Abstract
Strains of Enterococcus faecium express a cell wall-anchored protein, Acm, which mediates adherence to collagen. Here, we (i) identify the minimal and high-affinity binding subsegments of Acm and (ii) show that anti-Acm immunoglobulin Gs (IgGs) purified against these subsegments reduced E. faecium TX2535 strain collagen adherence up to 73 and 50%, respectively, significantly more than the total IgGs against the full-length Acm A domain (28%) (P < 0.0001). Blocking Acm adherence with functional subsegment-specific antibodies raises the possibility of their use as therapeutic or prophylactic agents.
Enterococcus faecium, a gram-positive commensal inhabiting normal human gut, has been recognized as an opportunistic pathogen capable of causing life-threatening infections, including infective endocarditis (3). The ability of E. faecium to colonize vascular tissue is believed to occur by MSCRAMM (7) interactions with ligand at the sites of endovascular injuries. We have recently reported that some clinically derived E. faecium strains can adhere to collagen and identified a gene named acm encoding an adhesin of collagen from E. faecium (6). Acm has characteristics typical of an MSCRAMM (7) with an N-terminal signal peptide, followed by a nonrepeated A domain, various numbers of B repeats depending on the strain, and C-terminal motifs required for surface sorting and covalent anchoring to peptidoglycan (Fig. 1A).
FIG. 1.
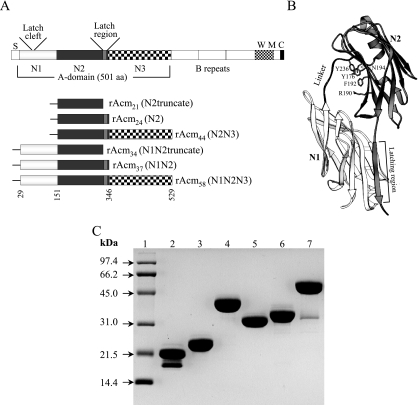
Recombinant constructs, purified proteins, and predicted model that adopts the previously identified DE variant of the Ig fold. (A) Schematic representation of the subdomains of E. faecium Acm and different constructs. The collagen-binding A domain is followed by B repeats. S, signal peptide; W, cell wall-anchoring region containing LPKTS; M, transmembrane segment; C, cytoplasmic tail. The three subdomains of the A domain are from residues 29 to 150 (N1), 151 to 346 (N2), and 347 to 529 (N3). The previously predicted minimum collagen-binding domain is from residues 151 to 320 (6, 10, 17). The predicted latch sequence (ASGGVNG) and the corresponding latch cleft region (VEGWGQF) of the N1 domain are shown. Recombinant proteins are indicated by the subdomain compositions. All constructed recombinant proteins contain an N-terminal His tag, as illustrated by “-.” (B) Ribbon representation of the model of Acm. A theoretical model of the structure of rAcm37 was obtained by homology modeling, using the crystal structure of Cna (Protein Data Bank identification no. 2F68) as a template. The HOMOLOGY module available in InsightII (Accelrys Inc., San Diego, CA) was used to build the model. The N1 and N2 subdomains are shown in light and dark gray shades, respectively. The five key residues predicted as potential contact points with the collagen in the N2 subdomain are shown as gray stick objects; these amino acids were shown to be critical for collagen binding by Cna of S. aureus (14). The three pairs of hydrogen bonds that would stabilize the closed conformation (latching event) of the “Collagen Hug” model (17) are marked as dotted lines. (C) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of recombinant His6-Acm constructs after purification. Lanes: 1, molecular mass standards; 2, Acm21; 3, Acm24; 4, Acm44; 5, Acm34; 6, Acm37; 7, Acm58.
Characterization of acm from diverse strains identified the predominance of a functional acm gene in clinically derived isolates versus a pseudogene in many fecal (6) and animal (S. R. Nallapareddy and B. E. Murray, unpublished results) isolates. Genetic analysis confirmed that Acm is necessary to mediate the attachment of E. faecium strains to collagen (5).
Our previous study localized the collagen type I binding activity of Acm to the 501-amino-acid (aa) A domain (6). The Acm A domain shares considerable sequence homology with a family of structurally related collagen-binding adhesins found in five gram-positive pathogens, namely, Staphylococcus aureus (8), Enterococcus faecalis (4), Streptococcus equi (2), Erysipelothrix rhusiopathiae (12), and Streptococcus mutans (11). Cna of S. aureus, the prototype member of this family, has been extensively characterized at the structural level (8, 10, 15, 17).
The ligand-binding A domain of Cna contains three subdomains, named N1, N2, and N3, each adopting an immunoglobulin (Ig)-like fold (9). It was recently shown that the protein construct corresponding to the N1N2 subdomains bound collagen with an affinity higher than that of the full-length A domain (17). Based on crystal structures of Cna N1N2 as an apoprotein and in complex with a synthetic collagen-like peptide, a multistep “Collagen Hug” binding model was proposed for the binding of Cna to collagen triple helices (17).
Subdomain organization of Acm A domain.
By comparing the amino acid sequences of Acm and Cna, and guided by molecular modeling, we found that the A domain of Acm also appears to be composed of three subdomains: N1, corresponding to aa 29 to 150, N2, corresponding to aa 151 to 346, and N3, corresponding to aa 347 to 529 (Fig. 1A); the previously predicted minimal region of Acm required for collagen binding (aa 151 to 320) would correspond to a truncated form of the N2 domain (6). Molecular modeling suggests that these domains could adopt the previously identified DE variant of the Ig fold (Fig. 1B), characteristic of staphylococcal MSCRAMMs (1). The predicted N1N2 region of Acm is almost the same size (318 aa) as the N1N2 region of Cna (314 aa), and they exhibit 46% identity and 56% similarity (6, 10, 17). In addition, the C-terminal latch region of the N2 domain (ASGGVNG), which is predicted to interact with the N1 domain to complement one of the β-sheets of the N1 domain in a proposed cleft formed by VEGWGQF (VSGFAEF in Cna) for securing the MSCRAMM-collagen complex, is conserved (Fig. 1B) (17). In the current work, we expressed six different recombinant proteins of Acm subregions, characterized their binding to collagen, and tested for alteration of adherence of E. faecium to collagen with subregion-specific antibodies.
Recombinant constructs.
The following recombinant constructs were made: (i) truncated N2, lacking the latch region, corresponding to aa 151 to 320, (ii) N2 (aa 151 to 346), (iii) combinations of tandem subdomains (i.e., N2N3 [residues 151 to 529], N1N2truncate [aa 29 to 320], and N1N2 [aa 29 to 346]), and (iv) the full-length A domain (N1N2N3 [aa 29 to 529]) (Fig. 1A). DNA fragments were PCR amplified from the previously generated pTEX5330 encoding the complete Acm A domain of the collagen-adhering E. faecium strain TX2555 (6), using primers listed in Table 1, cloned into the pQE30 expression vector as described previously (6, 13), and confirmed by DNA sequencing. The expression and large-scale purification of the recombinant fragments, using a nickel-charged HiTrap chelating HP column followed by a HiTrap Q-Sepharose column (Amersham), were as described previously (6, 13), and this method of using two different columns allowed for the isolation of essentially pure proteins that were estimated to be >95% pure. Purified recombinant proteins were named based on their molecular sizes (Fig. 1 and Table 1). Analysis of these recombinant proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed the migration of all proteins at their predicted molecular sizes (Fig. 1C). However, a second band of smaller molecular size, likely representing degradation, was observed in the preparations of proteins rAcm21 and rAcm58 upon overnight storage even under different conditions. Verification by mass spectrometry indicated that the bands of rAcm24, rAcm44, rAcm34, and rAcm37 proteins and larger bands of rAcm21 and rAcm58 were of full size (Table 1).
TABLE 1.
Recombinant constructs used in this study and oligonucleotide primers used to amplify the subsegments of the Acm A domain
| Expression construct | Subdomain | aa | Molecular mass (kDa)b | Recombinant protein | Sequencea |
|---|---|---|---|---|---|
| pTEX5411 | N2trun | 151-320 | 20.881 | rAcm21 | 5′-CGCGGATCCGTGACGAGCGGTGATAAAACAGCTACTG |
| 5′-CGGGGTACCTTAATTTTTAACTGTATGATTGAAACTTTC | |||||
| pTEX5412 | N2 | 151-346 | 23.667 | rAcm24 | 5′-CGCGGATCCGTGACGAGCGGTGATAAAACAGCTACTG |
| 5′-CGGGGTACCTTAAATCTCTGTGTCATTGATATATTTG | |||||
| pTEX5413 | N2N3 | 151-529 | 44.286 | rAcm44 | 5′-CGCGGATCCGTGACGAGCGGTGATAAAACAGCTACTG |
| 5′-CGGGGTACCTTATTTATTCTCATTTGTAACGACTAGC | |||||
| pTEX5414 | N1N2trun | 29-320 | 34.401 | rAcm34 | 5′-CGCGGATCCGATGCAGGCAGAGATATCAGCAGTAATG |
| 5′-CGGGGTACCTTAATTTTTAACTGTATGATTGAAACTTTC | |||||
| pTEX5415 | N1N2 | 29-346 | 37.147 | rAcm37 | 5′-CGCGGATCCGATGCAGGCAGAGATATCAGCAGTAATG |
| 5′-CGGGGTACCTTAAATCTCTGTGTCATTGATATATTTG | |||||
| pTEX5416 | N1N2N3 | 29-529 | 57.900 | rAcm58 | 5′-CGCGGATCCGATGCAGGCAGAGATATCAGCAGTAATG |
| 5′-CGGGGTACCTTATTTATTCTCATTTGTAACGACTAGC |
The introduced restriction sites are underlined, and stop codons are italicized.
Molecular masses were estimated by matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Identification of minimal and high-affinity binding subsegments of Acm A domain.
To test the interaction of each of the recombinant Acm A-domain subsegments with immobilized collagen, 20 μM concentrations of recombinant proteins were used in an enzyme-linked immunosorbent assay-type ligand-binding assay (16). In brief, purified recombinant Acm proteins were added to microtiter wells coated with 1 μg of collagen type I (Sigma) and the bound recombinant proteins were detected with mouse anti-His monoclonal antibody (Amersham), followed by alkaline phosphatase-conjugated rabbit anti-mouse IgG (Bio-Rad).
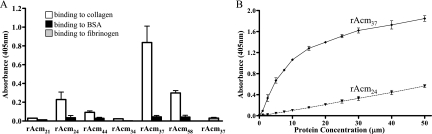
The full-length A domain (rAcm58) and three subsegments (rAcm24, N2; rAcm37, N1N2; and rAcm44, N2N3) each bound to immobilized collagen (Fig. 2A). rAcm37, containing the predicted N1N2 domains, bound collagen with a substantially higher apparent affinity than the full-length A domain (rAcm58) (Fig. 2A). A similar observation was previously reported for Cna (17). Recombinant protein that contained the complete N2 domain (rAcm24) bound collagen with a lower affinity but higher than that of rAcm44 (N2N3). Recombinant proteins representing the previously predicted minimal region required for collagen binding (rAcm21) and N1N2truncate (rAcm34) did not show any measurable binding for collagen, suggesting that the complete N2 domain, including the putative latch region, is critical for the binding activity of this protein. Further analyses using 1 to 50 μM of Acm proteins confirmed the relative affinities for rAcm24 and rAcm37 (Fig. 2B). As shown in Fig. 2B, the collagen-coated wells were saturated with rAcm37, but not with rAcm24, at the concentrations indicated. A concentration-dependent curve for a recombinant protein similar to rAcm58 (but expressed in pBADHisA vector) has previously been reported (6).
FIG. 2.
Binding characteristics of recombinant Acm A-domain subsegments. (A) Binding of 20 μM recombinant Acm A-domain subsegments to immobilized collagen type I. This experiment was repeated three times using different batches of protein purification. Values represent the means ± standard errors of the means from three independent experiments using different batches of protein purification. The binding of rAcm37 to fibrinogen (additional negative control) is also shown. (B) Binding curves of high-affinity binding subdomains (rAcm37) and the minimal region required for binding (rAcm24) to 1 μg of immobilized collagen as a function of their concentration. Values represent the means ± standard deviations from two independent experiments using the same batch of protein purification. BSA, bovine serum albumin.
Taken together, the relative binding abilities of different subsegments of Acm are comparable to the binding characteristics of similar subsegments of Cna, consistent with the hypothesis that Acm N1N2 subdomains may adopt similar two-domain structures, with N2 being the binding subdomain, and use the “Collagen Hug” mechanism for binding to collagen (17). In this model, the C-terminal region of the N2 subdomain is proposed to overlap with the N1 domain and function as a latch by complementing one of the β-sheets of the N1 domain for securing the Acm-collagen complex in the closed confirmation (Fig. 1B).
Inhibition of E. faecium cells adhering to collagen by recombinant Acm A-domain subsegments.
We have previously reported partial reduction in the adherence of the vancomycin-resistant endocarditis-derived E. faecium isolate TX2535 (6) to collagen upon preincubation of collagen-coated wells with the recombinant full-length Acm A domain but only at concentrations greater than that for the saturated binding of the recombinant Acm A domain. In this study, two different recombinant Acm subsegments (rAcm37 and rAcm24) that exhibit different collagen-binding affinities were examined for their abilities to inhibit the adherence of TX2535 to collagen. For inhibition experiments, collagen-coated wells were incubated with various concentrations (5 to 60 μM) of recombinant proteins for 2 h at 37°C. The wells were then washed in phosphate-buffered saline containing 0.1% Tween 80 and 0.1% bovine serum albumin to remove excess unbound proteins prior to the addition of labeled cells in a previously described adherence assay (6). Both of the recombinant proteins partially inhibited bacterial attachment at concentrations higher than 40 μM. At a 60 μM concentration, the collagen adherence reduction percentages for rAcm37, rAcm58, and rAcm24 were 55, 43, and 15%, respectively (data not shown). Thus, the differences seen between the subsegment constructs in their binding to collagen are reflected by a difference in their ability to inhibit Acm-dependent bacterial attachment to collagen. The observed partial inhibitory effect suggests that native Acm may have a higher affinity to immobilized collagen than recombinant subsegments.
Concentration-dependent inhibition of collagen adherence of two endocarditis-derived E. faecium isolates with specific antibodies against collagen-binding subsegments.
For inhibition studies, three sets of anti-Acm polyclonal IgGs were used: (i) total IgGs from rabbit antisera raised against the Acm A domain (6) purified by affinity chromatography on a protein G column (Pierce), (ii) specific IgGs eluted against the high-affinity binding segment, rAcm37, and (iii) specific IgGs eluted against the minimal region required for collagen binding, rAcm24. The last two specific IgGs were prepared using the respective recombinant proteins (coupled to cyanogen bromide-activated Sepharose 4B) as immunoabsorbents according to the manufacturer's protocol (Amersham). Of note, determination of the titers of total anti-Acm IgGs in an enzyme-linked immunosorbent assay showed that anti-Acm IgGs saturate 1 μg/ml recombinant Acm58 when added at a concentration of 3 μg/ml or higher (data not shown).
For inhibition by IgGs, labeled bacteria were preincubated with 10-μg/ml concentrations of the three anti-Acm IgGs mentioned above for 1 h at 37°C, and 50 μl of these cells was then added to the collagen- or fibrinogen-coated wells in the adherence assay described earlier (6). The cells used in this assay were grown to entry into stationary phase, the phase at which we detected Acm on the surface of ∼93% of cells of TX2535 by using flow cytometry (S. R. Nallapareddy et al., unpublished data). IgGs from preimmune rabbit serum served as a control. All of the data presented are means ± standard deviations of the results of three to four independent experiments. Statistical analysis was done by analysis of variance with Bonferroni's post hoc modification for multiple comparisons.
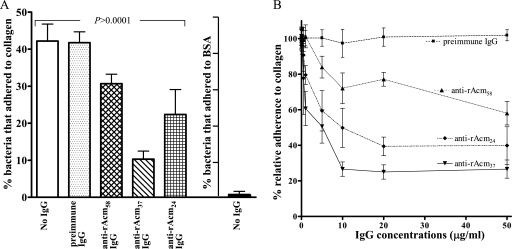
As shown in Fig. 3A, all three anti-Acm IgGs inhibited the adherence of TX2535 to collagen. However, the reduction in adherence seen with binding region-specific (anti-rAcm37 and anti-rAcm24) IgGs was considerably greater than that seen with total anti-rAcmA IgGs: 73.4%, 50.2%, and 27.9% reduction with anti-rAcm37 IgGs, anti-rAcm24 IgGs, and anti-Acm total IgGs, respectively, compared to 2.7% reduction with preimmune IgGs (P < 0.0001 and P < 0.001, respectively, for multiple comparisons). In addition, the reduction differences between anti-rAcm37 IgGs, anti-rAcm24 IgGs, and anti-Acm total IgGs were also statistically significant (P < 0.001 for anti-rAcm37 versus the other two and P < 0.05 for anti-rAcm24 IgGs versus anti-Acm total IgGs). As anticipated, anti-Acm antibodies did not inhibit the adherence of this strain to fibrinogen (data not shown). We have also tested the effects of these IgGs on the adherence of another endocarditis-derived E. faecium isolate, TX0082. The collagen reduction percentages after preincubation with anti-Acm IgGs for TX0082 were 80.0%, 49.5%, and 31.9% for anti-rAcm37 IgGs, anti-rAcm24 IgGs, and anti-Acm total IgGs, respectively, compared to a 10.6% increase for preimmune IgGs (P < 0.0001) (data not shown).
FIG. 3.
Inhibition of E. faecium strain TX2535 adherence to collagen by anti-Acm IgGs affinity purified against different segments of the Acm A domain. (A) Inhibition of adherence by 10 μg/ml of anti-rAcm A-domain total IgGs, specific anti-rAcm37 IgGs, and specific anti-rAcm24 IgGs. The adherence of TX2535 to bovine serum albumin (BSA) is also shown. Bars represent the means of the percentages of adhering cells ± standard deviations for six wells. Results are from three independent experiments. Anti-Acm antibodies did not inhibit the adherence of TX2535 to fibrinogen (data not shown). (B) Dose-dependent effects of anti-Acm IgGs. Data points were normalized with the respective data points for bacteria adhering in the absence of IgGs and expressed as percentages. Data points represent the means ± standard deviations for six wells (results are from three independent experiments, with two wells per experiment).
In subsequent inhibition experiments, we tested a range of concentrations (0.05 to 50 μg per ml) of each of these IgGs by using strains TX2535 and TX0082. The results for TX2535 showed that as little as 1 μg of specific anti-rAcm37 IgGs per ml was sufficient to inhibit 39.4% of the adherence to collagen, whereas preimmune serum had no effect on adherence over the range of concentrations tested (Fig. 3B). Furthermore, the inhibition effect was concentration dependent (Fig. 3B).
The results for TX0082 also indicate that anti-rAcm37 IgGs were the most effective in inhibiting cell adhesion, causing 80% inhibition of cell adhesion at 20 μg/ml (data not shown). Anti-Acm A-domain total IgGs also caused some reduction in adherence but were less potent than anti-rAcm24; the reduction differences were consistent over the concentrations tested (data not shown).
The finding that anti-Acm antibodies are able to reduce the adherence of E. faecium to collagen raises the possibility that these antibodies may have potential as therapeutic or prophylactic agents. Our ongoing studies using a rat endocarditis model may provide some evidence in this direction.
In summary, the biochemical analysis of recombinant subdomains of Acm showing that the high-affinity binding subsegment contains two Ig-like similarly folded (based on structural predictions) subdomains supports the hypothesis that Acm may mediate adhesion via the proposed “Collagen Hug” mechanism (17). Definitive proof of this hypothesis needs to be derived from biophysical and structural analyses of these recombinant proteins. We also found that specific antibodies against high-affinity binding subsegments were the most effective at inhibiting E. faecium adherence to collagen. Thus, blocking Acm with functional subsegment-specific antibodies could potentially reduce E. faecium colonization of collagen-enriched host endovascular tissues.
Acknowledgments
This work was supported by NIH grants R56 AI 42399 and R01 AI 67861 from the Division of Microbiology and Infectious Diseases to B. E. Murray.
Editor: F. C. Fang
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Deivanayagam, C. C., E. R. Wann, W. Chen, M. Carson, K. R. Rajashankar, M. Hook, and S. V. Narayana. 2002. A novel variant of the immunoglobulin fold in surface adhesins of Staphylococcus aureus: crystal structure of the fibrinogen-binding MSCRAMM, clumping factor A. EMBO J. 21:6660-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lannergård, J., L. Frykberg, and B. Guss. 2003. CNE, a collagen-binding protein of Streptococcus equi. FEMS Microbiol. Lett. 222:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 4.Nallapareddy, S. R., X. Qin, G. M. Weinstock, M. Höök, and B. E. Murray. 2000. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68:5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nallapareddy, S. R., K. V. Singh, and B. E. Murray. 2006. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl. Environ. Microbiol. 72:334-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nallapareddy, S. R., G. M. Weinstock, and B. E. Murray. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733-1747. [DOI] [PubMed] [Google Scholar]
- 7.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 8.Patti, J. M., J. O. Boles, and M. Hook. 1993. Identification and biochemical characterization of the ligand binding domain of the collagen adhesin from Staphylococcus aureus. Biochemistry 32:11428-11435. [DOI] [PubMed] [Google Scholar]
- 9.Ponnuraj, K., M. G. Bowden, S. Davis, S. Gurusiddappa, D. Moore, D. Choe, Y. Xu, M. Hook, and S. V. Narayana. 2003. A “dock, lock, and latch” structural model for a staphylococcal adhesin binding to fibrinogen. Cell 115:217-228. [DOI] [PubMed] [Google Scholar]
- 10.Rich, R. L., B. Demeler, K. Ashby, C. C. Deivanayagam, J. W. Petrich, J. M. Patti, S. V. Narayana, and M. Hook. 1998. Domain structure of the Staphylococcus aureus collagen adhesin. Biochemistry 37:15423-15433. [DOI] [PubMed] [Google Scholar]
- 11.Sato, Y., K. Okamoto, A. Kagami, Y. Yamamoto, T. Igarashi, and H. Kizaki. 2004. Streptococcus mutans strains harboring collagen-binding adhesin. J. Dent. Res. 83:534-539. [DOI] [PubMed] [Google Scholar]
- 12.Shimoji, Y., Y. Ogawa, M. Osaki, H. Kabeya, S. Maruyama, T. Mikami, and T. Sekizaki. 2003. Adhesive surface proteins of Erysipelothrix rhusiopathiae bind to polystyrene, fibronectin, and type I and IV collagens. J. Bacteriol. 185:2739-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sillanpää, J., Y. Xu, S. R. Nallapareddy, B. E. Murray, and M. Hook. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 150:2069-2078. [DOI] [PubMed] [Google Scholar]
- 14.Symersky, J., J. M. Patti, M. Carson, K. House-Pompeo, M. Teale, D. Moore, L. Jin, A. Schneider, L. J. DeLucas, M. Hook, and S. V. Narayana. 1997. Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat. Struct. Biol. 4:833-838. [DOI] [PubMed] [Google Scholar]
- 15.Visai, L., Y. Xu, F. Casolini, S. Rindi, M. Hook, and P. Speziale. 2000. Monoclonal antibodies to CNA, a collagen-binding microbial surface component recognizing adhesive matrix molecules, detach Staphylococcus aureus from a collagen substrate. J. Biol. Chem. 275:39837-39845. [DOI] [PubMed] [Google Scholar]
- 16.Wann, E. R., S. Gurusiddappa, and M. Hook. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 275:13863-13871. [DOI] [PubMed] [Google Scholar]
- 17.Zong, Y., Y. Xu, X. Liang, D. R. Keene, A. Hook, S. Gurusiddappa, M. Hook, and S. V. Narayana. 2005. A ‘Collagen Hug’ model for Staphylococcus aureus CNA binding to collagen. EMBO J. 24:4224-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]