Abstract
After phagocytosis, the intracellular pathogen Mycobacterium tuberculosis arrests the progression of the nascent phagosome into a phagolysosome, allowing for replication in a compartment that resembles early endosomes. To better understand the molecular mechanisms that govern phagosome maturation arrest, we performed a visual screen on a set of M. tuberculosis mutants specifically attenuated for growth in mice to identify strains that failed to arrest phagosome maturation and trafficked to late phagosomal compartments. We identified 10 such mutants that could be partitioned into two classes based on the kinetics of trafficking. Importantly, four of these mutants harbor mutations in genes that encode components of the ESX-1 secretion system, a pathway critical for M. tuberculosis virulence. Although ESX-1 is required, the known ESX-1 secreted proteins are dispensable for phagosome maturation arrest, suggesting that a novel effector required for phagosome maturation arrest is secreted by ESX-1. Other mutants identified in this screen had mutations in genes involved in lipid synthesis and secretion and in molybdopterin biosynthesis, as well as in genes with unknown functions. Most of these trafficking mutants exhibited a corresponding growth defect during macrophage infection, but two mutants grew like wild-type M. tuberculosis during macrophage infection. Our results support the emerging consensus that multiple factors from M. tuberculosis, including the ESX-1 secretion system, are involved in modulating trafficking within the host.
After phagocytosis by a host macrophage, Mycobacterium tuberculosis, the causative agent of tuberculosis disease, resides in a phagosomal compartment that resists maturation into to an acidic phagolysosome and maintains many characteristics of early endosomes (11, 12, 33). This phenomenon, termed phagosome maturation arrest (PMA), was first observed over three decades ago in landmark studies demonstrating that most (∼70%) M. tuberculosis-containing phagosomes (MCPs) failed to mix with lysosomal tracers (1). Since then, the phenomenon of PMA during M. tuberculosis infection has been well characterized. During infection of the macrophage, M. tuberculosis bacilli reside in transferrin-accessible (6), mildly acidic (pH 6.5) (28, 39), early phagosomal compartments even at very late time points postinfection. In contrast, phagosomes containing inert particles such as latex beads or killed M. tuberculosis gradually mature, usually within an hour of phagocytosis, into more acidic late phagosomal compartments that are enriched for late endosomal or lysosomal markers (5). These observed trafficking differences between live and killed M. tuberculosis suggests that there is a labile activity associated with live bacteria. Although the precise molecular details remain to be elucidated, evidence is mounting that multiple M. tuberculosis factors, including lipids (16, 17) and proteins (42), may be involved in arresting the maturation of the phagosome.
There are several reasons why PMA might benefit the pathogenesis of M. tuberculosis. Trafficking to the acidic and hydrolytic lysosomal environment could decrease intracellular viability. One study supporting this notion showed that coinfection with Coxiella burnetii resulted in acidification of MCPs, which coincided with inhibited intracellular growth of M. tuberculosis (20). In contrast, a recent study identified two mutants of M. tuberculosis that trafficked to late compartments without suffering a decrease in intracellular survival (28). In addition, it has been proposed that PMA could benefit M. tuberculosis pathogenesis by sequestering the bacteria away from antigen-presenting compartments, thereby altering the host immune response (14, 29, 36). Although the potential benefits of PMA to M. tuberculosis pathogenesis are numerous, understanding its precise virulence function has been complicated, since any strain with increased susceptibility to killing by the macrophage will traffic to late compartments as a secondary consequence of decreased viability (10, 27, 32).
Microbial pathogens often use specialized secretion pathways to export virulence factors that subvert host defense functions. Legionella pneumophila, for example, uses the Dot/Icm specialized secretion system to export effector proteins into host cells, some of which interfere with normal phagosome trafficking (35, 41). Likewise, the recently discovered ESX-1 protein secretion pathway in M. tuberculosis secretes effector proteins important for host interaction and pathogenesis. In addition to being attenuated for virulence in mice (21, 22, 37), mutants of the ESX-1 secretion pathway in M. tuberculosis exhibit a range of pathogenic defects, including attenuation of intracellular growth in macrophages (21, 37), altered immune modulation of host cells, and a diminished ability to lyse pneumocytes (22). Although several of the genes required for ESX-1 secretion in M. tuberculosis have been described (21, 22, 37), only a few proteins that require ESX-1 for export have been reported. These include ESAT-6 and CFP-10, two highly abundant culture filtrate proteins that interact to form a dimer (3, 31), and EspA (15). Currently, the function of these proteins is not known.
Interestingly, the live attenuated vaccine strain Mycobacterium bovis bacilli Calmette-Guerin (BCG), a species related to M. tuberculosis, arrests the maturation of the phagosome during macrophage infection (12) despite lacking a functional ESX-1 secretion system (2, 7, 24). This has led to the assumption that the ESX-1 pathway is dispensable for phagosome maturation arrest. In contrast, a recent study reported that ESX-1 secretion is required for phagosome maturation arrest during macrophage infection by Mycobacterium marinum (40), another species closely related to M. tuberculosis that causes similar disease in poikilothermic hosts. Currently, rigorous evidence defining the requirement of ESX-1 secretion in M. tuberculosis-mediated phagosome maturation arrest during macrophage infection is lacking.
Recently, two studies used forward genetics to identify mutants that fail to arrest phagosome maturation. One study used a magnetic selection methodology to identify mutants of M. tuberculosis that failed to arrest phagosome maturation, many of which were severely impaired for survival in macrophages (28). Another study identified trafficking mutants of BCG using flow cytometry to sort acidified MCPs and determined mutant representation by transposon hybridization to microarrays (38). These two studies identified mutually exclusive sets of genes with a range of predicted functions, including lipid synthesis and transport, metabolic enzymes, cell wall components, and genes of unknown function.
We adopted a different strategy to identify mutants of M. tuberculosis that fail to arrest phagosome maturation. Starting with a collection of 67 M. tuberculosis mutants specifically attenuated for growth during mouse infection, we used a fluorescence microscopy methodology to identify mutants with aberrant trafficking phenotypes. We reasoned that if PMA is required for growth during M. tuberculosis infection, a subset of these 67 mutants might exhibit trafficking defects. This visual screen identified 10 mutants that trafficked to late phagosomal compartments with various kinetics. Genes required for PMA encode proteins with a range of functions, including ESX-1 secretion. We show that while ESX-1 is required for PMA, known substrates of the pathway are dispensable for PMA, suggesting that novel ESX-1 effectors could play a role in modulating phagosome traffic in the host.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains of Mycobacterium tuberculosis (Erdman) used in the present study are listed in Table 1. All strains were grown as previously described (9). Briefly, cultures for macrophage infection were grown to mid-logarithmic phase in 7H9 media supplemented with 10% Middlebrook OADC (BD Biosciences), 0.5% glycerol, and 0.05% Tween 80. Heat-killed M. tuberculosis was prepared by boiling for 20 min.
TABLE 1.
Plasmids and strains used in this study
| Strain genotype or plasmid | Description | Source or reference |
|---|---|---|
| Plasmid | ||
| pYUB921 | pGroEL, eGFP, oriE, oriM, KanR | W. R. Jacobs, Jr. |
| M. tuberculosis strains | ||
| Erdman | Wild type | W. R. Jacobs, Jr. |
| Rv3870::Tn | HygR | 37 |
| Rv3871::Tn | HygR | 37 |
| Rv3877::Tn | HygR | 37 |
| ΔesxA | HygR | 37 |
| Rv3615c::Tn | HygR | 23 |
| pcaA::Tn | HygR | This study |
| Rv3887c::Tn | HygR | This study |
| Rv2693c::Tn | HygR | This study |
| Rv2206::Tn | HygR | This study |
| moeB1::Tn | HygR | This study |
| ΔmmpL9 | HygR | This study |
Antibodies and reagents.
Fixable 10,000-molecular-weight Alexa 488-conjugated dextran and Alexa 488-conjugated human transferrin were purchased from Molecular Probes. Mouse monoclonal lysobisphosphatidic acid (LBPA) antibody was a generous gift from Jean Gruenberg. Rat polyclonal LAMP-2 antibody (ABL-93) was purchased from the Developmental Studies Hybridoma Bank (NICHID, University of Iowa). Alexa 488-conjugated anti-mouse and anti-rat secondary antibodies were purchased from Molecular Probes.
Macrophage infection for colocalization.
The macrophages used in all experiments were derived from bone marrow cells isolated from C57BL/6 mice and differentiated for 6 days in BMM medium (Dulbecco modified Eagle medium with 30% L-cell supernatants, 20% fetal calf serum, 2 mM glutamine, and 0.11 mg of sodium pyruvate/ml). At 24 h prior to infection, 5 × 106 macrophages were seeded onto 10-cm tissue culture dishes containing sterile glass coverslips. For infection, mid-log-phase M. tuberculosis cultures were washed three times in phosphate-buffered saline (PBS), centrifuged at low speed to remove large clumps, and sonicated briefly to generate a single-cell suspension. Inocula were prepared by diluting bacteria to a multiplicity of infection (MOI) of 5 in Dulbecco modified Eagle medium containing 10% horse serum. Macrophage monolayers were inoculated for 2 h, washed three times in warm PBS, covered in warm BMM medium, and returned to the incubator.
At the indicated time points postinfection, coverslips were removed from tissue culture dishes and fixed in freshly prepared fixative (4% paraformaldehyde in PBS [pH 7.4]). For dextran or transferrin-labeled samples, coverslips were incubated in BMM medium containing fluorescent dextran (0.25 mg/ml) or fluorescent transferrin (0.05 mg/ml) for 10 min and washed in PBS immediately prior to fixation.
Immunofluorescence labeling of infected macrophages.
Fixed monolayers were permeabilized by incubation in fresh blocking solution (0.1% bovine serum albumin-0.05% saponin in PBS) for 20 min at room temperature. Lamp-2 immunofluorescence was determined by incubating coverslips in primary antibody (1:100 in blocking solution), followed by three washes in PBS and incubation in Alexa 488-conjugated anti-rat secondary antibody (1:200 in blocking solution). LBPA immunofluorescence was determined as previously described (26).
Modified acid-fast stain.
To visualize mycobacteria in infected macrophages, we modified traditional acid-fast staining protocols to improve compatibility with multicolor fluorescence imaging. Fixed, infected monolayers were stained with a solution of dye (0.55% Rhodamine B, 55% glycerol, 7.5% phenol) diluted 1:5,000 in PBS for 5 min and then washed extensively in PBS. Stained monolayers were then mounted and analyzed immediately after preparation.
Fluorescence microscopy.
Coverslips were mounted onto slides with SlowFade antifade reagent (Molecular Probes) and imaged using a DeltaVision DV3 restoration microscope (Applied Precision) using a MicroMax 5 MHz cooled charge-coupled device camera (Roper Scientific). For each infected monolayer, 5-μm z-stacks, centered at the mycobacteria bacillus, were collected for roughly 70 infected macrophages. Each stack was deconvolved and analyzed by using SoftWoRx software (Applied Precision).
For each strain and marker used in the present study, fraction colocalization was quantified from at least three separate infections totaling over 200 individual phagosomes. A Student t test was used to determine the statistical significance compared to the wild type.
Growth determination in vivo and in cultured macrophages.
Signature-tagged mutagenesis, mutant isolation, tag representation, and insertion determination were performed as previously described (9). Briefly, pools of 48 signature-tagged transposon mutants were pooled, cultured, and used to inoculate two C56B6 mice by tail vein injection. At 3 weeks postinfection, signature DNA tags from mutant mycobacteria harvested from inoculum pools or the lungs of two mice were amplified, radiolabeled, and hybridized to array filters. Tag representation was determined by measuring mean pixel intensity using ImageJ software (http://rsbweb.nih.gov/ij/).
CFU from macrophages infected with M. tuberculosis were determined as previously described (23). Briefly, three separate monolayers were inoculated at an MOI of 1 for 2 h. Infected monolayers were washed and lysed at 0, 24, 72, 120, and 168 h postinfection. Lysates were diluted and plated on 7H10 media. The growth curves shown are representative of at least two independent experiments.
RESULTS
Identification of M. tuberculosis strains that fail to mediate PMA.
To assess the ability of M. tuberculosis to mediate PMA, we infected naive murine bone marrow-derived macrophages with live or heat-killed M. tuberculosis at a low MOI (i.e., an MOI of 1) for 2 h. After inoculation, monolayers were washed extensively to remove extracellular bacilli, and the infection was allowed to proceed for 24 or 72 h postinoculation. At these time points, infected monolayers were pulsed for 10 min with fluorescent dextran to label early endosomes immediately prior to fixation. Fixed monolayers were processed by using a modified acid-fast procedure to stain the bacteria, and fluorescence deconvolution microscopy was used to collect three-dimensional images and quantify the fraction of MCPs that colocalized with the fluorescent dextran. At both 24 and 72 h postinfection, ∼65% of live MCPs, but only ∼30% of heat-killed MCPs, colocalized with the short pulse of dextran (Fig. 1A and C and Table 2). This is consistent with previously reported quantitation of PMA during M. tuberculosis infection (1, 6, 43).
FIG. 1.
Identification of trafficking mutants in M. tuberculosis. Bone-marrow derived macrophages were infected with strains of M. tuberculosis and, at 24 and 72 h postinfection, pulsed for 10 min with fluorescent dextran immediately prior to fixation. Fixed monolayers were imaged by using fluorescence deconvolution microscopy. Fluorescent dextran is green, while bacilli were stained red. White arrows point to individual bacilli. (A) Live and heat-killed M. tuberculosis were used as controls. (B) The screen identified several mutants exhibiting aberrant dextran colocalization. (C and D) For each strain, the fraction colocalization at 24 and 72 h postinfection was averaged over at least three separate infections totaling >200 phagosomes. A total of 10 mutants with colocalization defects were identified. Scale bars, 10 μm. **, P < 0.001.
TABLE 2.
Summary of fraction colocalization with markers used in this study
| Strain | Time postinfection (h) | Fraction colocalizationa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Dextran
|
Transferrin
|
LBPA
|
LAMP-2
|
||||||
| Avg | SD | Avg | SD | Avg | SD | Avg | SD | ||
| Controls | |||||||||
| Erdman live | 24 | 0.654 | 0.045 | 0.619 | 0.039 | 0.447 | 0.044 | 0.399 | 0.033 |
| 72 | 0.667 | 0.047 | 0.627 | 0.056 | 0.391 | 0.031 | 0.353 | 0.035 | |
| Erdman heat killed | 24 | 0.320 | 0.045 | 0.395 | 0.053 | 0.678 | 0.051 | 0.598 | 0.027 |
| 72 | 0.308 | 0.059 | 0.413 | 0.041 | 0.679 | 0.038 | 0.589 | 0.049 | |
| Class I mutants | |||||||||
| Rv3887::Tn | 24 | 0.314 | 0.108 | 0.402 | 0.043 | 0.583 | 0.128 | 0.560 | 0.023 |
| 72 | 0.356 | 0.050 | 0.381 | 0.015 | 0.687 | 0.007 | 0.559 | 0.070 | |
| Rv2206::Tn | 24 | 0.390 | 0.056 | 0.372 | 0.024 | 0.688 | 0.069 | 0.617 | 0.001 |
| 72 | 0.376 | 0.080 | 0.374 | 0.043 | 0.718 | 0.064 | 0.617 | 0.036 | |
| Rv2693c::Tn | 24 | 0.440 | 0.109 | 0.401 | 0.002 | 0.664 | 0.060 | 0.605 | 0.032 |
| 72 | 0.365 | 0.037 | 0.344 | 0.054 | 0.614 | 0.077 | 0.570 | 0.024 | |
| ΔmmpL9 | 24 | 0.464 | 0.074 | 0.330 | 0.042 | 0.638 | 0.086 | 0.519 | 0.087 |
| 72 | 0.404 | 0.076 | 0.353 | 0.002 | 0.614 | 0.075 | 0.587 | 0.009 | |
| Class II mutants | |||||||||
| pcaA::Tn | 24 | 0.637 | 0.044 | 0.589 | 0.038 | 0.387 | 0.046 | 0.429 | 0.069 |
| 72 | 0.338 | 0.037 | 0.442 | 0.060 | 0.580 | 0.010 | 0.571 | 0.014 | |
| moeB1::Tn | 24 | 0.657 | 0.048 | 0.632 | 0.083 | 0.433 | 0.060 | 0.382 | 0.061 |
| 72 | 0.319 | 0.074 | 0.393 | 0.027 | 0.622 | 0.042 | 0.589 | 0.062 | |
The average fraction colocalization with each marker was computed by using >200 scored phagosomes from at least three experiments.
Using this experimental approach, we screened 67 mutant strains that were previously identified in a genetic screen for attenuated virulence in mice (9). For primary characterization, >70 phagosomes were scored by using deconvolution microscopy at both 24 and 72 h postinfection for each strain. A total of 57 of the mutant strains tested, including several PDIM mutants which are severely attenuated for growth in vivo (9), showed intracellular trafficking that was indistinguishable from wild-type bacteria (data not shown). The remaining 10 mutants exhibited aberrant colocalization patterns (Fig. 1B). These 10 mutants were then characterized more extensively by scoring >200 phagosomes from at least three experiments to quantify the trafficking defects. We grouped these mutants into two kinetic classes: class I mutants (Fig. 1C), which exhibited aberrant dextran colocalization at both 24 and 72 h postinfection, and class II mutants (Fig. 1D), which were similar in appearance to the wild type at 24 h postinfection but aberrant by 72 h postinfection.
To confirm trafficking defects in these 10 mutants, we performed similar colocalization experiments using a different marker of early compartments, fluorescent transferrin (Fig. 2A). Similar to the short dextran pulse, ∼62% of live MCPs but only ∼40% of heat-killed MCPs colocalized with the fluorescent transferrin. Mutants defective for colocalization with the short dextran pulse were similarly defective for colocalization with the fluorescent transferrin (Table 2). Together, the dextran and transferrin colocalization data strongly suggest that these mutants are defective for arresting phagosome maturation.
FIG. 2.
Colocalization of M. tuberculosis strains with different maturation markers. Infections were performed and imaged as in Fig. 1. Representative images for 24 h and 72 h postinfection are shown from macrophages infected with live and heat-killed M. tuberculosis, as well as Rv3877::Tn and Rv3615c::Tn mutant strains. Maturation markers are green, while bacilli were stained red. White arrows point to individual bacilli. (A) For fluorescent transferrin colocalization, infected monolayers were pulsed with fluorescent transferrin for 10 min immediately prior to fixation. (B and C) Fixed monolayers were stained for late compartment markers LBPA and Lamp-2 by indirect immunofluorescence. Scale bars, 10 μm.
Of the 10 genes required for PMA during macrophage infection, 6 (Rv3870, Rv3871, Rv3877, Rv3615c, mmpL9, and pcaA) have been reported to be required for full virulence in mice (13, 19, 23, 37). For the remaining four genes (Rv2206, Rv2693c, moeB1, and Rv3887c), analysis of results from the signature-tagged mutagenesis screen indicated that growth in vivo defects of these mutants range from mild (Rv2693c::Tn) to severe (Rv2206::Tn) (Fig. 3).
FIG. 3.
In vivo growth defects of PMA mutant strains. (A) Array filters were used to determine tag representation within pools of signature-tagged transposon mutants from culture (input) and infected mice (output). WT, a strain in the pool of mutants that exhibited wild-type growth in mice at 3 weeks postinfection. (B) For each spot on the array filters, representation was determined by densitometry. For each mouse, the following equation was used to calculate a ratio of pixel intensities normalized to the wild type: (mutantmouse/wild typemouse)/(mutantinput/wild typeinput).
PMA mutants traffic to late phagosomal compartments.
To further characterize the trafficking defects of these mutants, we analyzed infected macrophage monolayers for localization of MCPs to late phagosomal compartments by using immunofluorescence microscopy. As expected, colocalization with late compartment markers such as LBPA (Fig. 2B) and Lamp-2 (Fig. 2C) was greater for heat-killed MCPs (ca. 60 to 70%) than for live wild-type MCPs (ca. 35 to 45%) at both 24 and 72 h postinfection (Table 2). Similar analysis of the trafficking mutants revealed that failure to mediate PMA correlated with increased colocalization with late compartment markers (Fig. 2B and C and Table 2). In summary, these data indicate that the mutants isolated in the present study fail to arrest phagosome maturation during macrophage infection, and instead traffic to late phagosomal compartments by either 24 h (class I) or 72 h (class II) postinfection.
Intracellular survival of trafficking mutants.
It is well documented that killed M. tuberculosis are unable to mediate PMA and instead traffic to late phagosomal compartments (5, 6, 25). Thus, there are two potential reasons that a mutant would traffic to late compartments: the mutant is killed by the macrophage and subsequently traffics to late compartments, or the mutant is defective for a mechanism that specifically mediates phagosome maturation arrest. To attempt to distinguish between these possibilities for the mutants identified in the present study, we assessed the ability of each mutant to grow during macrophage infection.
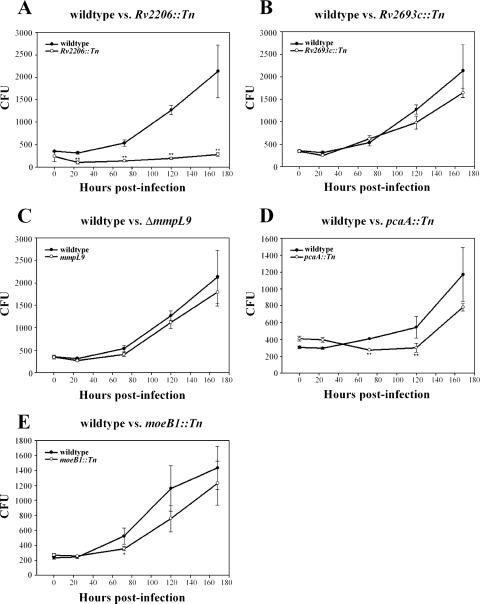
The class I mutant Rv2206::Tn (Fig. 4A) and the class II mutants pcaA::Tn (Fig. 4D) and moeB1::Tn (Fig. 4E) had growth defects in macrophages that corresponded to the observed trafficking defects. The same is true for all ESX-1 secretion mutants tested, which exhibited trafficking defects that correlated with growth defects during macrophage infection (21, 23, 37). In these cases, it is possible that the mutants trafficked to late compartments as a secondary consequence of loss of viability.
FIG. 4.
Analysis of intracellular survival of trafficking mutants. Bone marrow-derived macrophage monolayers were infected with M. tuberculosis strains at an MOI of 1 for 2 h and plated for CFU determination at 0, 24, 72, 120, and 168 h postinfection. CFU were determined for the Rv2206::Tn (A), Rv2693::Tn (B), ΔmmpL9 (C), pcaA::Tn (D), and moeB1::Tn (E) mutants. Values shown on the y axis are from a dilution of the monolayer lysate and represent 1/400 of the actual number of CFU in the monolayer. Growth curves shown are representative growth curves from at least two similar experiments. *, P < 0.05; **, P < 0.01.
In contrast, both Rv2693c::Tn (Fig. 4B) and ΔmmpL9 (Fig. 4C) mutants grew at approximately wild-type levels in macrophages despite trafficking to late compartments. These results indicate that trafficking to late compartments does not inherently attenuate the growth of M. tuberculosis at the level of the infected macrophage.
ESX-1 components, but not known substrates, are required for PMA.
Having isolated multiple ESX-1 mutants in our screen, we further investigated the role of ESX-1 secretion in modulating phagosome trafficking during infection. To confirm that loss of ESX-1 secretion was responsible for the mutant trafficking defect, we performed complementation analysis of the Rv3877::Tn mutant since Rv3877 is absolutely required for secretion of all known ESX-1 substrates. Introduction of a wild-type copy of Rv3877 which partially rescues ESX-1 secretion (37) similarly rescued the trafficking defect observed in the mutant (Fig. 5). We therefore attribute the trafficking defect in these mutants to the loss of ESX-1 secretion.
FIG. 5.
Quantification of trafficking defects of ESX-1 mutants. Several mutants known to be defective for ESX-1 secretion (Rv3877::Tn, Rv3870::Tn, Rv3871::Tn, ΔesxA, and Rv3615c::Tn) were scored for their ability to mediate PMA at 24 h and 72 h postinfection. (A to D) A short dextran pulse (A) and transferrin (B) were used as markers of early phagosomal compartments, whereas LBPA (C) and Lamp-2 (D) were used as markers of late phagosomal compartments. For each strain, fraction colocalization was averaged over at least three separate infections totaling >200 phagosomes. **, significant difference (P < 0.01) compared to both wild-type and ΔesxA strains.
The correlation between ESX-1 secretion and phagosome maturation arrest led us to hypothesize that substrates of the system, such as ESAT-6, CFP-10, or EspA, could be required for phagosome maturation arrest. To test this, we analyzed an ΔesxA mutant, which does not express ESAT-6 and CFP-10 (21, 37) and fails to secrete EspA (15), for the ability to arrest phagosome maturation. In contrast to the other ESX-1 secretion mutants, the ΔesxA strain showed wild-type colocalization patterns with each marker tested, significantly colocalizing with the short pulse of dextran (Fig. 5A) and transferrin (Fig. 6A and B) while exhibiting limited colocalization with the late markers LBPA (Fig. 6C and 5C) and Lamp-2 (Fig. 5D). To confirm this difference and to rule out any possibility of artifacts associated with the modified acid-fast stain, we tested green fluorescent protein-expressing Rv3877::Tn and ΔesxA mutants for colocalization with LBPA (Fig. 6D). Again, the data suggest that the ΔesxA mutant arrests the maturation of the phagosome, whereas the Rv3877::Tn mutant traffics to late phagosomal compartments. Thus, we conclude that essential components of the ESX-1 secretion system are required for normal trafficking in the host cell, while three known substrates of the system, ESAT-6, CFP-10, and EspA, are dispensable for arresting the maturation of the phagosome.
FIG. 6.
ΔesxA is not defective for PMA and does not traffic to a late phagosome. (A) Macrophages were infected with M. tuberculosis ΔesxA mutant and analyzed for colocalization with transferrin and LBPA by using fluorescence deconvolution microscopy. Fluorescent markers are green, while bacilli were stained red. White arrows point to individual bacilli. Scale bar, 10 μm. (B) differential interference contrast (left), deconvolved fluorescent z-slice (middle), and merged (right) images of individual macrophages infected with either Rv3877::Tn or ΔesxA mutant strains were stained for the late marker LBPA by indirect immunofluorescence. Images are from 24 h postinfection. Nuclei (blue) were stained with DAPI (4′,6′-diamidino-2-phenylindole). (C) Z-stacks from macrophages infected with either Rv3877::Tn or ΔesxA mutants (stained red) were deconvolved and rendered to visualize and determine colocalization in three dimensions. LBPA stain is green. (D) Rv3877::Tn and ΔesxA mutants (green) expressing green fluorescent protein were also used in some experiments as an alternative to postfixation staining of bacteria. The LBPA stain is red.
DISCUSSION
We screened a set of M. tuberculosis mutants attenuated for virulence in mice and identified 10 mutants (representing ca. 15% of the strains tested) defective for PMA. These mutants were classified into two kinetically distinct groups: six strains were class I mutants, trafficking to late compartments by 24 h postinfection, while four strains were class II mutants, trafficking to late compartments by 72 h. There is no overlap between our mutants and mutants identified in previous studies (28, 38), which could be due to differences in experimental design. Although other screens were unbiased, our screen was based on the assumption that PMA mutants would be represented in a set of mutants attenuated for virulence. In addition, while previous screens for PMA mutants focused on isolation of mutants with trafficking defects several hours postinfection, we focused on defects apparent on the order of days postinfection. Indeed, the isolation of class II mutants from our screen demonstrates that we were able to isolate mutants with trafficking defects at much later time points.
Overall, there was not a strong correlation between degree of attenuation in vivo and defects in PMA: mutants with comparable trafficking defects exhibited a range of in vivo growth defects from mild (Rv2693c::Tn) to severe (Rv2206::Tn), while other strains, like those defective for PDIM synthesis, are severely attenuated for growth in vivo (9) without consequence to PMA or growth in macrophages (data not shown) (32). Likewise, these mutants exhibited various growth phenotypes in a macrophage infection model: six had severe growth defects in macrophages, two showed moderate growth defects at late time points postinfection, and two grew with wild-type kinetics in macrophages. Since we did not perform complementation on any mutants outside the ESX-1 locus, we cannot exclude the possibility that the observed trafficking defects are due to unlinked mutations or polar effects on surrounding genes.
Interestingly, 4 of the 10 mutants identified in the screen were defective for ESX-1 secretion (37). Isolation of multiple ESX-1 secretion mutants with trafficking defects in the host, coupled with our complementation of the trafficking defect associated with mutation at the ESX-1 locus, lead us to conclude that this secretion pathway is important for M. tuberculosis-mediated PMA. Rv3877::Tn and Rv3615c::Tn were class I mutants, while Rv3870::Tn and Rv3871::Tn were class II mutants. It is unclear why these mutants have similar secretion defects and yet kinetically distinct trafficking defects. One possible explanation is that a putative ESX-1-dependent PMA mediator may be only partially affected by mutations in Rv3870 and Rv3871 but completely nullified by mutations in Rv3877 and Rv3615c. The observation that the ESX-1 pathway is required for phagosome maturation arrest is consistent with a recent study in M. marinum (40) but raises several questions about the trafficking phenotype of the attenuated vaccine strain, BCG. Although BCG lacks a functional ESX-1 secretion system, it still mediates PMA during macrophage infection (12). It is possible that BCG's ability to mediate PMA hinges largely on conserved lipid factors (4), whereas M. tuberculosis also uses protein factors secreted by ESX-1. It is also possible that, having lost the ESX-1 secretion system, BCG compensates by utilizing one or more of the ESX-1 paralogs (18) to arrest phagosome maturation during infection. More studies comparing PMA in M. tuberculosis- versus BCG-infected macrophages will be required to decipher this phenotypic puzzle.
Unexpectedly, the ΔesxA mutant was not required for phagosome maturation arrest, suggesting that expression of ESAT-6, CFP-10 and secretion of EspA are dispensable for wild-type trafficking in the host. It is possible that other substrates of the ESX-1 pathway could mediate PMA in an esxA-independent manner. Discovery of additional ESX-1 substrates will be critical to resolve this issue.
The remaining six mutants identified in the screen exhibited wild-type levels of ESAT-6 and CFP-10 secretion (data not shown), indicating that they are not defective for ESX-1 secretion. The Rv3887c gene is an integral membrane protein homologous to Rv3877, a component of the ESX-1 secretion system, and is situated downstream of an esx pair (esxC and esxD), suggesting that it might also be involved in the secretion of other Esx family member proteins. The Rv3887c::Tn mutant is attenuated for growth in macrophages at early time points (A. Lau and J. Cox, unpublished results) corresponding to the trafficking defects observed in the present study. Likewise, mutation of Rv2206, which encodes a conserved transmembrane protein with unknown function, resulted in a trafficking defect by 24 h postinfection. This mutant was severely attenuated for growth during macrophage infection and may be sensitive to early macrophage effector mechanisms.
The class II mutant pcaA::Tn is defective for cyclopropyl modification of mycolic acids, a lipid species crucial to the mycobacterial cell envelope structure. The mutant was reported to have a growth defect in macrophages by 48 h postinfection but not at 24 h postinfection (30), which is consistent with our results. Similarly, the class II moeB1::Tn mutant was attenuated for growth at later time points corresponding to the observed trafficking defect. The moeB1 gene encodes an enzyme involved in the synthesis of molybdopterin, a cofactor used in many enzymatic reactions, including nitrate reduction (34). Based on the moderate growth defects of the pcaA::Tn and moeB1::Tn mutants during macrophage infection, we hypothesize that these mutants are sensitive to macrophage effector mechanisms and thus are killed and traffic to late phagosomal compartments at later time points postinfection.
The Rv2693c::Tn and ΔmmpL9 mutants were interesting because they failed to arrest the maturation of the phagosome without consequence to survival during macrophage infection. Interestingly, the trafficking defects observed in the Rv2693c::Tn and ΔmmpL9 mutants at 24 h postinfection were slightly less severe than other mutants described here (Fig. 1C and Table 2). It is possible that these mutants have a moderate trafficking defect that does not critically affect growth in macrophages. However, the ΔmmpL9 mutant, which by 72 h postinfection exhibits a more severe trafficking defect, grew with wild-type growth kinetics even at very late time points postinfection. mmpL9 encodes a membrane protein that is part of a large family of homologs known to be involved in the secretion of lipids (8, 9, 13). Although a lipid substrate for mmpL9 has not yet been identified, this is consistent with previous observations that lipids play an important role in M. tuberculosis-mediated PMA (4, 28, 38). Interestingly, Pethe et al. isolated a mutant with a similar phenotype in their screen (Rv2930::Tn), which resided in an acidified phagosome but grew at wild-type levels in macrophages (28). Given the possibility of artifacts inherent to the macrophage infection model, we cannot rule out the possibility that trafficking or growth phenotypes in macrophages ex vivo might not mimic cellular pathogenesis in vivo. However, taken at face value, these results suggest that phagosome maturation arrest, while probably important for M. tuberculosis pathogenesis, is not directly required for growth or survival in naive macrophages. Instead, PMA may benefit M. tuberculosis during infection by sequestering the bacteria away from important immune response elements, such as the major histocompatibility complex class II antigen presentation machinery or pathogen recognition receptors. It is also possible that phagosome maturation arrest, while dispensable for growth in naive macrophages, is critical for survival in harsher, more acidified, and oxidative phagosomes of activated macrophages. Indeed, macrophage activation could explain the results of the previous study linking phagosome maturation to loss of M. tuberculosis viability in the context of coinfection with Coxiella burnetii (20), given the complexities of host response to multiple invading pathogens.
Acknowledgments
We thank P. Champion, Y. Ohol, and S. Raghavan for critical reading of the manuscript. We also thank E. MacGurn and members of the Cox laboratory for helpful comments throughout the course of this research.
J.A.M. is supported by a Microbial Pathogenesis Training Grant from the National Institutes of Health. J.S.C. gratefully acknowledges the support of the Sandler Family Supporting Foundation and the W. M. Keck Foundation. This study was supported by National Institutes of Health grant AI051667.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Armstrong, J. A., and P. D. Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134:713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 3.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144(Pt. 11):3195-3203. [DOI] [PubMed] [Google Scholar]
- 4.Chua, J., I. Vergne, S. Master, and V. Deretic. 2004. A tale of two lipids: Mycobacterium tuberculosis phagosome maturation arrest. Curr. Opin. Microbiol. 7:71-77. [DOI] [PubMed] [Google Scholar]
- 5.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens, D. L., and M. A. Horwitz. 1996. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 8.Converse, S. E., J. D. Mougous, M. D. Leavell, J. A. Leary, C. R. Bertozzi, and J. S. Cox. 2003. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. USA 100:6121-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79-83. [DOI] [PubMed] [Google Scholar]
- 10.Darwin, K. H., S. Ehrt, J. C. Gutierrez-Ramos, N. Weich, and C. F. Nathan. 2003. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302:1963-1966. [DOI] [PubMed] [Google Scholar]
- 11.Deretic, V., S. Singh, S. Master, J. Harris, E. Roberts, G. Kyei, A. Davis, S. de Haro, J. Naylor, H. H. Lee, and I. Vergne. 2006. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defense mechanism. Cell Microbiol. 8:719-727. [DOI] [PubMed] [Google Scholar]
- 12.Deretic, V., L. E. Via, R. A. Fratti, and D. Deretic. 1997. Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking. Electrophoresis 18:2542-2547. [DOI] [PubMed] [Google Scholar]
- 13.Domenech, P., M. B. Reed, and C. E. Barry III. 2005. Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infect. Immun. 73:3492-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn, J. L., and J. Chan. 2003. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr. Opin. Immunol. 15:450-455. [DOI] [PubMed] [Google Scholar]
- 15.Fortune, S. M., A. Jaeger, D. A. Sarracino, M. R. Chase, C. M. Sassetti, D. R. Sherman, B. R. Bloom, and E. J. Rubin. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc. Natl. Acad. Sci. USA 102:10676-10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fratti, R. A., J. Chua, I. Vergne, and V. Deretic. 2003. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 100:5437-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gey Van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 2001. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C gram-positive bacteria. Genome Biol. 2:RESEARCH0044. [DOI] [PMC free article] [PubMed]
- 19.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 20.Gomes, M. S., S. Paul, A. L. Moreira, R. Appelberg, M. Rabinovitch, and G. Kaplan. 1999. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect. Immun. 67:3199-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guinn, K. M., M. J. Hickey, S. K. Mathur, K. L. Zakel, J. E. Grotzke, D. M. Lewinsohn, S. Smith, and D. R. Sherman. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu, T., S. M. Hingley-Wilson, B. Chen, M. Chen, A. Z. Dai, P. M. Morin, C. B. Marks, J. Padiyar, C. Goulding, M. Gingery, D. Eisenberg, R. G. Russell, S. C. Derrick, F. M. Collins, S. L. Morris, C. H. King, and W. R. Jacobs, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 100:12420-12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacGurn, J. A., S. Raghavan, S. A. Stanley, and J. S. Cox. 2005. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol. Microbiol. 57:1653-1663. [DOI] [PubMed] [Google Scholar]
- 24.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik, Z. A., C. R. Thompson, S. Hashimi, B. Porter, S. S. Iyer, and D. J. Kusner. 2003. Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J. Immunol. 170:2811-2815. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo, H., J. Chevallier, N. Mayran, I. Le Blanc, C. Ferguson, J. Faure, N. S. Blanc, S. Matile, J. Dubochet, R. Sadoul, R. G. Parton, F. Vilbois, and J. Gruenberg. 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303:531-534. [DOI] [PubMed] [Google Scholar]
- 27.Ng, V. H., J. S. Cox, A. O. Sousa, J. D. MacMicking, and J. D. McKinney. 2004. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol. Microbiol. 52:1291-1302. [DOI] [PubMed] [Google Scholar]
- 28.Pethe, K., D. L. Swenson, S. Alonso, J. Anderson, C. Wang, and D. G. Russell. 2004. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. USA 101:13642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandra, L., E. Noss, W. H. Boom, and C. V. Harding. 2001. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J. Exp. Med. 194:1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao, V., N. Fujiwara, S. A. Porcelli, and M. S. Glickman. 2005. Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J. Exp. Med. 201:535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renshaw, P. S., P. Panagiotidou, A. Whelan, S. V. Gordon, R. G. Hewinson, R. A. Williamson, and M. D. Carr. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex: implications for pathogenesis and virulence. J. Biol. Chem. 277:21598-21603. [DOI] [PubMed] [Google Scholar]
- 32.Rousseau, C., N. Winter, E. Pivert, Y. Bordat, O. Neyrolles, P. Ave, M. Huerre, B. Gicquel, and M. Jackson. 2004. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell Microbiol. 6:277-287. [DOI] [PubMed] [Google Scholar]
- 33.Russell, D. G. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell. Biol. 2:569-577. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz, G. 2005. Molybdenum cofactor biosynthesis and deficiency. Cell Mol. Life Sci. 62:2792-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shohdy, N., J. A. Efe, S. D. Emr, and H. A. Shuman. 2005. Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc. Natl. Acad. Sci. USA 102:4866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh, C. R., R. A. Moulton, L. Y. Armitige, A. Bidani, M. Snuggs, S. Dhandayuthapani, R. L. Hunter, and C. Jagannath. 2006. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J. Immunol. 177:3250-3259. [DOI] [PubMed] [Google Scholar]
- 37.Stanley, S. A., S. Raghavan, W. W. Hwang, and J. S. Cox. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. USA 100:13001-13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart, G. R., J. Patel, B. D. Robertson, A. Rae, and D. B. Young. 2005. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 1:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 40.Tan, T., W. L. Lee, D. C. Alexander, S. Grinstein, and J. Liu. 2006. The ESAT-6/CFP-10 secretion system of Mycobacterium marinum modulates phagosome maturation. Cell Microbiol. 8:1417-1429. [DOI] [PubMed] [Google Scholar]
- 41.Vogel, J. P., and R. R. Isberg. 1999. Cell biology of Legionella pneumophila. Curr. Opin. Microbiol. 2:30-34. [DOI] [PubMed] [Google Scholar]
- 42.Walburger, A., A. Koul, G. Ferrari, L. Nguyen, C. Prescianotto-Baschong, K. Huygen, B. Klebl, C. Thompson, G. Bacher, and J. Pieters. 2004. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 304:1800-1804. [DOI] [PubMed] [Google Scholar]
- 43.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D. G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153:2568-2578. [PubMed] [Google Scholar]