Abstract
Leishmania spp. are obligate intracellular parasites, requiring a suitable microenvironment for their growth within host cells. We previously reported that the growth of Leishmania amazonensis amastigotes in murine macrophages (Mφs) was enhanced in the presence of gamma interferon (IFN-γ), a Th1 cytokine normally associated with classical Mφ activation and killing of intracellular pathogens. In this study, we provided several lines of evidence suggesting that IFN-γ-mediated parasite growth enhancement was associated with l-arginine transport via mouse cationic amino acid transporter 2B (mCAT-2B). (i) mRNA expression of Slc7A2, the gene encoding for mCAT-2B, as well as l-arginine transport was increased in IFN-γ-treated Mφs. (ii) Supplementation of l-arginine in Mφ cultures increased parasite growth. (iii) Parasite growth enhancement in wild-type Mφs was inhibited in the presence of nonmetabolized l-arginine analogues. (iv) IFN-γ-mediated parasite growth was absent in Mφs derived from mCAT-2B-deficient mice. Although we detected a clear upregulation of mCAT-2B and l-arginine transport, no measurable iNOS or arginase activities were observed in IFN-γ-treated, infected Mφs. Together, these data suggest an involvement of a novel l-arginine usage independent of iNOS and arginase activities during IFN-γ-mediated parasite growth enhancement. A possible role of mCAT-2B in supplying l-arginine directly to the parasites for their proliferation is discussed.
Protozoan parasites in the genus Leishmania are obligate intracellular pathogens that can cause a wide spectrum of diseases, ranging from benign cutaneous lesions to life-threatening visceral infections. Leishmania spp. exhibit a dimorphic life cycle, in which parasites are transmitted by a sand fly vector as flagellated promastigotes and reside within mammalian macrophages (Mφs) as amastigotes. The fate of intracellular amastigotes depends largely on the activation status of Mφs, which is greatly influenced by various cytokines and chemokines (19, 34, 35). In general, Th1 cytokines (e.g., gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α]) induce classical Mφ activation, leading to an upregulation of inducible NO synthase (iNOS) and production of leishmaniacidal nitric oxide (NO) (24, 32). Th2 cytokines (e.g., interleukin-4 [IL-4] and IL-10), on the other hand, induce arginase activation (19), which is involved in the production of polyamines needed for parasite proliferation (17).
Due to the critical role of iNOS in inducing parasite killing and of arginase in promoting parasite proliferation, it is not surprising that the activities of iNOS and arginase are tightly regulated, displaying counter-regulatory mechanisms on one another. For example, Nω-hydroxy-l-arginine (LOHA), an intermediate product in the iNOS pathway, is known to strongly inhibit arginase function (16). Conversely, arginase can reduce iNOS activity by competitively utilizing l-arginine, a common substrate for both the iNOS and the arginase pathways (13). The activities of iNOS and arginase are also dependent on l-arginine availability. It has been shown that l-arginine from the extracellular milieu is required for iNOS-dependent NO synthesis (31). A similar association of l-arginine transport was also documented for arginase activity, as suggested by a significant reduction of l-arginine conversion to l-ornithine and polyamines in Mφs lacking an l-arginine transporter (39). These studies suggest that l-arginine transport from the extracellular milieu is important in providing factors needed for parasite growth and killing within the host cells.
Several l-arginine transport systems, including systems y+, Bo+, bo+, and y+L, have been documented (36). The basal level of l-arginine is maintained primarily through the y+L system in nonactivated Mφs, while transport through the y+ system increases significantly in activated Mφs (39). The cationic amino acid transporter family (CAT) belongs to the y+ system transporter family that includes four members, CAT-1 to CAT-4, encoded by the Slc7A1 to -4 genes, respectively (36). While the first three members (CAT-1, -2, and -3) transport cationic amino acids such as l-arginine, the function of CAT-4 is unclear (36). CAT-1 is widely distributed in various tissues except in adult livers (20), while CAT-3 is expressed only in the brain (36). CAT-2 is expressed in two splice variants, with CAT-2A being constitutively expressed in the liver and muscle cells (5) and CAT-2B being induced by cytokines in many cell types, including Mφs (25). The expression of CAT-2B in Mφs is of particular interest in Leishmania research due to its ability to provide substrates for efficient iNOS and arginase activities, during Mφ classical activation (via IFN-γ + lipopolysaccharide [LPS]) and alternative activation (via IL-4 + IL-10), respectively (39). Despite this crucial role of CAT-2B in the regulation of iNOS and arginase activities (25), there is limited knowledge on how this transporter may directly influence the outcome of Leishmania infection.
Although it is important to examine l-arginine transport of Leishmania-infected cells, it is also crucial to understand the pathways by which l-arginine is utilized. Beside l-arginine usage by iNOS in classically activated Mφs (15, 23) and by arginase in alternatively activated Mφs (17), it has been reported that in vitro cultured Leishmania parasites can also directly acquire and utilize l-arginine from the extracellular milieu for polyamine synthesis (18, 30). Although the exact pathways by which Leishmania can access the l-arginine pool within the host Mφs remain unclear, it is possible that cellular l-arginine transport may be directed toward the parasites residing within the cells.
In our previous study, we reported that, instead of facilitating parasite killing, IFN-γ treatment actually increases the number of L. amazonensis amastigotes in murine Mφs, independently of IL-10 and transforming growth factor β (27). Since the understanding of mechanisms underlying IFN-γ-mediated parasite growth enhancement may provide new insights into treatment and vaccine strategies against New World cutaneous leishmaniasis, we sought to examine the potential mechanisms for this phenomenon with a focus on l-arginine transport and l-arginine usage. In the present study, we found that infection with L. amazonensis amastigotes in the presence of IFN-γ led to an upregulation of the Slc7A2 gene in infected Mφs. Moreover, l-arginine availability appeared to be a key regulator of IFN-γ-mediated amastigote growth enhancement, as evidenced by reduced parasite burdens in the presence of the nonmetabolized l-arginine analogues l-NMMA and l-SDMA. Using Mφs from CAT-2 knockout (KO) mice, we further demonstrated a requirement of mouse CAT-2B (mCAT-2B) in IFN-γ-mediated parasite growth enhancement. Together, our data strongly suggest the involvement of mCAT-2B and l-arginine availability in IFN-γ-mediated parasite growth enhancement. Since IFN-γ-treated, L. amazonensis-infected Mφs lack detectable activities for iNOS or arginase, we proposed that IFN-γ-mediated parasite growth enhancement was likely due to a novel l-arginine metabolic pathway independent of iNOS or arginase. The possibility of direct l-arginine usage by the parasites is discussed.
MATERIALS AND METHODS
Mice.
Female BALB/c and C57BL/6 mice (Harlan, Indianapolis, IN), as well as CAT-2 KO mice (6 weeks to 6 months old) on the C57BL/6 background (25, 39), were used in the present study. All mice were maintained under specific-pathogen-free conditions. All protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, TX).
Reagents.
Recombinant mouse IFN-γ and IL-4 were purchased from Leinco Technologies (St. Louis, MO). LPS from Salmonella enterica serovar Typhimurium, NG-methyl-l-arginine (l-NMMA), Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), and l-arginine were purchased from Sigma. NG,NG′-dimethyl-l-arginine dihydrochloride (l-SDMA) was purchased from Calbiochem (San Diego, CA).
Parasites.
L. amazonensis (RAT/BA/74/LV78) clone 12-1 (a gift from Kwang-Poo Chang, Department of Microbiology and Immunology, Chicago Medical School) was maintained as axenic amastigotes in complete Grace's insect cell culture medium (Invitrogen/Gibco, Carlsbad, CA), supplemented with 20% fetal bovine serum (pH 5.0; HyClone, Logan, UT) at 33°C (10). Lesion-derived amastigotes of L. amazonensis (MHOM/BR/77/LTB0016) obtained from foot tissues of infected BALB/c mice were cultured in Schneider's Drosophila media (Invitrogen/Gibco) supplemented with 20% fetal bovine serum (pH 5.0) at 33°C for 2 days prior to use (27). Lesion-derived amastigotes remained in the amastigote form under these culture conditions. L. mexicana (TG) axenic amastigotes were obtained from a patient in 2002 (37) and were maintained as the amastigote form in Schneider's Drosophila media (pH 5.0) at 33°C.
pMφ cultures.
Mice were injected intraperitoneally with 2 ml of 3% Brewer's thioglycolate broth for 5 to 7 days. Peritoneal Mφ (pMφs) were harvested using cold 2 mM EDTA in phosphate-buffered saline (PBS) solution and then plated at a concentration of 3 × 105 cells/well/ml in 24-well tissue culture plates for the determination of infection or at a concentration of 105 cells/well/200 μl in 96-well tissue culture plates for the arginase activity assays.
pMφ stimulation and parasite infection.
pMφs were either left untreated or were treated for 4 h with 20 ng of IFN-γ/ml alone, together with 10 ng of LPS/ml, or with 20 ng of IL-4/ml alone. As previously reported, IFN-γ at the concentration of 20 ng/ml was found to be the optimal dose, yielding high parasite growth enhancement (27). After incubation at 33°C for the indicated times, cells were infected with L. amazonensis amastigotes at 2:1 parasite/cell ratios. The culture plates were spun at 100 × g for 5 min to synchronize the binding of amastigotes to pMφs. The cultures were maintained at 33°C for an indicated additional time prior to each specific assay.
Determination of parasite burden in infected pMφs.
The number of amastigotes per well in infected pMφs was determined as previously described (27). Briefly, pMφs in 24-well plates were incubated for approximately 10 to 15 min with 200 μl of a sodium dodecyl sulfate-PBS solution (0.01% [wt/vol]) to release the intracellular amastigotes. The lysis process was monitored visually to verify adequate release of intact parasites. The reaction was stopped by adding 0.8 ml of PBS per well, the released parasites were resuspended thoroughly by repeated pipetting, and the parasites were counted by using a hemocytometer.
One-step real-time reverse transcription-PCR (RT-PCR).
Applied Biosystems (Foster City, CA) Assays-by-Design and Assays-on-Demand 20× assay mixes of primers and TaqMan MGB probes (FAM dye-labeled) were used for detection of the Slc7A2 and Slc7A1 genes, respectively. Predeveloped 18S rRNA (VIC-dye-labeled probe) TaqMan assay reagent (P/N 4319413E) was used as an endogenous control. The primers were designed to span exon-exon junctions to prevent the detection of genomic DNA. All primers and probe sequences were searched against the Celera database to confirm specificity. The primer and probe sequences for Slc7A2 are as follows: probe, 5′-CCCGATGACAAAGTAGCAAT; forward primer, 5′-TCAATTCCAAAACGAAGACACCAGTA; and reverse primer, 5′-AGGTCAAAAAGAAAGGCCATCACA. An Assay-on-Demand patented by Applied Biosystems (assay Mm00432019_m1) was used to analyze Slc7A1.
In separate tubes, real-time RT-PCR was performed with 80 ng of RNA for both the target genes and endogenous control using a TaqMan one-step RT-PCR master mix reagent kit (P/N 4309169). The cycling parameters for RT-PCR were set as follows: RT, 48°C for 30 min; AmpliTaq activation, 95°C for 10 min; denaturation, 95°C for 15 s; and annealing and/or extension, 60°C for 1 min (repeated 40 times) on ABI7000. Duplicates of the threshold cycle (CT) values were analyzed in Microsoft Excel using the comparative CT (ΔΔCT) method, as described by the manufacturer (Applied Biosystems). The amount of target (2−ΔΔCT) was obtained and normalized to an endogenous reference (18s rRNA) and a calibrator (one of the experimental samples).
Arginase activity assays.
The arginase activities from Mφ cell lysates were measured as previously described (8), with some modifications. Briefly, pMφs (105/well, in 96-well plates) were treated with cytokines for 4 h prior to infection with amastigotes at a 2:1 parasite/cell ratio. After removal of the culture supernatants, the cells were washed and incubated with 50 μl of 0.1% Triton X-100 containing a protease inhibitor cocktail (Sigma) for 30 min at room temperature. Arginase was activated with 50 μl of 10 mM MnCl2 and 50 mM Tris-HCl (pH 7.5) at 55°C for 10 min. After 25 μl of each sample was transferred into a 96-deep-well assay plate, the enzyme reaction was carried out by adding 25 μl of 0.5 M of l-arginine (pH 9.7) at 37°C for 60 min with shaking, and the reaction was stopped with 400 μl of a H2SO4-H3PO4-H2O (1:3:7) acid mixture. Subsequently, freshly prepared 9% 1-phenyl-1,2-propanedione-2-oxime (ISPF; Sigma) in 100% ethanol was added (25 μl/well), and the reaction plates were incubated at 95 to 100°C for 45 min for color development. After a 10-min incubation in the dark, 200 μl of each sample was transferred to transparent microplates for an absorbance reading at 540 nm. A calibration curve was prepared using urea standards (USB, Cleveland, OH) at concentrations of 1.5 to 30 μg.
Arginase activities in live, intact cells were also measured as previously described (3) with minor modifications. Briefly, Mφs (3 × 105 cells/well, in 24-well plates) were treated with various cytokines and infected with axenic amastigotes at a parasite/cell ratio of 2:1. At 28 h postinfection, cells were incubated with 1 μM l-[guanido-14C]-arginine (55 mCi/mmol; American Radiolabeled Chemical, Inc., St. Louis, MO), and 100 μM l-NMMA in fetal bovine serum-free Hanks balanced salt solution was added to the culture. In all experiments, an aliquot of l-[guanido-14C]-arginine was incubated without cells and used as a negative control. At 48 h postinfection, 0.15 ml of supernatant was removed and added to 0.8 ml of 250 mM acetic acid solution (pH 4.5) containing 100 mM urea and 10 mM l-arginine. After the addition of 0.3 ml (1g/ml) of Dowex resin (HCR-W2; Sigma), the tubes were mixed for 1 min and centrifuged at 120 × g for 5 min. Supernatants (500 μl) containing [14C]-urea were removed for the radioactive reading. The percent conversion of l-[14C]arginine to [14C]urea was calculated as follows: [(counts per minute [cpm] in 0.5 ml of supernatant − cpm in medium without cells)/(0.36 × cpm in 0.15 ml of unseparated medium)] × 100.
iNOS assay.
At 72 h postinfection, culture supernatants were collected for measurement of the end products from the iNOS pathway. Nitrite was measured by using a nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI). Griess reagents were used for color development.
l-Arginine uptake assay.
After a 4-h cytokine treatment and subsequent infection with amastigotes for 24 h, l-arginine uptake of infected and control pMφs was measured as described previously (39), with some modifications. Briefly, after two washes with 1 ml of prewarmed uptake solution (137 mM NaCl, 5.4 mM KCl, 1.2 mM MgSO4·7H2O, 2.8 mM CaCl2·2H2O, 10 mM HEPES, and 1 mM KH2PO4 [pH 7.4]), l-arginine uptake was initiated by adding 250 μl of 100 μM l-[14C(U)]arginine (360 mCi/mmol, 0.1 mCi/ml; Perkin-Elmer, Wellesley, MA) in prewarmed buffer in the presence of 5 mM concentration of l-leucine for 5 min at room temperature. The reaction was stopped, and samples were washed three times with stop solution (10 mM HEPES, 10 mM Tris, and 137 mM NaCl, and 10 mM nonradioactive l-arginine [pH 7.4]) to remove unincorporated substrates. Cells were then lysed with 1% sodium dodecyl sulfate, and the radioactive incorporation was determined with a liquid scintillation analyzer (Tri-CARB 2100TR; Packard). d-[1-3H(N)]mannitol (Perkin-Elmer) was used for the normalization of extracellular substrates. The protein concentrations were measured by using a BCA protein assay kit (Pierce, Rockford, IL). The amount of incorporated l-arginine per min was normalized to the total protein concentration of each sample.
Data analysis.
To evaluate the statistical significance of the difference between experimental groups, statistical analysis (Student t test and one-way and two-way analyses of variance [ANOVA]) was performed on a GraphPad Prism 4.0 program (GraphPad Software, Inc., San Diego, CA). For one-way ANOVA, the Dunnett post test was used to compare treatment groups to the medium controls. The Bonferroni post test was used following two-way ANOVA. The differences between groups were considered significant when the P value was ≤0.05.
RESULTS
IFN-γ enhanced the growth of lesion-derived and axenic L. amazonensis amastigotes in pMφs.
We previously reported that IFN-γ treatment induced significant growth enhancement of L. amazonensis in Mφs infected with lesion-derived amastigotes without altering parasite uptake by the cells (27). Given that lesion-derived amastigotes may be opsonized with antibodies that can induce alternative activation of Mφs (19), we extended our study by using axenic amastigotes that were maintained in vitro in the absence of host cells and antibodies. We found that in a manner similar to that of lesion-derived amastigotes (Fig. 1A) (27), the number of axenic amastigotes recovered from IFN-γ-treated Mφs was significantly increased compared to medium controls (P < 0.001, Fig. 1B). Similar results were observed when axenic amastigotes of L. mexicana were used (data not shown). Collectively, these results indicate that IFN-γ-mediated growth enhancement does not require antibody opsonization and is a common phenomenon for amastigotes in the L. mexicana complex. Axenic amastigotes of L. amazonensis were therefore used in the rest of the study. Since IFN-γ+LPS cotreatment and IL-4 routinely induce parasite killing and enhanced parasite growth, respectively, these treatments were included in subsequent experiments as positive controls to ensure adequate Mφ activation among independent repeats.
FIG. 1.
IFN-γ enhanced the growth of lesion-derived and axenic L. amazonensis amastigotes in pMφs. pMφs (3 × 105/well/ml) were left untreated or treated with 20 ng of IFN-γ alone/ml or together with 10 ng of LPS or 20 ng of IL-4/ml for 4 h and then infected with lesion-derived (A) or axenic L. amazonensis amastigotes (B). The parasite burden was determined at 72 h postinfection. The data are presented as means ± the standard deviation (SD) of triplicate wells for each condition. Similar results were observed from three independent experiments. Asterisks indicate values significantly different from those in the respective medium control (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Amastigote infection in the presence of IFN-γ induced the expression of Slc7A2 and l-arginine uptake.
To investigate the involvement of the l-arginine metabolic pathway in IFN-γ-mediated parasite growth enhancement, we first examined the expression of putative transporters for l-arginine, mCATs, via real-time RT-PCR. Since Slc7A2A (encoding CAT-2A) and Slc7A3 (encoding for CAT-3) are known to be only expressed in the brain and hepatocytes, respectively (36), we focused our attention on examining the expression of Slc7A1 (encoding for CAT-1) and Slc7A2 (encoding for CAT-2B in Mφs). As shown in Fig. 2A, we found that the expression of Slc7A1 in infected Mφs remained low and unaffected by cytokine treatments, confirming that mCAT-1 was not involved in differential Mφ activations. In contrast, the levels of Slc7A2 expression were markedly increased in cells treated with IFN-γ+LPS or IL-4 (positive controls). Although the increase in Slc7A2 expression was not as high as those of the positive controls, IFN-γ-treated, parasite-infected cells showed an approximately twofold increase in Slc7A2 expression compared to the medium controls (Fig. 2B). To examine whether the enhanced expression of mCAT-2B mRNA correlated with an increase in cationic amino acid transport into pMφs, we performed l-arginine uptake assays using l-[14C (U)]-arginine in the presence of 5 mM l-leucine to indicate the specificity of the y+ transport system (39). We detected a twofold increase in l-arginine uptake via the y+ system in IFN-γ-treated, parasite-infected pMφs (Fig. 2C). As expected, high levels of l-arginine uptake were detected in cells treated with IFN-γ+LPS or IL-4 (Fig. 2C), correlating with their Slc7A2 expressions. Since there were no significant differences in the expression patterns of Slc7A1 and Slc7A2 and l-arginine uptake in infected and uninfected cells (data not shown), it appeared that parasite infection alone was insufficient to induce l-arginine transport via mCAT-2B. Together, these data demonstrate that infection by L. amazonensis amastigotes in the presence of IFN-γ significantly increased mCAT-2B expression and cellular l-arginine transport.
FIG. 2.
Amastigote infection in the presence of IFN-γ induced the expression of the Slc7A2 gene. pMφs (3 × 105/well) were left untreated or treated with 20 ng of IFN-γ alone/ml or together with 10 ng of LPS or 20 ng of IL-4/ml for 4 h and then infected with axenic L. amazonensis amastigotes (a parasite/cell ratio of 2:1). Total RNA was extracted at 8 h postinfection. The expression of Slc7A1 (A) and Slc7A2 (B) was analyzed by using one-step real-time RT-PCR. The results for real-time RT-PCR are shown as an average ± the SD of samples obtained from three independent experiments. (C) An l-arginine uptake assay was performed at 24 h postinfection. The patterns of Slc7A1 and Slc7A2 expression and l-arginine uptake in infected cells were similar to those of uninfected cells (data not shown). The data are presented as mean ± the SD of triplicate samples. Similar results were observed from two independent experiments. Asterisks indicate values significantly different from those in the respective medium control (*, P < 0.05; **, P < 0.01).
l-Arginine was involved in IFN-γ-mediated growth of L. amazonensis amastigotes.
To assess the biological relevance of enhanced Slc7A2 expression in IFN-γ-treated, infected pMφs, we directly examined the involvement of l-arginine in IFN-γ-mediated parasite growth enhancement. Since l-NMMA, a nonmetabolized l-arginine analogue, has been shown to effectively block l-arginine transport (2), we treated Mφs with this compound in order to examine parasite growth in the absence of l-arginine transport. As shown in Fig. 3A, the addition of l-NMMA abolished IFN-γ-mediated growth enhancement in a dose-dependent manner with a minimum dose of 5 mM required for growth inhibition. l-NMMA also abrogated the effect of l-arginine-mediated parasite killing via IFN-γ+LPS and parasite growth enhancement by IL-4 (Fig. 3B), indicating the requirement of l-arginine substrate in both of these pathways. Although l-NMMA can effectively block l-arginine transport, it also functions as a direct inhibitor of iNOS. To further verify whether blockade of l-arginine transport can alter parasite infection, we also examined parasite growth in the presence of another l-arginine analogue, l-SDMA, which can compete with l-arginine transport but does not directly block enzymatic activity of iNOS (6). Similar to the observations with l-NMMA, the addition of l-SDMA inhibited IFN-γ-mediated parasite growth enhancement, as judged by parasite burdens (black bars in Fig. 3C). To further examine the role of l-arginine, we next tested whether excessive amounts of l-arginine would further enhance parasite growth. As shown in Fig. 3D, 10 mM l-arginine significantly increased parasite burdens in the presence or absence of IFN-γ, suggesting a direct correlation between l-arginine availability and parasite growth. As expected, l-arginine also enhanced parasite killing after IFN-γ+LPS treatment, as well as parasite growth enhancement after IL-4 treatment. These data suggest that there is a direct correlation between l-arginine availability and the increase in parasite growth in IFN-γ-treated Mφs.
FIG. 3.
l-Arginine was involved in the IFN-γ-mediated growth of L. amazonensis amastigotes. pMφs (3 × 105/well) were treated and infected as described in Fig. 2. As indicated in some groups, various concentrations of l-NMMA (A), 10 mM l-NMMA (B), 10 mM l-SDMA (C), or 10 mM l-arginine (D) were added at the time of cytokine treatment, and the parasite burden was determined at 72 h postinfection. The data are presented as the means ± the SD of triplicate samples. Similar results were observed from two independent experiments. Asterisks indicate values significantly different from those in the respective medium controls (*, P < 0.05; **, P < 0.01).
mCAT-2B regulated IFN-γ-mediated growth of L. amazonensis amastigotes.
To further verify that l-arginine transport via mCAT-2B was required in IFN-γ-mediated parasite growth, we infected pMφs derived from CAT-2 KO and wild-type C57BL/6 mice with L. amazonensis amastigotes, and parasite burdens were measured. In contrast to the wild-type counterparts that contained differential levels of parasite burdens after different cytokine treatments, pMφs from CAT-2 KO mice contained comparable parasite burdens in all treatment groups (Fig. 4A). These results indicated that the effects of IFN-γ- and IL-4-mediated parasite growth enhancement, as well as IFN-γ+LPS-mediated parasite killing, were all regulated by mCAT-2B. Consistent with a previous report (39), the nitrite levels in IFN-γ+LPS-treated CAT-2 KO cells were significantly reduced, indicating a complete absence of mCAT-2B (data not shown). A close examination of parasite numbers per cell revealed that 18% of IFN-γ-treated Mφs from wild-type mice contained heavily infected cells (≥9 parasites per cells), whereas only 10% of IFN-γ-treated Mφs from CAT-2 KO mice contained this high level of parasite burden (Fig. 4B), suggesting that the enhanced parasite growth could not be established in the absence of mCAT-2B. This reduction in parasite burdens was not due to a decrease in the susceptibility of Mφs from CAT-2 KO mice, as indicated by similar infection rates in all tested groups (Fig. 4C). Together, these data suggest that l-arginine transport via mCAT-2B plays a significant role in the enhancement of parasite growth in IFN-γ-treated cells.
FIG. 4.
IFN-γ-mediated growth enhancement of L. amazonensis amastigotes was abolished in pMφs from CAT-2 KO mice. pMφs (3 × 105/well) from C57BL/6 wild-type or CAT-2 KO mice were left untreated or treated with 20 ng of IFN-γ alone/ml or together with 10 ng of LPS or 20 ng of IL-4/ml for 4 h and then infected with axenic L. amazonensis amastigotes (at a 2:1 ratio). (A) At 72 h postinfection, the total number of parasites per well was determined. (B) A parallel set of infected cells was grown on glass coverslips, fixed, and stained with a Diff-Quik kit. Images of infected cells were taken at ×40 magnification, and the numbers of parasites per cells in 400 cells/group were counted. IFN-γ treatment only induced an increase in the percentage of cells containing high parasite numbers (≥9 parasites/cell) in wild-type groups but not in CAT-2 KO groups. (C) No significant differences in infection rates were observed between groups. The data are presented as means ± the SD of triplicate samples. Similar results were observed from three independent experiments. Asterisks indicate values significantly different from those in the respective medium control (*, P < 0.05; **, P < 0.01; ***, P < 0.001). WT, wild type.
IFN-γ-mediated treatment was insufficient to activate either iNOS or arginase in infected Mφs.
Having demonstrated that IFN-γ treatment enhanced the expression of Slc7A2 and uptake of l-arginine in amastigote-infected Mφs, we then investigated the potential utilization of l-arginine of infected Mφs under different stimulations. Since l-arginine is a shared substrate for both iNOS and arginase enzymes, we examined the activities of both enzymes. iNOS induction, as indicated by nitrite production at 72 h postinfection, was markedly upregulated by IFN-γ+LPS treatment (P < 0.01); however, treatment with IFN-γ alone did not induce any measurable iNOS activity above the basal levels (Fig. 5A). It is important to note that parasite infection alone also did not induce any measurable nitrite under these experimental settings (data not shown).
FIG. 5.
IFN-γ treatment was insufficient to activate either iNOS or arginase in infected pMφs. pMφs (105/well) were treated and infected as described in Fig. 2. (A) At 72 h postinfection, supernatants were collected for the measurement of nitrite concentrations. (B) Total cellular proteins were extracted at the indicated time points for the determination of arginase expression. (C) The levels of [14C]urea in culture supernatants were measured as an indication of l-14C-[guanido]arginine conversion by arginase. iNOS and arginase activities in infected cells were comparable to those in their respective uninfected controls (data not shown). The data are presented as means ± the SD of triplicate samples. Similar results were observed from three independent experiments. Asterisks indicate values significantly different from those in the respective medium control (**, P < 0.01; ***, P < 0.001).
We next examined arginase expression by providing substrate and cofactors of the enzymes and measuring the subsequent urea production in cell lysates. As expected, IL-4-treated, amastigote-infected Mφs showed a dramatic increase in arginase expression at 16 and 24 h postinfection (Fig. 5B); however, basal levels of arginase were observed in untreated cells, as well as in cells pretreated with IFN-γ or IFN-γ+LPS (Fig. 5B). The arginase activity in live, intact cells was also determined. Using 14C-l-[guanido]arginine, the release of [14C]urea was measured and percentage of l-arginine conversion by arginase was calculated (3). As shown in Fig. 5C, arginase-mediated l-arginine conversion was significantly increased in IL-4-treated Mφs, whereas the conversion rates in IFN-γ- or IFN-γ+LPS-treated cells remained comparable to that of medium controls. Collectively, these data suggest that although IFN-γ treatment can enhance Slc7A2 expression and the uptake of l-arginine of amastigote-infected Mφs, it did not significantly induce iNOS or arginase activities. Therefore, the mechanisms of IFN-γ-mediated growth enhancement of L. amazonensis amastigotes appeared to be independent of these two enzymatic activities.
Full activation of the iNOS pathway for effective amastigote killing was dependent on l-arginine availability.
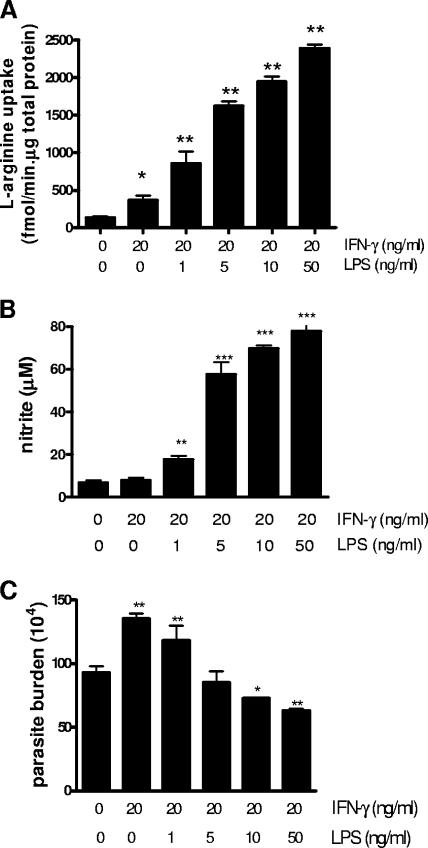
To better understand how l-arginine availability influences the fate of L. amazonensis amastigotes within infected Mφs, we examined the relationship between l-arginine uptake (Fig. 6A), nitrite concentrations (Fig. 6B), and the parasite burdens (Fig. 6C). We found that when 1 ng of LPS/ml was added together with 20 ng of IFN-γ/ml, parasite growth enhancement was still observed, suggesting that low levels of LPS were insufficient to reverse the parasite growth enhancement mediated by IFN-γ. A complete reversal of parasite growth enhancement and the establishment of effective parasite killing was not achieved until sufficiently high levels of LPS were added (≥10 ng/ml), which correlated with a nitrite level of ≥65 μM. Therefore, while an optimal activation of Mφs could be achieved for effective control of L. amazonensis amastigotes, suboptimal stimulation of Mφs actually promoted parasite survival and growth. Our data provided evidence for a direct correlation between l-arginine uptake and the iNOS activity needed for parasite killing, indicating a fine balance between parasite growth enhancement when cells were treated with IFN-γ alone, and parasite killing when IFN-γ was given together with LPS.
FIG. 6.
Full activation of the iNOS pathway for effective amastigote killing was dependent on l-arginine availability. pMφs (3 × 105/well) were left untreated or treated with 20 ng of IFN-γ/ml alone or together with various concentrations of LPS for 4 h and then infected with axenic L. amazonensis amastigotes (parasite/cell ratio of 2:1). (A) l-Arginine uptake was measured at 24 h postinfection. (B and C) At 72 h postinfection, nitrite production (B) and the parasite burden (C) were determined. The data are presented as means ± the SD of triplicate samples. Similar results were observed from two independent experiments. Asterisks indicate values significantly different from those in the respective medium control (*, P < 0.05; **, P < 0.01; ***, P < 0.01).
DISCUSSION
Since IFN-γ is one of the major cytokines consistently detected in nonhealing lesions caused by L. amazonensis infection (28), a better understanding of its involvement in immunopathogenesis would provide valuable insight into the requirements for design of vaccines and treatments against New World cutaneous leishmaniasis. Leishmania amastigotes live and multiply within the parasitophorous vacuoles (PVs) of infected Mφs. The fate of the parasite depends largely on the activation status of host cells. While IFN-γ coupled with LPS induces parasite killing via the iNOS pathway (14), IL-4 promotes parasite growth via the arginase-dependent, alternative activation of Mφs (17, 38). Consistent with our previous report (27), we showed here that IFN-γ alone not only failed to activate Mφs to kill parasites but actually enhanced the growth of L. amazonensis amastigotes, regardless whether they were isolated from lesions or axenically cultured in vitro (Fig. 1).
Since l-arginine is a crucial substrate for both iNOS and arginase, we focused our investigation of IFN-γ-mediated parasite growth enhancement on l-arginine transport and l-arginine metabolic pathways. Examination of l-arginine catabolism and its transport provided evidence suggesting that various cytokine stimulations can lead to differential cell activations. As illustrated in Fig. 7, our working models may explain the influence of cytokine stimulation on l-arginine availability and Leishmania infection outcomes. When Mφs are stimulated with IFN-γ together with LPS, the upregulation of mCAT-2B can induce l-arginine uptake, thereby providing substrates for iNOS activation and parasite killing. Conversely, when cells are stimulated with IL-4, the upregulation of mCAT-2B, l-arginine transport, and arginase activity directly correlate with high parasite burden, suggesting the promotion of parasite growth via arginase-mediated l-arginine utilization. Interestingly, when infected Mφs are stimulated with IFN-γ alone, mCAT-2B is significantly upregulated without an induction of iNOS or arginase (Fig. 3). IFN-γ alone, therefore, exerts a unique Mφ activation status that was independent of iNOS and arginase.
FIG. 7.
Working model for the influence of l-arginine, iNOS, and arginase on classical activation (A), alternative activation (B), and the IFN-γ-mediated enhancement (C) of L. amazonensis amastigote growth in Mφs.
In the present study, we demonstrated that both IFN-γ and IL-4 treatments induced L. amazonensis amastigote growth via l-arginine-dependent pathways (Fig. 3 and 4). However, the underlying mechanisms of IFN-γ-mediated parasite growth enhancement appeared to be different from that of IL-4. While IL-4 is known to promote parasite growth via the induction of arginase (16), IFN-γ-mediated growth enhancement was not linked to arginase upregulation (Fig. 5C). Moreover, IFN-γ and IL-4 could induce parasite growth enhancement to similar extents, although l-arginine transport was moderately induced by IFN-γ in comparison to IL-4 (Fig. 2C). These findings suggest that l-arginine-mediated parasite growth enhancement may operate through various mechanisms, either dependent on arginase (via IL-4 stimulation) or independent of arginase (via IFN-γ stimulation). Although the potential utilization of l-arginine in IFN-γ-treated, L. amazonensis-infected Mφs is unclear, it is possible that during IFN-γ stimulation, l-arginine is used directly by the parasites for their proliferation. This hypothesis is supported by the findings that in vitro-cultured Leishmania are capable of directly acquiring l-arginine from the culture media (18) and produce significant amounts of urea, a product of l-arginine conversion by arginase (40). Moreover, the presence of arginase enzyme was documented in several species of Leishmania, including L. major, L. amazonensis, and L. mexicana (9). Finally, targeted deletion of parasite-derived arginase was subsequently shown to inhibit parasite growth both in vitro and in vivo (30). Collectively, these reports suggest that Leishmania may have an ability to gain access to intracellular l-arginine pool while residing within the host Mφs. Since amastigotes reside within specialized, acidic subcellular PVs, it would be valuable to further identify the mechanisms by which l-arginine is transported across the PV membranes. Although mCAT2-B is predominantly expressed on the plasma membranes of Mφs (7), it may potentially be recruited to the PVs and facilitate cationic amino acid transport to the parasites. The recruitment of proteins normally expressed on plasma membranes to PVs has been previously reported. For example, major histocompatibility complex class II molecules are known to be present on 90% of the PVs of Leishmania-infected Mφs (1, 22). We are currently investigating whether mCAT-2B is recruited to the PVs and facilitates l-arginine transport for parasite replication.
l-Arginine plays an important role in iNOS-mediated control of intracellular pathogens. Conversely, l-arginine conversion to l-ornithine and urea and its involvement in polyamine synthesis has also been shown to promote growth of many pathogens, including Leishmania spp. (21, 30), Trypanosoma spp. (26), Helicobacter pylori (4), and Mycobacterium bovis (33). Similar to our study showing the association of l-arginine transport and the enhancement of L. amazonensis growth, previous studies have demonstrated that other pathogens also interfere with the l-arginine metabolic pathways to promote their survival within the host. For example, the improved survival capacity of AS-1 strain M. bovis BCG was found to be associated with its ability to induce mCAT-2A, mCAT-2B, l-arginine uptake, and arginase activity (33). Moreover, the ability of the lumen-dwelling protozoon, Giardia lamblia, to take up extracellular l-arginine and compete with iNOS for l-arginine substrate was suggested to be its strategy to evade host defense (11). Together, these studies strongly suggest that l-arginine metabolic pathways are likely targets for microbial virulence factors and that invasion of these pathways by microorganisms would directly promote their survival within the host.
L. amazonensis infection in mice displays distinct phenotypes compared to infection caused by L. major. While many inbred strains of mice display self-healing phenotypes after L. major infection (29), L. amazonensis infection results in the development of chronic, nonhealing lesions in most strains of mice (28). Several intrinsic, parasite-derived factors may contribute to the nonhealing phenotype of L. amazonensis infection. For example, IFN-γ treatment alone promotes growth, rather than killing, of L. amazonensis amastigotes (27). We also demonstrated here that L. amazonensis amastigotes are able to survive moderate Mφ activation, and high NO levels are required to trigger sufficient parasite killing (Fig. 6). Finally, since L. amazonensis infection does not trigger Mφs to produce TNF-α, a cytokine shown to act synergistically with IFN-γ to efficiently control L. major infection (12), there may be incomplete activation of the iNOS pathway during L. amazonensis infection. Therefore, the differential requirement for the induction of high iNOS activity for the killing of L. amazonensis may partially explain the general susceptibility of inbred strains of mice to L. amazonensis infection.
In summary, this study provides strong evidence that IFN-γ alone can induce a unique activation status in L. amazonensis-infected Mφs, a process that appears to use l-arginine as a key regulator to promote the growth of intracellular parasites. We also demonstrated a fine balance between host iNOS, arginase, and l-arginine transport via mCAT-2B, as well as their influences on the fate of intracellular parasites. This report highlights the importance of an optimal activation of the host immune system for effective parasite killing and potential challenges in the development of effective vaccines and treatment regimens for the control of New World cutaneous leishmaniasis.
Acknowledgments
This study is supported by James W. McLaughlin Predoctoral Fellowship to N.W., NIH grant AI043003 to L.S., and NIH grant K08 CA88035 to L.G.E.
We thank Kwang-Poo Chang for providing axenic amastigotes and valuable comments on the manuscript, Ana M. Pajor and Jittima Weerachayaphorn for technical assistance of the l-arginine uptake experiments, the University of Texas Medical Branch real-time PCR core lab for assistance in real-time PCR experiments, and Peter Kima and Hai Qi for critical review of the manuscript. We also thank Joseph Masterson and Mardelle Susman for their assistance in the preparation of the manuscript.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 26 March 2007.
REFERENCES
- 1.Antoine, J. C., T. Lang, E. Prina, N. Courret, and R. Hellio. 1999. H-2M molecules, like MHC class II molecules, are targeted to parasitophorous vacuoles of Leishmania-infected macrophages and internalized by amastigotes of L. amazonensis and L. mexicana. J. Cell Sci. 112(Pt. 15):2559-2570. [DOI] [PubMed] [Google Scholar]
- 2.Baydoun, A. R., and G. E. Mann. 1994. Selective targeting of nitric oxide synthase inhibitors to system y+ in activated macrophages. Biochem. Biophys. Res. Commun. 200:726-731. [DOI] [PubMed] [Google Scholar]
- 3.Boutard, V., R. Havouis, B. Fouqueray, C. Philippe, J. P. Moulinoux, and L. Baud. 1995. Transforming growth factor-beta stimulates arginase activity in macrophages: implications for the regulation of macrophage cytotoxicity. J. Immunol. 155:2077-2084. [PubMed] [Google Scholar]
- 4.Bussiere, F. I., R. Chaturvedi, Y. Cheng, A. P. Gobert, M. Asim, D. R. Blumberg, H. Xu, P. Y. Kim, A. Hacker, R. A. Casero, Jr., and K. T. Wilson. 2005. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J. Biol. Chem. 280:2409-2412. [DOI] [PubMed] [Google Scholar]
- 5.Closs, E. I., L. M. Albritton, J. W. Kim, and J. M. Cunningham. 1993. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J. Biol. Chem. 268:7538-7544. [PubMed] [Google Scholar]
- 6.Closs, E. I., F. Z. Basha, A. Habermeier, and U. Forstermann. 1997. Interference of l-arginine analogues with l-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide 1:65-73. [DOI] [PubMed] [Google Scholar]
- 7.Closs, E. I., A. Simon, N. Vekony, and A. Rotmann. 2004. Plasma membrane transporters for arginine. J. Nutr. 134:2752S-2759S; 2765S-2767S. [DOI] [PubMed] [Google Scholar]
- 8.Corraliza, I. M., M. L. Campo, G. Soler, and M. Modolell. 1994. Determination of arginase activity in macrophages: a micromethod. J. Immunol. Methods 174:231-235. [DOI] [PubMed] [Google Scholar]
- 9.da Silva, E. R., T. M. Castilho, F. C. Pioker, C. H. Tomich de Paula Silva, and L. M. Floeter-Winter. 2002. Genomic organisation and transcription characterisation of the gene encoding Leishmania (Leishmania) amazonensis arginase and its protein structure prediction. Int. J. Parasitol. 32:727-737. [DOI] [PubMed] [Google Scholar]
- 10.Dutta, S., D. Ray, B. K. Kolli, and K. P. Chang. 2005. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phthalocyanine chloride for photolysis in vitro. Antimicrob. Agents Chemother. 49:4474-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckmann, L., F. Laurent, T. D. Langford, M. L. Hetsko, J. R. Smith, M. F. Kagnoff, and F. D. Gillin. 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 164:1478-1487. [DOI] [PubMed] [Google Scholar]
- 12.Gomes, I. N., A. F. Calabrich, S. Tavares Rda, J. Wietzerbin, L. A. de Freitas, and P. S. Veras. 2003. Differential properties of CBA/J mononuclear phagocytes recovered from an inflammatory site and probed with two different species of Leishmania. Microbes Infect. 5:251-260. [DOI] [PubMed] [Google Scholar]
- 13.Gotoh, T., and M. Mori. 1999. Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. J. Cell Biol. 144:427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 15.Green, S. J., M. S. Meltzer, J. B. Hibbs, Jr., and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 16.Iniesta, V., L. C. Gomez-Nieto, and I. Corraliza. 2001. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J. Exp. Med. 193:777-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iniesta, V., L. C. Gomez-Nieto, I. Molano, A. Mohedano, J. Carcelen, C. Miron, C. Alonso, and I. Corraliza. 2002. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 24:113-118. [DOI] [PubMed] [Google Scholar]
- 18.Kandpal, M., R. B. Fouce, A. Pal, P. Y. Guru, and B. L. Tekwani. 1995. Kinetics and molecular characteristics of arginine transport by Leishmania donovani promastigotes. Mol. Biochem. Parasitol. 71:193-201. [DOI] [PubMed] [Google Scholar]
- 19.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 20.Kim, J. W., E. I. Closs, L. M. Albritton, and J. M. Cunningham. 1991. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 352:725-728. [DOI] [PubMed] [Google Scholar]
- 21.Kropf, P., J. M. Fuentes, E. Fahnrich, L. Arpa, S. Herath, V. Weber, G. Soler, A. Celada, M. Modolell, and I. Muller. 2005. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 19:1000-1002. [DOI] [PubMed] [Google Scholar]
- 22.Lang, T., C. de Chastellier, C. Frehel, R. Hellio, P. Metezeau, S. Leao Sde, and J. C. Antoine. 1994. Distribution of MHC class I and of MHC class II molecules in macrophages infected with Leishmania amazonensis. J. Cell Sci. 107(Pt. 1):69-82. [DOI] [PubMed] [Google Scholar]
- 23.Liew, F. Y., Y. Li, D. Moss, C. Parkinson, M. V. Rogers, and S. Moncada. 1991. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur. J. Immunol. 21:3009-3014. [DOI] [PubMed] [Google Scholar]
- 24.Lin, J. Y., R. Seguin, K. Keller, and K. Chadee. 1994. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect. Immun. 62:1534-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson, B., C. K. Manner, J. Kleeman, and C. L. MacLeod. 2001. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J. Biol. Chem. 276:15881-15885. [DOI] [PubMed] [Google Scholar]
- 26.Peluffo, G., L. Piacenza, F. Irigoin, M. N. Alvarez, and R. Radi. 2004. l-Arginine metabolism during interaction of Trypanosoma cruzi with host cells. Trends Parasitol. 20:363-369. [DOI] [PubMed] [Google Scholar]
- 27.Qi, H., J. Ji, N. Wanasen, and L. Soong. 2004. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect. Immun. 72:988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 29.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, S. C., M. J. Tancer, M. R. Polinsky, K. M. Gibson, O. Heby, and B. Ullman. 2004. Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J. Biol. Chem. 279:23668-23678. [DOI] [PubMed] [Google Scholar]
- 31.Stevens, B. R., D. K. Kakuda, K. Yu, M. Waters, C. B. Vo, and M. K. Raizada. 1996. Induced nitric oxide synthesis is dependent on induced alternatively spliced CAT-2 encoding l-arginine transport in brain astrocytes. J. Biol. Chem. 271:24017-24022. [DOI] [PubMed] [Google Scholar]
- 32.Stuehr, D. J., S. S. Gross, I. Sakuma, R. Levi, and C. F. Nathan. 1989. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J. Exp. Med. 169:1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talaue, M. T., V. Venketaraman, M. H. Hazbon, M. Peteroy-Kelly, A. Seth, R. Colangeli, D. Alland, and N. D. Connell. 2006. Arginine homeostasis in J774.1 macrophages in the context of Mycobacterium bovis BCG infection. J. Bacteriol. 188:4830-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teixeira, M. J., C. R. Teixeira, B. B. Andrade, M. Barral-Netto, and A. Barral. 2006. Chemokines in host-parasite interactions in leishmaniasis. Trends Parasitol. 22:32-40. [DOI] [PubMed] [Google Scholar]
- 35.Vasquez, R. E., and L. Soong. 2006. CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice. Infect. Immun. 74:6769-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verrey, F., E. I. Closs, C. A. Wagner, M. Palacin, H. Endou, and Y. Kanai. 2004. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 447:532-542. [DOI] [PubMed] [Google Scholar]
- 37.Vinetz, J. M., and L. Soong. 2006. Leishmania mexicana infection of the eyelid in a traveler to Belize. Braz. J. Infect. Dis. 10:304-307. [DOI] [PubMed] [Google Scholar]
- 38.Wu, G., and S. M. Morris, Jr. 1998. Arginine metabolism: nitric oxide and beyond. Biochem. J. 336(Pt. 1):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeramian, A., L. Martin, L. Arpa, J. Bertran, C. Soler, C. McLeod, M. Modolell, M. Palacin, J. Lloberas, and A. Celada. 2006. Macrophages require distinct arginine catabolism and transport systems for proliferation and for activation. Eur. J. Immunol. 36:1516-1526. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida, N., and E. P. Camargo. 1978. Ureotelism and ammonotelism in trypanosomatids. J. Bacteriol. 136:1184-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]