Abstract
The Neisseria gonorrhoeae pglA gene has two alleles, one of which is phase variable. A previous study reported that all disseminated gonococcal infection (DGI) isolates contained the phase-variable allele and proposed a causal link. In the present study of 81 strains no absolute correlation between DGI and the phase-variable pglA allele was observed.
Neisseria gonorrhoeae infections result in a spectrum of disease from asymptomatic carriage and mucosal gonococcal infection (MI) to disseminated gonococcal infection (DGI). Pili are a major virulence factor of N. gonorrhoeae, facilitating adhesion to the epithelial surface. Pili are posttranslationally modified by glycosylation, and a number of genes involved in the glycosylation of pili have been identified (5, 8, 9, 16, 17). pglA was the first of these genes to be identified (8) and is responsible for the transfer of the first galactose to the trideoxyhexose sugar in the pilin-linked Gal(β1-4) Gal(α1-3) 2,4-diacetamido-2,4,6-trideoxyhexose in N. meningitidis (see Fig. 1B). Subsequent work by Banerjee et al. (5) reported on the equivalent gene in N. gonorrhoeae (pgtA [96% identical at the nucleotide level]) (8). Hence, pgtA is referred to as pglA.
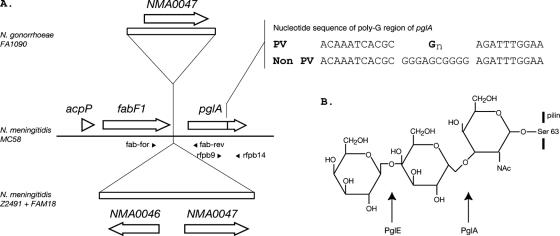
FIG. 1.
Genetic map of pglA region in Neisseria and structure of posttranslational modification of N. gonorrhoeae pilin. (A) Genetic map of pglA region in various Neisseria strains (as indicated on left). Open reading frames are represented by unfilled arrows and are named according to the N. meningitidis strain Z2491 genome annotation. The key region defining the phase-variable and non-phase-variable alleles of pglA is indicated in the top right of the figure. GN indicates that a different number of G residues may be observed in this position in different strains (range, 11 to 17). The positions of the primers are indicated by small black triangles, and their names are adjacent. (B) The predicted structure of the pilin-linked glycan from N. gonorrhoeae with both PglE and PglA expressed. Arrows indicate the linkages created by PglE and PglA.
Jennings et al. (8) and Banerjee et al. (5) have reported that the expression of pglA is phase variable. Phase variation is the reversible high-frequency on-off switching of the expression of a gene and is commonly associated with virulence factors in a number of host-adapted human pathogens (reviewed in reference 11). The N. gonorrhoeae pglA gene has two distinct alleles: one containing a homopolymeric tract that mediates phase variation (random on/off switching of expression) and another lacking the tract. The phase variation of pglA is mediated by a tract of G residues within the pglA coding sequence (see Fig. 1A). Variation in the number of G residues can result in a frameshift mutation and a nonfunctional pglA gene product. An analysis of the pglA gene in a number of strains conducted by Jennings et al. (8) revealed a wide variation in the number of G residues in the homopolymeric tract in pathogenic Neisseria.
Banerjee et al. (5) analyzed 52 strains of N. gonorrhoeae for the presence of this homopolymeric tract. All of the 24 strains from patients with DGI contained the pglA allele with the repeat tract. In contrast, only 8 of the 28 MI strains contained the pglA allele with the homopolymeric tract. This was an exciting observation since it identified the first example of a virulence factor where the potential for phase-variable expression was invariably associated with progression to a distinct pathology, and this has been subsequently cited in the literature (4, 7, 18). The reasons why some cases of gonorrhea progress from uncomplicated mucosal disease to DGI is not well characterized. Several prior studies (6, 12, 13) of the differences between MI and DGI strains have found no single factor that was predictive of whether an N. gonorrhoeae strain was a potential DGI strain (such as arginine, hypoxanthine, and uracil auxotrophy and the gonococcal pathogenicity island). Banerjee et al. (5) suggested that the phase variation of pglA is likely to be involved in the conversion of MI to DGI because of its absolute correlation of the phase-variable pglA allele with DGI.
In the present study, the observations made by Banerjee et al. (5) were tested in a larger, more diverse strain collection and are extended to examine the relationship between other phase-variable genes involved in the posttranslational modification of pili and the distinct pathologies of MI and DGI. The strain collection was derived from isolates referred from public and private sectors in two geographically distinct Australian jurisdictions, New South Wales and the Northern Territory, between 1988 and 2002. The sampling methods used were those of the Australian Gonococcal Surveillance Programme as detailed in annual reports (2, 3). Some cases were identified as having presented in Australia after being contracted overseas (i.e., in the Middle East, United Kingdom, Thailand, and Fiji). Strains were classified as being from DGI if they were isolated from blood or joint cultures or as MI if they were isolated from anogenital sites or the eye. The details of the strains are recorded in Table S1 in the supplemental material and include data on the isolation site and antibiotic resistances. In addition, an effort was made to exclude strains that were presumptively “clonal.” The criteria for exclusion included geographic, temporal, and antibiotic resistance clustering. The repeat region of the pglA gene in each strain was sequenced as previously described (as described in reference 8). Phase-variable and non-phase-variable alleles of pglA were defined as shown in Fig. 1A. Similar to the study of Banerjee et al., non-phase-variable alleles were defined as having the sequence 5′-GGGAGCGGG-3′ in place of the homopolymeric tract.
We observed no absolute correlation between the phase-variable pglA allele and DGI; only 37% of DGI strains examined in the present study had the PV tract in pglA (Table 1). Among the MI strains, 18% had a phase-variable allele. We conclude that there is no absolute correlation between the presence of the phase-variable allele and either pathology.
TABLE 1.
Strain survey of pglA allele from DGI and MI strains
| Strain | No. (%) of strains with pglA allele type present:
|
|
|---|---|---|
| Phase-variable | Non phase-variable | |
| DGI | 15 (37) | 26 (63) |
| MI | 7 (18) | 33 (82) |
Two characterized pglA-linked polymorphisms were described by Power and Jennings (15) (see Fig. 1A). There are up to two additional genes inserted between pglA and fabF (8), referred to by their N. meningitidis Z2491 annotation: NMA0047, a member of the PFAM sugar transporter family (10) (PFAM PF00083), and NMA0046, a member of a family of bile acid/Na+ symporter family (PFAM PF01758). The primers Fab-for (5′-ATGAGTCAGAGAAGAGTAG-3′) and Fab-rev (5′-TCAGCCTTTGAAGCGTTT-3′) were used to PCR amplify the region in which the polymorphisms had been identified. The size of the products was used to determine which allele was present. The results revealed that NMA0046 was present in all N. gonorrhoeae strains studied. The alternative genetic arrangements found in N. meningitidis MC58 (19) (no genes) and in N. meningitidis Z2491 (14) (two genes) were not found in any N. gonorrhoeae strains.
Two other phase-variable genes associated with pilin-linked posttranslational modifications were also examined to look for a correlation with MI or DGI. pglE is responsible for the addition of the terminal galactose in the N. meningitidis pilin-linked trisaccharide (17). pptA, is involved in the addition of ChoP to pilin in N. meningitidis and a similar role has been suggested for pptA of N. gonorrhoeae (1, 20). The potential for phase variation, as defined by the presence or absence or polymorphism in the size of the repeat tracts of pglA or pptA, did not correlate with either pathology (see Table S1 in the supplemental material).
In the present study we demonstrate that there is no absolute correlation between the potential of the pilin glycosylation gene pglA to phase vary and DGI. We assume that the correlation reported by Banerjee et al. (5) was due to the use of a clonal strain collection and indicates the importance of accessing a large, diverse, and well-characterized bacterial strain collection in undertaking such studies.
Supplementary Material
Acknowledgments
This study was supported by program grant 284214 from the National Health and Medical Research Council of Australia.
We acknowledge the Australian National Neisseria Network, who provided access to the strain collection used in this study.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 12 February 2007.
Supplemental material for this article may be found at http://iai.asm.org./.
REFERENCES
- 1.Aas, F. E., W. Egge-Jacobsen, H. C. Winther-Larsen, C. Lovold, P. G. Hitchen, A. Dell, and M. Koomey. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 281:27712-27723. [DOI] [PubMed] [Google Scholar]
- 2.Australian Gonococcal Surveillance Programme. 2005. Annual report of the Australian Gonococcal Surveillance Programme. Commun. Dis. Intell. 29:137-142. [PubMed] [Google Scholar]
- 3.Australian Gonococcal Surveillance Programme. 1984. Penicillin sensitivity of gonococci in Australia: development of the Australian Gonococcal Surveillance Programme. Br. J. Vener. Dis. 60:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee, A., and S. K. Ghosh. 2003. The role of pilin glycan in neisserial pathogenesis. Mol. Cell Biochem. 253:179-190. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee, A., R. Wang, S. L. Supernavage, S. K. Ghosh, J. Parker, N. F. Ganesh, P. G. Wang, S. Gulati, and P. A. Rice. 2002. Implications of phase variation of a gene (pgtA) encoding a pilin galactosyl transferase in gonococcal pathogenesis. J. Exp. Med. 196:147-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263-277. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, S. K., J. Zhao, M. C. Philogene, A. Alzaharani, S. Rane, and A. Banerjee. 2004. Pathogenic consequences of Neisseria gonorrhoeae pilin glycan variation. Microbes Infect. 6:693-701. [DOI] [PubMed] [Google Scholar]
- 8.Jennings, M. P., M. Virji, D. Evans, V. Foster, Y. N. Srikhanta, L. Steeghs, P. van der Ley, and E. R. Moxon. 1998. Identification of a novel gene involved in pilin glycosylation in Neisseria meningitidis. Mol. Microbiol. 29:975-984. [DOI] [PubMed] [Google Scholar]
- 9.Kahler, C. M., L. E. Martin, Y. L. Tzeng, Y. K. Miller, K. Sharkey, D. S. Stephens, and J. K. Davies. 2001. Polymorphisms in pilin glycosylation locus of Neisseria meningitidis expressing class II pili. Infect. Immun. 69:3597-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, E. H., and W. M. Shafer. 1999. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33:839-845. [DOI] [PubMed] [Google Scholar]
- 11.Moxon, E. R., R. E. Lenski, and P. B. Rainey. 1998. Adaptive evolution of highly mutable loci in pathogenic bacteria. Perspect. Biol. Med. 42:154-155. [DOI] [PubMed] [Google Scholar]
- 12.Noble, R. C., R. R. Reyes, M. C. Parekh, and J. V. Haley. 1984. Incidence of disseminated gonococcal infection correlated with the presence of AHU auxotype of Neisseria gonorrhoeae in a community. Sex Transm. Dis. 11:68-71. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien, J. P., D. L. Goldenberg, and P. A. Rice. 1983. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine 62:395-406. [PubMed] [Google Scholar]
- 14.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 15.Power, P. M., and M. P. Jennings. 2003. The genetics of glycosylation in gram-negative bacteria. FEMS Microbiol. Lett. 218:211-222. [DOI] [PubMed] [Google Scholar]
- 16.Power, P. M., L. F. Roddam, M. Dieckelmann, Y. N. Srikhanta, Y. C. Tan, A. W. Berrington, and M. P. Jennings. 2000. Genetic characterization of pilin glycosylation in Neisseria meningitidis. Microbiology 146:967-979. [DOI] [PubMed] [Google Scholar]
- 17.Power, P. M., L. F. Roddam, K. Rutter, S. Z. Fitzpatrick, Y. N. Srikhanta, and M. P. Jennings. 2003. Genetic characterization of pilin glycosylation and phase variation in Neisseria meningitidis. Mol. Microbiol. 49:833-847. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt, M. A., L. W. Riley, and I. Benz. 2003. Sweet new world: glycoproteins in bacterial pathogens. Trends Microbiol. 11:554-561. [DOI] [PubMed] [Google Scholar]
- 19.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 20.Warren, M. J., and M. P. Jennings. 2003. Identification and characterization of pptA: a gene involved in the phase-variable expression of phosphorylcholine on pili of Neisseria meningitidis. Infect. Immun. 71:6892-6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.