Abstract
Stratified epithelium contains a mitotically active basal layer of cells that cease proliferating, then migrate outwards and undergo terminal differentiation. The control of this process, which is abnormal in cutaneous neoplasia and inflammation, is not well understood. In normal epidermis, NF-κB proteins were found to exist in the cytoplasm of basal cells and then to localize in the nuclei of suprabasal cells, suggesting a role for NF-κB in the switch from proliferation to growth arrest and differentiation. Functional blockade of NF-κB by expressing dominant-negative NF-κB inhibitory proteins in transgenic murine and human epidermis produced hyperplastic epithelium in vivo. Consistent with this, application of a pharmacologic inhibitor of NF-κB to intact skin induced epidermal hyperplasia. In contrast, overexpression of active p50 and p65 NF-κB subunits in transgenic epithelium produced hypoplasia and growth inhibition. These data suggest that spatially restricted NF-κB activation occurs in stratified epithelium and indicate that NF-κB activation in this tissue, in contrast to its role in other settings, is important for cellular growth inhibition.
NF-κB gene regulatory proteins are activated in a range of conditions involving cellular stress and injury (1–3). Stratified epithelial tissues must respond to such frequent environmental stresses while maintaining a precise balance between cellular proliferation and cell loss via desquamation. In stratified epithelium, proliferative basal cells adherent to the underlying basement membrane undergo cell cycle arrest associated with outward migration and activation of terminal differentiation genes (4, 5). Abnormalities in this process disrupt epithelial homeostasis and are characteristic of cutaneous neoplasms as well as a wide array of inflammatory skin diseases. The gene regulatory transcription factors mediating control of epithelial growth and differentiation are not fully known. A number of such transcription regulators, however, may influence keratinocyte gene expression (6), including AP-1 (7–9), c-myc (10), AP-2 (11), LEF-1 (12), steroid family receptors, retinoid receptors, (13–16), and homeodomain proteins (17) such as Skin1a/i (18), Oct6 (19) and distal-less 3 (20). Although there is evidence for activation of each of these gene regulatory factors in specific settings in epithelium, the mechanisms linking gene regulation to cellular growth control have not been clearly elucidated.
NF-κB/Rel proteins are potent inducible gene regulatory factors expressed in a wide array of tissues. NF-κB subunits function as dimeric DNA-binding transcription factors with mammalian family members that include RelA(p65), RelB, c-Rel, p50, and p52, with Dif and Dorsal identified as homologs in Drosophila (1, 2). Studies of NF-κB in lymphoid tissues have revealed potent effects in stimulating proliferation, preventing apoptosis, activating the immune response, and triggering cellular stress response genes. NF-κB activity is controlled at a number of levels, prominent among these being the regulation of its transition from an inactive preexisting cytoplasmic form to an active nuclear protein. IκB family proteins mediate this inhibitory cytoplasmic anchoring effect and functional mammalian members include IκBα, IκBβ, p105/IκBγ, p100/IκBδ, and IκBɛ, with Cactus an IκB family member identified in Drosophila. NF-κB activation involves phosphorylation and degradation of IκB proteins (21–23), a process that releases NF-κB dimers to translocate to the nucleus and alter expression of target genes (1, 2).
The role of NF-κB in epithelium is not well studied. For some time it has been known that NF-κB, consistent with its role as a primary transcription factor, is rapidly activated after UV injury in many cell types, and recent work has confirmed the rapid induction of NF-κB DNA-binding activity in nuclear extracts prepared from cutaneous tissue irradiated with UV light (24). The expression pattern of NF-κB and its effects on global cellular processes that have been characterized in the immune system, however, have not yet been defined in epithelium. We wished to investigate whether NF-κB—as a prototype primary transcription factor controlling important cell fate decisions—may be important in stratified epithelium, a tissue possessing an array of rapidly inducible genetic programs mediating response to external injury, growth, and differentiation.
Here we report that NF-κB undergoes a translocation from cytoplasm to nucleus in a pattern that coincides spatially with the differentiation-associated cell cycle arrest in stratified epithelium. To examine a functional role for NF-κB in this setting, we generated murine and human epidermis transgenic for proteins activating or inhibiting NF-κB function. The results of these studies indicate that NF-κB function in stratified epithelium, in contrast to its role in other tissues, is important for growth arrest.
METHODS
Production of Transgenic Skin Tissue.
Sequences encoding the IκBαM mutant (25), the constitutively nuclear p50 XbaI mutant (26) and p65 (27) were subcloned downstream of a 2,075-bp human keratin 14 promoter (28) construct containing a 5′ intron from the β-globin gene and used to produce transgenic mice. Transgene integration was confirmed both by Southern blot analysis as well as PCR of genomic DNA. For the latter, primers specific for the 3′ end of the K14 promoter and the 5′ end of the expressed cDNA, either the mutant IκBα dominant-negative for NF-κB function (IκBαM), the constitutively nuclear p50 XbaI mutant or p65 were used to amplify an 850-bp fragment. Fourteen independently generated IκBαM[+], 13 CN.p50[+], and 9 p65[+] mice were characterized.
Genetically engineered human epidermis was regenerated on CB.17 scid/scid mice from early passage keratinocytes after high efficiency retroviral gene transfer by previously described methods (29, 30). Briefly, transduced keratinocytes were plated on devitalized human dermis and grown in vitro with growth media for 7–10 days followed by grafting to the backs of CB.17 scid/scid mice. Analysis of grafted human tissue was performed 3 weeks postgrafting.
Immunoblotting.
Whole cell extracts were prepared from keratinocytes grown in vitro and immunoblotted as described (31) after separation by SDS/PAGE on an 8% gel. In addition to antibodies for p50, p65, and IκBα (Santa Cruz Biotechnology), immunoblots were also incubated simultaneously with antibodies to BRG1 (32), a constitutively expressed protein that served as an internal control of extract quality and protein transfer efficiency. Immunoblots were performed on transgenic skin tissue extracts as an additional confirmation of transgene expression. Following skin biopsy, tissue was incubated for 1 h at 37°C with dispase (Becton Dickinson) (25 units/ml) to separate the epidermis from underlying dermis. Epidermal extracts were then prepared and analyzed as above.
Cell Culture and Gene Transfer.
Primary human keratinocytes were isolated from skin of normal human donors as described (33) and grown in a 1:1 mixture of SFM (GIBCO/BRL) and 154 medium (Cascade Biologics, Portland, OR), optimal conditions for proliferation. cDNA sequences corresponding to the coding regions of human p50 (amino acids 1–502, XbaI truncation) (36), p65 (27), and the dominant-negative mutants IκBαM (25) and mutant p50 dominant-negative for NF-κB function (ΔSP) (34) were subcloned into the EcoRI site of the LZRS retroviral vector (35). Amphotropic retrovirus production and gene transfer to passage 1 human keratinocytes was performed as previously described (29, 35); >99% gene transfer efficiency was confirmed for each vector by immunofluorescence analysis by using antibodies to detect cells overexpressing the transduced gene product. Transient transfections were performed by the modified polybrene shock method, as described (36). Briefly, 30% confluent normal human keratinocytes were transfected with 2 μg of p50, p65, IκBαM or ΔSP expression plasmid, 2 μg of NF-κB-luciferase reporter plasmid, and 1 μg of Rous sarcoma virus–chloramphenicol acetyltransferase internal control. For assessment of IκBαM and ΔSP dominant-negative effects, NF-κB activity was induced for 4 h with 30 ng/ml of phorbol 12-myristate 13-acetate prior to reporter gene analysis (32).
Immunohistochemistry.
Immunohistochemical stainings were performed as described (29, 31) with antibodies to NF-κB subunits (Santa Cruz Biotechnology) as well as to involucrin (Biomedical Technologies, Stoughton, MA), filaggrin (Babco, Richmond, CA), keratin 10 (Babco), transglutaminase 1 (Biomedical Technologies), and laminin 5 (gift of P. Marinkovich, Stanford University). Briefly, 5-μM-thick skin cryosections were air-dried, fixed 10 min in acetone, and blocked with 5% normal goat serum. For staining, slides were incubated with primary antibodies for 30 min, followed by PBS washing and incubation with fluorescein isothiocyanate-conjugated secondary antibodies (Sigma) and mounted with Vectashield mounting media (Vector Laboratories). Slides were then analyzed by fluorescence microscopy and, where indicated, by laser confocal microscopy. For immunoperoxidase staining, a horseradish peroxidase-conjugated secondary antibody (Dako) was used, followed by visualization with dihydroxyacetone phosphate (Boehringer Mannheim) and counterstaining with methyl green.
Analysis of Mitotic Activity.
For in vivo BrdU labeling, mice were injected i.p. with BrdU (250 mg/kg body weight) and then sacrificed 2 h later; tissue sections were subjected to immunofluorescence staining with fluorescein isothiocyanate-conjugated antibody to BrdU.
RESULTS
Expression of NF-κB in Stratified Epithelium.
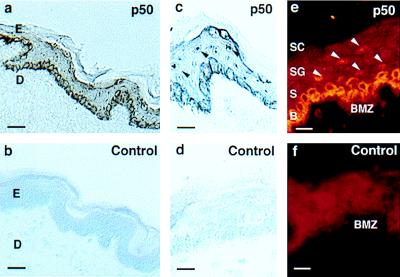
To examine a potential role for NF-κB in a stratified epithelial tissue, we analyzed expression of NF-κB subunits in non-sun-exposed human epidermis. In cells of the basal epithelial layer, p105/p50 is strongly expressed in the cytoplasm. In contrast, in the nonproliferative cells of the suprabasal layers this cytoplasmic expression is entirely absent, and cells demonstrate nuclear expression (Fig. 1). Given the well-characterized regulation of NF-κB via control of nuclear translocation (1, 2, 37, 38), these data suggest that NF-κB activation may play a role in the switch from the proliferative basal cell phenotype to the nonproliferative, terminal differentiation program of the suprabasal layers.
Figure 1.
Expression of NF-κB in stratified epithelium in vivo. Frozen tissue sections of non-sun-exposed adult human abdominal skin were immunostained with antibody to p50. Immunoperoxidase staining for (a and c) p50 and (b and d) secondary antibody alone control. Immunofluorescence staining for (e) p50 and (f) secondary antibody alone control. E, epidermis; D, dermis; SC, stratum corneum; SG, stratum granulosum; S, squamous layer; B, basal layer; BMZ, basement membrane zone. Arrows denote suprabasal nuclei. [Bars = 75 μM (a and b) and 25 μM (c–f).]
Transgenic Mice with Alterations in Epidermal NF-κB Function.
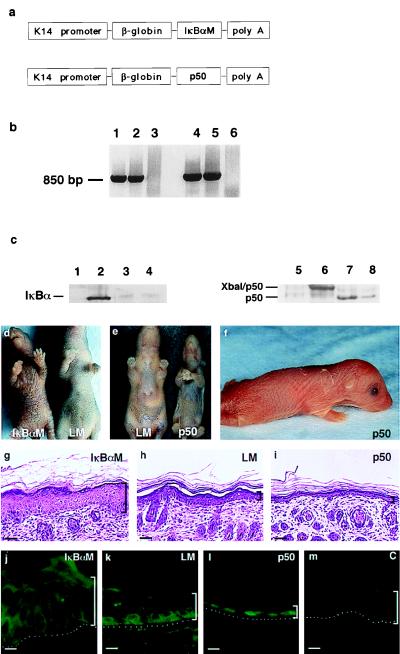
To investigate the role of NF-κB in stratified epithelium, we generated transgenic mice expressing proteins for dominant-negative inhibition as well as for constitutive activation of NF-κB function (Fig. 2). For blockade of epidermal NF-κB function, we targeted expression of the dominant-negative IκBαM mutant to epidermis via the keratin 14 promoter (13, 28, 39) (Fig. 2a). IκBαM contains substitutions at serines 32 and 36 of IκBα along with deletion of COOH-terminal PEST sequences (25) and was chosen because it has been shown to abolish nuclear DNA binding activity by any of the 5 NF-κB subunits in a range of mammalian cells (1, 25). Fourteen IκBαM[+] transgenic mice were generated with transgene integration confirmed by Southern blot analysis and PCR (Fig. 2b). IκBαM[+] mice express IκBαM on Western blot analysis of epidermal tissue extracts, as detected by antibodies to IκBα that recognize both the wild type and mutant proteins (Fig. 2c). IκBαM[+] mice also demonstrate markedly increased immunostaining with IκBα-specific antibodies throughout all layers of transgenic epidermis (data not shown), consistent with the expected resistance to degradation by this mutant protein. To test that IκBαM blocked nuclear localization of NF-κB in transgenic epidermis, immunostaining for p50 was performed and demonstrated a complete absence of nuclear NF-κB subunit expression in the suprabasal layers (Fig. 2j). All IκBαM[+] transgenic mice develop epidermal hyperplasia clinically and histologically within 4 days after birth (Fig. 2 d and g). This hyperplasia appeared to be caused by an increase in the thickness of the suprabasal squamous layer (Fig. 2g). In addition, these mice lack both clinical and histologic evidence of normal hair formation, exhibit growth retardation, and die within 5–7 days; no abnormalities in any internal organs are seen on either histologic or macroscopic evaluation.
Figure 2.
Transgenic mice engineered for gain and loss of epidermal NF-κB function. (a) Schematic of the K14-IκBαM and K14-p50 transgenes for targeted expression to murine epidermis. (b) Confirmation of transgene integration. Genomic DNA extracted from tail specimens of IκBαM[+] (lanes 1 and 2) and p50[+] (lanes 4 and 5) mice was subjected to PCR by using transgene-specific primers. Data obtained from unaffected littermates (lanes 3 and 6) with each primer set are also shown. (c) Western blot analysis of tissue protein extracts prepared from p50[+], IκBαM[+], and control mice. Lanes 1–4 are tissue extracts blotted with antibodies to IκBα. Lanes: 1, normal littermate skin; 2, IκBαM[+] skin; 3, normal littermate liver; 4, IκBαM[+] liver. Lanes 5–8 are blotted with antibodies to p50. Lanes: 5, normal littermate skin; 6, p50[+] skin; 7, normal littermate liver; 8, p50[+] liver. (d) Clinical appearance of K14-IκBαM transgenic mice. Five-day-old IκBαM[+] transgenic animals displayed clinical evidence of epidermal hyperplasia with markedly enhanced skin markings and a lack of visible hair growth compared with nontransgenic littermates. (e and f) Clinical appearance of 5-day-old p50[+] transgenic mice. p50[+] mice displayed clinical evidence of thin, slack skin compared with nontransgenic control. (g–i) Histology of IκBαM[+], age and site-matched control, and p50[+] mice; brackets define the thickness of the epidermis. Note increased epidermal thickness relative to normal in IκBαM[+] transgenic tissue compared with decreased epidermal thickness in p50[+] transgenic skin. (Bars = 75 μM.) (j–m) Epidermal expression pattern of p50 in (j) IκBαM[+] and (l) p50[+] transgenic mice along with (k) littermate and (m) secondary antibody alone controls.
These data suggest that nuclear translocation of NF-κB subunits may be necessary for growth control in stratified epithelium, raising the possibility that improper activation of NF-κB in the proliferative basal compartment of the epidermis could lead to premature growth inhibition. To test this hypothesis, we generated transgenic mice expressing constitutively nuclear NF-κB subunits in this location (p50 construct, Fig. 2a). This mutant p50 construct encodes a protein longer than the endogenous processed p50 and displays well characterized transactivating properties (26). Constitutive nuclear expression of p50 in transgenic skin was confirmed by immunofluorescence (Fig. 2l) and results in epidermal hypoplasia at the clinical and histologic levels (Fig. 2 e, f, and i); similar findings were observed in mice transgenic for K14-targeted p65 (data not shown). The markedly thin epidermis of p50[+] mice, consists of as little as two viable cell layers (Fig. 2i). In addition, p50[+] mice fail to gain weight normally, appear to become cachectic and die within 5 days after birth. The most severely affected mice demonstrate open eyes at birth accompanied by extreme skin fragility and death within hours after being born (Fig. 2f). Consistent with the specificity of K14-directed gene expression, no transgene expression or tissue abnormalities were observed in any internal organs, including liver, kidney, heart, and lung in either IκBαM[+] or p50[+] mice (Fig. 2c and data not shown), leaving the basis for the lethality associated with altered expression of NF-κB in the epidermis unclear.
Effect of a Pharmacologic Inhibitor of NF-κB on Normal Murine Skin.
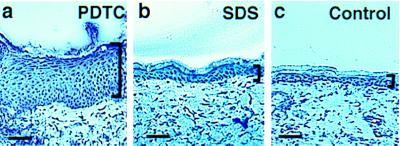
In an alternative approach to alter NF-κB function in epidermis, we applied a pharmacologic inhibitor of NF-κB, pyrrolidine dithiocarbamate (PDTC) (40), topically to the skin of normal adult C57BL/6J mice. SDS (0.1%) was applied under occlusion for the same period as an additional control for nonspecific reactive hyperplasia to irritant stimuli. Application of 10 mM PDTC for 1 week induces significant epidermal thickening over controls (Fig. 3), indicating that topical application of an agent that blocks NF-κB function to normal intact adult skin is associated with epidermal hyperplasia.
Figure 3.
Effect of a pharmacologic inhibitor of NF-κB function on normal murine skin. Histologic appearance of adult C57BL/6J mouse skin is shown after 7 days of twice daily topical application of (a) 10 mM PDTC in PBS versus (b) 0.1% SDS in PBS and (c) PBS alone (control). Brackets define the thickness of the epidermis (Bars = 100 μM.)
Growth Characteristics of Murine Epidermis Transgenic for Alterations in NF-κB Function.
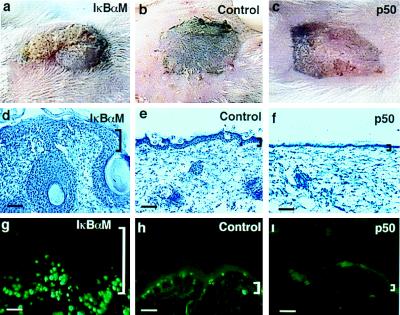
To follow epithelial growth characteristics beyond the time of perinatal mortality observed in both IκBαM[+] and p50[+] mice, skin from transgenic mice as well as nontransgenic littermate controls was grafted onto immune-deficient mice in an approach recently shown to preserve growth characteristics of donor skin (39). Grafted IκBαM[+] epidermis demonstrates pronounced epidermal hyperplasia clinically and histologically at 14 days postgrafting, with epidermal invaginations penetrating deeply into the underlying dermis (Fig. 4 a and d). p50[+] epidermis, in contrast, remains hypoplastic at this time point clinically and histologically (Fig. 4 c and f).
Figure 4.
Growth characteristics of murine epidermis transgenic for gain and loss of NF-κB function. (a–c) Clinical appearance of IκBαM[+], littermate control, and p50[+] skin harvested from 3-day-old transgenic mice and grafted to CB.17 scid/scid recipients, shown at 14 days postgrafting. (d–f) Histologic appearance of grafted skin from IκBαM[+], control, and p50[+] mice (scale bars = 150 μM). (g–i) The proportion of epithelial cells actively synthesizing DNA in vivo as a function of altered NF-κB activity. CB.17 scid/scid mice bearing IκBαM[+], p50[+], and nontransgenic control skin were injected with BrdU (250 mg/kg of body weight) i.p. Skin biopsy specimens were obtained 2 h later, and tissue sections were stained with antibody to BrdU. Shown are representative immunofluorescence micrographs. Note the increased labeling activity in IκBαM[+] skin that extends into cells of the suprabasal layers and the marked decrease in labeling activity in p50[+] skin compared with control. (Bars = 150 μM.)
To further examine the basis for these alterations in epidermal homeostasis, DNA synthetic activity was studied in transgenic and control epidermis by BrdU incorporation (Fig. 4 g–i). IκBαM[+] epidermis demonstrates a marked increase in proportion of cells actively synthesizing DNA. BrdU-positive cells in IκBαM[+] transgenic epidermis include those well above the basal layer (Fig. 4g), indicating a possible failure of the cell cycle arrest that is normally associated with outward migration. p50[+] epidermis, in contrast, demonstrates a near absence in cells incorporating BrdU over the 2-h time period analyzed, consistent with inhibition of proliferation (Fig. 4i). These findings suggest that functional loss of NF-κB leads to hyperproliferation in stratified epithelium caused by failure of growth arrest, in marked contrast to the proliferative defect seen with NF-κB blockade in lymphoid cells (41), and suggest that premature expression of activated NF-κB subunits in mitotically active basal epithelial cells leads to growth inhibition.
Impact of Altering NF-κB Activity in Human Skin Tissue in Vivo.
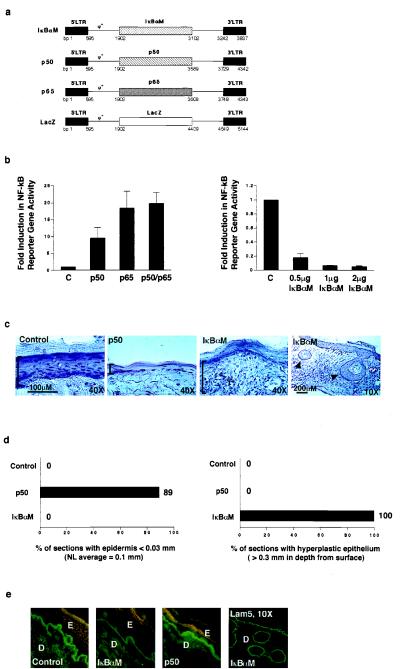
Epithelial neoplasia is much more readily induced in many strains of mice than in humans (42). To study the functional relevance of NF-κB in growth control of human stratified epithelium as distinct from murine tissue as well as to further examine NF-κB effects in postnatal, developmentally mature epithelium, we produced amphotropic retroviral expression vectors for mutant proteins exerting either activating or inhibitory effects on NF-κB function (Fig. 5). These retroviral vectors included dominant-negative IκBαM (25), constitutively nuclear mutant p50 (26), and p65/RelA (27); transduction with a lacZ vector (35); as well as mock transduction served as controls. These vectors effectively express full-length mutant proteins in primary cultures of human epithelial cells (data not shown). Expression of these proteins alters the subcellular distribution pattern of NF-κB subunits, with either high levels of constitutive nuclear expression (p50, p65) or a complete absence of nuclear NF-κB subunit expression (IκBαM), as anticipated. p50 combined with p65, as well as either subunit alone, produce consistent activation of NF-κB-directed gene expression (Fig. 5b); IκBαM, in contrast, blocks phorbol ester induced NF-κB-directed reporter gene expression in a dose-dependent manner (Fig. 5b), as expected.
Figure 5.
Impact of NF-κB on human epithelial growth in vivo. (a) Retroviral expression vectors for proteins exerting inhibitory and activating effects on NF-κB function. Diagrammed are vectors for dominant-negative mutant IκBαM, constitutively nuclear p50 (p50), and p65. The LZRS lacZ vector (35) served as a control for these studies. (b) Impact of inhibitory and activating NF-κB proteins on NF-κB-driven gene expression in epithelial cells in vitro. The panel of vectors above was expressed in human keratinocytes along with a reporter construct containing three copies of NF-κB DNA consensus binding sites driving expression of the luciferase reporter gene. All transfections were performed in triplicate. Twenty-four hours following gene transfer, cell extracts were prepared and analyzed for reporter gene activity. Data are reported as fold induction in reporter gene activity, normalized for transfection efficiency by using a cotransfected Rous sarcoma virus–chloramphenicol acetyltransferase internal control. C, lacZ control. For assessment of IκBαM dominant-negative effects, NF-κB activity was induced for 4 h with 30 ng/ml of the phorbol ester phorbol 12-myristate 13-acetate prior to performance of reporter gene analysis. (c) Tissue architecture of human skin expressing either activating or inhibitory NF-κB subunits and lacZ control. Retroviral expression vectors for proteins activating or inhibiting NF-κB function were utilized to transduce primary human keratinocytes in vitro. These cells were then used to regenerate human skin on CB.17 scid/scid mice. Histologic appearance is shown; immunostaining of each tissue section with species-specific antibodies to human involucrin was used to confirm human tissue origin prior to hematoxylin/eosin staining. Brackets define epidermal thickness; arrows in the low power (×10) field of IκBαM skin denote focal areas of deep hyperplasia. (d) Frequency of histologic abnormalities in human epidermis in vivo. Multiple 5-μM sections were obtained in a stepwise fashion through tissue biopsies that spanned the full 1.5 cm thickness of each regenerated human graft. Atrophic changes were defined as less than 30% thickness of viable epidermis as compared with normal average value of 0.1 mm found in controls. Deep hyperplasia was defined as epithelial tissue penetrating underlying dermis to a depth of at least 0.3 mm. For p50, a total of 9 representative tissue sections were analyzed from all grafted mice; for IκBαM, n =12; and for lacZ and unengineered control, n = 6. The data are expressed as the percent of individual tissue sections displaying the given histologic abnormality. (e) Expression of involucrin in human skin expressing either activating or inhibitory NF-κB subunits and lacZ control. Double immunostaining was performed with human species-specific antibodies to involucrin and laminin 5, a basement membrane zone protein used to highlight the inferior boundary of the basal epidermal layer; involucrin (rhodamine), and laminin 5 (fluorescein isothiocyanate). Low power field of laminin 5 immunostained IκBαM[+] skin is also shown to highlight the boundaries of deep human epithelium. E, epidermis; D, dermis.
These vectors were then used to examine the impact of altering NF-κB function in human epithelial tissue in vivo. Keratinocytes expressing p50, IκBαM, and lacZ control were used to regenerate human epidermis on severe combined immunodeficient (SCID) mice. Six mice were grafted for each vector in two separate sets of experiments and analyzed 3 weeks later. Similar to transgenic mice, p50 localization was appropriately altered in engineered epidermis compared with controls. Consistent with findings in transgenic murine tissue, histologic analysis showed p50[+] human epidermis to be markedly atrophic whereas IκBαM[+] specimens display epithelial hyperplasia (Fig. 5 c and d). This hyperplasia is characterized by epidermal thickening as well as formation of invaginations penetrating deeper in the dermis (Fig. 5c), similar to those observed in IκBαM[+] transgenic mice. An additional trans-dominant repressor for NF-κB function, the p50 ΔSP mutant (34), produces uniformly hyperplastic changes in vivo as well; however, these are less deeply invasive (data not shown), consistent with its less potent spectrum of inhibitory effects on NF-κB function than those of IκBαM (1, 25, 34). These changes in tissue architecture in vivo confirm NF-κB effects in an alternative model system to transgenic mice that uses postnatal human tissue.
In addition to growth arrest, suprabasal cells in stratified epithelium activate expression of proteins necessary for terminal differentiation. To study the involvement of NF-κB in the expression of such differentiation genes, we analyzed the keratin 10, involucrin, transglutaminase 1, and filaggrin differentiation markers in all vector groups. These proteins are expressed in normal suprabasal distribution in epidermis engineered for either loss or gain of NF-κB function, suggesting that NF-κB does not exert a dominant role in the regulation of differentiation gene expression in stratified epithelium (Fig. 5e and data not shown).
DISCUSSION
Although NF-κB/Rel proteins have been extensively studied in lymphoid tissue, little is known about their expression and function in epithelia. In lymphocytes and other nonepithelial cell types, NF-κB proteins promote cellular proliferation, inhibit apoptosis, and activate cellular stress responses (1, 2). Targeted inactivation of murine NF-κB/Rel genes has not yet revealed a dramatic phenotype in stratified epithelium; however, this may be due to the embryonic lethality observed in some cases or to compensation by other NF-κB proteins in others. The inflammatory infiltrate seen in skin of IκBα knock-out mice (43) suggests a role for NF-κB in skin but does not support a primary role in epithelial homeostasis as distinct from the secondary changes commonly observed during cutaneous inflammation. Therefore, we expressed proteins exerting both dominant-negative and constitutively active effects on NF-κB function to examine a possible role for NF-κB in stratified epithelium. This general strategy has also identified key roles for other transcription factors in epithelia that were undetected in knock-out models, including retinoid receptors (13). Recently, this approach has also been used to help confirm a previously suggested role for NF-κB proteins in promoting lymphocyte proliferation that had not been as evident in prior knock-out models (41). Our findings suggest that functional nuclear NF-κB may be necessary for the growth inhibition characteristic of upward cellular migration and differentiation in stratified epithelium. In such a setting, inactive NF-κB subunits sequestered in the cytoplasm of proliferative basal cells would undergo nuclear translocation and activation in a manner spatially restricted to cells preparing to undergo terminal differentiation. This model would predict that premature activation of NF-κB in mitotically active basal cells would lead to growth inhibition and epithelial hypoplasia, similar to what we have observed. Direct cell cycle inhibition by NF-κB has not been previously reported. Given the pleiotropic effects of NF-κB in other tissues, NF-κB could alter epithelial growth via either a direct impact on cell cycle regulators or by a more indirect process. Such indirect effects could include the induction of growth inhibitory cytokines.
In spite of their opposite effects on epidermal growth, IκBαM[+] and p50[+] mice share common features of growth retardation and death within the first week after birth, suggesting that proper epidermal growth regulation may be necessary for normal postnatal survival and development. A clear gene dosage-dependent severity of phenotype could not be confirmed in any of the transgenic mice analyzed. Although it is possible that posttranslational control of transgene protein expression may have contributed to this observation, the fact that higher levels of mutant proteins in skin could lead to embryonic lethality in mice expressing high levels of transgenes is also a formal possibility. Our findings are also consistent with several other murine genetic models characterized by abnormal stratified epithelium. Transgenic mice with K14 promoter targeted expression of tumor necrosis factor α (TNF-α) in epidermis also demonstrate epidermal hypoplasia, inhibition of proliferation, and cachexia (44), a finding that may be explained by the fact that TNF-α is a potent activator of NF-κB function. Interestingly, the most severely affected p50 transgenic mice present with open eyes at birth. This resembles the phenotype of another series of mice generated for targeted disruption of the epidermal growth receptor who also show epidermal hypoplasia (45–47). Thus far, a clear role for NF-κB in epidermal growth factor signaling pathways has not been established; however, these phenotypic similarities suggest either possible direct linkage in effector pathways controlling epithelial growth, as may be the case for TNF-α activation of NF-κB, or common defects in epithelial growth control caused by altering distinct pathways.
We have shown that topical application of the NF-κB inhibitor PDTC induces hyperplasia of normal epidermis within one week. PDTC, however, is not an entirely NF-κB-selective compound, and the development of more potent and specific compounds will be necessary to extend this finding with greater precision. Our data raise the possibility that the opposite approach, namely application of a pharmacologic inducer of NF-κB function could be used to treat hyperproliferative human skin disorders such as psoriasis. Interestingly, it has recently been shown that anthralin, a topical agent used for decades in the treatment of psoriasis, activates NF-κB in a dose-dependent fashion (48). Consistent with this, UV light—a mainstay of current treatment for severe psoriasis—is also a potent inducer of NF-κB in skin (24). The complexity of such proliferative disorders of epithelium, however, requires careful future studies to determine any actual potential utility for direct topical regulation of NF-κB function in this setting.
Based on our findings using both genetic and pharmacologic approaches, it appears that spatially restricted nuclear translocation and activation of NF-κB contribute to growth inhibition in stratified epithelium.
Acknowledgments
We thank P. Jackson for helpful early discussions and advice. We thank G. Crabtree, G. Nolan, P. Jackson, E. Bauer, A. Oro, M. Scott, and J. Healy for presubmission review; G. Nolan for LZRS and p65 plasmids; I. Verma for the IκBαM plasmid; A. Israel for the XbaI p105 plasmid and p50 ΔSP mutant; G. Crabtree for the NF-κB reporter plasmid; E. Fuchs for the K14 promoter plasmid; P. Marinkovich for Laminin 5 antibody; and N. Griffiths and P. Bernstein for administrative support. This work was supported by the Office of Research and Development, Department of Veterans Affairs, and National Institutes of Health Grant AR43799 (to P.A.K.). C.S.S is the recipient of a postdoctoral fellowship award from Deutsche Forschungsgemeinschaft.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: IκBαM, mutant IκBα dominant-negative for NF-κB function; ΔSP, mutant p50 dominant-negative for NF-κB function; PDTC, pyrrolidine dithiocarbamate.
References
- 1.Baeuerle P A, Baltimore D. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A S., Jr Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs E. J Cell Sci Suppl. 1993;17:197–208. doi: 10.1242/jcs.1993.supplement_17.28. [DOI] [PubMed] [Google Scholar]
- 5.Jones P H, Harper S, Watt F M. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 6.Byrne C, Tainsky M, Fuchs E. Development (Cambridge, UK) 1994;120:2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- 7.Briata P, D’Anna F, Franzi A T, Gherzi R. Exp Cell Res. 1993;204:136–146. doi: 10.1006/excr.1993.1018. [DOI] [PubMed] [Google Scholar]
- 8.Welter J F, Crish J F, Agarwal C, Eckert R L. J Biol Chem. 1995;270:12614–12622. doi: 10.1074/jbc.270.21.12614. [DOI] [PubMed] [Google Scholar]
- 9.Lu B, Rothnagel J A, Longley M A, Tsai S Y, Roop D R. J Biol Chem. 1994;269:7443–7449. [PubMed] [Google Scholar]
- 10.Gandarillas A, Watt F M. Oncogene. 1995;11:1403–1407. [PubMed] [Google Scholar]
- 11.Magnaldo T, Vidal R G, Ohtsuki M, Freedberg I M, Blumenberg M. Gene Exp. 1993;3:307–315. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou P, Byrne C, Jacobs J, Fuchs E. Genes Dev. 1995;9:700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]
- 13.Saitou M, Sugai S, Tanaka T, Shimouchi K, Fuchs E, Narumiya S, Kakizuka A. Nature (London) 1995;374:159–162. doi: 10.1038/374159a0. [DOI] [PubMed] [Google Scholar]
- 14.Roop D R, Huitfeldt H, Kilkenny A, Yuspa S H. Differentiation. 1987;35:143–150. doi: 10.1111/j.1432-0436.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 15.Vollberg T M, Sr, Nervi C, George M D, Fujimoto W, Krust A, Jetten A M. Mol Endocrinol. 1992;6:667–676. doi: 10.1210/mend.6.5.1318502. [DOI] [PubMed] [Google Scholar]
- 16.Fisher G J, Reddy A P, Datta S C, Kang S, Yi J Y, Chambon P, Voorhees J J. J Invest Dermatol. 1995;105:80–86. doi: 10.1111/1523-1747.ep12313352. [DOI] [PubMed] [Google Scholar]
- 17.Scott G A, Goldsmith L A. J Invest Dermatol. 1993;101:3–8. doi: 10.1111/1523-1747.ep12358258. [DOI] [PubMed] [Google Scholar]
- 18.Andersen B, Schonemann M D, Flynn S E, Pearse R V d, Singh H, Rosenfeld M G. Science. 1993;260:78–82. doi: 10.1126/science.7682011. [DOI] [PubMed] [Google Scholar]
- 19.Faus I, Hsu H J, Fuchs E. Mol Cell Biol. 1994;14:3263–3275. doi: 10.1128/mcb.14.5.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morasso M I, Markova N G, Sargent T D. J Cell Biol. 1996;135:1879–1887. doi: 10.1083/jcb.135.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. Nature (London) 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 22.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 23.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 24.Fisher G J, Datta S C, Talwar H S, Wang Z Q, Varani J, Kang S, Voorhees J J. Nature (London) 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 25.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 26.Blank V, Kourilsky P, Israel A. EMBO J. 1991;10:4159–4167. doi: 10.1002/j.1460-2075.1991.tb04994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan G P, Ghosh S, Liou H C, Tempst P, Baltimore D. Cell. 1991;64:961–996. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- 28.Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Proc Natl Acad Sci USA. 1989;86:1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choate K A, Medalie D A, Morgan J R, Khavari P A. Nat Med. 1996;2:1263–1267. doi: 10.1038/nm1196-1263. [DOI] [PubMed] [Google Scholar]
- 30.Medalie D A, Eming S A, Tompkins R G, Yarmush M L, Krueger G G, Morgan J R. J Invest Dermatol. 1996;107:121–127. doi: 10.1111/1523-1747.ep12298363. [DOI] [PubMed] [Google Scholar]
- 31.Choate K A, Kinsella T M, Williams M L, Nolan G P, Khavari P A. Hum Gene Ther. 1996;7:2247–2253. doi: 10.1089/hum.1996.7.18-2247. [DOI] [PubMed] [Google Scholar]
- 32.Khavari P A, Peterson C L, Tamkun J W, Mendel D B, Crabtree G R. Nature (London) 1993;366:170–174. doi: 10.1038/366170a0. [DOI] [PubMed] [Google Scholar]
- 33.Rheinwald J G, Green H. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 34.Logeat F, Israel N, Ten R, Blank V, Le Bail O, Kourilsky P, Israel A. EMBO J. 1991;10:1827–1832. doi: 10.1002/j.1460-2075.1991.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinsella T M, Nolan G P. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 36.Freiberg R A, Spencer D M, Choate K A, Peng P D, Schreiber S L, Crabtree G R, Khavari P A. J Biol Chem. 1996;271:31666–31669. doi: 10.1074/jbc.271.49.31666. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh S, Gifford A M, Riviere L R, Tempst P, Nolan G P, Baltimore D. Cell. 1990;62:1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- 38.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 39.Oro A E, Higgins K M, Hu Z, Bonifas J M, Epstein E H, Scott M P. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 40.Schreck R, Meier B, Mannel D N, Droge W, Baeuerle P A. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boothby M R, Mora A L, Scherer D C, Brockman J A, Ballard D W. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Horst G T J, van Steeg H, Berg R J W, van Gool A J, de Wit J, et al. Cell. 1997;89:425–435. doi: 10.1016/s0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- 43.Beg A A, Sha W C, Bronson R T, Baltimore D. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J, Turksen K, Yu Q C, Schreiber H, Teng M, Fuchs E. Genes Dev. 1992;6:1444–1456. doi: 10.1101/gad.6.8.1444. [DOI] [PubMed] [Google Scholar]
- 45.Miettinen P J, Berger J E, Meneses J, Phung Y, Pedersen R A, Werb Z, Derynck R. Nature (London) 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 46.Sibilia M, Wagner E F. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 47.Threadgill D W, Dlugosz A A, Hansen L A, Tennenbaum T, Lichti U, et al. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt K N, Podda M, Packer L, Baeuerle P A. J Immunol. 1996;156:4514–4519. [PubMed] [Google Scholar]