Abstract
Although Moraxella catarrhalis continues to be a significant cause of disease in both children and adults, the steps involved in pathogenesis remain poorly understood. We have identified three open reading frames in the M. catarrhalis genome that encode homologues of the two-partner secretion system (TPS). The sequenced M. catarrhalis hemagglutinin-like locus of strain 7169 has a unique gene organization composed in the order of mchA1, mchB, and mchA2, where mchA1 is divergent. MchA1 and MchA2 are 74% identical at the amino acid level and diverge only in the C-terminal regions. The TPS motif identified in the common N-terminal regions of MchA1 and MchA2 was found to be homologous to the filamentous hemagglutinin of Bordetella pertussis, and MchB has homology to other TpsB transporters. The presence of MchA1 and MchA2 in outer membrane protein preparations and concentrated culture supernatants (CCSs) of strain 7169 was confirmed by immunoblotting using specific antisera. Nanoscale liquid chromatography-tandem mass spectrometry peptide sequencing of the antibody-reactive bands from the CCSs was performed and demonstrated that 13 different peptides mapped to identical regions of MchA1 and MchA2. Quantitative adherence assays revealed a decrease of binding to primary normal human bronchial epithelial cells by the mch mutants 7169mchB and 7169mchA1A2B compared to that by the wild-type strain. These studies show that MchA1, MchA2, and MchB are components of a novel TPS identified in M. catarrhalis and suggest that these proteins may be involved in colonization.
Moraxella catarrhalis is an important gram-negative human mucosal pathogen. It is one of the three major causes of acute otitis media, along with Streptococcus pneumoniae and nontypeable Haemophilus influenzae (10, 43). In adults with chronic bronchitis and chronic obstructive pulmonary disease (COPD), M. catarrhalis causes lower respiratory tract infections that often lead to acute exacerbations (33, 34). In addition, M. catarrhalis can cause sinusitis in infants and young children (23, 43). Because 90% of M. catarrhalis clinical isolates are beta-lactamase positive (23, 43) and no protective vaccine is available (29, 30), M. catarrhalis continues to be a major source of human disease.
Most of the research involving M. catarrhalis pathogenesis has focused largely on the identification and characterization of outer membrane proteins (OMPs) on the bacterial surface, although most of these have an undefined role in virulence (23, 32, 43). M. catarrhalis expresses some OMPs, including the transferrin binding protein TbpB (28) and the hemin and hemoglobin utilization proteins HumA and MhuA (11, 12), to obtain iron from the human host. In addition, other OMPs, such as the ubiquitous surface protein UspA2, can be involved in serum resistance (1) or in natural competence, as described for the type IV pilus (26). To date, only a few OMPs, including UspA1 and UspA2H (1, 24), the M. catarrhalis adherence protein McaP (41), and the hemagglutinating protein Hag/M. catarrhalis immunoglobulin D-binding protein (3, 14, 19), have been reported to directly mediate binding to cell lines in vitro. Therefore, it is clear that like many other gram-negative pathogens, M. catarrhalis has developed multiple virulence mechanisms to successfully colonize the human mucosal surface.
In this report, we have identified a locus in M. catarrhalis 7169 containing three open reading frames (ORFs) that encode homologues of the previously described two-partner secretion systems (TPS) in various other pathogens, including Bordetella pertussis (21, 22). The B. pertussis TPS pathway is composed of the filamentous hemagglutinin FhaB (generically named TpsA) and the transporter FhaC (TpsB) (25). FhaB is the major adhesin involved in bacterial attachment and colonization of the human upper respiratory tract, and this protein is also a component of the acellular diphtheria-pertussis vaccine (25, 36). The M. catarrhalis hemagglutinin-like locus described in this study contains three ORFs, termed mchA1, mchA2, and mchB. The TPS motif identified in MchA1 and MchA2 was found to be homologous to FhaB of B. pertussis. MchB has homology to FhaC of B. pertussis (22). This is the first report of a TPS in M. catarrhalis, and our data demonstrate that this system is likely to be conserved among clinical isolates. The proteins expressed by this TPS may represent an important M. catarrhalis virulence mechanism involved in adherence to primary normal human bronchial epithelial (NHBE) cells. Furthermore, although the M. catarrhalis TPS locus shows some homology to other TPS loci, the arrangement of the genes that encode MchA1, MchA2, and MchB appears to be unique.
MATERIALS AND METHODS
Bacterial strains, cell lines, and culture conditions.
The bacterial strains used in this study are listed in Table 1 and were cultured as previously described (26). The M. catarrhalis mutants were cultured on brain heart infusion plates with the addition of kanamycin (KAN; 30 μg per ml), zeocin (ZEO; 3 μg per ml), and/or spectinomycin (SPC; 15 μg per ml) as required. The non-Chelex-treated chemically defined medium has been described previously (6). Escherichia coli XL1-Blue was used as the host strain for plasmid DNA manipulation, and E. coli Rosetta(DE3)pRARE (Novagen, Madison, WI) was used for fusion protein expression. E. coli strains were cultured at 37°C on Luria-Bertani agar plates or in broth containing the appropriate antibiotic (50 μg of carbenicillin per ml, 100 μg of ampicillin per ml, 30 μg of KAN per ml, 100 μg of SPC per ml, or 25 μg of ZEO per ml).
TABLE 1.
Strains used in this study
| Strain | Description | Source or reference(s)a |
|---|---|---|
| M. catarrhalis strains | ||
| 7169 | Pediatric middle-ear isolate | 26, 28 |
| 7169mchB | mchB isogenic mutant of strain 7169 | This study |
| 7169mchB-R | 7169mchB wild-type revertant | This study |
| 7169mchA1A2B | mchA1-mchA2-mchB isogenic mutant of strain 7169 | This study |
| 43617 | Transtracheal aspirate isolate | ATCC |
| O12E | Middle-ear fluid isolate | 17 |
| O35E | Middle-ear fluid isolate | 17 |
| 7966 | Isolate from Australia | Mark Achtman |
| 7315 | Isolate from France | Mark Achtman |
| 7477 | Isolate from Japan | Mark Achtman |
| 7984 | Isolate from Australia | Mark Achtman |
| 10059 | Isolate from Angola | Mark Achtman |
| 3P8B1 | Adult COPD sputum isolate | Timothy Murphy |
| 5P26B1 | Adult COPD sputum isolate | Timothy Murphy |
| 7P94B1 | Adult COPD sputum isolate | Timothy Murphy |
| 39P10 | Adult COPD sputum isolate | Timothy Murphy |
| 74P10B1 | Adult COPD sputum isolate | Timothy Murphy |
| 103P14 | Adult COPD sputum isolate | Timothy Murphy |
| HF-006 | Pediatric middle-ear isolate | Howard Faden |
| HF-084 | Pediatric middle-ear isolate | Howard Faden |
| HF-165 | Pediatric middle-ear isolate | Howard Faden |
| HF-297 | Pediatric middle-ear isolate | Howard Faden |
| HF-3615 | Pediatric middle-ear isolate | Howard Faden |
| Other Moraxella strains | ||
| M. cuniculi 14688 | ATCC | |
| M. caviae 14659 | ATCC | |
| M. nonliquefaciens 17953 | ATCC | |
| M. osloensis 15276 | ATCC | |
| M. bovis 10900 | ATCC | |
| Non-Moraxella strains | ||
| Neisseria gonorrhoeae GC1 | Dave Dyer | |
| H. influenzae 7891 | Timothy Murphy | |
| Pseudomonas aeruginosa 94-343-0448 | Thomas Russo | |
| H. ducreyi 35000HP | 2 | |
| P. mirabilis 94-341-0610 | Thomas Russo |
The affiliations of the providers of the strains listed here are as follows: Mark Achtman, Max Planck Institute, Berlin, Germany; Timothy Murphy, Veterans Affairs Medical Center, Buffalo, NY; Howard Faden, Women's and Children's Hospital, Buffalo, NY; Dave Dyer, University of Oklahoma Health Sciences Center, Oklahoma City; and Thomas Russo, Veterans Affairs Medical Center, Buffalo, NY. ATCC, American Type Culture Collection.
The NHBE cells were purchased as a complete culture system and propagated as defined in the instructions from the manufacturer (Lonza Walkersville, Walkersville, MD). The monolayers were maintained at 37°C in an atmosphere of 5% CO2.
General DNA and RNA manipulations.
M. catarrhalis chromosomal DNA was prepared as previously described (37). PCR amplifications were performed with the GeneAMP PCR system 9700 (PE Applied Biosystems, Foster City, CA) by using genomic M. catarrhalis 7169 DNA with Platinum Taq high-fidelity polymerase (Invitrogen Life Technologies, Carlsbad, CA). All PCR products and plasmid constructs were purified by using MinElute kits or QIAprep spin kits (QIAGEN, Santa Clarita, CA). DNA sequencing was performed by personnel at the Roswell Park Cancer Institute Biopolymer Facility, Roswell Park Cancer Institute, Buffalo, NY, and the results were analyzed with MacVector software (version 7.2; Genetic Computer Group). Total RNA was isolated by using the RNeasy mini kit (QIAGEN), and transcriptional analysis was performed with the OneStep reverse transcription-PCR kit (QIAGEN). Restriction endonucleases and standard molecular biology reagents were obtained from New England Biolabs, Inc. (Beverly, MA). Restriction enzyme digestions, ligations, and transformations were performed by standard methods, and all primers are listed in Table 2.
TABLE 2.
Nucleotide sequences of oligonucleotide primers
| Primer | Sequence (5′→3′)a | Restriction endonuclease |
|---|---|---|
| 340 | TATACTAGTGGGTGACTAACTAGGAGGAATAAATGGCTA | SpeI |
| 481 | GCGGGTACCGTCGACTCTAGAGGATCCCCGGGTCATTA | KpnI |
| 639 | CACACCCATATGAATGAGGTTCGTAGCAGC | NdeI |
| 640 | ATGGATCCTGCCGTCTTGACCGATTTC | BamHI |
| 651 | GGGGTGGTCAGCCTAAAAAGTAAC | |
| 652 | AGTGGTGATTTGGTCGTTCTGG | |
| 659 | TATCTTTTACTGGCTCCTCC | |
| 660 | TACCTCATACCAACCGCTC | |
| 661 | CGCCTCGAGCACTAAAAGCAAAGCCTGC | XhoI |
| 662 | ATATAGATCTCCTCAATCACAAAGTCAATCTC | BglII |
| 788 | ATAGGTACCAGGGTATCAACCAATCTAATCCGC | KpnI |
| 789 | ATTCCACCTCACCACCTCTG | |
| 790 | ATACTGCAGACGATGGCGAAGTGGTTTATTG | PstI |
| 791 | GTGACTAGTGGTGGACTGTCCTTGTTGTTTTAAG | SpeI |
| 798 | TTTTGGCTCAATACCAGCATTCGG | |
| 799 | CAGGCACACTCATTCACACCATCC | |
| 802 | CGCATCAGCATCATCTTCACCGAG | |
| 803 | TACCTCATACCAACCGCTCCAGCC | |
| 814 | TATAAGATCTACGTGTTGACAATTAATCATCG | BglII |
| 815 | TATACTCGAGATTCTCAGTCCTGCTCCTCG | XhoI |
| 858 | GTGCAGCCGACTTTTCTTCC | |
| 965 | AGGTATCGCTAATGCCACTTCAAC | |
| 966 | ACTCAAGACCATAAGAGCCGTCAG | |
| 1058 | GCTTCATCAAGTTTTGCCTCC | |
| 1059 | TTTGCTTTGGTTGGGTCTG | |
| 1062 | TCTCAGATCTACAATGAATAGGTTTACAC | BglII |
| 1063 | TGTGCTCGAGGCAAGGGTTTATTGTTTTC | XhoI |
| 1213 | ACACCTCGAGCCACCATCTATGTTCAAGGCA | XhoI |
| 1214 | TCTCAGATCTGGGTTGTAAAGGTTGTGTTGG | BglII |
| 1243 | ATTACCATCACCGCAGAT | |
| 1417 | ATACCCGGGTTGACAATGGTGTGTGCCTC | |
| 1418 | ATAACTAGTTTTAGCACCGATTTTTCGC |
Engineered restriction endonuclease sites are underlined.
Identification of mchA1, mchA2, and mchB.
The M. catarrhalis homologues of the filamentous-hemagglutinin genes were identified by BLAST searches of the patented M. catarrhalis genome (deposited under patent WO0078968 and patent W09958685 in the National Center for Biotechnology Information [NCBI] nucleotide database). The two large ORFs that are homologous to fhaB of B. pertussis were named mchA1 and mchA2. A third ORF, homologous to fhaC of B. pertussis, was named mchB. The nucleotide sequence of the entire 17.6-kb hemagglutinin-like region of M. catarrhalis 7169 was obtained, analyzed, and used for all subsequent DNA manipulations.
Construction of the isogenic mutants defective in the expression of mchA1, mchA2, and mchB.
Three different resistance cassettes were used to inactivate mchB, mchA2, and mchA1 in strain 7169 by using a previously described inverse-PCR strategy (26). An isogenic 7169mchB mutant was produced using primers 659 and 660 to amplify a 1.8-kb fragment containing an internal portion of the mchB gene. The resulting amplicon was ligated into pGEM-T Easy (Promega, Madison, WI), subjected to PCR with primers 661 and 662, and restriction enzyme digested to allow for the insertion of a ZEO cassette amplified from plasmid pEM7/ZEO (Invitrogen Life Technologies) by using primers 814 and 815. A 1.3-kb mchB mutagenesis construct, containing a ZEO cassette within an internal mchB deletion, was generated with the primers 659 and 660 and used for the natural transformation of M. catarrhalis as described previously (26) to generate 7169mchB. To inactivate mchA2, a 1-kb DNA fragment upstream of the coding region was amplified using primers 788 and 789 and cloned into pGEM-T Easy. The nonpolar KAN resistance cassette aphA-3 (31) was amplified using primers 340 and 481 and directionally cloned downstream. A 1.3-kb DNA fragment downstream of mchA2 was amplified using primers 790 and 791 and directionally cloned into the above-mentioned construct. Primers 789 and 858 were used to generate a 3.2-kb mchA2 mutagenesis construct, and the purified amplicon was used for the natural transformation of 7169mchB to generate 7169mchA2B. To delete mchA1, a 2.4-kb DNA fragment containing an internal portion of the 5′ end of mchA1 was amplified from the chromosomal DNA of mutant 7169mchA2B by using primers 1058 and 1059. This fragment was cloned into pGEM-T Easy. An SPC cassette was amplified by PCR from pCR-Blunt (45) by using primers 1062 and 1063 and was inserted into the above-named construct by using inverse PCR with primers 1213 and 1214 as described above for 7169mchB. Primers 1058 and 1243 were used to amplify the linear mutagenesis construct used for the natural transformation of 7169mchA2B to generate 7169mchA1A2B. All constructs and chromosomal mutations were confirmed by sequencing. Reverse transcription-PCR was performed to show the absence of RNA transcripts in all mutants by using the gene-specific primers indicated in Table 3 (data not shown).
TABLE 3.
Presence of mchA1, mchA2, and mchB in strains used in this studya
| Strain | Detection of:
|
|||
|---|---|---|---|---|
| mchA1 | mchA2 | mchA1 and mchA2 regions corresponding to N termini | mchB | |
| M. catarrhalis strains | ||||
| 7169 | + | + | + | + |
| 43617 | + | + | + | + |
| O12E | − | + | + | + |
| O35E | − | + | + | + |
| 7966 | − | + | + | + |
| 7315 | − | + | + | + |
| 7477 | − | − | + | + |
| 7984 | − | − | + | + |
| 10059 | + | + | + | + |
| 3P8B1 | − | − | + | + |
| 5P26B1 | + | − | + | + |
| 7P94B1 | − | + | + | + |
| 39P10 | − | + | + | + |
| 74P10B1 | − | + | + | + |
| 103P14 | − | + | + | + |
| HF-006 | − | + | + | + |
| HF-084 | − | − | + | + |
| HF-165 | + | + | + | + |
| HF-297 | − | + | + | + |
| HF-3615 | − | − | + | + |
| Other Moraxella strains | ||||
| M. cuniculi 14688 | − | − | − | − |
| M. caviae 14659 | − | − | − | − |
| M. nonliquefaciens 17953 | − | − | − | − |
| M. osloensis 15276 | − | − | − | − |
| M. bovis 10900 | − | − | − | − |
| Non-Moraxella strains | ||||
| Neisseria gonorrhea GC1 | − | − | − | − |
| H. influenzae 7891 | − | − | − | − |
| Pseudomonas aeruginosa 94-343-0448 | − | − | − | − |
| H. ducreyi 35000HP | − | − | − | − |
| P. mirabilis 94-341-0610 | − | − | − | − |
The detection of mchA1, mchA2, and mchB was performed by PCR with chromosomal DNA from a series of bacterial isolates by using gene-specific primer pairs 798-799 (mchA1), 965-966 (mchA2), 651-652 (mchA1 and mchA2 regions corresponding to N termini), and 802-803 (mchB). +, present; −, absent.
Reversion of 7169mchB to the wild type.
The wild-type mchB gene was introduced into the 7169mchB mutant to generate the revertant 7169mchB-R by using methods previously described (11, 27). Primers 1417 and 1418 were used to amplify the native mchB gene from M. catarrhalis 7169 chromosomal DNA. The purified amplicon was used to naturally transform 7169mchB. Revertant clones were screened for the loss of ZEO resistance. The restoration of the wild-type mchB gene was verified by sequence analysis.
Construction and purification of fusion protein rMchA-His.
A 2,062-nucleotide fragment was amplified from the chromosomal DNA of M. catarrhalis 7169 by using primers 639 and 640 and cloned into pET-16b (Novagen) (see Fig. 1). Competent E. coli Rosetta(DE3)pRARE cells (Novagen) were transformed with the recombinant plasmid, and recombinant His-tagged MchA (rMchA-His) was overexpressed and purified using the specifications for the pET expression system (Novagen) with the following modifications. The cultures were grown overnight at 25°C, and the bacteria were harvested and the inclusion bodies were purified using BugBuster protein extraction reagent (Novagen). The approximately 80-kDa rMchA-His fusion protein was further purified by gel elution following separation by sodium dodecyl-sulfate-7% polyacrylamide gel electrophoresis. The eluted protein was concentrated using Amicon Centricon XM-30 filtration devices, quantitated using a protein assay kit (Sigma-Aldrich, St. Louis, MO), and analyzed for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
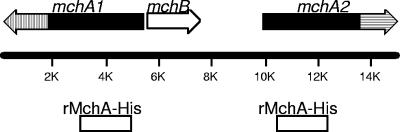
FIG. 1.
Genetic organization and orientation of the M. catarrhalis 7169 hemagglutinin-like locus. The solid areas in the maps of mchA1 and mchA2 define the homologous regions of the two genes corresponding to the N termini of the products (100% identical), while the hatched areas represent the divergent regions corresponding to the C termini (24% identical). The 2-kb region common to the portion of mchA1 and mchA2 corresponding to the N termini that is used to express rMchA-His is also shown.
Preparation of WCLs, OMPs, and CCSs.
Whole-cell lysates (WCLs) and OMPs were prepared and analyzed as previously described (5, 6, 26). To prepare concentrated culture supernatant (CCS), the bacteria were grown in non-Chelex-treated chemically defined medium 100 for 6 h and centrifuged at 8,000 × g for 10 min. The culture supernatant was treated with a complete protease inhibitor cocktail (Roche, Mannheim, Germany), passed through a 0.22-μm-pore-size filter, centrifuged at 125,000 × g for 1.5 h, and concentrated 100-fold by ultrafiltration by using a Centricon Plus-70 Ultracel PL-30 filtration unit (Millipore, Inc., Billerica, MA).
Immunoblot analysis.
Rabbit polyclonal antibodies to rMchA-His were developed by Proteintech Group Inc. (Chicago, IL) and used at a dilution of 1:50. Peroxidase-labeled goat anti-rabbit immunoglobulin G (heavy and light chains; KPL, Gaithersburg, MD) or peroxidase-labeled protein A from Staphylococcus aureus (KPL) was used as the secondary antibody. The immunoreactive bands were detected by using either the 4-chloro-1-naphthol substrate (Sigma-Aldrich) or the Supersignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). The monoclonal antibody 3F5-5E5 used to detect MhuA was previously described (12).
Adherence assays.
Quantitative adherence assays were performed as previously described with the following modifications (3, 19). Each strain of bacteria was harvested at the early log phase of growth, resuspended into sterile culture medium without antibiotics, and inoculated (106 CFU per well) in triplicate onto monolayers of human cells (104 cells per well). The 24-well tissue culture plates were centrifuged at 220 × g for 5 min to facilitate contact between bacteria and human cells and incubated for 5 min at 37°C in a 5% CO2 atmosphere. The wells were rinsed three times with HEPES-buffered saline solution to remove nonadherent bacteria, the contents of the wells were harvested with trypsin, and the wells were washed once with HEPES. The cells were pooled, serially diluted, and plated onto brain heart infusion agar to determine the number of viable bacterial cells attached to human cells. The negative control included bacteria incubated in the absence of NHBE cells which did not exhibit adherence to the plastic (data not shown). The level of adherence is expressed as the percentage of bacteria attached to the human cells relative to the original inoculum added to the well. Adherence assays were repeated at least three separate times on different days. Statistical analyses were performed with Prism software (version 4; GraphPad Software, Inc.) using one-way analysis of variance.
Nucleotide sequence accession number.
The M. catarrhalis 7169 hemagglutinin-like locus sequence has been deposited in GenBank under accession number DQ923126.
RESULTS
Identification of the M. catarrhalis 7169 chromosomal locus encoding filamentous hemagglutinin homologues.
Two 5-kb ORFs were identified in the patented M. catarrhalis genome deposited through the NCBI. The deduced amino acid sequences corresponding to these large ORFs were used as queries for BLAST searches, which revealed limited overall sequence similarity to other bacterial TpsA proteins. Regions of similarity that include both the secretion and functional domains were found in the adhesin FhaB of B. pertussis (23% similar) (36), the hemolysin HhdA of Haemophilus ducreyi (25% similar) (35), the hemolysin HpmA of Proteus mirabilis (31% similar) (42), and the high-molecular-weight adhesin HMW1A of nontypeable H. influenzae (26% similar) (16). A potential hemagglutination activity domain reported in the Protein Families Database (PF05860) was found in the N-terminal portions of the amino acid sequences of MchA1 and MchA2. Clantin et al. have proposed to redefine this region, which corresponds to residues 1 to 130 of FhaB of B. pertussis, as the TPS domain of TpsA proteins (7). The product of a third ORF identified in the same locus of the genome had homology to the transporter FhaC of B. pertussis (31% similar) (46), as well as other TpsB transporter proteins such as the large supernatant protein LspB of H. ducreyi (31% similar) (44) and HpmB of P. mirabilis (29% similar) (42). To determine if these three genes encode filamentous hemagglutinin homologues, the 17.6-kb chromosomal hemagglutinin-like region of M. catarrhalis 7169 was cloned and sequenced. The organization of the hemagglutinin-like region of strain 7169, consisting of mchA1 (5.4 kb), mchB (2.1 kb), and mchA2 (5.2 kb), is shown in Fig. 1. These data demonstrate that the hemagglutinin-like region of M. catarrhalis 7169 has a unique gene organization compared to regions corresponding to previously described TPS (22).
Amino acid analysis of MchA1, MchA2, and MchB.
M. catarrhalis MchA1 and MchA2 have estimated molecular masses of 191 kDa (1,795 amino acids [aa]) and 183 kDa (1,727 aa), respectively. The deduced amino acid sequences of MchA1 and MchA2 were aligned, demonstrating that the first 1,200 amino acids are identical. MchA1 and MchA2 are 74% identical and 79% similar at the amino acid level over their entire sequences, but the level of similarity decreases to 41% in the divergent C-terminal regions (Fig. 1). An analysis of the first 200 amino acids, common to MchA1 and MchA2, identified the TPS domain, which is a distinct feature of the TpsA proteins transported by the TPS pathway (22). The homology to the N-terminal secretion motif of B. pertussis FhaB and other high-molecular-weight protein members of the TpsA family was also significant (similarity, 28 to 48%). Further analysis of the TPS domains of both MchA1 and MchA2 revealed an N-terminal extension linked to the signal peptide. The N-terminal extension begins with the conserved sequence MNR, where M is the first residue of the proteins, and terminates with the motif LVVVSEITK, similar to the consensus motif LIAVSELAR found in many TpsA proteins (21). The N-terminal extension is followed by a Sec-dependent signal sequence with a putative cleavage site after residue 69 (AFA-N, where the hyphen represents the cleavage site). The TPS domains of MchA1 and MchA2 contain the two motifs NPFL (residues 143 to 146) and NPSGI (residues 183 to 187). These motifs are similar to the conserved secretion motifs NPNL and NPNGI of B. pertussis FhaB, which are prime candidates for the molecular interaction between TpsA and TpsB at the periplasmic side of the outer membrane during the secretion process (18, 20, 22).
The M. catarrhalis MchB protein has 26 to 31% similarity to other bacterial TpsB transporter homologues and has an estimated molecular mass of 78 kDa (705 aa). MchB contains one polypeptide transport-associated domain (POTRA_2; Protein Families Database codename, PF08479), localized between aa 112 and 203. The polypeptide transport-associated extramembrane N-terminal domain is a molecular feature of the Omp85 family, of which the TpsB proteins are members (13). The N-terminal segment of MchB carries two conserved cysteines (residues 111 and 156) also present in the TpsB transporters HpmB of P. mirabilis, ShlB of Serratia marcescens, and HecB of Erwinia chrysanthemi (22). Further analysis of the amino acid sequence of MchB using SignaIP 3.0 revealed a cleavable Sec-dependent signal sequence with a putative cleavage site between residues 50 and 51 (AKA-QI).
Detection of mchA1, mchA2, and mchB in other M. catarrhalis strains.
Chromosomal DNA purified from a series of diverse clinical isolates from various geographical areas was subjected to PCR analysis in order to detect mchA1, mchA2, and mchB (Table 3). Amplicons for mchB and also for the conserved regions of mchA1 and mchA2 corresponding to the N termini of the gene products were derived from all strains of M. catarrhalis tested. In contrast, the regions of mchA1 and mchA2 corresponding to the C termini of the gene products appeared to be divergent among the various strains. These PCR analyses were also performed with other Moraxella species as well as five other gram-negative bacteria. Amplicons corresponding to the three hemagglutinin-like genes were not detected in any of these other species.
MchA1 and MchA2 are present in the outer membrane and in the culture supernatant.
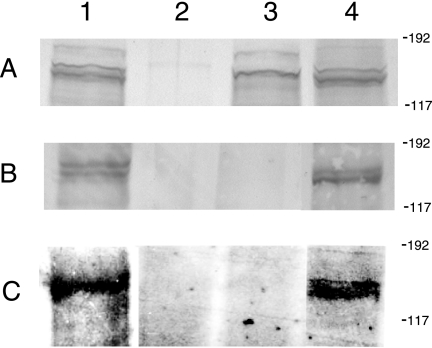
Following secretion by TpsB, some TpsA proteins are noncovalently associated with the bacterial surface, with small amounts released into the culture supernatant (4, 15, 38, 40). The presence of a putative Sec-dependent signal peptide and a TPS domain containing the motifs NPFL and NPSGI at the N termini of MchA1 and MchA2 suggested that these proteins were translocated across the bacterial inner membrane and may also be exported via the putative outer membrane transporter MchB. To evaluate the expression of MchA1 and MchA2 by M. catarrhalis, the isogenic mutants 7169mchA1A2B and 7169mchB were constructed as described in Materials and Methods. In addition, the 7169mchB mutant was transformed with the wild-type mchB gene, creating the 7169mchB-R revertant. These constructs were analyzed by immunoblotting to determine if MchA1 and MchA2 were localized in the outer membrane. Figure 2 is a composite of immunoblots probed with the rMchA-His polyclonal antisera raised to the conserved region of MchA1 and MchA2. Panel A shows immunoreactive bands in the molecular mass range corresponding to MchA1 (184 kDa) and MchA2 (176 kDa) detected in the WCLs of the wild type (lane 1), the 7169mchB mutant (lane 3), and the revertant 7169mchB-R (lane 4). MchA1 and MchA2 were not detected in the WCL of the 7169mchA1A2B mutant (lane 2). Panel B demonstrates that MchA1 and MchA2 were detected in the OMPs of the wild type and the revertant 7169mchB-R. However, these bands were not detected in the OMPs of the 7169mchA1A2B or the 7169mchB mutant. These results suggest that MchA1 and MchA2 are localized in the outer membrane of M. catarrhalis and that MchB is involved in the extracellular transport of these proteins.
FIG. 2.
Comparative analysis of M. catarrhalis 7169 and mutant WCLs (A), OMPs (B), and CCSs (C) by immunoblotting using the rMchA-His polyclonal antisera. Lane 1, 7169; lane 2, 7169mchA1A2B; lane 3, 7169mchB; lane 4, 7169mchB-R. Molecular size standards are shown in kilodaltons.
To determine if MchA1 and MchA2 were present in the extracellular milieu, CCSs were analyzed by immunoblotting using the rMchA-His antisera. Figure 2C demonstrates that the antibodies to rMchA-His recognized multiple bands in the supernatants of the wild type and the revertant 7169mchB-R. No reactivity was detected in the supernatant from the 7169mchA1A2B or the 7169mchB mutant. To confirm that the MchA proteins present in the supernatant fractions were not part of membrane debris, we verified the absence of the integral OMP MhuA (data not shown) (12). The localization of MchA1 and MchA2 into the CCS specifically involved MchB since the MchA proteins present in the WCL of the 7169mchB strain were not found in the respective CCS of this strain. Together, these results suggest that MchA1 and/or MchA2 is localized in the extracellular milieu in a soluble form and further support the idea that MchB is involved in the transport of both proteins.
Proteomic analysis of the secreted high-molecular-weight proteins detected in the CCS.
While the immunoblot studies described above provided strong evidence that MchA1 and MchA2 were secreted, nanoscale liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) peptide sequencing of the digested bands extracted from the polyacrylamide gel was performed to confirm our data. The antibody-reactive protein bands in the CCS, corresponding to the predicted molecular weights of the mature MchA1 and MchA2, were subjected to tryptic digestion and mass spectrometry analysis, and the resulting peptide sequences were used to search the nonredundant protein databases (ProtTech, Inc., Norristown, PA). The results of these studies demonstrated that 13 different peptides mapped to various regions within M. catarrhalis 7169 MchA1 and MchA2 (Table 4). In addition, these peptides mapped to identical regions of MchA1 and MchA2 in M. catarrhalis 7169, spanning a majority of the conserved sequence. None of the peptides matched the C-terminal divergent regions of either MchA1 or MchA2, and thus we could not determine conclusively if both proteins are secreted into the CCS. Nevertheless, this protein identification data taken together with the protein bands detected by immunoblotting provide strong support that either MchA1 or MchA2, and likely both, is secreted by M. catarrhalis and can be localized in the external milieu.
TABLE 4.
Characteristics of internal peptide fragments of MchA1 and MchA2 identified by nanoLC-MS/MS analysis of CCSs
| Peptide mass (Da) | Peptide sequence | Amino acid start-end position |
|---|---|---|
| 1,947.98 | YTQFDVPNQGIVLNNAR | 113-129 |
| 1,713.94 | TGAGTQLVGAVAANPFLK | 130-147 |
| 1,357.72 | ASQLAGSIEVAGQK | 163-176 |
| 1,956.99 | SANVATQTTTNNIGLGGPNK | 230-249 |
| 1,135.59 | NADLVNLYSK | 250-259 |
| 1,261.64 | GFGVNQAGSLNAK | 328-340 |
| 1,753.93 | ITHSGQSSVQQGVVSLK | 351-367 |
| 1,470.81 | KIEAIVFDNPTPK | 430-442 |
| 1,614.83 | NAQVLNGSLSIQTDR | 595-609 |
| 1,229.67 | AASIILTGSQNR | 824-835 |
| 1,914.99 | AATDKQDETPTLVLQGTK | 870-887 |
| 1,513.85 | LLAALSELQTATQR | 1070-1083 |
| 2,820.47 | QLKDEIITLASQPQYSYLNDLVNR | 1137-1160 |
Conservation of MchA1 and MchA2 expression by M. catarrhalis clinical isolates.
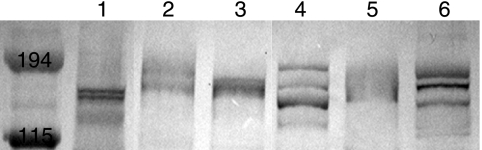
To determine the level of conservation of MchA1 and MchA2, OMPs were prepared from a series of M. catarrhalis isolates. Figure 3 is an immunoblot probed with rMchA-His polyclonal antibodies, demonstrating that all of these isolates expressed antibody-reactive protein bands corresponding to molecular weights similar to those of MchA1 and MchA2 of strain 7169. This finding suggests that the expression of these proteins in the outer membrane is conserved among M. catarrhalis strains.
FIG. 3.
Conservation of MchA1 and MchA2 in the OMPs from a panel of M. catarrhalis clinical isolates. Lane 1, 7169; lane 2, HF-084; lane 3, HF-165; lane 4, 3P8B1; lane 5, 5P26B1; lane 6, 7P94B1. Molecular size standards are shown in kilodaltons.
The M. catarrhalis hemagglutinin-like locus is associated with adherence to NHBE cells.
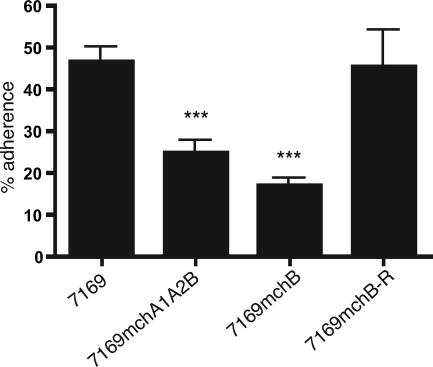
There have been a number of reports demonstrating that many of the TpsA proteins are adhesins and are likely to be involved in the early steps of colonization (25, 39). As MchA1 and MchA2 are homologous to many TpsA adhesins, we compared the wild-type strain 7169, the revertant, and the mutants in adherence studies using NHBE cell monolayers in vitro. Figure 4 is a summary of the adherence data demonstrating that the 7169mchA1A2B and the 7169mchB mutants were significantly deficient in adherence to NHBE cells (24.9 and 16.9% of the inoculated mutant cells adhered, respectively) compared to the wild-type M. catarrhalis 7169 strain (46.6% of the wild-type cells adhered). In addition, the reintroduction of the wild-type mchB gene into the 7169mchB mutant restored wild-type levels of adherence (45.4%). These data indicate that the MchA proteins are adhesins specifically involved in M. catarrhalis 7169 attachment to NHBE cells.
FIG. 4.
Adherence to NHBE cells. The values represent the means of results from separate experiments plus the standard errors. ***, P < 0.001.
DISCUSSION
In this report, we have described the identification and characterization of a unique TPS in M. catarrhalis 7169. In comparison to previously reported TPS loci, the M. catarrhalis TPS locus is organized in the order of mchA1, mchB, and mchA2, where mchA1 is divergent. To our knowledge, this gene arrangement has not been described for any other TPS reported in the literature. The TPS is a pathway that involves a single accessory protein (TpsB) specifically devoted to the translocation of very large virulence proteins (TpsA) across the outer membrane (20, 22). The TpsA proteins, mostly adhesins or cytolysins, are large proteins with masses ranging between 100 and 500 kDa (21). TpsB proteins are part of a large family of channel-forming outer membrane porin-like proteins called Omp85 (13). As stated previously, one of the best-studied TPS is the one expressed by B. pertussis, and the M. catarrhalis TPS described in this work shows many important similarities with this as well as other known TPS.
Our data demonstrate that MchA1 and MchA2 are secreted into the outer membrane, and a fraction of MchA1/MchA2 is also located in the extracellular milieu. According to the literature, secretion applies to extracellular proteins that are entirely outside of the outermost lipid bilayer, including soluble (free) and surface-associated proteins and surface appendages (9). HMWA and ShlA, two well-characterized TpsA proteins that are homologous to MchA1 and MchA2, are secreted via their respective TpsB transporters and remain noncovalently associated with the bacterial surface (4, 15, 38, 40). However, previous investigators also reported that a small amount of ShlA and HMWA (less than 5%) is then released into the extracellular milieu (38, 40). This finding is completely consistent with our data, which suggest that MchB is involved in the transport of MchA1 and MchA2, as the disruption of mchB alone completely abolished the extracellular localization of either MchA1 or MchA2. This is the first molecular characterization of a TPS in M. catarrhalis, and the results from this study lead us to propose that MchB, a member of the TpsB family, serves a functional role similar to that of other TpsB proteins as a transporter required for the secretion of the high-molecular-weight TpsA proteins MchA1 and MchA2.
We observed that MchA1 and/or MchA2 was present in the extracellular milieu by using an immunologic and proteomic approach combined with genetics. The 13 peptides identified by nanoLC-MS/MS mapped to the conserved first two-thirds of MchA1 and MchA2, from residues 113 to 1083. However, we were unable to determine if both MchA1 and MchA2 were present in the CCS. One likely explanation is that the C termini of MchA1 and MchA2 are exposed to the extracellular milieu and may be cleaved due to a maturation process similar to that of FhaB of B. pertussis, which is cleaved by a specific protease, SphB1, upon translocation across the outer membrane via FhaC (8). A second possible explanation is that the C-terminal ends of MchA1 and MchA2 are anchored to the bacterial surface inside MchB in a process similar to the recently described interaction between HMW1A and HMW1B in H. influenzae (4). However, this is the initial report of a TPS identified in M. catarrhalis, and more studies are needed in order to characterize the overall mechanism of this unique system.
Some TpsA proteins, such as HMW1A and HMW1B of H. influenzae (39) and FhaB of B. pertussis (25), are known adhesins which are involved in the colonization of the human mucosal surface. Since these two mucosal pathogens share a niche similar to that of M. catarrhalis, we hypothesized that either MchA1 or MchA2 may be involved in attachment to host tissues. NHBE cells were chosen for the adherence assays because they are primary cells obtained from normal human tissue and represent the human respiratory mucosal epithelium in vitro. Our data demonstrate that the MchA proteins of M. catarrhalis 7169 are associated with adherence to NHBE cells. While the 7169mchB and 7169mchA1A2B mutants exhibited a significantly lower level of adherence in these studies, it is important that attachment was not completely abolished. This finding can be explained by the presence of other M. catarrhalis adhesins, defined in previous studies, that may account for the detected adherence of our mutants. Nevertheless, the M. catarrhalis hemagglutinin-like locus represents a novel adherence mechanism for M. catarrhalis that may be involved in the early steps of colonization of the human respiratory tract.
Our studies also suggest that this TPS may be conserved among clinical isolates of M. catarrhalis. This is a potentially important observation, as both MchA1 and MchA2 show homology to FhaB of B. pertussis, which is a critical component of the new version of the acellular pertussis vaccine against whooping cough. MchA1, MchA2, and MchB constitute a novel system described for M. catarrhalis, and our data provide a foundation for further studies designed to determine the role and the function of this TPS in the pathogenesis of this important human pathogen.
Acknowledgments
This research was supported by grants DC005837 and DC007153 from the National Institutes of Health (NIDCD) awarded to A.A.C.
We thank R. Balder and E. R. Lafontaine for their help in the preparation of the manuscript.
Editor: D. L. Burns
Footnotes
Published ahead of print on 9 April 2007.
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 3.Bullard, B., S. L. Lipski, and E. R. Lafontaine. 2005. Hag directly mediates the adherence of Moraxella catarrhalis to human middle ear cells. Infect. Immun. 73:5127-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscher, A. Z., S. Grass, J. Heuser, R. Roth, and J. W. St. Geme III. 2006. Surface anchoring of a bacterial adhesin secreted by the two-partner secretion pathway. Mol. Microbiol. 61:470-483. [DOI] [PubMed] [Google Scholar]
- 5.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 64:3920-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campagnari, A. A., K. L. Shanks, and D. W. Dyer. 1994. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect. Immun. 62:4909-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clantin, B., H. Hodak, E. Willery, C. Locht, F. Jacob-Dubuisson, and V. Villeret. 2004. The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway. Proc. Natl. Acad. Sci. USA 101:6194-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutte, L., R. Antoine, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2001. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J. 20:5040-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Economou, A., P. J. Christie, R. C. Fernandez, T. Palmer, G. V. Plano, and A. P. Pugsley. 2006. Secretion by numbers: protein traffic in prokaryotes. Mol. Microbiol. 62:308-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enright, M. C., and H. McKenzie. 1997. Moraxella (Branhamella) catarrhalis: clinical and molecular aspects of a rediscovered pathogen. J. Med. Microbiol. 46:360-371. [DOI] [PubMed] [Google Scholar]
- 11.Furano, K., and A. A. Campagnari. 2004. Identification of a hemin utilization protein of Moraxella catarrhalis (HumA). Infect. Immun. 72:6426-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furano, K., N. R. Luke, A. J. Howlett, and A. A. Campagnari. 2005. Identification of a conserved Moraxella catarrhalis haemoglobin-utilization protein, MhuA. Microbiology 151:1151-1158. [DOI] [PubMed] [Google Scholar]
- 13.Gentle, I. E., L. Burri, and T. Lithgow. 2005. Molecular architecture and function of the Omp85 family of proteins. Mol. Microbiol. 58:1216-1225. [DOI] [PubMed] [Google Scholar]
- 14.Gjorloff Wingren, A., R. Hadzic, A. Forsgren, and K. Riesbeck. 2002. The novel IgD binding protein from Moraxella catarrhalis induces human B lymphocyte activation and Ig secretion in the presence of Th2 cytokines. J. Immunol. 168:5582-5588. [DOI] [PubMed] [Google Scholar]
- 15.Grass, S., and J. W. St. Geme III. 2000. Maturation and secretion of the non-typable Haemophilus influenzae HMW1 adhesin: roles of the N-terminal and C-terminal domains. Mol. Microbiol. 36:55-67. [DOI] [PubMed] [Google Scholar]
- 16.Harrison, A., D. W. Dyer, A. Gillaspy, W. C. Ray, R. Mungur, M. B. Carson, H. Zhong, J. Gipson, M. Gipson, L. S. Johnson, L. Lewis, L. O. Bakaletz, and R. S. Munson, Jr. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187:4627-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helminen, M. E., I. Maciver, M. Paris, J. L. Latimer, S. L. Lumbley, L. D. Cope, G. H. McCracken, Jr., and E. J. Hansen. 1993. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival in vivo. J. Infect. Dis. 168:1194-1201. [DOI] [PubMed] [Google Scholar]
- 18.Hodak, H., B. Clantin, E. Willery, V. Villeret, C. Locht, and F. Jacob-Dubuisson. 2006. Secretion signal of the filamentous haemagglutinin, a model two-partner secretion substrate. Mol. Microbiol. 61:368-382. [DOI] [PubMed] [Google Scholar]
- 19.Holm, M. M., S. L. Vanlerberg, D. D. Sledjeski, and E. R. Lafontaine. 2003. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 71:4977-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob-Dubuisson, F., C. El-Hamel, N. Saint, S. Guedin, E. Willery, G. Molle, and C. Locht. 1999. Channel formation by FhaC, the outer membrane protein involved in the secretion of the Bordetella pertussis filamentous hemagglutinin. J. Biol. Chem. 274:37731-37735. [DOI] [PubMed] [Google Scholar]
- 21.Jacob-Dubuisson, F., R. Fernandez, and L. Coutte. 2004. Protein secretion through autotransporter and two-partner pathways. Biochim. Biophys. Acta 1694:235-257. [DOI] [PubMed] [Google Scholar]
- 22.Jacob-Dubuisson, F., C. Loch, and R. Antoine. 2001. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 40:306-313. [DOI] [PubMed] [Google Scholar]
- 23.Karalus, R., and A. Campagnari. 2000. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2:547-559. [DOI] [PubMed] [Google Scholar]
- 24.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locht, C., P. Bertin, F. D. Menozzi, and G. Renauld. 1993. The filamentous haemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol. Microbiol. 9:653-660. [DOI] [PubMed] [Google Scholar]
- 26.Luke, N. R., A. J. Howlett, J. Shao, and A. A. Campagnari. 2004. Expression of type IV pili by Moraxella catarrhalis is essential for natural competence and is affected by iron limitation. Infect. Immun. 72:6262-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luke, N. R., R. J. Karalus, and A. A. Campagnari. 2002. Inactivation of the Moraxella catarrhalis superoxide dismutase SodA induces constitutive expression of iron-repressible outer membrane proteins. Infect. Immun. 70:1889-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luke, N. R., T. A. Russo, N. Luther, and A. A. Campagnari. 1999. Use of an isogenic mutant constructed in Moraxella catarrhalis to identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6. Infect. Immun. 67:681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMichael, J. C. 2000. Progress toward the development of a vaccine to prevent Moraxella (Branhamella) catarrhalis infections. Microbes Infect. 2:561-568. [DOI] [PubMed] [Google Scholar]
- 30.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 19(Suppl. 1):S101-S107. [DOI] [PubMed] [Google Scholar]
- 31.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy, T. F. 1996. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol. Rev. 60:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, T. F. 1998. Lung infections. 2. Branhamella catarrhalis: epidemiological and clinical aspects of a human respiratory tract pathogen. Thorax 53:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy, T. F., and S. Sethi. 2002. Chronic obstructive pulmonary disease: role of bacteria and guide to antibacterial selection in the older patient. Drugs Aging 19:761-775. [DOI] [PubMed] [Google Scholar]
- 35.Palmer, K. L., and R. S. Munson, Jr. 1995. Cloning and characterization of the genes encoding the hemolysin of Haemophilus ducreyi. Mol. Microbiol. 18:821-830. [DOI] [PubMed] [Google Scholar]
- 36.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo, T. A., J. E. Guenther, S. Wenderoth, and M. M. Frank. 1993. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated disruption. Mol. Microbiol. 9:357-364. [DOI] [PubMed] [Google Scholar]
- 38.Schiebel, E., H. Schwarz, and V. Braun. 1989. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J. Biol. Chem. 264:16311-16320. [PubMed] [Google Scholar]
- 39.St. Geme, J. W., III, S. Falkow, and S. J. Barenkamp. 1993. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc. Natl. Acad. Sci. USA 90:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St. Geme, J. W., III, and S. Grass. 1998. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplasmic intermediate and requires the HMWB and HMWC proteins. Mol. Microbiol. 27:617-630. [DOI] [PubMed] [Google Scholar]
- 41.Timpe, J. M., M. M. Holm, S. L. Vanlerberg, V. Basrur, and E. R. Lafontaine. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect. Immun. 71:4341-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uphoff, T. S., and R. A. Welch. 1990. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J. Bacteriol. 172:1206-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verduin, C. M., C. Hol, A. Fleer, H. van Dijk, and A. van Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward, C. K., J. R. Mock, and E. J. Hansen. 2004. The LspB protein is involved in the secretion of the LspA1 and LspA2 proteins by Haemophilus ducreyi. Infect. Immun. 72:1874-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitby, P. W., D. J. Morton, and T. L. Stull. 1998. Construction of antibiotic resistance cassettes with multiple paired restriction sites for insertional mutagenesis of Haemophilus influenzae. FEMS Microbiol. Lett. 158:57-60. [DOI] [PubMed] [Google Scholar]
- 46.Willems, R. J., H. G. van der Heide, and F. R. Mooi. 1992. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol. Microbiol. 6:2661-2671. [DOI] [PubMed] [Google Scholar]