Abstract
CD4+ CD45RBhi CD25− effector T cells (TE) promote Helicobacter pylori gastritis in mice, and CD4+ CD45RBlo CD25+ regulatory T cells (TR) are anti-inflammatory. Using adoptive transfer into H. pylori-infected Rag2−/− mice, we evaluated effects of wild-type (wt) C57BL/6 or congenic interleukin-10-deficient (IL-10−/−) TR cells on gastritis, gastric cytokines, and H. pylori colonization. Infected Rag2−/− mice colonized in the corpus and antrum with 105 to 106 H. pylori CFU/gram without associated gastritis. TE cell transfer caused morbidity and an H. pylori-independent pangastritis and duodenitis (gastroduodenitis) associated with increased expression of gamma interferon (IFN-γ) and tumor necrosis factor alpha. TE cell transfer to H. pylori-infected mice led to additive corpus gastritis associated with inflammatory cytokine expression and reduced colonization. wt TR cells reduced morbidity, H. pylori corpus gastritis, gastroduodenitis, and inflammatory cytokine expression and reversed the decline in H. pylori colonization attributable to TE cells. Although less effective than wt TR cells, IL-10−/− TR cells also reduced morbidity and gastroduodenitis but did not reduce H. pylori corpus gastritis or impact TE cell inhibition of colonization. Gastric tissues from mice receiving wt TR cells expressed higher levels of Foxp3 compared to recipients of IL-10−/− TR cells, consistent with lower regulatory activity of IL-10−/− TR cells. These results demonstrate that wt TR cells suppressed TE-cell-mediated H. pylori-independent gastroduodenitis and H. pylori-dependent corpus gastritis more effectively than IL-10−/− TR cells. Compartmental differences in TE-cell- and H. pylori-mediated inflammation and in regulatory effects between wt TR and IL-10−/− TR cells suggest that IL-10 expression by wt TR cells is important to regulatory suppression of gastric inflammation.
Helicobacter pylori infects the human stomach and causes gastritis, with a subset of patients developing peptic ulcer disease, gastric carcinoma, and gastric mucosa-associated lymphoid tissue lymphoma (20, 32). Infection of C57BL/6 mice with H. pylori or Helicobacter felis results in chronic active gastritis (26, 27) and has been used to study the immune basis of H. pylori-induced gastritis. Helicobacter infections in humans and mice induce a Th1-predominant immune response with activation of CD4+ T lymphocytes and expression of proinflammatory cytokines such as gamma interferon (IFN-γ) (24, 30). This cell-mediated immunity results in gastritis characterized by mononuclear cell infiltrates, mucosal hyperplasia, and intestinal metaplasia. In contrast, immunodeficient B6.129S7-Rag1tm1Mom mice that lack mature B and T cells colonized at high density with H. felis but developed only minimal gastritis (37), indicating the importance of the adaptive immune response to helicobacter-associated gastric disease.
Recent data have demonstrated that different subpopulations of CD4+ T lymphocytes play diverse roles in mediating and regulating H. pylori-induced gastritis. In B6.CB17-Prkdcscid mice, adoptive transfer of wild-type (wt) CD4+ CD45RBhi effector T (TE) cells from naïve donors caused severe gastritis in H. pylori-infected recipients, while cotransfer of wt CD4+ CD45RBlo regulatory T (TR) cells protected against development of gastritis (11). TR cells have also been defined by expression of the interleukin-2 (IL-2) receptor α chain and Foxp3 (4), the forkhead transcription factor critical to thymic selection of CD4+ CD25+ TR cells (21). Depletion of CD25+ Foxp3+ TR cells in H. pylori-infected C57BL/6 mice led to loss of immune regulation and more severe gastritis (34), as did adoptive transfer of lymphocytes depleted of CD4+ CD25+ cells into H. pylori-infected B6.Cg-Foxn1nu (nu/nu) recipients (35). Of the many T-cell subsets with ascribed regulatory function (22), cell sorting experiments commonly use CD4+ CD25+ CD45RBlo as naturally occurring TR cells. However, the mechanism(s) for regulation by this type of TR cell is not fully understood.
In adoptive transfer models using coadministration of wt TE cells and wt or IL-10-deficient (IL-10−/−) TR cells, IL-10 has been shown to be essential for the function of TR cells in suppressing inflammatory bowel disease, dysplasia, and cancer of the colon (3, 13). Based on this evidence, we evaluated H. pylori gastritis in B6.129S6-Rag2tm1Fwa (Rag2−/−) mice that had received wt CD4+ CD25− CD45RBhi TE cells and CD4+ CD25+ CD45RBlo TR cells from either wt C57BL/6 or congenic IL-10−/− mice. The results demonstrate that TE cells mediated a gastroduodenitis in Rag2−/− mice independently of H. pylori infection and that wt TR cells suppressed this lesion to a greater extent than IL-10−/− TR cells. Only wt TR cells suppressed additive corpus gastritis attributable to H. pylori infection. The data support compartmental differences in TE and H. pylori-mediated inflammation of the stomach and differences in regulatory effects between wt and IL-10−/− TR cells, suggesting that IL-10 expression by wt TR cells is important to regulatory suppression of gastric inflammation.
MATERIALS AND METHODS
Experimental groups.
C57BL/6 wt cell donor mice and adoptive transfer recipient Rag2 gene knockout mice (B6.129S6-Rag2tm1Fwa or Rag2−/−), backcrossed 12 generations to B6 wt mice, were originally from Taconic Farms (Germantown, NY). IL-10 knockout cell donor mice (B6.129P2-Il10tm1Cgn/J or IL-10−/−), backcrossed 10 generations to B6 wt mice, were originally from the Jackson Laboratory (Bar Harbor, ME). Mice were bred and maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care in static microisolator cages under specific-pathogen-free conditions as previously described (16). Male and female, 6- to 8-week old, helicobacter-free Rag2−/− mice were randomly assigned to uninfected or H. pylori-infected groups that were further subdivided 4 weeks later into groups that received either no T cells, wt TE cells, or wt TE cells in combination with wt or IL-10−/− TR cells. Helicobacter-free C57BL/6 mice were also dosed with the same inoculum of H. pylori to confirm that the mouse-adapted strain induced robust gastritis in wt mice. Some mice died acutely or were euthanized at earlier time points due to declining body condition. Only data from mice surviving to the predetermined necropsy time points of 16 and 20 weeks postinfection with H. pylori (12 and 16 weeks postadoptive transfer, respectively) were analyzed. The protocol was approved by the Committee on Animal Care of the Massachusetts Institute of Technology.
H. pylori infection.
H. pylori Sydney strain 1 was used for oral inoculation as described previously (16, 19). H. pylori was grown for 24 h at 37°C under microaerobic conditions in brucella broth with 10% fetal bovine serum. The inoculum was suspended in phosphate-buffered saline to an optical density at 600 nm of 1.000 and then assessed by Gram stain and phase microscopy for purity, morphology, and motility as well as for urease, catalase, and oxidase activity. Mice were gavaged with 0.2 ml every other day for three doses. Control groups were given 0.2 ml of phosphate-buffered saline.
Cell sorting and adoptive transfer.
Single-cell suspensions from spleens and mesenteric lymph nodes of wt or IL-10−/− mice were prepared as described previously (12). In brief, CD4+ cells were isolated by using CD4 Dynabeads and CD4 DETACHaBEAD (Dynal, Oslo, Norway). Cells were then labeled with anti-CD4-Cy, anti-CD45RB-fluorescein isothiocyanate, and anti-CD25-phycoerythrin antibodies (Pharmingen, La Jolla, CA) and then sorted by flow cytometry (model Mo-flo; Cytomation Inc., Fort Collins, CO) to a purity of >95% for wt TE cells (CD4+ CD25− CD45RBhi) and wt or IL-10−/− TR cells (CD4+ CD25+ CD45RBlo). Anesthetized Rag2−/− mice were injected in the retro-orbital sinus with 3 × 105 TE cells alone or in combination with 3 × 105 wt or IL-10−/− TR cells suspended in 200 μl of Hanks balanced salt solution.
Histological evaluation.
At necropsy, the stomach and proximal duodenum were removed and cut along the greater curvature. Linear gastric strips from the lesser curvature were fixed overnight in 10% neutral-buffered formalin, embedded, cut at 4 μm, and stained with hematoxylin and eosin (H-E). Lesions were scored by a veterinary pathologist blinded to sample identity, as described previously (36). Total lesion indices were calculated by the addition of individual scores for each assessment described in Table 1 except for mucous metaplasia and hyalinosis, which have been observed to develop spontaneously in mice (23) as well as from H. felis infection (15, 43).
TABLE 1.
Gastric lesions at 16 and 20 WPI with H. pylori
| Group (n)a | WPI | H. pylori | TE cells | TR cells | Median (range) of lesion indexb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corpus
|
Antrum
|
||||||||||||||
| I | ED | H | IM | A | MM | Total | I | BGD | D | Total | |||||
| wt (2/2) | 20 | − | − | − | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| wt (3/3) | 20 | + | − | − | 2 (2-2.5) | 1 (1-2) | 1 (0.5-1) | 1 (0.5-1) | 2.5 (2.5) | 0 (0-1) | 8 (6.5-9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rag (7/7) | 20 | − | − | − | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H. pylori (11/11) | 20 | + | − | − | 0.5 (0-1.5) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0-1) | 0 (0-1.5) | 0.5 (0-2.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TE (3/10) | 16 | − | + | − | 1.5 (1.5-2)f | 0.5 (0.5)f | 1.5 (0.5-2) | 1 (0.5-1) | 3 (2-3) | 2 (2) | 8 (5-8) | 2 (2-2.5) | 4 (4) | 1 (1)f | 9 (8-9) |
| H. pylori+TE (9/12) | 16 | + | + | − | 2.5 (2-3)d,f | 1 (0.5-2)c,f | 1 (1-2)d | 0.5 (0-1.5) | 3 (2-3.5) | 1.5 (0.5-3)d | 8.5 (6-11.5)e | 1.5 (1.5-2.5)c | 3.5 (2.5-4)c,d | 0.5 (0.5-1)c,d,f | 7.5 (6.5-10)d,e |
| H. pylori+TE+wt TR (6/7)e | 20 | + | + | wt | 1 (0.5-1.5)d | 0.5 (0.5-1)c | 1 (0.5-1)d,g | 0.5 (0-0.5) | 3 (2.5-2.5) | 3 (2.5-3.5)d | 5 (4.5-5.5)e | 1 (0.5-2)c | 2.5 (0.5-3)d | 0 (0-0.5)d | 4.5 (3-6.5)e |
| H. pylori+TE+IL-10−/−TR (4/6)d | 20 | + | + | IL-10−/− | 2 (1.5-3) | 0.5 (0.5-1) | 1.25 (1-2)g | 0.25 (0-0.5) | 2.75 (2-3) | 2.75 (2-3) | 7 (5-9) | 1.25 (1-1.5) | 2.25 (2-3)c | 0 (0-0.5)c | 5.25 (4-6)d |
Number of mice necropsied/number of mice in group. Differences represent early morbidity.
I, inflammation; ED, epithelial defect; H, hyperplasia; IM, intestinal metaplasia; A, atrophy; MM, mucous metaplasia; BGD, Bruner's gland destruction; D, dysplasia.
Significant influence of TR cells at a P of < 0.05.
Significant influence of TR cells at a P of < 0.01.
Significant influence of TR cells at a P of < 0.001.
Significant influence of H. pylori infection at a P of < 0.05.
Significant difference between wt and IL-10−/− TR cells at a P of < 0.05.
Special stains and immunohistochemistry.
Selective tissues were characterized using special stains and immunohistochemistry. Acidic (intestinal type) mucins were demonstrated using pH 2.5 Alcian blue followed by periodic acid-Schiff to stain remaining neutral (gastric type) mucins (36). Macrophages were stained with monoclonal antibody F4/80 (1:150; Caltag Laboratories, Burlingame, CA) and with an avidin-biotin-peroxidase complex kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions.
RNA extraction and quantitative PCR for cytokine expression and Foxp3.
Stomach tissue was harvested and snap-frozen in liquid nitrogen. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized from 5 μg of total RNA using a High Capacity cDNA Archive kit (Applied Biosystems, Forster City, CA). Levels of IFN-γ, tumor necrosis factor alpha (TNF-α), IL-4, IL-10, and Foxp3 were quantified with SYBR Green PCR reagent (QIAGEN, Valencia, CA) in an ABI Prism Sequence Detection System 7700 (Applied Biosystems) per the manufacturer's instructions. Primers were designed by Lasergene software (DNASTAR, Madison, WI). Sequences of primers were as follows: for IFN-γ, CATGGCTGTTTCTGGCTGTTACTG (forward [F]) and GTTGCTGATGGCCTGATTGTCTTT (reverse [R]) annealing at 56°C; for TNF-α, CATCTTCTCAAAATTCGAGTGACAA (F) and TGGGAGTAGACAAGGTACAACCC (R) annealing at 60°C; for IL-4, ACAGGAGAAGGGACGCCAT (F) and GAAGCCCTACAGACGAGCTCA (R) annealing at 60°C; for IL-10, GGTTGCCAAGCCTTATCGGA (F) and ACCTGCTCCACTGCCTTGCT (R) annealing at 60°C; for Foxp3, CCCAGGAAAGACAGCAACCTT (F) and CTCACAACCAGGCCACTTGCA (R) annealing at 60°C; for glyceraldehyde-3-phosphate dehydrogenase (GAPDH),TCCATGACAACTTTGGCATTG (F) and TCACGCCACAGCTTTCCA (R) annealing at 60°C. The final concentration of each primer was 0.3 μM. Tenfold dilutions (107 to 101 copies) of each cytokine cDNA plasmid were used to generate standard curves. Expression levels, presented as cytokine copy numbers or as ratios to GAPDH (Foxp3), were normalized to the internal control (GAPDH).
Quantitative culture of H. pylori.
Colonization of H. pylori in the stomach was assessed by quantitative culture as described previously (16). Briefly, tissues from corpus and antrum were weighed and homogenized in 250 μl of brucella broth using a sterile glass tissue grinder. The homogenate was serially diluted 10- and 100-fold in brucella broth and plated onto selection plates containing 5% horse blood, 250 μg/ml amphotericin B, 7 μg/ml bacitracin, 10.7 μg/ml nalidixic acid, 3.3 μg/ml polymyxin, and 100 μg/ml vancomycin. Plates were incubated microaerobically at 37°C for 7 days. Bacterial colonies were counted, and the number of CFU per gram of tissue was calculated.
Statistical analysis.
Morbidity between groups was compared by the log rank test. Lesion scores were compared by a Mann-Whitney U test or by a Kruskal-Wallis one-way analysis of variance with Dunnett's test. Cytokine and Foxp3 expression levels and H. pylori colonization data (after log transformation) were compared using the Newman-Keuls test. Statistical analysis was performed using commercial software (Graphpad Prism 4.0; San Diego, CA) with significance at a P value of <0.05.
RESULTS
Rag2−/− TE-cell-recipient mice developed morbidity and gastroduodenitis independent of H. pylori infection.
H. pylori-infected and helicobacter-free control Rag2−/− mice that did not receive cell transfers were clinically normal throughout the 20-week study period. In contrast, 7 of 10 uninfected TE-cell-recipient and 3 of 12 H. pylori-infected TE-cell-recipient Rag2−/− mice died acutely or developed diarrhea and declining body condition, necessitating early euthanasia between 4 and 12 weeks posttransfer of T cells (Table 1). All remaining uninfected and H. pylori-infected Rag2−/− mice that had received TE cells alone were necropsied at 12 weeks posttransfer of T cells, which was also 16 weeks postinfection (wpi) with H. pylori.
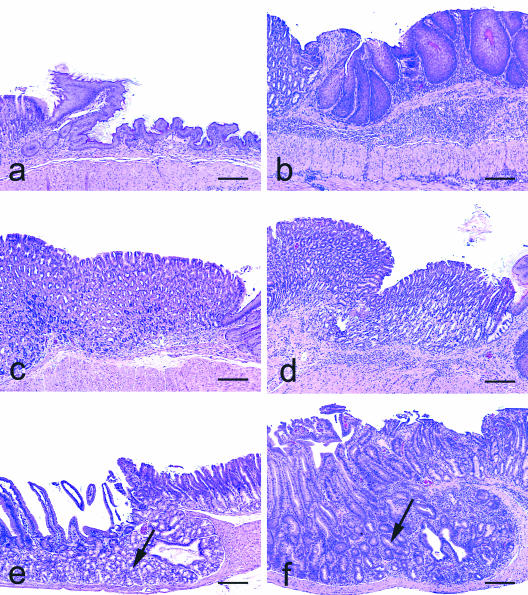
In all uninfected TE-cell-recipient Rag2−/− mice, the entire alimentary tract including the stomach through the colon was grossly edematous. The stomach and proximal duodenum from all mice were evaluated histologically, and there was severe pangastritis that involved the squamous and glandular compartments of the stomach with inflammation extending into the proximal duodenum (Table 1 and Fig. 1). This TE-cell-mediated gastroduodenitis was characterized by extensive infiltration of the mucosa and submucosa with lymphocytes, macrophages, eosinophils, and neutrophils. Other histological changes included hypertrophy and orthokeratotic hyperkeratosis of the squamous portion of the stomach, as well as epithelial defects, hyalinosis, and mucous metaplasia of the corpus, along with mild blunting, atrophy, and fusion of duodenal villi (Table 1 and Fig. 1). A consistent feature of the gastroduodenitis was loss of Brunner's glands through a combination of atrophy and dysplasia, accompanied by metaplasia to a tubuloductular phenotype (Fig. 1). The bowel distal to the proximal duodenum was examined histologically in three of these mice and had inflammatory infiltrates in the cecum and colon (data not shown), which is consistent with a previous report of TE-cell transfer-associated colitis in Rag2−/− mice (8).
FIG. 1.
Transfer of wt TE cells into Rag2−/− mice resulted in immune-mediated pangastritis and gastroduodenitis. (a) Normal squamous gastric compartment of Rag2−/− mouse that did not receive TE cells. (b) TE-cell transfer resulted in inflammation of the squamous stomach with reactive epithelial cell hypertrophy and plication of the surface mucosa. (c) TE-cell transfer also caused inflammation in the cardia and corpus. (d) TE-cell-mediated gastritis of the glandular and oxyntic mucosa resulted in oxyntic atrophy characterized by loss of parietal and chief cells. (e) Normal Brunner's glands (arrow) in the proximal duodenum. (f) TE-cell transfer produced duodenitis with near-complete loss of Brunner's glands due to atrophy and tubuloductular metaplasia (arrow). H-E staining was used for these samples. Bar, 200 μm.
Adaptive immunity is required to develop H. pylori-associated gastritis.
When evaluated at 20 wpi, the stomach and intestinal tract of H. pylori-infected Rag2−/− mice appeared grossly normal and were similar to those from uninfected Rag2−/− mice. Histologically, H. pylori-infected Rag2−/− mice developed only minimal corpus gastritis with scattered neutrophils (Table 1 and Fig. 2) and F4/80+ macrophages in the submucosa (not shown). H. pylori-infected wt mice were evaluated to confirm robust gastritis from infection with H. pylori Sydney strain 1. These mice had moderate gross thickening of the stomach, accompanied by histological lesions consisting of significant infiltration with mononuclear cells and neutrophils, epithelial defects, oxyntic atrophy, intestinal metaplasia, and hyperplasia (Table 1) (P < 0.001), as previously reported (27). These findings indicated that H. pylori-associated gastritis in mice was promoted by adaptive immunity and that inflammation observed in H. pylori-infected, TE-cell-recipient Rag2−/− mice was attributable to the inflammatory activity of donor TE cells.
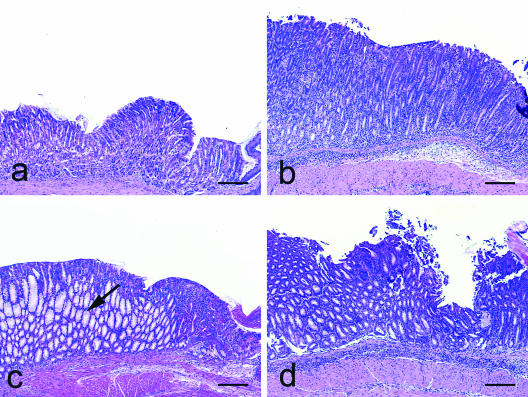
FIG. 2.
H. pylori infection in Rag2−/− mice with or without subsequent transfer of wt TE cells alone or in combination with wt or IL-10−/− TR cells. (a) In the absence of T cells, H. pylori infection produced minimal to no gastritis. (b) H. pylori infection followed by transfer of TE cells produced moderate to severe gastritis. (c) Cotransfer of wt TR cells ameliorated severity of gastric inflammation in H. pylori-infected, TE-cell-recipient mice, but for unknown reasons, marked mucous metaplasia of parietal cells developed (arrow). (d) Cotransfer of IL-10−/− TR cells into H. pylori-infected TE-cell-recipient mice decreased TE-cell-mediated antral gastritis, Brunner cell loss, and epithelial dysplasia but did not reduce H. pylori-induced corpus gastritis. H-E staining was used for these samples. Bar, 200 μm.
H. pylori infection exacerbated corpus gastritis in Rag2−/− TE-cell-recipient mice.
H. pylori-infected Rag2−/− mice that received TE cells at 4 wpi and were necropsied at 16 wpi were clinically affected to a similar degree as uninfected Rag2−/− mice that received TE cells alone. Unexpectedly, H. pylori-infected, TE-cell-recipient Rag2−/− mice had less mortality than uninfected TE-cell recipients, with 9 of 12 mice surviving to the time point of 16 wpi (P < 0.05) (Table 1). The infected TE-recipient mice developed similar gross changes in the stomach and intestinal tract with similar histological evidence of gastroduodenitis (Table 1 and Fig. 2). However, corpus gastritis was notably more severe in the H. pylori-infected TE-recipient mice compared to the uninfected TE recipients (P < 0.05) (Table 1). Otherwise, total lesion indices were similar (P = 0.20) (Table 1) between H. pylori-infected and uninfected TE-cell-recipient Rag2−/− mice, suggesting that TE cells mediated the antral gastritis, duodenitis, and destruction of Brunner's glands. These results also indicate that H. pylori infection further promoted the corpus gastritis that was superimposed on H. pylori-independent gastroduodenitis.
Adoptive transfer of wt or IL-10−/− TR cells reduced morbidity and gastroduodenitis, but only wt TR cells reduced severity of H. pylori corpus gastritis.
Clinical morbidity posttransfer of TE cells into H. pylori-infected Rag2−/− mice was less severe when either wt (P < 0.001) or IL-10−/− TR cells (P < 0.01) were cotransferred (Table 1). Total lesion indices for TE-cell-associated gastroduodenitis that developed independently of H. pylori infection were lower in Rag2−/− mice cotransferred with either wt TR (P < 0.001) or IL-10−/− TR cells (P < 0.01) (Table 1). wt TR cells were more efficacious than IL-10−/− TR cells in ameliorating antral gastritis (P < 0.01 and P < 0.10, respectively), Brunner's gland destruction (P < 0.01 and P < 0.05, respectively), and antral dysplasia (P < 0.01 and P < 0.05, respectively). Comparable to H. pylori-infected wt mice, gastritis in the H. pylori-infected Rag2−/− mice that received TE cells and either type of TR cells was concentrated in the corpus (Table 1 and Fig. 2), with mild inflammation in adjacent compartments. Notably, the TE-cell-mediated corpus pathology in H. pylori-infected Rag2−/− mice was diminished to the greatest extent in mice that were cotransferred with wt TR cells (P < 0.001), in contrast to the lack of a significant effect of IL-10−/− TR cells on total lesion indices. None of the individual pathology parameters characterizing corpus gastritis were significantly different in H. pylori-infected TE-cell-recipient mice given IL-10−/− TR cells from infected mice that received TE cells alone (P = 0.26 and higher) (Table 1 and Fig. 2). Interestingly, cotransfer of wt TR cells resulted in significantly greater mucous metaplasia of parietal cells than the H. pylori-infected Rag2−/− mice that received TE cells alone (P < 0.01) (Fig. 2).
Th1 cytokine responses were down-regulated to a greater extent by wt than IL-10−/− TR cells.
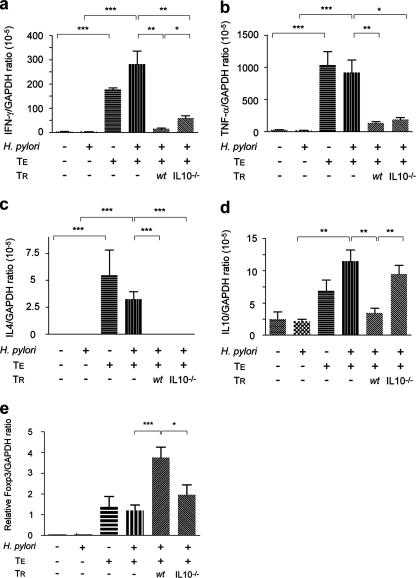
Consistent with the absence of gastritis, low levels of expression for IFN-γ, TNF-α, IL-4, and IL-10 were observed in gastric samples from uninfected and H. pylori-infected Rag2−/− mice that had not received T cells (Fig. 3). TE-cell-associated gastroduodenitis was accompanied by a significant up-regulation of IFN-γ, TNF-α, IL-4, and IL-10 (P < 0.001), regardless of H. pylori infection status (P = 0.2 and higher), indicating that TE cells were the main stimulus for cytokine expression. Notably, Th1-associated IFN-γ and TNF-α expression levels in gastric samples from TE-cell-recipient, H. pylori-infected Rag2−/− mice were 2 logs higher than Th2-associated IL-4 and IL-10 mRNA levels, consistent with the proinflammatory response to gastric helicobacters (11, 14). H. pylori-infected Rag2−/− mice that received TE plus wt TR cells had significantly lower expression levels of IFN-γ, TNF-α, IL-4, and IL-10 (P < 0.01 and lower) than infected Rag2−/− mice that received TE cells only. Consistent with amelioration of TE-cell-mediated gastroduodenitis, cotransfer of IL-10−/− TR cells also lowered mRNA expression for IFN-γ, TNF-α, and IL-4 (P < 0.005 and lower), but a decrease in IL-10 was not observed (P = 0.51). The ability of wt TR cells, and not IL-10−/− TR cells, to suppress corpus gastritis was consistent with higher expression levels for IFN-γ (P < 0.05) (Fig. 3a) in recipients of IL-10−/− TR cells. Additionally, suppression of TNF-α mRNA levels was more significant in mice receiving wt than IL-10−/− TR cells (P < 0.01 and P < 0.05, respectively) (Fig. 3b). Thus, Th1-predominant cytokine responses in H. pylori-infected TE-cell-recipients were suppressed by both wt and IL-10−/− TR cells, but suppression was greatest in recipients of wt TR cells.
FIG. 3.
Mean expression levels of mRNA for IFN-γ (a), TNF-α (b), IL-4 (c), IL-10 (d), and Foxp3 (e) were determined by quantitative PCR in gastric tissues from uninfected and H. pylori-infected Rag2−/− mice subsequently transferred with wt TE cells alone or in combination with wt or IL-10−/− TR cells. TE cells up-regulated the expression of Th1 cytokines (IFN-γ and TNF-α) and Th2 cytokine (IL-4) with mRNA for Th1 cytokines expressed 1 to 2 logs higher than for Th2 cytokines. Cotransfer of TE plus wt or IL-10−/− TR cells suppressed expression of IFN-γ, TNF-α, and IL-4. IL-10 expression was up-regulated in TE-cell-recipient mice while concurrent transfer of wt TR but not IL-10−/− TR cells down-regulated IL-10 expression. Foxp3 expression was significantly higher in mice that received TE cells. Cotransfer of wt TR cells resulted in a fourfold up-regulation of Foxp3 expression which was significantly higher than that induced by IL-10−/− TR cells (P < 0.05), which was unchanged from mice receiving TE cells alone. Bars represent standard error. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Foxp3 expression in gastric tissues was promoted to a greater extent by wt than IL-10−/− TR cells.
Uninfected and H. pylori-infected Rag2−/− mice that did not receive TE cells had no detectable expression of Foxp3 in gastric tissues (Fig. 3e). Foxp3 expression levels were elevated in mice that received TE cells (P < 0.05), and H. pylori infection did not further stimulate expression. Levels of Foxp3 were significantly higher in H. pylori-infected mice that received wt TR cells (P < 0.001). Foxp3 expression in tissues from recipients of IL-10−/− TR cells was not further elevated over levels observed for H. pylori-infected mice that received TE cells alone.
H. pylori colonization levels were reduced by TE cells and maintained when wt TR cells were cotransferred.
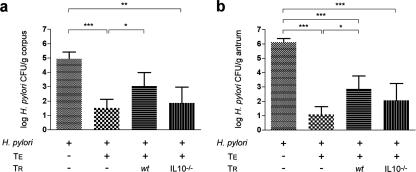
H. pylori colonization levels have been reported to be inversely related to the severity of associated chronic gastritis in mice (38). Consistent with the absence of an inflammatory response, H. pylori-infected Rag2−/− mice that did not receive T cells were colonized at levels 3 to 5 logs higher in the corpus and antrum, respectively, than in infected, TE-cell-recipient Rag2−/− mice (Fig. 4) (P < 0.001). H. pylori-infected mice that received TE and wt TR cells maintained colonization in the corpus at levels similar to H. pylori-infected mice that did not receive TE cells (P = 0.18) (Fig. 4a). In contrast, H. pylori-infected mice that received TE plus IL-10−/− TR cells had reduced corpus colonization similar to infected mice that received TE cells alone (P = 0.43), suggesting that IL-10−/− TR cells failed to influence a TE-cell-mediated inhibition of H. pylori. In the antrum, wt TR cells maintained higher colonization levels than in infected mice that received TE cells alone (P < 0.05), whereas colonization in mice receiving TE and IL-10−/− TR cells was similar to infected mice receiving TE cells alone (P = 0.19) (Fig. 4b).
FIG. 4.
Mean H. pylori colonization in the corpus (a) and antrum (b) of Rag2−/− mice receiving either no T cells, wt TE cells, or TE cells in combination with TR cells from wt or IL-10−/− mice. H. pylori colonization in the corpus was lower in mice receiving TE or TE plus IL-10−/− TR cells. Cotransfer of wt TR cells maintained colonization levels in the corpus (only), whereas TE plus IL-10−/− TR cells significantly diminished H. pylori colonization in the corpus and antrum. Bars represent standard error. * P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Using T-cell transfer into H. pylori-infected Rag2−/− mice, this study demonstrated that wt TR cells reduced TE cell-mediated morbidity, H. pylori-dependent corpus gastritis, and H. pylori-independent gastroduodenitis. The H. pylori-independent gastroduodenitis was characterized by antral gastritis, duodenitis, Brunner cell loss, and epithelial dysplasia. These lesions have not been previously described in mice with helicobacter infections and are consistent with autoimmune-like disease (22). IL-10−/− TR cells also reduced TE-cell-mediated morbidity and, to a lesser extent, H. pylori-independent gastroduodenitis. Notably, IL-10−/− TR cells did not reduce corpus gastritis exacerbated by H. pylori infection, nor did IL-10−/− TR cells reverse the TE-cell-mediated reduction in H. pylori colonization levels. TE cells mediated a sufficiently robust proinflammatory cytokine response that IFN-γ and TNF-α expression levels were similar irrespective of H. pylori infection status. This Th1-predominant response was suppressed by both wt and IL-10−/− TR cells, but reductions in proinflammatory cytokine levels were greatest in recipients of wt TR cells. Gastric tissues from mice receiving wt TR cells expressed higher levels of Foxp3 than recipients of IL-10−/− TR cells, consistent with lower regulatory activity of IL-10−/− TR cells. These results suggest compartmental differences in TE-cell- and H. pylori-mediated gastritis and indicate that IL-10 expression by wt TR cells is important to regulatory suppression of gastric inflammation.
Similar to H. felis infection in Rag1−/− mice (37) and despite high H. pylori colonization levels, Rag2−/− mice that did not receive TE cells did not develop morbidity or gastrointestinal lesions. TE-cell-recipient mice developed morbidity and gastroduodenitis independent of H. pylori infection, consistent with previous studies of TE cell transfer into Rag2−/− mice (2, 8) and mouse models of TR deficiency (31, 33). Observations of TE-cell-mediated inflammation in the upper gastrointestinal tract have been reported (10, 11) but not as frequently as TE-cell-mediated colitis (31, 33) and may be impacted by the number of cells transferred (V. P. Rao, personal communication). Extraintestinal inflammation attributable to TE-cell infiltrates in sites such as the liver and Harderian gland have also been observed (42) and may contribute to morbidity. At these sites, TE cells likely proliferate in response to host or, more likely, bacterial antigens either absorbed through the local epithelium or distributed systemically by vascular and lymphatic circulation. TE-cell-mediated morbidity may also be attributable to systemic effects of up-regulation of IFN-γ, TNF-α, IL-4, IL-10, and other mediators released from gastrointestinal and extraintestinal tissues. Interestingly, preexisting H. pylori infection resulted in a higher survival rate in mice that subsequently received TE cells, potentially by promoting homing of TE cells to mucosal sites, resulting in less extraintestinal inflammation.
In uninfected TE-cell-recipient Rag2−/− mice, reactive TE cells in the gastrointestinal mucosa most likely expanded in response to dietary antigens and antigens of normal microbiota known to colonize all regions of the bowel, including the upper gastrointestinal tract of mice (39). TE-cell-mediated pangastritis involved the squamous and glandular compartments of the stomach and extended into the duodenum. Lymphocytic infiltration of the antrum and duodenum promoted antral dysplasia, villus atrophy, and destruction of Brunner glands and was an overlapping but distinct disease pattern compared to the H. pylori corpus gastritis as described in this study and by others (2, 8, 41). As expected, severity of corpus gastritis was inversely correlated with H. pylori colonization levels, and this is indirect evidence that TE cells were responding to H. pylori antigens. Both wt and IL-10−/− TR cells reduced the H. pylori-independent gastroduodenitis, but only wt TR cells reduced the H. pylori corpus gastritis and maintained higher levels of H. pylori colonization, suggesting compartmental or other differences in the stomach that may favor a protective role for IL-10. The anti-inflammatory effect of IL-10 is implied by the observations that IL-10−/− mice developed more severe H. felis (5) and H. pylori gastritis (28, 44) than congenic wt mice, as did H. pylori-infected B6.CB17-Prkdcscid mice injected with IL-10−/− splenocytes (11). Additionally, a requirement for IL-10 competency of TR cells has been shown in a mouse model of inflammatory bowel disease using Helicobacter hepaticus infection. In this model, only wt TR cells, and notably not IL-10−/− TR cells, reduced inflammation, dysplasia, and cancer lesions (13).
IL-10−/− TR cells reduced morbidity and down-regulated TE-cell-mediated gastroduodenitis along with lower IFN-γ and TNF-α expression. This observation is consistent with the absence of this distinct gastroduodenitis in uninfected or H. pylori-infected IL-10−/− mice (44) that lack IL-10-competent TR cells. Interestingly, IL-10 expression in the Rag2−/− gastric tissues was not reduced in IL-10−/− TR-cell-recipient mice as observed in mice that received wt TR cells. This result suggests that IL-10 message was expressed by donor TE or host epithelial or other inflammatory cells (6), possibly as a compensatory response of inflamed gastric tissue. Similarly, IL-10 protein levels in the H. pylori-infected gastric mucosa of humans have been positively associated with the severity of gastritis (6). Although the gastric IL-10 level was not sufficient to dampen H. pylori-mediated corpus gastritis, our findings suggest that IL-10 competency of TR cells, but not local IL-10 per se, directly or indirectly reduces severity of H. pylori gastritis. Importantly, although IL-10 competence appears necessary for TR-cell suppression of H. pylori gastritis, our results do not rule out other mechanisms. For example, CTLA-4 engagement induced and maintained anergy of H. pylori-specific T cells in a TR-cell-independent manner (1). Our data also show that Foxp3 expression in gastric tissues was promoted by TE cells, suggesting differentiation of TE cells into TR cells in peripheral tissues as reported by others (7). Lastly, Foxp3 expression was promoted only by wt and not IL-10−/− TR cells, consistent with the finding that the wt has more potent regulatory activity than IL-10−/− TR cells. Further studies are necessary to clarify these issues.
In humans, H. pylori gastritis and progression to carcinoma have been associated with atrophy and intestinal and mucous metaplasia of fundic glands (29), consistent with the hypothesis that gastritis, gastric atrophy, and gastric cancer represent a continuum of progressive disease (18). Irrespective of H. pylori infection, Rag2−/− mice that received TE cells developed marked mucous metaplasia in the corpus, which was more prominent in mice that received cotransfer of wt TR cells. Fundic atrophy from chronic H. felis infection in mice has been associated with mucous metaplasia characterized by increased expression of trefoil factor 2 (TFF2), also known as spasmolytic polypeptide, by gastric mucous neck cells (15, 43). This lesion appears to be a replacement of parietal and chief cells with TFF2-secreting mucous cells that are similar in morphology to antral or Brunner's glands and has been suggested to be a precursor lesion of gastric cancer in mice (19) and in humans (9, 40). Alternatively, because TFF2−/− mice infected with H. pylori (17) or H. felis (25) developed more severe gastritis and hyperresponsiveness to IL-1β than wt mice, TFF2 may be a negative regulator of gastritis, and the promotion of mucous metaplasia by wt TR cells may reflect a protective TFF2-associated response through an unknown mechanism.
In summary, our data demonstrate the role of TE cells as one component of adaptive immunity that can trigger and sustain gastroduodenal inflammation in the absence of TR cells. H. pylori infection coupled with TE-cell transfer resulted in a more severe corpus gastritis that was compartmentally distinct from TE-cell-mediated gastroduodenitis. Further, our results demonstrate that IL-10 competence of TR cells appeared to be a requirement for suppression of H. pylori-induced gastritis but was not as critical for amelioration of TE-cell-mediated gastroduodenitis or protection against associated morbidity. These results are compatible with previous reports that H. pylori-induced gastritis in mice is regulated by an IL-10-dependent, Th1-type immune response (5, 44), likely via the function of CD25+ Foxp3+ TR cells (34), and is consistent with increased Foxp3 expression in wt TR-cell recipients. Compartmental differences in TE-cell- and H. pylori-mediated inflammation and differential regulation by wt or IL-10−/− TR cells should prove valuable for study of bacterial and immune-mediated gastritis.
Acknowledgments
This work was supported by grants R01AI37750, R01AI50952, P01CA26T31, and P30ES02109 (J.G.F.).
We thank Vivian Ng and Philip Lee for animal care and necropsy, Kathy Cormier for histology and immunohistochemistry, Sandy Xu and Nancy Taylor for quantitative culture, Kuo-Liong Chien for assistance with statistical analysis, and Glenn A. Paradis and Michele Perry for their expert assistance with cell sorting.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Anderson, K. M., S. J. Czinn, R. W. Redline, and T. G. Blanchard. 2006. Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection. J. Immunol. 176:5306-5313. [DOI] [PubMed] [Google Scholar]
- 2.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, T. C. Barbosa, A. Cumano, and A. Bandeira. 2001. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 166:3008-3018. [DOI] [PubMed] [Google Scholar]
- 3.Asseman, C., S. Mauze, M. W. Leach, R. L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banham, A. H., F. M. Powrie, and E. Suri-Payer. 2006. FOXP3+ regulatory T cells: current controversies and future perspectives. Eur. J. Immunol. 36:2832-2836. [DOI] [PubMed] [Google Scholar]
- 5.Berg, D. J., N. A. Lynch, R. G. Lynch, and D. M. Lauricella. 1998. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10−/− mice. Am. J. Pathol. 152:1377-1386. [PMC free article] [PubMed] [Google Scholar]
- 6.Bodger, K., J. I. Wyatt, and R. V. Heatley. 1997. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut 40:739-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, W., W. Jin, N. Hardegen, K. J. Lei, L. Li, N. Marinos, G. McGrady, and S. M. Wahl. 2003. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198:1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corazza, N., S. Eichenberger, H. P. Eugster, and C. Mueller. 1999. Nonlymphocyte-derived tumor necrosis factor is required for induction of colitis in recombination activating gene RAG2−/− mice upon transfer of CD4+ CD45RBhi T cells. J. Exp. Med. 190:1479-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhar, D. K., T. C. Wang, R. Maruyama, J. Udagawa, H. Kubota, T. Fuji, M. Tachibana, T. Ono, H. Otani, and N. Nagasue. 2003. Expression of cytoplasmic TFF2 is a marker of tumor metastasis and negative prognostic factor in gastric cancer. Lab. Investig. 83:1343-1352. [DOI] [PubMed] [Google Scholar]
- 10.Dohi, T., K. Fujihashi, T. Koga, Y. Etani, N. Yoshino, Y. I. Kawamura, and J. R. McGhee. 2004. CD4+ CD45RBHi interleukin-4 defective T cells elicit antral gastritis and duodenitis. Am. J. Pathol. 165:1257-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456-7461. [DOI] [PubMed] [Google Scholar]
- 12.Erdman, S. E., T. Poutahidis, M. Tomczak, A. B. Rogers, K. Cormier, B. Plank, B. H. Horwitz, and J. G. Fox. 2003. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 162:691-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdman, S. E., V. P. Rao, T. Poutahidis, M. M. Ihrig, Z. Ge, Y. Feng, M. Tomczak, A. B. Rogers, B. H. Horwitz, and J. G. Fox. 2003. CD4+ CD25+ regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 63:6042-6050. [PubMed] [Google Scholar]
- 14.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 15.Fox, J. G., X. Li, R. J. Cahill, K. Andrutis, A. K. Rustgi, R. Odze, and T. C. Wang. 1996. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 110:155-166. [DOI] [PubMed] [Google Scholar]
- 16.Fox, J. G., A. B. Rogers, M. Ihrig, N. S. Taylor, M. T. Whary, G. Dockray, A. Varro, and T. C. Wang. 2003. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 63:942-950. [PubMed] [Google Scholar]
- 17.Fox, J. G., A. B. Rogers, E. A. Kurt-Jones, and T. C. Wang. 2006. H. pylori accelerates the progression of dysplasia of the pylori antrum in Tff2−/− C57BL/6 × Sv129 infected mice. Gastroenterology 130:A9. [Google Scholar]
- 18.Fox, J. G., and T. C. Wang. 2007. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 117:60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox, J. G., T. C. Wang, A. B. Rogers, T. Poutahidis, Z. Ge, N. Taylor, C. A. Dangler, D. A. Israel, U. Krishna, K. Gaus, and R. M. Peek, Jr. 2003. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology 124:1879-1890. [DOI] [PubMed] [Google Scholar]
- 20.Hansson, L. E., O. Nyren, A. W. Hsing, R. Bergstrom, S. Josefsson, W. H. Chow, J. F. Fraumeni, Jr., and H. O. Adami. 1996. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 335:242-249. [DOI] [PubMed] [Google Scholar]
- 21.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, S., R. I. Lechler, X. S. He, and J. F. Huang. 2006. Regulatory T cells and transplantation tolerance. Hum. Immunol. 67:765-776. [DOI] [PubMed] [Google Scholar]
- 23.Kang, W., S. Rathinavelu, L. C. Samuelson, and J. L. Merchant. 2005. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab. Investig. 85:702-715. [DOI] [PubMed] [Google Scholar]
- 24.Karttunen, R. 1991. Blood lymphocyte proliferation, cytokine secretion and appearance of T cells with activation surface markers in cultures with Helicobacter pylori. Comparison of the responses of subjects with and without antibodies to H. pylori. Clin. Exp. Immunol. 83:396-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurt-Jones, E. A., L. Cao, F. Sandor, A. B. Rogers, M. T. Whary, P. R. Nambiar, A. Cerny, G. Bowen, J. Yan, S. Takaishi, A. L. Chi, G. Reed, J. Houghton, J. G. Fox, and T. C. Wang. 2007. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 75:471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, A., J. G. Fox, G. Otto, and J. Murphy. 1990. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology 99:1315-1323. [DOI] [PubMed] [Google Scholar]
- 27.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto, Y., T. G. Blanchard, M. L. Drakes, M. Basu, R. W. Redline, A. D. Levine, and S. J. Czinn. 2005. Eradication of Helicobacter pylori and resolution of gastritis in the gastric mucosa of IL-10-deficient mice. Helicobacter 10:407-415. [DOI] [PubMed] [Google Scholar]
- 29.Mera, R., E. T. Fontham, L. E. Bravo, J. C. Bravo, M. B. Piazuelo, M. C. Camargo, and P. Correa. 2005. Long-term follow up of patients treated for Helicobacter pylori infection. Gut 54:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 31.Morrissey, P. J., K. Charrier, S. Braddy, D. Liggitt, and J. D. Watson. 1993. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 178:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisani, P., D. M. Parkin, F. Bray, and J. Ferlay. 1999. Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer 83:18-29. [DOI] [PubMed] [Google Scholar]
- 33.Powrie, F., M. W. Leach, S. Mauze, L. B. Caddle, and R. L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 SCID mice. Int. Immunol. 5:1461-1471. [DOI] [PubMed] [Google Scholar]
- 34.Rad, R., L. Brenner, S. Bauer, S. Schwendy, L. Layland, C. P. da Costa, W. Reindl, A. Dossumbekova, M. Friedrich, D. Saur, H. Wagner, R. M. Schmid, and C. Prinz. 2006. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 131:525-537. [DOI] [PubMed] [Google Scholar]
- 35.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers, A. B., N. S. Taylor, M. T. Whary, E. D. Stefanich, T. C. Wang, and J. G. Fox. 2005. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 65:10709-10715. [DOI] [PubMed] [Google Scholar]
- 37.Roth, K. A., S. B. Kapadia, S. M. Martin, and R. G. Lorenz. 1999. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J. Immunol. 163:1490-1497. [PubMed] [Google Scholar]
- 38.Sakagami, T., M. Dixon, J. O'Rourke, R. Howlett, F. Alderuccio, J. Vella, T. Shimoyama, and A. Lee. 1996. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut 39:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarma-Rupavtarm, R. B., Z. Ge, D. B. Schauer, J. G. Fox, and M. F. Polz. 2004. Spatial distribution and stability of the eight microbial species of the altered Schaedler flora in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 70:2791-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt, P. H., J. R. Lee, V. Joshi, R. J. Playford, R. Poulsom, N. A. Wright, and J. R. Goldenring. 1999. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab. Investig. 79:639-646. [PubMed] [Google Scholar]
- 41.Suri-Payer, E., and H. Cantor. 2001. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+ CD25+ T cells. J. Autoimmun. 16:115-123. [DOI] [PubMed] [Google Scholar]
- 42.Theve, E., J. Fox, and A. Rogers. 2005. Multiple autoimmune syndrome in 129/SvEv Rag2−/− mice receiving adoptive transfer of T effector cells. Vet. Pathol. 42:707. [Google Scholar]
- 43.Wang, T. C., J. R. Goldenring, C. Dangler, S. Ito, A. Mueller, W. K. Jeon, T. J. Koh, and J. G. Fox. 1998. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114:675-689. [DOI] [PubMed] [Google Scholar]
- 44.Whary, M., B. Sheppard, J. Cline, S. Xu, and J. Fox. 2001. IL-10 deficiency reduces Helicobacter pylori gastric colonization in C57BL/6 mice. Gastroenterology 120:A99-A100. [Google Scholar]