Abstract
Leishmanization is the inoculation of live Leishmania into the host to vaccinate against subsequent infections. This approach has been largely discontinued due to safety concerns. We have previously shown that combining CD40 ligand (CD40L) with Leishmania antigen preferentially induces a type 1 immune response and provides some protection to vaccinated mice (G. Chen, P. A. Darrah, and D. M. Mosser, Infect. Immun. 69:3255-3263, 2001). In the present study, we developed transgenic L. major organisms which express and secrete the extracellular portion of CD40L (L. major CD40LE). We hypothesized that these organisms would be less virulent but more immunogenic than wild-type organisms and therefore be more effective at leishmanization. Transgenic parasites expressing CD40L mRNA and protein were developed. BALB/c mice infected with these parasites developed significantly smaller lesions containing fewer parasites than animals infected with wild-type organisms. Infection of resistant C57BL/6 mice with low doses of transgenic parasites induced a significant amount of protection against subsequent high-dose infection with wild-type organisms. These results demonstrate that transgenic organisms expressing CD40L are less virulent than wild-type organisms while retaining full immunogenicity.
Leishmaniasis is a vector-borne parasitic disease endemic in nearly 100 countries worldwide (8, 33). The disease is contracted when an infected female phlebotamine sandfly takes a blood meal, injecting promastigotes into the skin of the host. Host tissue macrophages rapidly take up the parasite, where Leishmania spp. transform into the amastigote stage to survive and replicate in macrophage phagolysosomes (36). Clinical manifestations of the disease range from localized, self-healing skin lesions to a potentially fatal disease where parasites disseminate to the visceral organs (8, 33). Leishmania major causes cutaneous leishmaniasis. Disease resolution typically depends on the development of a Th1 response and the production of gamma interferon (IFN-γ) by activated CD4+ T cells. IFN-γ activates macrophages to effectively kill the parasites (32). Early production of interleukin-12 (IL-12) is essential to mount an effective response, and this is thought to be a CD40-CD40 ligand (CD40L)-dependent process (26, 39).
CD40L is a member of the tumor necrosis family (Tnf5) (10). It is a type II glycoprotein primarily expressed on activated T cells. This molecule forms biologically active trimers on the cell surface that induce signaling through CD40 on macrophages, dendritic cells, and B cells. CD40L was first implicated in class switching in B cells. A mutation leading to nonfunctional CD40L results in hyperimmunoglobulin M syndrome in humans (34, 43). CD40-CD40L interactions have also been shown to be important for cell-mediated responses. CD40L can induce IL-12 from macrophages and DCs (21, 22, 35). Mice deficient in CD40L are defective in T-cell activation and IFN-γ production (13, 41).
Mice lacking CD40 or CD40L have been shown to be more susceptible to Leishmania infection (4, 20, 30, 31, 41). Furthermore, administration of anti-CD40 antibodies was shown to induce IL-12 and protect mice from Leishmania challenge (12). Anti-CD40 antibodies were also shown to induce macrophage killing of parasites and act synergistically with antimony therapy to improve disease outcome (30). Previously, we demonstrated that the addition of CD40L to leishmanial antigens provided protection against challenge with virulent organisms (7). In addition, CD40L trimer DNA has been successfully used as an adjuvant in the BALB/c model of L. major infection (14).
Leishmanization remains the only effective vaccine against leishmaniasis. Vaccination of humans with virulent organisms has been largely discontinued due to safety concerns (28). We hypothesized that transgenic organisms expressing CD40L would be attenuated and induce less pathology while still providing protection against wild-type challenge. In this study, we developed a transgenic Leishmania major strain that expresses the extracellular domain of CD40L (L. major CD40LE) and determined that these organisms caused reduced disease and conferred protection against virulent organisms. Because this organism is more immunogenic and less virulent than the wild-type organisms, we propose that transgenic organisms expressing CD40L, or other immunomodulatory molecules, may provide a unique alternative to leishmanization that is safer and more effective than traditional methods.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old BALB/c or C57BL/6 female mice were purchased from Charles River Laboratories (Wilmington, MA) and Taconic, Inc. (Rockville, MD), respectively. SCID mice on a BALB/c background and CD40−/− and CD40L−/− mice on a C57BL/6 background were obtained from Jackson Labs (Bar Harbor, ME). All mice were maintained in high-efficiency particulate air-filtered caging units (Thoren Caging Systems, Hazelton, PA) at the University of Maryland (College Park, MD). Mice were used for experiments at 6 to 10 weeks of age. All protocols were approved by the University of Maryland Institutional Animal Care and Use Committee (IACUC).
Parasite culture.
Promastigotes of the Leishmania major Friedlin strain, clone V1 (MHOM/IL/80/Friedlin), were used throughout. Parasites were maintained in BALB/c mice and cultured in vitro. Promastigotes were cultured in 50:50 medium, which is composed of 50% M199 medium (CellGro) and 50% Schneider's Drosophila medium (Sigma Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS), 100 U of penicillin G per ml, 100 μg of streptomycin per ml, and 2 mM glutamine. Nourseothricin (100 μg/ml) (Werner BioAgents, Germany) was added to transgenic cultures to select for resistant parasites.
Infections, immunizations, and parasite quantitation.
For infections, parasites were washed in Hanks’ balanced salt solution (HBSS) (CellGro, Lawrence, KS), centrifuged at 1,000 x g for 10 min, and resuspended in HBSS for subcutaneous injection into the footpad in a volume of approximately 20 μl. For standard BALB/c infections, 1 × 105 organisms were injected into the right hind footpad. A total of 5 × 105 parasites were used for C57BL/6 mice. For immunizations with live parasites, mice were infected with 5 × 104 wild-type, empty vector, or CD40LE L. major parasites in the right hind footpad and 3 to 5 weeks later were challenged with wild-type L. major in the contralateral footpad (1 × 105 for BALB/c and 5 × 105 for C57BL/6). When mice were infected in the ear dermis, 1 × 104 parasites were injected in a 10-μl volume.
Disease progression was monitored twice weekly using a digital caliper to measure footpad thickness. The lesion size for standard infections was calculated as the difference between the thicknesses of the infected and uninfected footpads as previously described (29). For immunizations where both footpads are infected, lesion size was reported as the thickness of the infected footpad only. Ear lesions were measured as the diameter of the lesion. Parasite burdens were determined by serial dilution of single-cell suspensions made from excised footpads, lymph nodes, or spleens as previously described (1).
Gene splicing by SOE PCR.
The 126-bp gp63 signal sequence from Leishmania amazonensis was amplified from plasmid U11 using the forward primer 5′-TAACCCGGGATGTCCGTCGACAGCAG-3′ (F1) and the reverse primer 5′-AAATTGCCTTCTCATGGCGTGTGCCCACGC-3′ (R1) from IDT DNA Technologies, Inc. (Coralville, IA). The CD40L extracellular domain (amino acids 87 to 260) was amplified from the plasmid pORF-mCD40L from Invivogen (San Diego, CA) using the forward primer 5′-TGGGCACACGCCATGAGAAGGCAATTT-3′ (F2) and the reverse primer 5′-TTAGCTAGCGAAGACTGCCAGCATCAGC-3′ (R2), also from IDT. The hybrid gp63CD40LE product was amplified using splice overlap extension (SOE) PCR as previously described (9). The gp63CD40LE product was then ligated into the TA cloning vector pCRII (Invitrogen, San Diego, CA) and grown in DH10B competent Escherichia coli (Invitrogen). Colonies were grown on LB plates containing ampicillin and X-Gal, 10 white colonies were chosen, and plasmid was extracted using the mini-prep kit (QIAGEN). Purified plasmid was then digested with XmaI and NheI (New England BioLabs [NEB], Beverly, MA) to detect clones containing the insert. Clones positive for the insert by enzymatic digest were then sent for sequencing (Core Sequencing Facility, University of Maryland, College Park). One clone with the correct sequence was excised from the TA vector using the restriction enzymes XmaI and NheI and ligated into the Leishmania vector pIR1SAT (pSAT) (a kind gift from Stephen Beverley, Washington University, St. Louis, MO), which had been digested with XmaI and XbaI (NEB). This plasmid contains 5′ and 3′ portions of the 18S rRNA gene of L. major to allow for insertion into the genome, as well as intergenic regions necessary for mini-exon splicing and polyadenylation. This allowed for stable transgenic constructs to be generated through homologous recombination. The plasmid also contains a streptothricin acetyltransferase (SAT) gene for selection with antibiotics from the streptothricin family (we used nourseothricin). Insertion into the pSAT vector was confirmed using XmaI and BglIII (NEB) (another downstream site in pSAT). pSAT CD40LE was linearized using the restriction enzyme SwaI (NEB) and gel purified (kit from QIAGEN).
Electroporation.
Leishmania major parasites were transformed with 10 μg of linearized plasmid using electroporation as previously described (9). Briefly, 1 × 108 parasites were resuspended in 400 μl of electroporation buffer (21 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2PO4) and mixed with plasmid. Following electroporation, cells were plated on blood agar plates containing 100 μg/ml of nourseothricin. Single colonies were selected and grown in 50:50 broth culture containing 100 μg/ml nourseothricin. Clones were labeled with no. 13 for the clone of pSAT-CD40LE used for the transfection and sequentially numbered from colonies chosen at random from blood agar plates, resulting in the nomenclature L. major CD40LE 13.2 (no. 13 for the pSAT clone and no. 2 for the colony from the blood agar plate).
RT-PCR.
For reverse transcription-PCR (RT-PCR), RNA was isolated using the TRIzol method according to the manufacturer's protocol (Invitrogen) as previously described (9). RNA was then converted to cDNA using random hexamer primers (Invitrogen). Then gp63 was amplified using the forward primer 5′-ATCCTCACCGACGAGAAGCGCGAC-3′ (F3) and the reverse primer 5′-ACGGAGGCGACGTACAACACGAAG-3′ (R3) from IDT. CD40L was amplified using the same primers used to generate the original PCR product (F2 and R2).
Western blotting.
A total of 1 × 108 parasites were harvested from late-log/stationary-phase cultures, washed once with HBSS, and resuspended in 1 ml of ice-cold lysis buffer as previously described (25). Lysates were cleared by centrifugation (13,000 rpm, 10 min, 4°C). Equal amounts were then loaded on 18% sodium dodecyl sulfate-polyacrylamide precast gels (Bio-Rad). After separation, proteins were transferred onto a polyvinylidene difluoride membrane for 1 h at 100 V. Membranes were then blocked in 5% nonfat milk in Tris-buffered saline-Tween (TBS-T) for 1 h at room temperature, washed briefly, and incubated with the primary antibody (1:200) (polyclonal goat anti-murine CD40L; R&D Systems catalog no. AF1163) overnight at 4°C. Membranes were washed in TBS-T and incubated with secondary antibody (anti-goat horseradish peroxidase conjugate diluted 1:5,000) for 1 h at room temperature. Membranes were then developed using ECL enhanced chemiluminescence substrate (Amersham Biosciences) according to the manufacturer's instructions.
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were done using standard protocols as described with the following antibody pair from R&D Systems: anti-CD40L (catalog no. AF1163) and biotinylated anti-CD40L (catalog no. BAF1163) (2).
MTT assay.
A total of 1 × 106 L. major, L. major pSAT, or L. major CD40LE 13.2 parasites were inoculated into 5 ml of 50:50 medium. One hundred microliters of culture was harvested at each time point and loaded onto a 96-well plate. Twenty microliters of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (stock, 5 mg/ml; Sigma, St. Louis, MO) was added to wells for 2 h at room temperature, followed by 100 μl of dimethyl sulfoxide (DMSO) to lyse cells for 30 min at room temperature in the dark. The plate was read on an ELISA plate reader at 550 nm.
Macrophages.
Bone marrow-derived macrophages (BMMφ) were generated as previously described (25). Briefly, bone marrow was flushed from BALB/c femurs and tibias and the cells were plated in Dulbecco's modified Eagle's medium supplemented with F12 (DMEM-F12) (DIFCO) supplemented with 10% HI-FBS (HyClone, Logan, UT), 100 U/ml of penicillin (Fisher Scientific), 100 μg/ml of streptomycin (Fisher Scientific), 2 mM glutamine (Fisher Scientific), and 20% L-cell conditioned medium. Cells were fed with 10 ml of this medium on day 2, and adherent cells were used on days 7 to 14.
Immunofluorescent staining of amastigotes.
Bone marrow-derived macrophages from BALB/c mice were plated 1 × 105 cells/coverslip and infected with a 20:1 ratio of L. major, L. major pSAT, or L. major CD40LE parasites for 2 h. Monolayers were thoroughly washed and fixed with methanol for 15 min at 4°C. Coverslips were stained with murine anti-L. major serum (1:250). A fluorescein isothiocyanate-conjugated goat anti-murine secondary antibody (1:100) was added, and cells were counterstained with propidium iodide. Coverslips were mounted with Mowiol and viewed using a fluorescent microscope (Zeiss).
Real-time PCR.
Real-time PCR was performed on draining lymph nodes from ear infections. Lymph nodes were placed in TRIzol immediately following removal. Tissue was disrupted with small pestles (QIAGEN) in 1.5-ml microcentrifuge tubes. RNA was quantified by spectrophotometry, and cDNA was generated using the ThermoScript kit from Invitrogen according to the manufacturer's instructions. Real-time PCR was performed for cytokines IL-10, IL-4, and IFN-γ using the following primer sets from IDT DNA: IL-10 forward, 5′-AAGGACCAGCTGGACAACAT-3′; IL-10 reverse, 5′-TCTCACCCAGGGAATTCAAA-3′; IL-4 forward, 5′-TCAACCCCCACGTAGTTGTC-3′; IL-4 reverse, 5′-ACGTTTGGCACATCCATCTC-3′; IFN-γ forward, 5′-GCGTCATTGAATCACACCTC-3′; and IFN-γ reverse, 5′-TGAGCTCATTGAATGCTTGG-3′. The hypoxanthine phosphoribosyltransferase (HPRT) gene was used as a housekeeping gene for normalization with the primers 5′-AAGCTTGCTGGTGAAAAGGA-3′ (forward) and 5′-TTGCGCTCATCTTAGGCTTT-3′ (reverse). Dissociation curves were performed for every run, and data were only analyzed if the curve showed a single peak. Samples from all primer sets were also run on a gel initially to ensure singe band products.
Statistical analysis.
For statistical analysis, Student's t test was performed. Values with P ≤ 0.05 were considered significant.
RESULTS
Transgenic parasites express CD40L.
Transgenic parasites expressing the extracellular domain of CD40L were developed using SOE PCR (9, 18, 19). This technique allowed us to attach the gp63 signal sequence from L. major to the extracellular domain of CD40L (CD40LE). It has previously been shown that the extracellular domain of CD40L can be expressed and secreted in a biologically active trimer by E. coli, and we used the same amino acids (87 to 260) from the 18-kDa protein described in that study for our molecule (27).
Antibiotic-resistant clones were assayed for expression of CD40L using a variety of methods. First, we examined mRNA expression by RT-PCR. CD40L mRNA was present in three clones of CD40LE L. major, designated 13.1, 13.2, and 13.4 (Fig. 1A, lanes 2 to 4). No CD40L mRNA was detected in wild-type organisms (Fig. 1A, lane 1). A leishmanial surface protein, gp63, was used as a loading control. It was detected in both wild-type and transgenic Leishmania major promastigotes (Fig. 1A, lower panel). To confirm the mRNA data, a Western blot was performed on whole-cell lysates (Fig. 1B). L929 cells stably transfected with full-length CD40L were used as a positive control (Fig. 1B, lane 1). Wild-type L. major organisms were used as a negative control (Fig. 1B, lane 2). All three clones were also shown to express CD40L protein by Western blotting. CD40L from transgenic L. major CD40LE was smaller than CD40L detected in L929 cells because L929 cells expressed full-length CD40L. L. major CD40LE lacks the transmembrane domain, allowing for the secretion of the protein. Secretion of CD40L was measured by ELISA (Fig. 1C). CD40L protein was detected in the supernatants of cultures of transgenic parasites in a dose-dependent manner (Fig. 1C), but not in the wild-type cultures (data not shown), indicating that CD40L was secreted from the parasite.
FIG. 1.
Expression of CD40L in transgenic parasites. (A) Promastigote mRNA was isolated from wild-type L. major (lane 1) and three clones of L. major CD40LE (lanes 2 to 4) and assayed for CD40L (upper panel) and gp63 (lower panel) by RT-PCR. gp63 was used as a loading control. (B) CD40L protein was detected by Western blotting of cell lysates from L929 cells stably transfected with CD40L (lane 1), wild-type L. major (lane 2), and L. major CD40LE (lanes 3 to 5). (C) Promastigotes were plated in 1:2 serial dilutions for 24 h. Secretion of CD40L into the supernatant was measured by ELISA. sCD40L (soluble CD40L). (D) BMMφ were infected for 24, 48, or 72 h with wild-type or CD40L organisms or left uninfected. At each time point, RNA was isolated and RT-PCR was performed for CD40L (top panel), gp63 (middle panel), and HPRT (bottom panel). Transgenic promastigotes were used as a positive control (lane 1). Results in panels A, B, and D are representative of at least three independent experiments. Results in panel C are combined from three independent experiments (mean ± standard error of the mean). (E) Macrophages were plated at 2 × 105 cells/well in a 48-well dish and infected with a 30:1 ratio of CD40L transgenic parasites. Two hours later, unbound parasites were washed away and 300 μl of medium was added. At indicated time points, supernatants were harvested and sCD40L was detected by ELISA.
After determining that promastigotes expressed CD40L, we examined expression by the amastigote form of the organism. BMMφ were infected at a 20:1 ratio of parasites to macrophages with either wild-type or CD40LE organisms. Two hours after infection, monolayers were washed thoroughly to remove unbound parasites and visually checked to ensure the removal of promastigotes. At indicated times following infection, RNA was harvested and assayed for CD40L. CD40L was detected from monolayers infected with transgenic parasites, but not from uninfected BMMφ or from BMMφ infected with wild-type L. major (Fig. 1D, top panel). The presence of parasites was confirmed by the detection of gp63 mRNA (Fig. 1D, middle panel). HPRT was used as an indicator of equal loading of macrophage cDNA (Fig. 1D, lower panel). L. major CD40LE promastigotes were used as a positive control. CD40L protein was also detected in the supernatants of BMMφ infected with transgenic parasites (Fig. 1E). Taken together, these data demonstrate that the transgenic Leishmania organisms we have generated are able to express and secrete CD40L. Clone 13.2 was chosen for the remainder of the study.
Transgenic parasites are as healthy as the wild type.
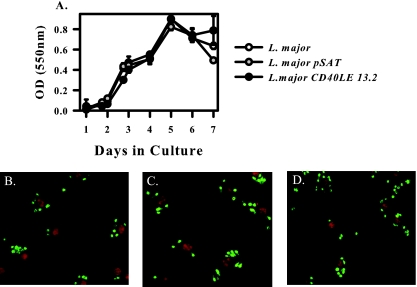
We next determined whether the insertion in the 18S rRNA gene had any effect on the viability and growth of the parasite. To assay the growth rate of the organisms, an MTT assay was performed. This assay measures the metabolic rate of the organism by the conversion of MTT to formazan. All three organisms have the same growth curve, indicating that the insertion has no effect on replication and metabolic rate of the parasite (Fig. 2A). Next, we infected bone marrow-derived macrophages with the transgenic organism to ensure that the parasites were still able to invade host cells and turn into amastigotes. Unstimulated BMMφ were plated on coverslips and infected for 2 h with either L. major (Fig. 2B), L. major pSAT (Fig. 2C), or L. major CD40LE 13.2 (Fig. 2D). Cells were fixed with methanol and stained with murine polyclonal anti-L. major serum. There was no difference between the numbers of wild-type or transgenic organisms at 2 h postinfection (data not shown), indicating that there is no defect in the ability of transgenic organisms to invade host cells and transform into amastigotes.
FIG. 2.
Transgenic organisms show the same growth characteristics as the wild type. A total of 1 × 106 L. major, L. major pSAT, or L. major CD40LE 13.2 organisms were inoculated into 5 ml of 50:50 medium. One hundred microliters of culture was harvested at each indicated time point, and metabolic activity was measured using an MTT assay. (A) Growth curves of wild-type (open circles), pSAT (gray circles), or CD40LE organisms (black circles) were compared (mean ± standard deviation). OD, optical density. (B to D) BMMφ were infected for 2 h with wild-type (B), empty vector (C), or CD40LE (D) promastigotes and stained with anti-L. major serum to visualize parasites. Propidium iodide was used to counterstain the nucleus. Results are representative of at least three independent experiments. No differences among samples were observed.
To confirm the viability of the transgenic organisms, we infected SCID mice on a BALB/c background with 1 × 105 wild-type or transgenic promastigotes in the hind footpad. Disease progression was monitored using calipers to measure footpad thickness. Lesion size was determined as the thickness of the infected foot minus the thickness of the uninfected foot. Transgenic CD40LE organisms generated lesions that were comparable to those of wild-type organisms (Fig. 3). Lesions contained similar parasite numbers as determined by limiting dilution assay (Fig. 3, inset). These data indicate that the insertion of the transgene does not affect parasite viability nor does it affect their ability to infect and replicate in immunocompromised hosts.
FIG. 3.
CD40LE parasites cause lesions in SCID mice. SCID mice on a BALB/c background were infected with 1 × 105 parasites in the right hind footpad. Lesion size was monitored, and parasite burdens were determined at the completion of the experiment (inset). Wild-type (open circles/bars) and CD40LE organisms (filled circles/bars) induced comparable lesions with similar numbers of parasites within them.
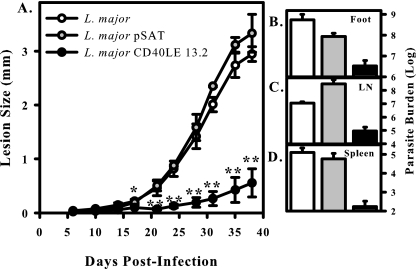
Transgenic L. major CD40LE induces less pathology in susceptible BALB/c mice.
To examine whether or not L. major CD40LE had an effect on disease outcome, BALB/c mice were infected with 1 × 105 wild-type, pSAT, or CD40LE 13.2 L. major parasites in the hind footpad. Lesion size was monitored over the course of the next 6 weeks. BALB/c mice are susceptible to L. major infections, and therefore mice infected with wild-type organisms developed large lesions (Fig. 4A). Littermate mice infected with L. major CD40LE 13.2 developed significantly smaller lesions (Fig. 4A) with fewer parasites within the lesions (Fig. 4B). Dissemination to the lymph nodes was also reduced (Fig. 4C), and parasites were virtually undetectable in the spleens of animals receiving CD40LE-expressing organisms (Fig. 4D). Mice infected with empty vector organisms developed lesions comparable to those of mice infected with wild-type organisms. These data indicate that L. major CD40LE 13.2 parasites are less virulent than wild-type organisms in the susceptible BALB/c host.
FIG. 4.
CD40LE parasites exhibit reduced pathology in BALB/c mice. BALB/c mice were infected in the hind footpad with 1 × 105 L. major (open circles/bars), L. major pSAT (gray circles/bars), or L. major CD40LE 13.2 (black circles/bars) organisms. (A) Lesion development was measured with calipers twice weekly. (B to D) Parasite burdens were determined in the footpad (B), spleen (C), and popliteal lymph node (LN) (D) at the end of the infection. Results are compiled from three independent experiments, with a minimum of five mice per group, and are expressed as the mean ± standard error of the mean. Asterisks indicate significance (*, P < 0.05; **, P < 0.01).
Transgenic organisms induce lower levels of Th2 cytokines in susceptible hosts.
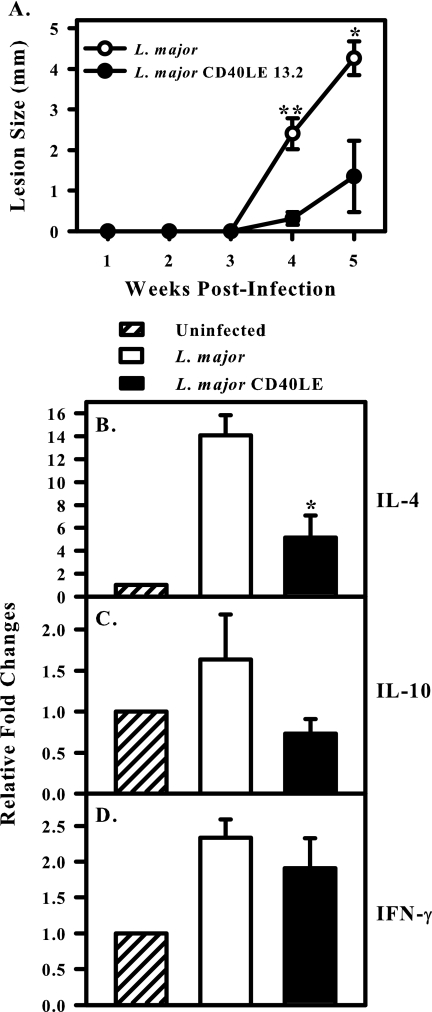
To examine cytokine induction in draining lymph nodes, we used an ear model of infection and isolated mRNA from lymph nodes. Disease progression was monitored using calipers, and lesion size was determined as the diameter of the ear lesion. As in the footpad model, wild-type L major parasites caused large, nonhealing lesions (Fig. 5A), whereas transgenic organisms induced significantly smaller lesions (Fig. 5A). At week 4 postinfection, draining lymph nodes were harvested directly into TRIzol to assay in vivo cytokine mRNA levels (Fig. 5B to D). Cytokine mRNA levels were determined using real-time PCR and normalized to HPRT. Data are expressed as relative changes (fold) compared to uninfected lymph nodes, which were normalized to 1. The transgenic parasites induced significantly less IL-4 than wild-type parasites (P < 0.05) (Fig. 5B). In three separate experiments, IL-4 levels were significantly lower in the nodes of transgenic organism-infected mice relative to wild-type-infected mice. IL-10 transcripts in the two groups were also compared. In two of three experiments, IL-10 levels were also reduced in the transgenic organism-infected mice (P < 0.05), but when the three experiments were compiled and compared to the wild type, the differences were not significant (Fig. 5C). Wild-type and transgenic parasites induced comparable levels of IFN-γ (Fig. 5D).
FIG. 5.
L. major CD40LE induce lower levels of IL-4 in BALB/c lymph nodes. BALB/c mice were infected in the ear dermis with 1 × 104 promastigotes. (A) Lesion progression was followed by measuring lesion diameter with calipers. L. major (open circles) induce large, nonhealing lesions. CD40LE organisms (filled circles) induce smaller lesions in this low-dose model. Asterisks indicate significance (*, P < 0.05; **, P < 0.01). (B) IL-4, (C) IL-10, and (D) IFN-γ levels were assayed by real-time PCR at week 4 postinfection. The asterisk indicates significantly less IL-4 (P < 0.05) induced in response to transgenic relative to wild-type infection. Data were normalized to HPRT and are expressed as change (fold) relative to uninfected controls. Data are compiled from three independent experiments and are expressed as the mean ± standard error of the mean.
Parasite-derived CD40L is biologically active.
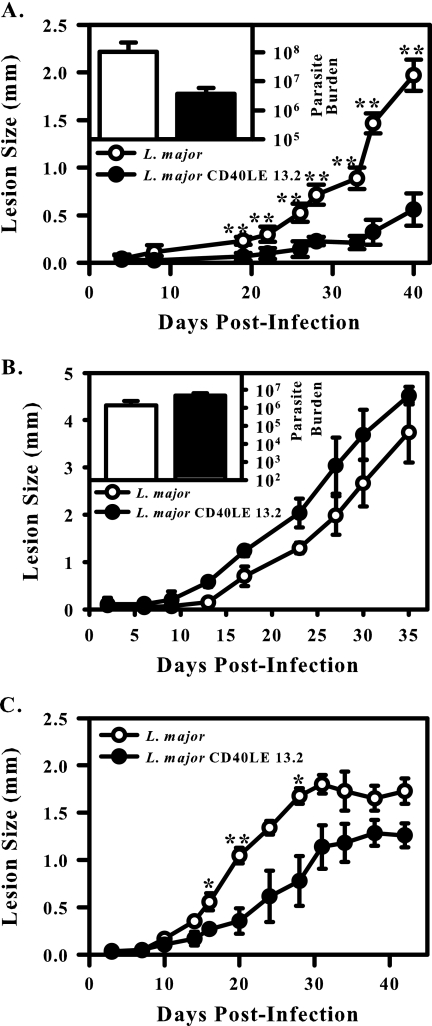
To demonstrate that the parasite-derived CD40L was active, we infected CD40L−/− mice on a C57BL/6 background with 1 × 105 wild-type or transgenic organisms. Lesion development was monitored over the course of the next 6 weeks. C57BL/6 mice lacking CD40L were relatively susceptible to wild-type L. major infection and developed progressive lesions (Fig. 6A, open circles). Mice infected with transgenic organisms developed significantly smaller lesions (P < 0.01) (Fig. 6A, filled circles), indicating that the CD40L produced by the parasite was biologically active in vivo.
FIG. 6.
Parasite-derived CD40L is biologically active. (A) CD40L−/− mice on a C57BL/6 background were infected with 1 × 105 wild-type (open circles/bars) or transgenic (filled circles/bars) parasites. Asterisks indicate significance (**, P < 0.01). (B) CD40−/− mice on a C57BL/6 background were infected with 5 × 105 wild-type (open circles/bars) or transgenic (filled circles/bars) parasites. Disease progression was monitored, and burdens were determined at the end of the experiment (inset). (C) Wild-type C57BL/6 mice were infected with 5 × 105 wild-type (open circles) or transgenic (filled circles) parasites. Asterisks indicate significance (*, P < 0.05; **, P < 0.01). Results are compiled from three independent experiments and are expressed as the mean ± standard error of the mean.
We also examined disease progression in CD40−/− mice on a C57BL/6 background to confirm that the attenuated phenotype was due to parasite-derived CD40L. We would expect that parasites expressing CD40L would have no phenotype in mice lacking CD40, and that is what we observed. CD40−/− mice infected with transgenic parasites developed lesions that were similar in size to those caused by wild-type parasites (Fig. 6B). Taken together, these data suggest that parasite-derived CD40L is biologically active and responsible for the attenuated phenotype observed.
As a control for these experiments, wild-type C57BL/6 mice were infected with 5 × 105 wild-type or transgenic parasites (Fig. 6C). Mice infected with transgenic parasites developed significantly smaller lesions than those infected with wild-type organisms (Fig. 6C). However, this phenotype was less dramatic than that observed in the CD40L−/− and BALB/c mice.
Live L. major CD40LE organisms establish protection against challenge with wild-type organisms.
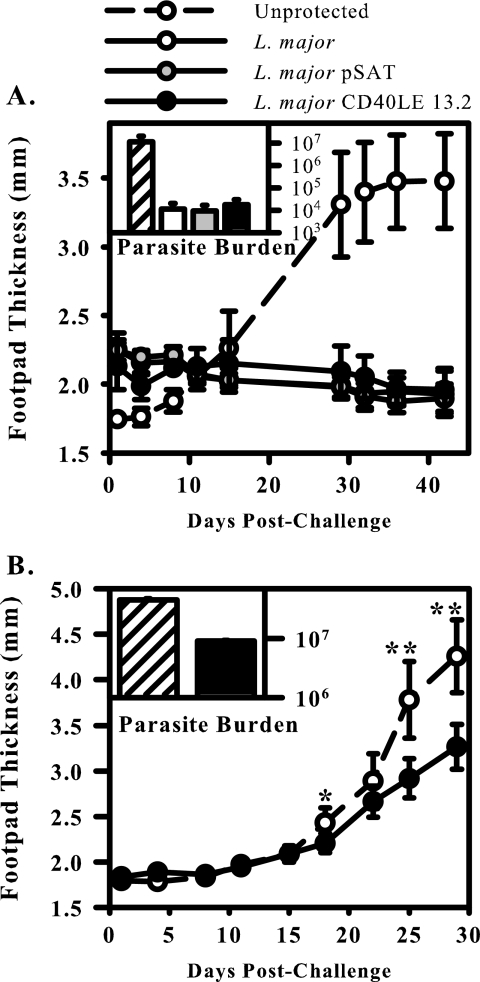
We examined the ability of the transgenic organisms to protect in a vaccination model of leishmaniasis. For these studies, the resistant C57BL/6 strain of mice was used. Several groups have demonstrated that these mice resolve infection with wild-type parasites and develop immunity to reinfection (15, 40). C57BL/6 mice were infected with a low dose (5 × 104) of either wild-type L. major, L. major pSAT, or L. major CD40LE 13.2 in the right hind footpad. Four to 5 weeks following initial infection, mice were rechallenged with 5 × 105 wild-type L. major parasites in the contralateral footpad and disease progression was monitored as described above. As previously reported, mice that had previously cleared an infection with wild-type parasites were resistant to reinfection (Fig. 7A) (38). Mice that were previously infected with transgenic parasites were similarly protected from reinfection. Mice that were not previously infected developed larger lesions with higher parasite numbers within them (Fig. 7A, inset). These data suggest that the attenuated transgenic organisms retain the immunogenicity of their wild-type counterparts.
FIG. 7.
L. major CD40LE 13.2 induce protection against wild-type challenge. (A) C57BL/6 mice were infected with 5 × 104 L. major (open circles/bars), L. major pSAT (gray circles/bars), or L. major CD40LE 13.2 organisms (black circles/bars) in the right hind footpad. Lesions were allowed to heal, and then mice were challenged in the contralateral footpad with 5 × 105 wild-type L. major parasites. Lesion development was monitored and burdens were determined as previously described. A group of unprotected mice were used as a control for lesion development (hatched lines/bars). Results are compiled from three independent experiments (mean ± standard error of the mean). (B) BALB/c mice were infected with 5 × 104 transgenic parasites (black circles/bars), and 4 to 5 weeks later were challenged with 1 × 105 wild-type parasites. Unprotected mice were used as a control for lesion development (hatched lines/bars). Insets in panels A and B show parasite burden. Asterisks indicate significance (*, P ≤ 0.05; **, P < 0.01). Results are representative of three independent experiments.
Next we wanted to ascertain if the transgenic parasites would be able to provide protection to the susceptible BALB/c animal. Vaccination with viable wild-type organisms is not possible in this model, because the animals develop large, nonhealing lesions and eventually succumb to infection. BALB/c animals were infected with a low dose (5 × 104) of CD40LE 13.2 organisms in the right hind footpad. Four to five weeks later, mice were rechallenged with 1 × 105 wild-type L. major promastigotes in the contralateral footpad, and lesions were monitored. Vaccination of susceptible BALB/c animals with L. major CD40LE 13.2 resulted in a significant, albeit modest, reduction of lesion size in challenged animals (Fig. 7B). These lesions contained approximately 1 log fewer parasites than unvaccinated animals (Fig. 7B, inset), indicating that vaccination with the transgenic organism was unable to completely overcome the Th2 bias in these animals and induce full protection.
DISCUSSION
Leishmaniasis is a disease that is endemic in nations with populations that cannot easily afford the costly drugs used to treat it. An effective vaccine is needed to prevent infection and improve the quality of life for at-risk people. The costimulatory molecule CD40L is known to be important for T-cell activation and antigen presentation through binding its receptor, CD40. Originally implicated in antibody class switching (3), it has been shown to play a role in cell-mediated immunity as well. It has an important role in many diseases, including leishmaniasis (4, 20, 41). Mice lacking either CD40 or CD40L have been shown to be more susceptible to infection with Leishmania spp. (4, 20, 31, 41). Previously, we have shown that combining CD40L with leishmanial antigens can improve disease outcome in susceptible animal models (7). Also, vaccination with CD40 ligand trimer DNA was shown to provide protection in the BALB/c host (14). In this study, we have developed transgenic L. major organisms expressing the extracellular domain of CD40L. We hypothesized that the transgenic organisms would induce less pathology in susceptible mice and would provide protection against rechallenge with wild-type organisms.
Our parasites were shown to express CD40L mRNA and protein and secrete CD40L into the media. CD40LE was stably integrated into the genome, and expression was detected in parasites isolated from footpads of mice infected for 6 weeks by both Western blotting and ELISA (data not shown). Using a variety of methods, we examined whether this insertion had any effect on the fitness of the organism. By all criteria, the transgenic organisms appear to be as healthy as wild-type organisms. The growth curves for these organisms were virtually identical, and parasites were able to infect macrophages and convert into the amastigote form within equally well. In addition, infection of SCID or CD40−/− mice with transgenic parasites resulted in lesions that were comparable in size to wild-type-infected mice, with similar numbers of parasites within them. From these data, we conclude that the insertion has no discernible effect on the growth and infectivity of the parasite.
We examined the ability of transgenic parasites to influence disease outcome in the susceptible BALB/c mouse. We found that L. major CD40LE caused substantially reduced disease in this model of infection. These animals developed significantly smaller lesions than those infected with either wild-type or empty vector organisms. It is important to note that mice infected with empty vector organisms developed lesions comparable to those of mice infected with wild-type organisms. This is another indication that the insertion of pSAT into the genome has no effect on the fitness of the organism. Thus, we conclude that transgenic organisms expressing CD40L are less virulent than wild-type organisms in a susceptible BALB/c mouse model.
We attempted to determine a possible explanation for the improved disease progression in BALB/c mice. This led us to examine in vivo cytokine mRNA levels in draining lymph nodes from lesions. We found that the wild-type organisms were better inducers of the anti-inflammatory cytokine IL-4 in BALB/c animals. Transgenic organisms induced a significantly lower level of this cytokine. This appeared to be specific to IL-4 because IFN-γ levels were not significantly altered. We also observed a trend toward reduced IL-10 induction in transgenic parasite-infected animals. In two of three independent experiments, transgenic parasite-infected animals induced significantly lower levels of IL-10, but when the three experiments were compiled, this difference was no longer significant. It is well known that in BALB/c mice, IL-4 and IL-10 are induced following infection with L. major (16, 24). In the resistant C57BL/6 strain of mice, however, IFN-γ is the dominant cytokine that is produced in response to this infection (17, 24, 38). The decreased lesion induction by the transgenic parasites correlates with the decrease in IL-4 production that occurs. IL-4 is thought to be a major contributor in the generation of a detrimental Th2 response in BALB/c animals, and treatment with anti-IL-4 antibody early in infection can confer a resistant phenotype on these genetically susceptible animals (6, 16, 37). In addition, BALB/c mice deficient in the IL-4 gene were resistant to L. major infection (23). C57BL/6 mice preferentially mount a Th1 response to Leishmania and express lower levels of IL-4 than BALB/c mice. Thus, differences in T-cell biasing may explain the reduced phenotype of the transgenic parasites in C57BL/6 mice (Fig. 6C) relative to BALB/c.
Many groups have shown that the disruption of CD40-CD40L interactions can result in susceptibility in the otherwise resistant C57BL/6 mouse (4, 20, 31, 41). To confirm that parasite-derived CD40L was active, we infected CD40L−/− and CD40−/− mice on a C57BL/6 background with wild-type or transgenic parasites. In CD40L−/− mice, transgenic parasites caused reduced disease. Mice infected with transgenic organisms developed significantly smaller lesions with fewer parasites than mice infected with wild-type organisms. From these data, we conclude that the CD40L produced by the parasite was biologically active and complemented the CD40L deficiency in these animals. In CD40-deficient mice, the opposite occurred. Transgenic parasites induced progressive disease with lesions at least as large as those observed in mice infected with wild-type parasites. These data suggest that parasite-derived CD40L is responsible for the attenuation seen in wild-type C57BL/6 mice.
We also examined the ability of the transgenic organism to vaccinate C57BL/6 mice. Animals that were vaccinated with wild-type or transgenic organisms were equally protected against rechallenge, indicating that these organisms, although reduced in virulence, retain immunogenicity required to induce an adaptive immune response. Previous studies in our laboratory demonstrated that the combination of L929 cells expressing CD40L with parasite antigens was able to provide significant protection in the susceptible BALB/c mouse (7). Also, CD40L trimer DNA has been used successfully to vaccinate BALB/c mice against L. major (14). Therefore, we hypothesized that the CD40LE organism would be able to provide protection in the BALB/c model. We found that immunization with transgenic L. major CD40LE was able to induce partial protection in susceptible BALB/c mice, with vaccinated animals developing slightly but significantly smaller lesions. This suggests that CD40L-expressing organisms were unable to overcome the overwhelming Th2 bias in these animals to provide complete protection against wild-type challenge. The differences seen in our study compared to those mentioned above might be explained by differences in study design. In our previous study, transfected cells expressing CD40L were mixed with Leishmania antigen and mice were vaccinated three times prior to challenge with wild-type organisms. The transgenic parasites in the present study make relatively small amounts of CD40L compared to these previous doses. If the expression level of CD40L were increased, we would expect improved protection in BALB/c mice.
The question of how the CD40L secreted by the parasite is able to access CD40+ cells remains unanswered. We provide evidence that some CD40L is released from monolayers of infected cells (Fig. 1E), but we have not formally determined whether it was secreted from these cells or released upon parasite destruction of the monolayer. Previous work in our laboratory demonstrated that MCP-1 secreted by transgenic parasites was unable to escape the macrophage until the cell was lysed (9). Our observation that some CD40L was released from infected macrophages is consistent with the work of Dumas et al., who developed L. major organisms secreting granulocyte-macrophage colony-stimulating factor (GM-CSF) and reported 25% escape of GM-CSF from the macrophage (11). Further studies must be done to determine the extent and mechanism of CD40L release from infected macrophages.
Others have attempted to generate transgenic “suicide” Leishmania organisms with varying degrees of success. Tobin et al. generated L. major organisms expressing IFN-γ (42). These parasites were able to delay lesion development in nude mice but had no effect on disease outcome in BALB/c animals (42). Dumas et al. developed GM-CSF-secreting organisms with more success (11). These parasites were able to delay lesion development in BALB/c animals, and there was evidence that GM-CSF could be combined with traditional treatments to improve disease outcome (11). However, this group did not examine the protective capabilities of the GM-CSF-secreting organism. The L. major organisms we have developed expand upon these previous studies generating transgenic Leishmania spp. to manipulate disease outcome. To our knowledge, this is the first report of the development of a transgenic Leishmania major strain to produce a murine costimulatory molecule. Our data correlate well with data obtained by Chamekh et al. that showed that Trypanosoma cruzi organisms transfected with CD40L are able to reduce disease and induce protection in an animal model (5). Our data confirm the ability of CD40L to act as an adjuvant to generate an attenuated organism that can induce a protective response in the host. Thus, this method of generating less-virulent organisms using the host's immunostimulatory molecules can have merit in a variety of infections.
In summary, previous observations that CD40L could improve disease outcome and provide protection in leishmaniasis led us to develop transgenic organisms secreting the extracellular domain of this protein. We observed that these transgenic organisms are less virulent than wild-type organisms, while retaining immunogenicity to induce a protective response. We conclude that transgenic parasites expressing immunostimulatory molecules may provide a better alternative to traditional leishmanization, resulting in improved vaccines.
Acknowledgments
We thank Stephen Beverley of Washington University in St. Louis, MO, for the generous gift of the pIR1SAT cloning vector.
This study was supported by National Institutes of Health grant AI055576 and by a grant from the Howard Hughes Medical Institute through the Undergraduate Biological Sciences Education Program.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 2 May 2007.
REFERENCES
- 1.Afonso, L. C. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, C. F., A. E. Field, L. Gutirrez-Kobeh, D. M. Mosser, and M. Lucas. 2004. T cell biasing by activated dendritic cells. J. Immunol. 173:955-961. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Bazan, D. Blanchard, F. Briere, J. P. Galizzi, K. C. Van, Y. J. Liu, F. Rousset, and S. Saeland. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12:881-922. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, K. A., W. C. Fanslow, S. G. Reed, P. J. Ovendale, M. K. Kennedy, and C. R. Maliszewski. 1996. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4:283-289. [DOI] [PubMed] [Google Scholar]
- 5.Chamekh, M., V. Vercruysse, M. Habib, M. Lorent, M. Goldman, A. Allaoui, and B. Vray. 2005. Transfection of Trypanosoma cruzi with host CD40 ligand results in improved control of parasite infection. Infect. Immun. 73:6552-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatelain, R., K. Varkila, and R. L. Coffman. 1992. IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 148:1182-1187. [PubMed] [Google Scholar]
- 7.Chen, G., P. A. Darrah, and D. M. Mosser. 2001. Vaccination against the intracellular pathogens Leishmania major and L. amazonensis by directing CD40 ligand to macrophages. Infect. Immun. 69:3255-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, C. M., and E. A. Lerner. 2002. Leishmaniasis: recognition and management with a focus on the immunocompromised patient. Am. J. Clin. Dermatol. 3:91-105. [DOI] [PubMed] [Google Scholar]
- 9.Conrad, S. M., D. Strauss-Ayali, A. E. Field, M. Mack, and D. M. Mosser. 2007. Leishmania-derived murine MCP-1 enhances the recruitment of a restrictive population of CCR2-positive macrophages. Infect. Immun. 75:653-665. (First published 6 November 2006; doi: 10.1128/IAI.01314-06.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosman, D. 1994. A family of ligands for the TNF receptor superfamily. Stem Cells 12:440-455. [DOI] [PubMed] [Google Scholar]
- 11.Dumas, C., A. Muyombwe, G. Roy, C. Matte, M. Olivier, M. Ouellette, and B. Papadopoulou. 2003. Recombinant Leishmania major secreting biologically active granulocyte-macrophage colony-stimulating factor survives poorly in macrophages in vitro and delays disease development in mice. Infect. Immun. 71:6499-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlin, W. G., D. A. Ferrick, R. L. Coffman, T. der Weid, F. Cottrez, and M. C. Howard. 1998. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur. J. Immunol. 28:525-531. [DOI] [PubMed] [Google Scholar]
- 13.Grewal, I. S., M. B. Oldstone, R. A. Flavell, P. Borrow, and E. G. Pamer. 1997. The CD40-CD154 system in anti-infective host defense. Curr. Opin. Immunol. 9:491-497. [DOI] [PubMed] [Google Scholar]
- 14.Gurunathan, S., E. Thomas, J. I. Cohen, N. P. Restifo, K. R. Irvine, C. Y. Wu, C. Prussin, and R. A. Seder. 1998. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J. Immunol. 161:4563-4571. [PMC free article] [PubMed] [Google Scholar]
- 15.Handman, E., R. Ceredig, and G. F. Mitchell. 1979. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust. J. Exp. Biol. Med. Sci. 57:9-29. [DOI] [PubMed] [Google Scholar]
- 16.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzel, F. P., M. D. Sadick, S. S. Mutha, and R. M. Locksley. 1991. Production of interferon γ, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. USA 88:7011-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton, R. M., L. R. Pease, Z. L. Cai, and S. N. Ho. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 19.Horton, R. M., J. K. Pullen, L. R. Pease, H. D. Hunt, and S. N. Ho. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 20.Kamanaka, M., K. Yoshida, T. Kishimoto, T. Kawabe, P. Yu, T. Yasui, T. Horii, and H. Kikutani. 1996. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity 4:275-281. [DOI] [PubMed] [Google Scholar]
- 21.Kato, T., R. Hakamada, H. Yamane, and H. Nariuchi. 1996. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J. Immunol. 156:3932-3938. [PubMed] [Google Scholar]
- 22.Kelsall, B. L., W. Strober, E. Stuber, and M. Neurath. 1997. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann. N. Y. Acad. Sci. 795:116-126. [DOI] [PubMed] [Google Scholar]
- 23.Kopf, M., F. Brombacher, G. Kohler, G. Kienzle, K. H. Widmann, K. Lefrang, C. Humborg, B. Ledermann, and W. Solbach. 1996. IL-4-deficient Balb/c mice resist infection with Leishmania major. J. Exp. Med. 184:1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locksley, R. M., F. P. Heinzel, M. D. Sadick, B. J. Holaday, and K. D. Gardner, Jr. 1987. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann. Inst. Pasteur Immunol. 138:744-749. [DOI] [PubMed] [Google Scholar]
- 25.Lucas, M., X. Zhang, V. Prasanna, and D. M. Mosser. 2005. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J. Immunol. 175:469-477. [DOI] [PubMed] [Google Scholar]
- 26.Marovich, M. A., T. B. Nutman, M. A. McDowell, and E. K. Thomas. 2000. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J. Immunol. 164:5858-5865. [DOI] [PubMed] [Google Scholar]
- 27.Mazzei, G. J., P. Graber, B. Allet, S. Lecoanet-Henchoz, J. F. Gauchat, M. D. Edgerton, C. Losberger, A. Durandy, and A. Bernard. 1995. Recombinant soluble trimeric CD40 ligand is biologically active. J. Biol. Chem. 270:7025-7028. [DOI] [PubMed] [Google Scholar]
- 28.Melby, P. C. 2002. Vaccination against cutaneous leishmaniasis: current status. Am. J. Clin. Dermatol. 3:557-570. [DOI] [PubMed] [Google Scholar]
- 29.Miles, S. A., S. M. Conrad, R. G. Alves, S. M. Jeronimo, and D. M. Mosser. 2005. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 201:747-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray, H. W., E. B. Brooks, J. L. DeVecchio, and F. P. Heinzel. 2003. Immunoenhancement combined with amphotericin B as treatment for experimental visceral leishmaniasis. Antimicrob. Agents Chemother. 47:2513-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray, H. W., C. M. Lu, E. B. Brooks, R. E. Fichtl, J. L. DeVecchio, and F. P. Heinzel. 2003. Modulation of T-cell costimulation as immunotherapy or immunochemotherapy in experimental visceral leishmaniasis. Infect. Immun. 71:6453-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray, H. W., B. Y. Rubin, and C. D. Rothermel. 1983. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J. Clin. Investig. 72:1506-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson, R. D., S. M. Lareau, and S. M. Jeronimo. 1999. Leishmaniasis at the End of the Millennium. Curr. Infect. Dis. Rep. 1:448-452. [DOI] [PubMed] [Google Scholar]
- 34.Ramesh, N., S. Lederman, L. Chess, M. J. Yellin, R. Fuleihan, V. Ramesh, R. S. Geha, S. Sharma, and F. S. Rosen. 1993. Deletions in the ligand for CD40 in X-linked immunoglobulin deficiency with normal or elevated IgM (HIGMX-1). Int. Immunol. 5:769-773. [DOI] [PubMed] [Google Scholar]
- 35.Reis e Sousa, H. Charest, D. Jankovic, A. Sher, S. Hieny, T. Scharton-Kersten, and R. N. Germain. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacks, D., J. A. Louis, and D. F. Wirth. 1993. Leishmaniasis, p. 237-268. In K. S. Warren (ed.), Immunology and molecular biology of parasitic infections. Blackwell Science, Inc., Malden, MA.
- 37.Sadick, M. D., F. P. Heinzel, B. J. Holaday, R. T. Pu, R. S. Dawkins, and R. M. Locksley. 1990. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J. Exp. Med. 171:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott, P. 2005. Immunologic memory in cutaneous leishmaniasis. Cell Microbiol. 7:1707-1713. [DOI] [PubMed] [Google Scholar]
- 39.Scott, P., and C. A. Hunter. 2002. Dendritic cells and immunity to leishmaniasis and toxoplasmosis. Curr. Opin. Immunol. 14:466-470. [DOI] [PubMed] [Google Scholar]
- 40.Sher, A., D. L. Sacks, and P. A. Scott. 1983. Host and parasite factors influencing the expression of cutaneous leishmaniasis. Ciba Found. Symp. 99:174-189. [DOI] [PubMed] [Google Scholar]
- 41.Soong, L., P. Kima, N. H. Ruddle, J. Sun, J. C. Xu, I. S. Grewal, R. A. Flavell, B. J. Longley, and D. McMahon-Pratt. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 42.Tobin, J. F., S. Zheng, R. M. Locksley, C. L. Leptak, S. L. Reiner, F. Hatam, and D. F. Wirth. 1993. Transfected Leishmania expressing biologically active IFN-gamma. J. Immunol. 150:5059-5069. [PubMed] [Google Scholar]
- 43.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]