Abstract
Moraxella catarrhalis is an important bacterial cause of otitis media in children and respiratory tract infections in the elderly. Lipooligosaccharide (LOS), a major surface antigen of this bacterium, is a potential vaccine component against the organism. There are three major LOS serotypes (serotypes A, B, and C) in clinical isolates of M. catarrhalis. Our previous studies demonstrated that serotype A and B LOS-based conjugates were immunogenic in animals and elicited bactericidal antibodies. In this study, LOS from serotype C strain 26404 was isolated, detoxified, and conjugated to tetanus toxoid (TT) or the cross-reactive mutant (CRM) of diphtheria toxin to form detoxified LOS (dLOS)-TT, dLOS-CRM-1, and dLOS-CRM-2 vaccine candidates. The molar ratios (dLOS/protein) of the resulting conjugates were 47:1, 19:1, and 32:1, respectively, while the weight ratios were 0.94, 0.84 and 1.44, respectively. All conjugates were highly immunogenic in both mouse and rabbit models. Three subcutaneous injections of each conjugate formulated with the Ribi adjuvant elicited >700-fold increases in serum anti-LOS immunoglobulin G levels in mice (5 μg of dLOS) and >2,000-fold increases in rabbits (50 μg of dLOS). The resulting mouse and rabbit antisera showed complement-mediated bactericidal activity against the homologous strain. In addition, a representative rabbit antiserum showed bactericidal activity against 14 of 18 testable strains, and this bactericidal activity could be 100% inhibited by the serotype C or A LOS but only 30% inhibited by the serotype B LOS. These results indicate that the serotype C LOS-based conjugates can be used as vaccine components for further investigation in humans.
Moraxella catarrhalis is a gram-negative aerobic dipolococcus and is currently the third most frequent cause of bacterial otitis media (OM) and sinusitis in children (3, 14, 21). More than 90% of all children develop OM before the age of 7 years (35); 15 to 20% of these middle ear infections are caused by M. catarrhalis (9, 31), and it is estimated that there are 3 to 4 million cases in the United States annually (23, 26). The chronic forms of OM in these young patients may result in hearing loss and are associated with developmental and learning problems as children reach school age (2, 22). Sinusitis, however, accounts for 5 to 10% of upper respiratory tract infections in early childhood (40). In addition, M. catarrhalis often causes lower respiratory tract infections in adults with chronic obstructive pulmonary disease (3, 14, 26) and occasionally causes severe infections, including pneumonia, endocarditis, septicemia, and meningitis (3, 5, 6). Currently, treatment of the diseases has relied mainly on antimicrobial agents. However, with growing antibiotic resistance observed in clinical isolates all over the world (15, 40), attention has been focused on the possibility of vaccination against M. catarrhalis infection (4, 27).
The research on vaccine antigens is presently based on the hypothesis that humoral immunity provides protection due to antibodies transudating into the middle ear cavity (24), although there is insufficient information about the protective antigens or an in vitro correlate of immunity against M. catarrhalis in humans. Since M. catarrhalis neither expresses a capsule nor secrets an exotoxin, the search for vaccine antigens has focused mainly on the conserved epitopes exposed on the bacterial surface. So far, a number of antigens have been identified; these include the adhesins UspA1, UspA2, Hag, CD, Mcap, and MID, the virulence factor UspA2, and the nutrient uptake-related proteins CD, E, LbpA, LbpB, TbpA, TbpB, and CopB (10, 24, 25, 36), as well as newly identified and highly conserved G1 and M35 proteins (1, 8).
Lipooligosaccharide (LOS) is another prominent surface component of M. catarrhalis, and it has been implicated as a virulence factor important in the pathogenesis of this organism (9, 17, 28). Rahman et al. (30) reported that serum antibodies to LOS developed in patients with M. catarrhalis infections, while Tanaka et al. (34) discovered that the bactericidal activity of convalescent-phase anti-LOS immunoglobulin G (IgG) from patients was against M. catarrhalis. Our study showed that a specific anti-LOS mouse monoclonal antibody was bactericidal and able to inhibit M. catarrhalis adherence to human epithelia and promoted clearance in a mouse pulmonary model after an aerosol challenge (18). In addition, the serological properties of LOS in humans have revealed a less variable structure among three serotypes of LOS accounting for 95% of clinical isolates (serotype A, 61%; serotype B, 29%; and serotype C, 5%) (39). Structural studies showed that the LOSs of the three serotypes were all branched, with a common inner core and a lipid A portion which is similar to that of other gram-negative bacteria (16). Thus, the LOS is becoming an attractive vaccine candidate.
We previously synthesized immunogenic conjugates from serotype A and B LOSs by detoxification of the LOSs and conjugation of the detoxified LOSs (dLOSs) to protein carriers. Both mice and rabbits immunized with the conjugates developed anti-LOS IgG antibodies with bactericidal activity (11, 43). Active or passive immunization with the serotype A conjugates or their antiserum generated protection against homologous and heterologous strains in a mouse model of pulmonary clearance (17). Similar protection was further demonstrated by mucosal immunization with the conjugates (20). These lines of evidence indicate that the immune responses against LOS may play an important role in the elimination of bacteria interacting with the host, resulting in resolution of infections. Since three LOS serotypes are found in clinical isolates, new serotype C LOS conjugates were developed and evaluated in this study.
MATERIALS AND METHODS
Bacterial strains.
M. catarrhalis strains CCUG 26404 (serotype C; LOS source strain), 26391 (serotype C), 26394 (serotype A), 26395 (serotype A), 26397 (serotype B; LOS source strain), 3292 (serotype B), and 26400 (serotype B) were obtained from the Culture Collection of the University of Goteborg (CCUG), Department of Clinical Bacteriology, Goteborg, Sweden. M. catarrhalis strains ATCC 8176, 8193, 23246, 25238 (serotype A; LOS source strain), 25239, 25240, 43167, 43618, 43627, 43628, and 49143 were purchased from the American Type Culture Collection (ATCC), Manassas, VA. Strains O35E and TTA24 (38) were provided by E. J. Hansen of the University of Texas, Dallas. Ten other clinical isolates (M1 to M10) were provided by G. Mogi of Oita Medical University, Oita, Japan.
Bacterial culture and LOS purification.
Strains were grown on chocolate agar plates at 37°C in 5% CO2 for 18 h. Four or five isolated colonies of each strain were transferred to new plates and incubated for 3.5 to 4 h (mid-logarithmic phase) for bactericidal and bactericidal inhibition assays. For LOS production, the bacterial seeds from agar plates were transferred and cultured in six flasks (1,400 ml each) with 3% tryptic soy broth (BD, Sparks, MD) at 37°C and 110 rpm for 24 h (11). Cells were collected and washed, and the LOS was purified from the cells by phenol-water extraction (11). The yields of the LOS ranged from 13 to 17 mg per liter of bacterial culture. The protein and nucleic acid contents of the purified LOS were shown to be around 2% (33, 41).
Synthesis of LOS-based conjugates.
Detoxification of LOS, derivatization of dLOS, and then conjugation of the dLOS to tetanus toxoid (TT) or the cross-reactive mutant (CRM) of diphtheria toxin were performed as described previously (43). Briefly, 240 mg of LOS was detoxified by using anhydrous hydrazine (Sigma, St. Louis, MO), and the dLOS was purified. Then adipic acid dihydrazide (Aldrich Chemical Co., Milwaukee, WI) was conjugated to the dLOS (96 mg) in 12 ml of a 287 mM adipic acid dihydrazide suspension to form adipic hydrazide (AH)-dLOS derivatives, using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide HCl and N-hydroxysulfosuccinimide (Pierce, Rockford, IL). The resulting AH-dLOS was finally coupled to TT or CRM. TT (15 mg/ml) was obtained from Pasteur Merieux Connaught, Swiftwater, PA (lot 45453), and CRM (23 mg/ml) was isolated from Corynebacterium diphtheriae by J. B. Robbin's laboratory at the National Institute of Child Health and Human Development, Bethesda, MD (32). Briefly, 10 mg of TT (Mr, 150,000) was reacted with 20 mg of AH-dLOS (10 mg/ml) at a molar ratio of AH-dLOS to TT of 100:1 using 0.05 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide HCl. Ten or five milligrams of CRM (Mr, 67,000) was also reacted with 20 mg of AH-dLOS (10 mg/ml) at a molar ratio of AH-dLOS to CRM of 45:1 or 90:1. All reaction mixtures were maintained at pH 5.0 to 5.2 for 4 h at 4°C, and the reactions were stopped by adjusting the pH to 7.0. The reaction mixtures were dialyzed against 0.9% NaCl for 2 to 3 days, centrifuged, and passed through a Sephacryl S-300 column (2.6 by 90 cm) in 0.9% NaCl. Peaks that contained both protein and carbohydrate were pooled and designated dLOS-TT, dLOS-CRM-1, and dLOS-CRM-2. The three conjugates were analyzed to determine their carbohydrate and protein contents using dLOS and bovine serum albumin as standards (7, 33).
Preparation of reference sera.
Ten female BALB/c mice were inoculated subcutaneously three times at 3-week intervals with 0.2 ml of a solution at two injection sites; the solution contained 108 CFU of strain 26404 and Ribi adjuvant (R-700; 50 μg of monophosphoryl lipid A [MPL] and 50 μg of synthetic trehalose dicorynomycolate [STD]; Corixa, Hamilton, MT), which were mixed at a ratio of 1:1 (by volume). Blood samples were collected and pooled 2 weeks after the third injection to obtain a reference serum.
Two New Zealand White rabbits (female, 2 to 3 kg) were inoculated subcutaneously three times at 4-week intervals with 1 ml of a solution at two injection sites; the solution contained 109 CFU of strain 26404 and Ribi adjuvant (R-700; 250 μg of MPL and 250 μg of STD), which were mixed at a ratio of 1:1 (by volume). Blood samples were collected 2 weeks after the third injection to obtain a reference serum.
ELISA.
Binding activities of the dLOS and conjugates with rabbit antiserum against strain 26404 were tested by using an enzyme-linked immunosorbent assay (ELISA) (43). The reactions were read by using a microplate autoreader at A405 after 1 h of incubation with a substrate.
Immunogenicity assays.
Conjugates were tested for immunogenicity in mice and rabbits. Five-week-old female BALB/c mice (eight mice per group) were inoculated at two sites subcutaneously with 5 μg of each conjugate (sugar content) or with a mixture of dLOS, TT, and CRM (5 μg each) in 0.2 ml of 0.9% NaCl with Ribi adjuvant (50 μg of MPL and 50 μg of STD). The injections were given three times at 3-week intervals, and the mice were bled 2 weeks after each injection.
Female New Zealand White rabbits (two rabbits per group) were inoculated at two sites subcutaneously with 50 μg of dLOS-TT or dLOS-CRM (sugar content) or with a mixture of dLOS, TT, and CRM (50 μg each) in 1 ml of 0.9% NaCl with Ribi adjuvant (250 μg of MPL and 250 μg of STD). The injections were given three times at 4-week intervals, and the rabbits were bled 2 weeks after each injection.
Serum anti-LOS levels were expressed in ELISA units using 26404 LOS as a coating antigen along with reference sera. The mouse reference serum was assigned values of 19,683 and 81 ELISA U/ml for IgG and IgM, respectively, and the rabbit reference serum was assigned values of 177,147 and 81 ELISA U/ml for IgG and IgM, respectively.
Bactericidal assay and bactericidal inhibition assay.
Mouse and rabbit antisera were inactivated at 56°C for 30 min and tested for bactericidal activity against M. catarrhalis by a microbactericidal assay as described previously (43). The antiserum was diluted at 1:5, and then twofold continuous dilution with Dulbecco's phosphate-buffered saline containing calcium, magnesium, and 0.1% gelatin was performed. Based on our preliminary tests, we selected guinea pig complement sera (1:5; Calbiochem, La Jolla, CA) or rabbit complement sera (1:8; Sigma) as the source of complement for mouse or rabbit antiserum detection because the rabbit complement sera did not show killing capacity on the detection of mouse antisera. Each serum sample was tested three times. For the inhibition assay, each inactivated rabbit antiserum elicited by dLOS-TT (25 μl at a 1:5 dilution) was incubated with equal volumes of LOS or lipopolysaccharide (LPS) inhibitor from strain 25238 (serotype A), 26397 (serotype B), or 26404 (serotype C), or Salmonella enterica serovar Minnesota Ra (Sigma) at concentrations of 25, 12.5, 6.3, 3.2, 1.6, and 0 μg/ml at 37°C for 60 min before the bactericidal assay. The level of inhibition was calculated as follows: (CFU from serum with LOS inhibitor − CFU from serum without inhibitor)/(CFU from complement only − CFU from serum without inhibitor) × 100.
LAL assay.
The LOS and dLOS were tested to determine their endotoxin reactivities using a Limulus amebocyte lysate (LAL) Pyrogent Plus reagent kit (24 single test vials; Biowhittaker, Inc. Walkersville, MD). The sensitivity of the LAL assay is 0.12 endotoxin unit (EU)/ml.
SDS-PAGE and silver staining.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and silver staining were performed as described previously (37).
Statistical analysis.
Antibody levels are expressed below in ELISA units or titers (reciprocal) (geometric mean ± standard deviation of n independent observations). Significance was determined with the two-tailed independent Student t test, and P values of <0.05 were considered significant. Correlations between anti-LOS antibody levels and bactericidal antibody levels were evaluated by a two-tailed linear regression analysis.
RESULTS
Characterization of dLOS, AH-dLOS, and conjugates.
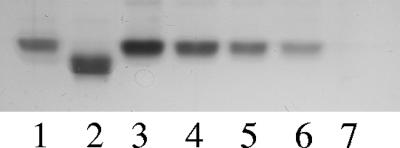
SDS-PAGE followed by silver staining for carbohydrates was performed to investigate the residual LOS in the dLOS preparation. The results revealed that 25 ng of LOS produced a single band; however, 20 μg of dLOS produced no detectable band (Fig. 1). This suggests that the percentage of the residual LOS in the dLOS preparation was less than 0.13%. In addition, the dLOS level was <0.12 EU/μg, whereas the LOS level was 10,000 EU/μg, a >80,000-fold reduction in toxicity as determined by an LAL assay.
FIG. 1.
Silver-stained SDS-PAGE patterns of LOS and dLOS from M. catarrhalis strain 26404. Lanes 1 and 2, 200 ng each of S. enterica serovar Minnesota LPS Ra and Rc, respectively; lanes 3 through 6, 200, 100, 50, and 25 ng of LOS, respectively; lane 7, 20 μg of dLOS from M. catarrhalis strain 26404.
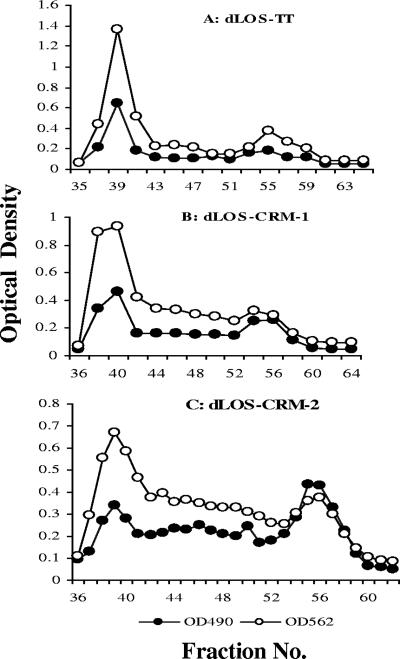
The molar ratio of AH to dLOS in AH-dLOS was 0.36, and the yield, based on the carbohydrate content, was 84.1%. Figure 2 presents chromatograms of samples after the coupling reaction between AH-dLOS and TT or CRM, and the final conjugate products eluted in the void volume. All conjugates produced similar yields but had different molar ratios and weight ratios due to different sizes and quantities of the protein carriers used (Table 1). All conjugates showed similar binding activities with a rabbit antiserum against whole cells of strain 26404 in ELISA.
FIG. 2.
Chromatographic profiles of three conjugates on a Sephacryl S-300 column (1.6 by 95 cm). The first peak around the void volume that contained both protein and carbohydrate was collected and designated dLOS-TT (A), dLOS-CRM-1 (B), or dLOS-CRM-2 (C). Unconjugated carrier proteins or AH-dLOS was eluted from fractions 53 to 58 on the same column. The void volume was around fraction 39, and the total volume was around fraction 83. Each fraction contained 2 ml eluent. The optical density at 490 (OD490) and optical density at 562 (OD562) were the optical density of the carbohydrate determined by the phenol-sulfuric acid assay (7) and the optical density of the protein determined by the micro bicinchoninic acid assay (33), respectively.
TABLE 1.
Composition, yield, and antibody binding activities of conjugates
| Conjugate | Amt (μg/ml) of:
|
dLOS/protein ratio
|
Yield (%)b | A405 (hyperimmune sera)c | ||
|---|---|---|---|---|---|---|
| dLOS | Protein | Molara | Wt | |||
| dLOS-TT | 460 | 489 | 47 | 0.94 | 25 | 1.0; 1.7 |
| dLOS-CRM-1 | 376 | 446 | 19 | 0.84 | 26 | 1.1; 1.5 |
| dLOS-CRM-2 | 456 | 315 | 32 | 1.44 | 27 | 1.1; 1.6 |
Molar ratios are expressed as moles of dLOS per mole of protein, and the estimated molecular weights were 3,000 for dLOS, 150,000 for TT, and 67,000 for CRM.
Based on the starting amount of the AH-dLOS preparation and the dLOS in the conjugates as measured by the phenol-sulfuric acid method.
Binding activities of conjugates were expressed as ELISA reactivity at A405 when the conjugates were used as coating antigens (10 μg/ml) and a rabbit immune serum was used as a binding antibody (1/16,000; 1/8,000). LOS (10 μg/ml) showed A405 values of 0.9 and 1.6 under the same conditions.
LOS antibodies in mice.
A mixture of dLOS, TT, CRM, and Ribi adjuvant did not elicit a significant increase in the levels of anti-LOS antibodies after three injections (Table 2). However, all three conjugates showed 95- to 162-fold increases in anti-LOS IgG after the third injection compared to the level after the first injection (162, 115, or 95 ELISA units versus 1 ELISA unit; P < 0.01). Formulation of the conjugates with Ribi adjuvant further enhanced their immunogenicities; there was an 11-fold increase in anti-LOS IgG after the third injection with dLOS-TT, and there were 16- to 32-fold increases with dLOS-CRM-1 and dLOS-CRM-2 (compared to three injections without Ribi adjuvant; P < 0.01). All conjugates elicited similar levels of anti-LOS IgG and low levels of anti-LOS IgM after three injections.
TABLE 2.
Murine antibody responses to M. catarrhalis serotype C LOS elicited by conjugates
| Immunogena | Injection no. | Geometric mean (range) (ELISA units)b
|
|
|---|---|---|---|
| IgG | IgM | ||
| dLOS-TT | 1 | 1 (1-2) | 4 (2-5) |
| 2 | 21 (2-267) | 6 (3-10) | |
| 3 | 162 (24-1,074)c | 15 (4-51) | |
| dLOS-CRM-1 | 1 | 1 | 3 |
| 2 | 19 (5-84) | 4 (2-8) | |
| 3 | 115 (65-202)c | 13 (6-29) | |
| dLOS-CRM-2 | 1 | 1 | 3 (3-5) |
| 2 | 8 (2-26) | 6 (1-28) | |
| 3 | 95 (16-572)c | 21 (6-68) | |
| dLOS-TT, Ribi | 1 | 2 (1-3) | 8 (5-11) |
| 2 | 491 (65-3,685) | 7 (3-18) | |
| 3 | 1,718 (171-17,212)c | 15 (2-98) | |
| dLOS-CRM-1, Ribi | 1 | 2 (1-5) | 6 (3-10) |
| 2 | 947 (88-10,253) | 18 (3-110) | |
| 3 | 1,887 (300-11,862)c | 14 (5-39) | |
| dLOS-CRM-2, Ribi | 1 | 4 (2-6) | 7 (4-11) |
| 2 | 2,558 (295-22,191) | 36 (10-132) | |
| 3 | 3,050 (1,244-7,479)c | 20 (12-33) | |
| dLOS, CRM, TT, Ribi | 1 | 1 (1-4) | 2 (1-3) |
| 2 | 2 (1-5) | 2 (1-5) | |
| 3 | 5 (4-7) | 2 (1-4) | |
Eight mice in each group were given a total of three subcutaneous injections at 3-week intervals consisting of 5 μg of conjugates (sugar content), conjugates with Ribi adjuvant, or a mixture of dLOS, TT, CRM, and Ribi adjuvant (5 μg of each). Blood samples were collected 2 weeks after each injection.
The number of ELISA units was based on the data obtained with a mouse reference serum against strain 26404, and the LOS from strain 26404 was used as a coating antigen.
P < 0.01 for a comparison with the corresponding group after one injection.
LOS antibodies in rabbits.
A mixture of dLOS, TT, CRM, and Ribi adjuvant elicited low levels of anti-LOS IgG antibodies after three injections (Table 3). However, all conjugates elicited significant increases in anti-LOS IgG in rabbits after three injections (the levels were 207- to 622-fold greater than the preimmune serum levels). Formulation of these conjugates with Ribi adjuvant enhanced their immunogenicities; there was a 107-fold increase in anti-LOS IgG after three injections of dLOS-TT, and there were 48- to 245-fold increases with dLOS-CRM compared with the level for each group without Ribi adjuvant. All conjugates elicited similar levels of anti-LOS IgG and low levels of anti-LOS IgM after three injections.
TABLE 3.
Rabbit antibody responses to M. catarrhalis serotype C LOS elicited by conjugates
| Immunogena | Injection no. | Geometric mean (ELISA units) (rabbit 1 value; rabbit 2 value)b
|
|
|---|---|---|---|
| IgG | IgM | ||
| dLOS-TT | 1 | 9 (9; 9) | 1 (1; 1) |
| 2 | 553 (81; 6,561) | 1 (1; 1) | |
| 3 | 553 (81; 6,561) | 8 (3; 27) | |
| dLOS-CRM-1 | 1 | 3 (3; 3) | 1 (1; 1) |
| 2 | 413 (243; 729) | 1 (1; 1) | |
| 3 | 1,243 (729; 2,187) | 8 (3; 27) | |
| dLOS-CRM-2 | 1 | 5 (3; 9) | 1 (1; 1) |
| 2 | 74 (27; 243) | 1 (1; 1) | |
| 3 | 413 (243; 729) | 8 (3; 27) | |
| dLOS-TT, Ribi | 1 | 24 (9; 81) | 3 (3; 3) |
| 2 | 33,690 (19,683; 59,049) | 15 (9; 27) | |
| 3 | 59,049 (59,049; 59,049) | 15 (9; 27) | |
| dLOS-CRM-1, Ribi | 1 | 12 (3; 81) | 2 (1; 3) |
| 2 | 33,690 (19,683; 59,049) | 9 (9; 9) | |
| 3 | 59,049 (59,049; 59,049) | 15 (27; 9) | |
| dLOS-CRM-2, Ribi | 1 | 5 (3; 9) | 5 (3; 9) |
| 2 | 33,690 (19,683; 59,049) | 5 (3; 9) | |
| 3 | 101,166 (59,049; 177,147) | 9 (9; 9) | |
| dLOS, TT, CRM, Ribi | 1 | 5 (3; 9) | 3 (3; 3) |
| 2 | 15 (27; 9) | 5 (3; 9) | |
| 3 | 15 (27; 9) | 5 (3; 9) | |
Two rabbits from each group were immunized subcutaneously three times at 4-week intervals with 50 μg of conjugates (sugar content), conjugates with Ribi adjuvant, or a mixture of dLOS (50 μg), TT (50 μg), CRM (50 μg), and Ribi adjuvant. Blood samples were collected 2 weeks after each injection.
The number of ELISA units was based on a rabbit reference serum against strain 26404, and the LOS from strain 26404 was used as a coating antigen. Preimmune serum gave 2 (1 to 5) or 1 (1 to 2) ELISA units of IgG or IgM.
Bactericidal activities of mouse and rabbit antisera.
All mouse antiserum elicited by three injections of the conjugates showed bactericidal activity against the homologous strain at titers ranging from 1:10 to 1:40 without Ribi adjuvant or at titers ranging from 1:10 to 1:80 with Ribi adjuvant. The levels of bactericidal activity were higher for the groups of the conjugates with Ribi adjuvant (P < 0.05 or P < 0.01) (Table 4). There was no correlation between the anti-LOS antibody levels and the bactericidal antibody levels in mouse sera (P > 0.05; n = 42).
TABLE 4.
Bactericidal activity against serotype C M. catarrhalis with mouse and rabbit antisera elicited by conjugates
| Immunogena | Geometric mean mouse bactericidal titer (range)b | Geometric mean rabbit bactericidal titer (rabbit 1 value; rabbit 2 value)b |
|---|---|---|
| dLOS-TT | 13 (9-18) | <5 |
| dLOS-CRM-1 | 14 (10-20) | <5 |
| dLOS-CRM-2 | 23 (17-32) | <5 |
| dLOS-TT, Ribi | 23 (14-37)c | 37 (20; 80) |
| dLOS-CRM-1, Ribi | 29 (16-56)d | 40 (40; 40) |
| dLOS-CRM-2, Ribi | 51 (36-73)d | 28 (20; 40) |
| dLOS, TT, CRM, Ribi | <5 | <5 |
See Table 2, footnote a, for mice and Table 3, footnote a, for rabbits. Test antisera were collected after three injections.
The reciprocal of the highest serum dilution causing >50% killing was expressed as the bactericidal titer.
P < 0.05 for a comparison with the corresponding group without Ribi adjuvant.
P < 0.01 for a comparison with the corresponding group without Ribi adjuvant.
In the rabbit model, only the antiserum elicited by the conjugate plus Ribi adjuvant showed bactericidal activity at titers ranging from 1:20 to 1:80 (Table 4). There were medium correlations between anti-LOS antibody levels and bactericidal titers for 14 rabbits (for IgG, r = 0.655 and P = 0.0109; for IgM, r = 0.563 and P = 0.0052).
The bactericidal activity of a rabbit antiserum elicited by dLOS-TT plus Ribi adjuvant was further detected with eight known serotype strains and 22 clinical isolates. Besides homologous strain 26404, the rabbit antiserum exhibited bactericidal activities against 13 of the 17 testable strains, including two serotype A strains, two serotype B strains, and 9 clinical isolates from the ATCC, United States, and Japan, and the titers ranged from 1:10 to 1:320 (Table 5). Strains 8176, 8193, 23246, 25240, 43167, 43618, 43628, M2, M5, and M8 were complement serum sensitive, and complement serum itself killed the strains without the presence of the antiserum.
TABLE 5.
Cross-bactericidal activity of a representative rabbit serum elicited by serotype C dLOS-TT conjugate
| Straina | Serotype | Bactericidal titerb |
|---|---|---|
| 25238 | A | 1:160 |
| 26395 | A | 1:320 |
| 26394 | A | CKc |
| 3292 | B | 1:20 |
| 26397 | B | <1:5 |
| 26400 | B | 1:80 |
| 26391 | C | CK |
| 26404 | C (homologous) | 1:80 |
| O35E | A/C′d | 1:40 |
| TTA24 | B′d | 1:20 |
| 25239 | B′ | <1:5 |
| 43627 | B′ | 1:20 |
| 49143 | B′ | 1:10 |
| M1 | A/C′ | 1:80 |
| M3 | A/C′ | 1:80 |
| M4 | B′ | <1:5 |
| M6 | A/C′ | 1:80 |
| M7 | B′ | 1:10 |
| M9 | A/C′ | 1:320 |
| M10 | B′ | <1:5 |
The concentration of each strain was adjusted to 6 × 103 CFU/ml for the bactericidal assay.
See Table 4, footnote b.
CK, complement itself killed the strains without the presence of antiserum.
Assigned serotypes were based on binding activities with monoclonal antibody 8E7 against serotype A/C LOS (18) and a rabbit serum against serotype B LOS (43) as determined by whole-cell ELISA and Western blotting. Strains that bound to only monoclonal antibody 8E7 or showed strong binding activity to monoclonal antibody 8E7 were assigned to serotype A/C. In contrast, strains that bound only to the rabbit serum against serotype B or showed strong binding activity to the rabbit serum were assigned to serotype B.
Inhibition of bactericidal activities on the rabbit antiserum by various LOSs.
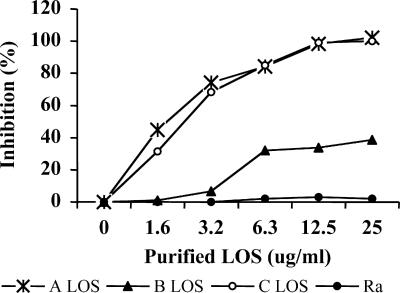
To determine the specificity of the bactericidal activity of the rabbit antiserum, inhibition with LOSs from homologous strain 26404 (serotype C) and strains 25238 (serotype A) and 26397 (serotype B) was examined, as was inhibition with a LOS from an unrelated S. enterica serovar Minnesota strain (Ra) (Fig. 3). The bactericidal activity was 100% inhibited by the homologous LOS and the serotype A LOS and 30% inhibited by the serotype B LOS but was not inhibited by the S. enterica serovar Minnesota LOS at a concentration of 12.5 μg/ml.
FIG. 3.
Inhibition of bactericidal activity of a rabbit antiserum elicited by serotype C dLOS-TT conjugate with M. catarrhalis LOSs, serotypes A (25238), B (26397), and C (26404), and S. enterica serovar Minnesota LPS (Ra). Each inhibition value was calculated as follows: (CFU from serum with LOS inhibitor − CFU from serum without inhibitor)/(CFU from complement only − CFU from serum without inhibitor) × 100.
DISCUSSION
We successfully chemically detoxified serotype C LOS from M. catarrhalis strain 26404. The resulting dLOS had at least an 80,000-fold-lower toxicity than the original LOS as determined by an LAL assay, which makes it clinically acceptable (42). We also conjugated the dLOS with the protein carrier TT or CRM at different ratios to form three conjugates. All the conjugates showed similar antigen binding activities in ELISA and elicited high levels of serum anti-LOS IgG in both mouse and rabbit models. The immunogenicity of the conjugates was better in rabbits than in mice, especially with an addition of Ribi adjuvant. These data are for the most part consistent with our previous studies with M. catarrhalis serotype A and B, meningococcal, and nontypeable Haemophilus influenzae LOS conjugate vaccines (11, 12, 13, 43). We believe that rabbits may respond better to the LOS-based conjugates, as well as Ribi adjuvant, than mice. Further comparison studies using different LOS-based conjugates and dose-dependent patterns are necessary to confirm this in both models. In addition, the two conjugates with different ratios of dLOS to CRM resulted in similar immunogenicities in both animal models.
To further investigate the protective capacity of the animal antiserum elicited by the conjugates, a complement-mediated bactericidal assay was performed. The bactericidal assay revealed that 100% of the BALB/c mouse antiserum elicited by the serotype C conjugates with or without Ribi adjuvant showed bactericidal activity against the homologous strain. However, in the case of serotype B, only 50 to 62.5% of the BALB/c mouse antiserum elicited by conjugates with Ribi adjuvant appeared to have bactericidal activity (43). We previously reported that 45% of outbred mouse antiserum (11) and all BALB/c mouse antiserum elicited by serotype A conjugates with various protein carriers showed bactericidal activity (19). It is not clear if the difference is due to different LOS serotypes, protein carriers, or mouse strains or the assays themselves. Based on the similar conjugates, protein carriers, mouse strains, and bactericidal assays used for serotype C and B conjugates, we believe that the high rate of bactericidal activity with the serotype C conjugate in mice is due to distinct chemical structures of the serotype C and B LOSs. In addition, we found that there appeared to be a poor correlation between the binding IgG antibody detected and the homologous bactericidal titers observed, indicating that the conjugates might induce nonbactericidal antibodies in mice.
In the rabbit model, all antiserum elicited by serotype C conjugate plus Ribi adjuvant showed bactericidal activity against the homologous strain, and the bactericidal activity correlated with both anti-LOS IgG and IgM antibody levels (P < 0.05). The data were consistent with serotype A and B conjugate data. However, unlike the serotype A and B conjugates, the serotype C conjugates alone did not generate any detectable bactericidal activity. A representative rabbit antiserum showed cross-bactericidal activity against five of six known serotype strains and 9 of 12 testable clinical and ATCC strains without complement self-killing (Table 5). This is similar to the results obtained for serotype A conjugates (9 of 10 conjugates) (11) and serotype B conjugates (9 of 12 conjugates) (43) which have different specificities. Interestingly, the representative rabbit antiserum showed higher bactericidal titers to serotype A strains 25238 and 26395 than to the homologous serotype C strain. Our explanations for this include the following observation: (i) there is a high level of cross-reaction between serotype A and C LOSs (strains); (ii) the quantities of LOS or LOS epitopes expressed on the surface of the strains can be different regardless of the serotype; and (iii) there may be different interruptions of other outer membrane components than the LOS molecule in different strains. A bactericidal inhibition study further suggested that the bactericidal activity of the rabbit antiserum elicited by the serotype C conjugate was specific to both serotype C and A LOSs, while there were some cross-reactions with serotype B LOS. The structural similarity among the three serotypes may be the reason for such cross-reactivity, which presents a common oligosaccharide inner core and the terminal tetrasaccharide α-d-Galp-(1→4)-β-d-Galp-(1→4)-α-d-Glcp-(1→2)-β-d-Glcp-(1→ at the branch substituting position 6 of the trisubstituted Glc residue (16). The differences in the three serotype LOSs are mainly limited to the branch substituting position 4 of the trisubstituted Glc residue; serotype C or A LOS contains α-d-GlcpNAc, while serotype B contains α-d-Glcp in its place (16). Previous studies showed significant cross-reactivity between serotypes, especially between serotypes A and C (39), while the predominant antibody response to the LOS of M. catarrhalis was the response to the serotype-specific epitopes (29). It is not known which specific protective anti-LOS antibody is generated from which epitope(s) at which chain branch of the LOS moiety. We plan to elucidate the potential epitopes by creating knockout LOS mutants.
In conclusion, novel serotype C LOS conjugate vaccines were developed along with biological and immunological functions. We suggest that a future conjugate vaccine for M. catarrhalis should contain two LOS components, serotypes A and B or serotypes B and C, in order to cover most disease strains. To test this, a series of studies of the cross-LOS antibody response, the cross-bactericidal activity, and the cross-active protection in animal models are planned with the two combination conjugates as candidate vaccines for human use.
Acknowledgments
We are grateful to E. J. Hansen and G. Mogi for providing clinical isolates and to J. C. McMichael (retired from Wyeth Vaccines, New York) for supplying strains and some LOS material.
This research was supported by the Intramural Research Program of the NIH, NIDCD.
Editor: D. L. Burns
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Adlowitz, D. G., T. Hiltke, A. J. Lesse, and T. F. Murphy. 2004. Identification and characterization of outer membrane proteins G1a and G1b of Moraxella catarrhalis. Vaccine 22:2533-2540. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, K. E., M. P. Haggard, P. A. Silva, and I. A. Stewart. 2001. Behaviour and developmental effects of otitis media with effusion into the teens. Arch. Dis. Child. 85:91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catlin, B. W. 1990. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin. Microbiol. Rev. 3:293-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cripps, A. W., and D. C. Otczyk. 2006. Prospects for a vaccine against otitis media. Expert Rev. Vaccines 5:517-534. [DOI] [PubMed] [Google Scholar]
- 5.Daoud, A., F. Abuekteish, and H. Masaadeh. 1996. Neonatal meningitis due to Moraxella catarrhalis and review of the literature. Ann. Trop. Paediatr. 16:199-201. [DOI] [PubMed] [Google Scholar]
- 6.Doern, G. V. 1986. Branhamella catarrhalis—an emerging human pathogen. Diagn. Microbiol. Infect. Dis. 4:191-201. [DOI] [PubMed] [Google Scholar]
- 7.Dubois, M., H. Gillis, J. K. Hamilton, A. A. Rebers, and R. Smith. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Biochem. 28:250-256. [Google Scholar]
- 8.Easton D. M., A. Smith, S. G. Gallego, A. R. Foxwell, A. W. Cripps, and J. M. Kyd. 2005. Characterization of a novel porin protein from Moraxella catarrhalis and identification of an immunodominant surface loop. J. Bacteriol. 187:6528-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faden, H., J. Hong, and T. F. Murphy. 1992. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect. Immune. 60:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsgren, A., M. Brant, M. Karamehmedovic, and K. Riesbeck. 2003. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect. Immun. 71:3302-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu, X.-X., J. Chen, S. J. Barenkamp, J. B. Robbins, C.-M. Tsai, D. J. Lim, and J. Battery. 1998. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect. Immun. 66:1891-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu, X.-X., and C.-M. Tsai. 1993. Preparation, characterization, and immunogenicity of meningococcal lipooligosaccharide-derived oligosaccharide-protein conjugate. Infect. Immun. 61:1873-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu, X.-X., C.-M. Tsai, T. Ueyama, S. J. Barenkamp, J. B. Robbins, and D. J. Lim. 1996. Synthesis, characterization, and immunological properties of detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins. Infect. Immun. 64:4047-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hager, H., A. Verghese, S. Alvarez, and S. L. Berk. 1987. Branhamella catarrhalis respiratory infections. Rev. Infect. Dis. 9:1140-1149. [DOI] [PubMed] [Google Scholar]
- 15.Hoi-Dang, A. B., B. C. Brive-Le, M. Barthelemy, and R. Labia. 1978. Novel beta-lactamase from Branbamella catarrhalis. Ann. Microbiol. (Paris) 129B:397-406. [PubMed] [Google Scholar]
- 16.Holme, T., M. Rahman, P. E. Jansson, and G. Widmalm. 1999. The lipopolysaccharide of Moraxella catarrhalis structural relationships and antigenic properties. Eur. J. Biochem. 265:524-529. [DOI] [PubMed] [Google Scholar]
- 17.Hu, W.-G., J. Chen, J. F. Battey, and X.-X. Gu. 2000. Enhancement of clearance of bacteria from murine lungs by immunization with detoxified lipooligosaccharide from Moraxella catarrhalis conjugated to proteins. Infect. Immun. 68:4980-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu, W.-G., J. Chen, J. C. Mc Michael, and X.-X. Gu. 2001. Functional characteristics of a protective monoclonal antibody against serotype A and C lipooligosaccharides from Moraxella catarrhalis. Infect. Immun. 69:1358-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, W.-G., J. Berry, J. Chen, and X.-X. Gu. 2004. Exploration of Moraxella catarrhalis outer membrane proteins, CD and UspA, as new carriers for lipooligosaccharide-based conjugates. FEMS Immunol. Med. Mic. 41:109-115. [DOI] [PubMed] [Google Scholar]
- 20.Jiao, X., H. Takashi, Y. Hou, and X.-X. Gu. 2002. Specific immune responses and enhancement of murine pulmonary clearance of Moraxella catarrhalis by intranasal immunization with a detoxified lipooligosaccharide conjugate vaccine. Infect. Immun. 70:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karalus, R., and A. Campagnari. 2000. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2:547-559. [DOI] [PubMed] [Google Scholar]
- 22.Klein, J. O. 1994. Current issues in upper respiratory tract infections in infants and children: rationale for antibacterial therapy. Pediatr. Infect. Dis. J. 13:S5-S9. (Discussion, 13:S20-S22.) [DOI] [PubMed] [Google Scholar]
- 23.Klein, J. O. 1994. Otitis media. Clin. Infect. Dis. 19:823-833. [DOI] [PubMed] [Google Scholar]
- 24.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 19:S101-S107. [DOI] [PubMed] [Google Scholar]
- 25.McMichael, J. C., and B. A. Green. 2003. Vaccines for Moraxella catarrhalis and non-typeable Haemophilus influenzae. Curr. Opin. Investig. Drugs 4:953-958. [PubMed] [Google Scholar]
- 26.Murphy, T. F. 1996. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol. Rev. 60:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy, T. F. 2005. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Rev. Vaccines 4:843-853. [DOI] [PubMed] [Google Scholar]
- 28.Peng, D., B. P. Choudhury, R. S. Petralia, R. W. Carlson, and X.-X. Gu. 2005. Roles of 3-deoxy-d-manno-2-octulosonic acid transferase from Moraxella catarrhalis in lipooligosaccharide biosynthesis and virulence. Infect. Immun. 73:4222-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman, M., and T. Holme. 1996. Antibody response in rabbits to serotype-specific determinants in lipopolysaccharides from Moraxella catarrhalis. J. Med. Microbiol. 44:348-354. [DOI] [PubMed] [Google Scholar]
- 30.Rahman, M., T. Holme, I. Jonsson, and A. Krook. 1995. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 14:297-304. [DOI] [PubMed] [Google Scholar]
- 31.Ruuskanen, O., and T. Heikkinen. 1994. Otitis media: etiology and diagnosis. Pediatr. Infect. Dis. J. 13:S23-S26. [PubMed] [Google Scholar]
- 32.Shiloach, J., and J. B. Kaufman. 1999. The combined use of expanded bed volume adsorption and gradient elution for capture and partial purification of mutant diphtheria toxin (CRM9) from Corynebacterium diphtheriae. Sep. Sci. Technol. 34:29-40. [Google Scholar]
- 33.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka, H., K. Oishi, F. Sonoda, A. Iwagaki, T. Nagatake, and K. Matsumoto. 1992. Biochemical analysis of lipopolysaccharides from respiratory pathogenic Branhamella catarrhalis strains and the role of anti-LPS antibodies in Branhamella respiratory infections. J. Jpn. Assoc. Infect. Dis. 66:709-715. [DOI] [PubMed] [Google Scholar]
- 35.Teele, D. W., J. O. Klein, and B. Rosner. 1989. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 160:83-94. [DOI] [PubMed] [Google Scholar]
- 36.Timpe, J. M., M. M. Holm, S. L. Vanlerberg, V. Basrur, and E. R. Lafontaine. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect. Immun. 71:4341-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 38.Unhanand, M., I. Maciver, O. Ramilo, O. Arencibia-Mireles, J. C. Argyle, G. H. McCracken, and E. J. Hansen. 1992. Pulmonary clearance of Moraxella catarrhalis in an animal model. J. Infect. Dis. 165:644-650. [DOI] [PubMed] [Google Scholar]
- 39.Vaneechoutte, M., G. Verschraegen, G. Claeys, and A. M. van den Abeele. 1990. Serological typing of Branhamella catarrhalis strains on the basis of lipooligosaccharide antigens. J. Clin. Microbiol. 28:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verduin C. M., C. Hol, A. Fleer, H. van Dijk, and A. van Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warburg, O., and W. Christian. 1942. Isolation and crystallization of enolase. Biochem. Z. 310:384-421. [Google Scholar]
- 42.W.H.O. Expert Committee on Biological Standardization. 1991. Requirements for Haemophilus type b conjugate vaccines. WHO Tech. Rep. Ser. 814:15-37. [Google Scholar]
- 43.Yu, S., and X.-X. Gu. 2005. Synthesis and characterization of lipooligosaccharide-based conjugate vaccines for serotype B Moraxella catarrhalis. Infect. Immun. 73:2790-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]