LPS activated mouse hepatocytes and macrophages slightly more than it activated m-ICcl2 cells. There is conflicting evidence concerning whether LPS of the closely related bacterium H. pylori activates via TLR4/CD14 or via TLR2 (11, 37, 52, 57). Convincing results of studies of mouse embryonal fibroblasts, derived from mice deficient in either TLR4 or TLR2, have indicated that H. pylori LPS activates via TLR4 (39), which may also be true for the LPS of other Helicobacter species.
One very important property of H. hepaticus and its soluble component LPS emerged when our model of intestinal epithelial cells was used: innate immune responses to the known TLR4 ligand E. coli LPS were inhibited by H. hepaticus live intact bacteria, bacterial lysates, and isolated H. hepaticus LPS. The inhibition of TLR4-induced responses in m-ICcl2 cells by live bacteria and lysates was independent of the known H. hepaticus virulence factor CDT (60) and the pathogenicity island HHGI1, a 70-kb genomic island containing genes of a type IV secretion system that has been implicated in disease severity (12, 60;T. Sterzenbach, Z. Ge, S. Suerbaum, and J. G. Fox, unpublished data). In conclusion, we identified H. hepaticus LPS (a highly purified preparation) as an active soluble molecule that can inhibit TLR4 activation. Strikingly, preincubation with H. hepaticus lysate and LPS prevented induction of endotoxin tolerance (27, 41) in our intestinal cell model or even enhanced the response to a second challenge with E. coli LPS. In addition, H. hepaticus LPS, in the absence of externally added serum, inhibited TLR5-mediated innate responses by intestinal epithelial cells of human origin, which do not possess active TLR4. This result suggests that the inhibitory capacity inherent in H. hepaticus LPS is not restricted to TLR4 activation. The inhibition of FliC/TLR5 signaling in the absence of serum but not in the presence of serum and the stronger inhibition of TLR4 responses in the absence of serum suggested that serum components may be able to bind and neutralize H. hepaticus LPS, thereby preventing its inhibitory activity. This may also imply that a cell-bound component that is similar to a soluble serum factor may be a receptor for H. hepaticus LPS.
At present, the mechanism of inhibition of TLR4 and TLR5 signaling by H. hepaticus LPS is not clear. The inhibition of TLR4-mediated responses of cells may occur either via competitive inhibition of binding/activation of E. coli LPS, TLR4, or other components of the TLR4 signaling complex or via a TLR4-independent inhibitory effect on innate immune responses. The hypothesis that there is competitive binding to components of the TLR4 signaling complex by H. hepaticus LPS in the absence of activation is not supported by the inhibition of TLR5 activation in Caco-2 cells, which do not possess active TLR4. We currently favor the hypothesis that the innate immune inhibition by H. hepaticus LPS and possibly other soluble components is mediated by an alternative receptor independent of TLRs or simply by changes in cell membrane signaling properties after incorporation of H. hepaticus LPS. For instance, H. hepaticus LPS may bind to and inhibit through lectin-type PRRs, similar to H. pylori LPS, which binds to the PRRs DC-SIGN and collectin in a strain-specific and variable manner (8, 31). Among the other gastrointestinal pathogenic bacteria, only H. pylori has been found to contain LPS that is able to inhibit TLR4 signaling by an as-yet-unknown mechanism, which is strain dependent (37). Better-studied examples of bacteria whose LPS or other soluble components suppress TLR4 signaling include dental bacterial pathogens. Porphyromonas gingivalis LPS inhibits the TLR4 signaling complex primarily by competitive binding of the cofactor MD2 (16). Treponema socranskii and Treponema medium use molecules similar to LPS or glycolipids for inhibition of TLR4 activation mainly through LBP/CD14-dependent mechanisms (5, 35, 50). A known structural feature of LPS of both H. pylori and dental pathogens that very likely contributes to low activity is a hypoacylated lipid A (penta- or tetraacylated). PRRs that recognize bacterial peptidoglycan, particularly NOD2, which has been implicated in chronic inflammatory diseases, such as Crohn's disease (1, 15, 25), apparently did not play a decisive role in our mouse intestinal epithelial cell model system.
In the natural setting, the inhibitory activity of H. hepaticus could indicate that innate beneficial activating responses to the commensal flora, which are thought to promote intestinal epithelial homeostasis and induction of tolerance by downmodulation of excessive inflammation (47), may be dampened. This may be one mechanism that triggers the onset of chronic inflammatory disease of the intestinal epithelium after infection by certain bacteria. In support of this hypothesis, innate m-ICcl2 responses to a challenge with heat-inactivated E. coli K-12 whole bacteria were also inhibited by H. hepaticus. A similar perturbation of the homeostasis of intestinal epithelia was observed by Horwitz and colleagues (18, 56), who used an inverse strategy and showed that suppression of innate immune responses by genetic NF-κB deficiency or by NF-κB inhibition (in RAG(−/−) mice) promoted typhlocolitis and colitis in mice. This concept is also consistent with the observed activity of H. hepaticus infection in animal models, particularly in immunocompromised mice which lack IL-10 or T/B cells (resulting in a lack of adaptive immune responses), in which H. hepaticus infection leads to colitis, inflammatory bowel syndrome-like disease, and even colon cancer (13, 19, 58). There was sustained upregulation of the immunomodulatory cytokines IL-10 and IL-12p40 in our model cells by H. hepaticus soluble factors, and downmodulation of IL-12p40 by E. coli LPS was counteracted by H. hepaticus lysate. This is a very interesting finding in light of recently described evidence that IL-12p40, as a subunit of both IL-12 and IL-23, plays a dominant role during development of experimental inflammatory bowel disease in mice caused by H. hepaticus (28, 32) and may also have a role in human inflammatory bowel disease (17). H. hepaticus has not been isolated from humans, but other species of enterohepatic helicobacters, such as Helicobacter bilis, Helicobacter fennelliae, and Helicobacter pullorum, do colonize humans and may contribute to chronic inflammatory conditions of the intestinal tract by similar mechanisms (2, 40). Indeed, we found that lysates of H. bilis and several H. pylori strains inhibited TLR4-induced responses in the m-ICcl2 cell model (unpublished results). So far, no particular bacterium has been identified as the sole infectious agent in chronic inflammation of the intestinal tract, but a variety of bacteria which may inhibit beneficial activities of the commensal flora and promote inflammatory disease have been implicated. By using similar mechanisms for innate immune evasion and suppression, different bacteria in the oral cavity and in the respiratory and intestinal tracts which can persist close to epithelial cells due to their properties may be able to change regulatory T-cell responses towards a Th1-type response in order to persist and at the same time may be able to trigger chronic inflammation.
Acknowledgments
We are very grateful to Alain Vandewalle for the kind gift of the m-ICcl2 mouse intestinal crypt cells and to Stefan Odenbreit for sharing the J774 macrophage line. Patrick Olbermann and Mathias Hornef are acknowledged for fruitful discussions.
Financial support by the German Ministry for Education and Research (Network PathoGenoMik), the German Research Council (grant SFB621/B8), and the U.S. National Institutes of Health (grant NIH RO1CA67529) is gratefully acknowledged.
Editor: F. C. Fang
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 174:4453-4460. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, L. P. 2001. New Helicobacter species in humans. Dig. Dis. 19:112-115. [DOI] [PubMed] [Google Scholar]
- 3.Andersen-Nissen, E., K. D. Smith, K. L. Strobe, S. L. Barrett, B. T. Cookson, S. M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA 102:9247-9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apostolov, E., W. A. Al Soud, I. Nilsson, I. Kornilovska, V. Usenko, V. Lyzogubov, Y. Gaydar, T. Wadstrom, and A. Ljungh. 2005. Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand. J. Gastroenterol. 40:96-102. [DOI] [PubMed] [Google Scholar]
- 5.Asai, Y., M. Hashimoto, and T. Ogawa. 2003. Treponemal glycoconjugate inhibits Toll-like receptor ligand-induced cell activation by blocking LPS-binding protein and CD14 functions. Eur. J. Immunol. 33:3196-3204. [DOI] [PubMed] [Google Scholar]
- 6.Backhed, F., B. Rokbi, E. Torstensson, Y. Zhao, C. Nilsson, D. Seguin, S. Normark, A. M. Buchan, and A. Richter-Dahlfors. 2003. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J. Infect. Dis. 187:829-836. [DOI] [PubMed] [Google Scholar]
- 7.Bens, M., A. Bogdanova, F. Cluzeaud, L. Miquerol, S. Kerneis, J. P. Kraehenbuhl, A. Kahn, E. Pringault, and A. Vandewalle. 1996. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am. J. Physiol. 270:C1666-C1674. [DOI] [PubMed] [Google Scholar]
- 8.Bergman, M. P., A. Engering, H. H. Smits, S. J. van Vliet, A. A. van Bodegraven, H. P. Wirth, M. L. Kapsenberg, C. M. Vandenbroucke-Grauls, Y. van Kooyk, and B. J. Appelmelk. 2004. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200:979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielaszewska, M., B. Sinha, T. Kuczius, and H. Karch. 2005. Cytolethal distending toxin from Shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation, and death of human endothelial cells. Infect. Immun. 73:552-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkholz, S., U. Knipp, C. Nietzki, R. J. Adamek, and W. Opferkuch. 1993. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol. Med. Microbiol. 6:317-324. [DOI] [PubMed] [Google Scholar]
- 11.Bliss, C. M., Jr., D. T. Golenbock, S. Keates, J. K. Linevsky, and C. P. Kelly. 1998. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect. Immun. 66:5357-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutin, S. R., Z. Shen, A. B. Rogers, Y. Feng, Z. Ge, S. Xu, T. Sterzenbach, C. Josenhans, D. B. Schauer, S. Suerbaum, and J. G. Fox. 2005. Different Helicobacter hepaticus strains with variable genomic content induce various degrees of hepatitis. Infect. Immun. 73:8449-8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burich, A., R. Hershberg, K. Waggie, W. Zeng, T. Brabb, G. Westrich, J. L. Viney, and L. Maggio-Price. 2001. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G764-G778. [DOI] [PubMed] [Google Scholar]
- 14.Cahill, R. J., C. J. Foltz, J. G. Fox, C. A. Dangler, F. Powrie, and D. B. Schauer. 1997. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect. Immun. 65:3126-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cario, E. 2005. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut 54:1182-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coats, S. R., T. T. Pham, B. W. Bainbridge, R. A. Reife, and R. P. Darveau. 2005. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J. Immunol. 175:4490-4498. [DOI] [PubMed] [Google Scholar]
- 17.Duerr, R. H., K. D. Taylor, S. R. Brant, J. D. Rioux, M. S. Silverberg, M. J. Daly, A. H. Steinhart, C. Abraham, M. Regueiro, A. Griffiths, T. Dassopoulos, A. Bitton, H. Yang, S. Targan, L. W. Datta, E. O. Kistner, L. P. Schumm, A. T. Lee, P. K. Gregersen, M. M. Barmada, J. I. Rotter, D. L. Nicolae, and J. H. Cho. 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314:1461-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdman, S., J. G. Fox, C. A. Dangler, D. Feldman, and B. H. Horwitz. 2001. Typhlocolitis in NF-kappa B-deficient mice. J. Immunol. 166:1443-1447. [DOI] [PubMed] [Google Scholar]
- 19.Erdman, S. E., T. Poutahidis, M. Tomczak, A. B. Rogers, K. Cormier, B. Plank, B. H. Horwitz, and J. G. Fox. 2003. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 162:691-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox, J. G. 2002. The non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut 50:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox, J. G., F. E. Dewhirst, Z. Shen, Y. Feng, N. S. Taylor, B. J. Paster, R. L. Ericson, C. N. Lau, P. Correa, J. C. Araya, and I. Roa. 1998. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology 114:755-763. [DOI] [PubMed] [Google Scholar]
- 22.Fox, J. G., F. E. Dewhirst, J. G. Tully, B. J. Paster, L. Yan, N. S. Taylor, M. J. J. Collins, P. L. Gorelick, and J. M. Ward. 1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 32:1238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horwitz, and S. E. Erdman. 2004. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge, Z., Y. Feng, M. T. Whary, P. R. Nambiar, S. Xu, V. Ng, N. S. Taylor, and J. G. Fox. 2005. Cytolethal distending toxin is essential for Helicobacter hepaticus colonization in outbred Swiss Webster mice. Infect. Immun. 73:3559-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helio, T., L. Halme, M. Lappalainen, H. Fodstad, P. Paavola-Sakki, U. Turunen, M. Farkkila, T. Krusius, and K. Kontula. 2003. CARD15/NOD2 gene variants are associated with familially occurring and complicated forms of Crohn's disease. Gut 52:558-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 27.Hornef, M. W., B. H. Normark, A. Vandewalle, and S. Normark. 2003. Intracellular recognition of lipopolysaccharide by Toll-like receptor 4 in intestinal epithelial cells. J. Exp. Med. 198:1225-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hue, S., P. Ahern, S. Buonocore, M. C. Kullberg, D. J. Cua, B. S. McKenzie, F. Powrie, and K. J. Maloy. 2006. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203:2473-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynes, S. O., J. A. Ferris, B. Szponar, T. Wadstrom, J. G. Fox, J. O'Rourke, L. Larsson, E. Yaquian, A. Ljungh, M. Clyne, L. P. Andersen, and A. P. Moran. 2004. Comparative chemical and biological characterization of the lipopolysaccharides of gastric and enterohepatic helicobacters. Helicobacter 9:313-323. [DOI] [PubMed] [Google Scholar]
- 30.Josenhans, C., K. A. Eaton, T. Thevenot, and S. Suerbaum. 2000. Switching of flagellar motility in Helicobacter pylori by reversible length variation of a short homopolymeric sequence repeat in fliP, a gene encoding a basal body protein. Infect. Immun. 68:4598-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khamri, W., A. P. Moran, M. L. Worku, Q. N. Karim, M. M. Walker, H. Annuk, J. A. Ferris, B. J. Appelmelk, P. Eggleton, K. B. Reid, and M. R. Thursz. 2005. Variations in Helicobacter pylori lipopolysaccharide to evade the innate immune component surfactant protein D. Infect. Immun. 73:7677-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullberg, M. C., D. Jankovic, C. G. Feng, S. Hue, P. L. Gorelick, B. S. McKenzie, D. J. Cua, F. Powrie, A. W. Cheever, K. J. Maloy, and A. Sher. 2006. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203:2485-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kullberg, M. C., A. G. Rothfuchs, D. Jankovic, P. Caspar, T. A. Wynn, P. L. Gorelick, A. W. Cheever, and A. Sher. 2001. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect. Immun. 69:4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 35.Lee, S. H., K. K. Kim, I. C. Rhyu, S. Koh, D. S. Lee, and B. K. Choi. 2006. Phenol/water extract of Treponema socranskii subsp. socranskii as an antagonist of Toll-like receptor 4 signalling. Microbiology 152:535-546. [DOI] [PubMed] [Google Scholar]
- 36.Lee, S. K., A. Stack, E. Katzowitsch, S. I. Aizawa, S. Suerbaum, and C. Josenhans. 2003. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 5:1345-1356. [DOI] [PubMed] [Google Scholar]
- 37.Lepper, P. M., M. Triantafilou, C. Schumann, E. M. Schneider, and K. Triantafilou. 2005. Lipopolysaccharides from Helicobacter pylori can act as antagonists for Toll-like receptor 4. Cell. Microbiol. 7:519-528. [DOI] [PubMed] [Google Scholar]
- 38.Maloy, K. J., L. R. Antonelli, M. Lefevre, and F. Powrie. 2005. Cure of innate intestinal immune pathology by CD4+ CD25+ regulatory T cells. Immunol. Lett. 97:189-192. [DOI] [PubMed] [Google Scholar]
- 39.Mandell, L., A. P. Moran, A. Cocchiarella, J. Houghton, N. Taylor, J. G. Fox, T. C. Wang, and E. A. Kurt-Jones. 2004. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via Toll-like receptor 2 but not Toll-like receptor 4. Infect. Immun. 72:6446-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsukura, N., S. Yokomuro, S. Yamada, T. Tajiri, T. Sundo, T. Hadama, S. Kamiya, Z. Naito, and J. G. Fox. 2002. Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn. J. Cancer Res. 93:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medvedev, A. E., A. Lentschat, L. M. Wahl, D. T. Golenbock, and S. N. Vogel. 2002. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 169:5209-5216. [DOI] [PubMed] [Google Scholar]
- 42.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 43.Mennechet, F. J., L. H. Kasper, N. Rachinel, W. Li, A. Vandewalle, and D. Buzoni-Gatel. 2002. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J. Immunol. 168:2988-2996. [DOI] [PubMed] [Google Scholar]
- 44.Moran, A. P., I. M. Helander, and T. U. Kosunen. 1992. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J. Bacteriol. 174:1370-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naik, S., E. J. Kelly, L. Meijer, S. Pettersson, and I. R. Sanderson. 2001. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J. Pediatr. Gastroenterol. Nutr. 32:449-453. [DOI] [PubMed] [Google Scholar]
- 46.Pierik, M., S. Joossens, K. Van Steen, N. Van Schuerbeek, R. Vlietinck, P. Rutgeerts, and S. Vermeire. 2006. Toll-Like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm. Bowel Dis. 12:1-8. [DOI] [PubMed] [Google Scholar]
- 47.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229-241. [DOI] [PubMed] [Google Scholar]
- 48.Rimoldi, M., M. Chieppa, V. Salucci, F. Avogadri, A. Sonzogni, G. M. Sampietro, A. Nespoli, G. Viale, P. Allavena, and M. Rescigno. 2005. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6:507-514. [DOI] [PubMed] [Google Scholar]
- 49.Sarma-Rupavtarm, R. B., Z. Ge, D. B. Schauer, J. G. Fox, and M. F. Polz. 2004. Spatial distribution and stability of the eight microbial species of the altered Schaedler flora in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 70:2791-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroder, N. W., B. Opitz, N. Lamping, K. S. Michelsen, U. Zahringer, U. B. Gobel, and R. R. Schumann. 2000. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J. Immunol. 165:2683-2693. [DOI] [PubMed] [Google Scholar]
- 51.Silva, C. P., J. C. Pereira-Lima, A. G. Oliveira, J. B. Guerra, D. L. Marques, L. Sarmanho, M. M. Cabral, and D. M. Queiroz. 2003. Association of the presence of Helicobacter in gallbladder tissue with cholelithiasis and cholecystitis. J. Clin. Microbiol. 41:5615-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552-32560. [DOI] [PubMed] [Google Scholar]
- 53.Solnick, J. V., and D. B. Schauer. 2001. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin. Microbiol. Rev. 14:59-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suerbaum, S., C. Josenhans, T. Sterzenbach, B. Drescher, P. Brandt, M. Bell, M. Droege, B. Fartmann, H.-P. Fischer, Z. Ge, A. Hörster, R. Holland, K. Klein, J. König, L. Macko, G. L. Mendz, G. Nyakatura, D. B. Schauer, Z. Shen, J. Weber, M. Frosch, and J. G. Fox. 2003. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 100:7901-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tada, H., S. Aiba, K. Shibata, T. Ohteki, and H. Takada. 2005. Synergistic effect of Nod1 and Nod2 agonists with Toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 73:7967-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomczak, M. F., S. E. Erdman, T. Poutahidis, A. B. Rogers, H. Holcombe, B. Plank, J. G. Fox, and B. H. Horwitz. 2003. NF-kappa B is required within the innate immune system to inhibit microflora-induced colitis and expression of IL-12 p40. J. Immunol. 171:1484-1492. [DOI] [PubMed] [Google Scholar]
- 57.Torok, A. M., A. H. Bouton, and J. B. Goldberg. 2005. Helicobacter pylori induces interleukin-8 secretion by Toll-like receptor 2- and Toll-like receptor 5-dependent and -independent pathways. Infect. Immun. 73:1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whary, M. T., and J. G. Fox. 2004. Natural and experimental Helicobacter infections. Comp. Med. 54:128-158. [PubMed] [Google Scholar]
- 59.Yokoyama, K., H. Higashi, S. Ishikawa, Y. Fujii, S. Kondo, H. Kato, T. Azuma, A. Wada, T. Hirayama, H. Aburatani, and M. Hatakeyama. 2005. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc. Natl. Acad. Sci. USA 102:9661-9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young, V. B., K. A. Knox, J. S. Pratt, J. S. Cortez, L. S. Mansfield, A. B. Rogers, J. G. Fox, and D. B. Schauer. 2004. In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infect. Immun. 72:2521-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]