Abstract
Extraintestinal pathogenic Escherichia coli (ExPEC) strains possess several siderophore-dependent iron uptake systems. In this study we demonstrated that the salmochelin siderophore receptor IroN is involved in the invasion of urothelial cells by ExPEC in vitro. Thus, IroN may play a dual role in the establishment of urinary tract infections, displaying an iron uptake receptor as well as an internalization factor.
Extraintestinal pathogenic Escherichia coli (ExPEC) strains possess an array of specific virulence factors that enable them to cause infections outside the gastrointestinal tract. These infections range from asymptomatic urinary tract infections (UTIs) to life-threatening diseases, such as pyelonephritis or sepsis (12, 17). The acquisition of iron (Fe3+) is a critical step in the pathogenesis of UTIs, as the concentration of free Fe3+ is extremely limited at the sites of infection in mammalian hosts. In order to acquire iron from the host organism, ExPEC strains have developed a variety of iron uptake mechanisms, such as the synthesis and transport of low-molecular-weight iron chelators, called siderophores (7). Recent studies have revealed that several siderophore systems are more prevalent in ExPEC than in commensal strains (10, 23) and play an important role in the pathogenesis of UTIs (11, 21). Two of these systems are the yersiniabactin siderophore system with the outer membrane receptor FyuA (4, 6, 22, 23) and the salmochelin system, which is characterized by the novel catecholate receptor IroN (2, 9, 19). The presence of these different iron uptake systems in ExPEC strains prompted us to look for further functions of the siderophore systems in the pathogenicity of ExPEC strains. Recent studies have provided evidence that siderophore systems of ExPEC strains may contribute to other virulence traits, such as adherence and invasion (18, 20). This is of particular interest, as it has recently been shown that ExPEC strains are able to form intracellular, biofilm-like structures in epithelial cells of the bladder in mice (1).
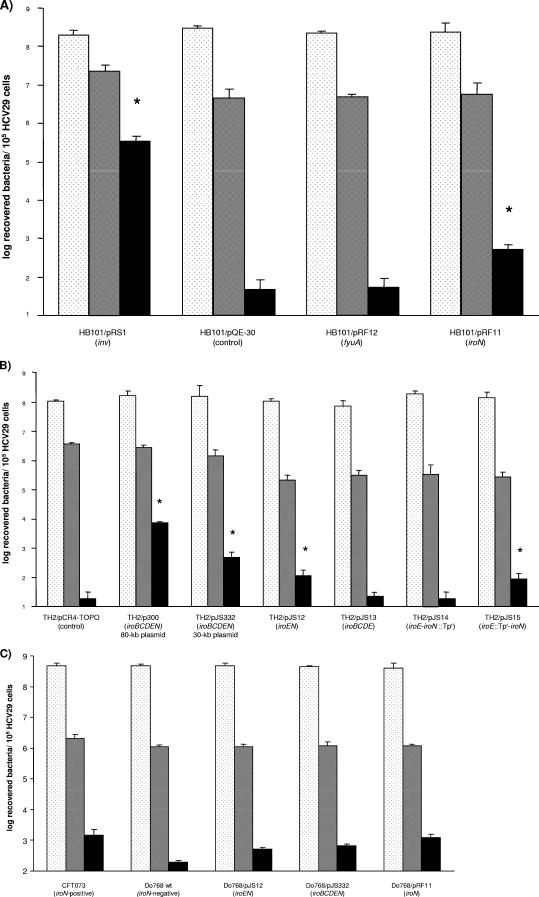
The aim of this study was to investigate the possible involvement of FyuA or IroN in mediating the invasion of urothelial cells by ExPEC strains. For this, IroN and FyuA proteins were recombinantly expressed in E. coli laboratory strain HB101 (3), and the invasion of urothelial cell line HCV29 by the resulting strains was assessed (14). In brief, the iroN gene of E. coli uropathogenic strain CFT073 and the fyuA gene of E. coli uropathogenic strain 536 were each cloned into the expression plasmid pQE-30 (QIAGEN, Hilden, Germany), resulting in recombinant plasmids pRF11 and pRF12, respectively. These plasmids were subsequently transformed into E. coli strain HB101, and the protein expression was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. For the in vitro invasion studies, bacterial strains, were grown at 37°C in Luria-Bertani medium to the logarithmic phase, and protein expression was induced. The invasion of HCV29 cells by recombinant HB101 strains was studied by means of the gentamicin protection assay (15). The premise of this assay is that the bacteria which are able to invade host cells can be identified by adding gentamicin, which kills all extracellular bacteria but not the intracellular bacteria since gentamicin is unable to enter host cells. In brief, HCV29 cells were infected at a multiplicity of infection of 50 to 100 bacteria per host cell. After 1 h of incubation, three fractions of bacteria were separated. First, the total number of infecting bacteria was determined after direct lysis of the host cells. The second fraction of cells was washed prior to lysis and represented the adherent and invaded bacteria. For the third fraction gentamicin was added, and the cells were lysed after an additional 1 h of incubation, resulting in the number of invaded bacteria. For each panel, the experiments were repeated at least three times, and comparable results were obtained in all experiments. The levels of invading E. coli HB101 were expressed as the means and standard deviations of six independent samples. The Student t test for unpaired data was used to test the significance of differences between the means, and P values of <0.05 were considered statistically significant. The expression of IroN was associated with a 5- to 10-fold increase in the rate of invasion by the E. coli HB101 host strain (Fig. 1A). Interestingly, FyuA did not increase the rate of invasion of HCV29 by the HB101 host strain. E. coli HB101 expressing the Yersinia enterocolitica O:8 WA-C invasin Inv (24) [HB101(pRS1)] was used as a positive control, and the number of invaded bacteria was 103-fold greater than the number obtained with HB101 carrying the pQE-30 vector alone. In addition, the level of invasion of IroN-expressing strain HB101(pRF11) was considerably lower after preincubation with a polyclonal antibody raised against IroN (data not shown).
FIG. 1.
Gentamicin protection assays using recombinant E. coli and HCV29 urothelial cells. (A) E. coli HB101 carrying expression plasmids for the Inv (pRS1), IroN (pRF11), and FyuA (pRF12) proteins. E. coli HB101 carrying the empty vector (pQE-30) was used as a negative control. (B) Results of the protection assay using E. coli TH2 with the entire iro region or with parts of the iro gene cluster, all expressed under control of the native promoter. E. coli TH2 carrying the empty vector (pCR4-TOPO) was used as a negative control. (C) Complementation of iroN-negative ExPEC strain Do768 with iroN-containing plasmids (pJS12, pJS332, and pRF11) led to levels of invasion comparable to the level observed for ExPEC strain CFT073. The bars indicate the total numbers of bacteria added (dotted bars), the numbers of adherent bacteria (gray bars), and the numbers of invaded bacteria (solid bars). The data are means and standard errors of the means (error bars) obtained from six wells. An asterisk indicates that the value is statistically significantly different than the value for the vector control (P < 0.05).
In order to exclude artifacts caused by the high level of IroN expression of the recombinant plasmid pRF11, we determined the invasion phenotype mediated by IroN expressed under the control of a native promoter. To do this, invasion assays were performed using E. coli laboratory strain TH2 (Takara, Bio Inc., Shiga, Japan) transformed with a recently identified 80-kb wild-type plasmid of ExPEC (p300) that carried the entire iroBCDEN gene cluster (25). To exclude the possibility that an invasive phenotype was caused by unknown genes present on p300, we examined E. coli TH2 strains containing plasmids carrying the subcloned iroBCDEN gene cluster (pJS332), as well as parts of the iro gene cluster (pJS12 for iroEN and pJS13 for iroBCDE). Consistent with the results obtained with recombinantly expressed IroN, we observed a significant difference between the invasion rates of the TH2 strains carrying plasmids with the entire iro gene cluster (p300 and pJS332) and the invasion rate of the TH2 strain carrying only the pCR4-TOPO vector (control) (Fig. 1B). We found that the plasmid which carried the iroEN genes (pJS12) consistently gave rise to the invasive phenotype, whereas plasmid pJS13 carrying the iroBCDE genes did not. To confirm that invasion was mediated by IroN, we compared the invasion rate of the E. coli TH2 strain carrying the intact iroN and iroE genes (plasmid pJS12) with the invasion rates of TH2 strains carrying either an intact iroN gene (pJS14) or an iroE gene (pJS15). Plasmids pJS14 and pJS15 were derived from plasmid pJS12 (iroEN) by EZ::TN transposon mutagenesis of the iroN and iroE genes, respectively. Concordantly, the transposon insertion in the iroE gene did not influence the invasive phenotype of TH2(pJS15), whereas mutation of iroN led to a significant decrease in the invasion rate of TH2(pJS14) (Fig. 1B). It is noteworthy that the IroN-positive E. coli strains [TH2(pJS12) and TH2(pJS15)] and the IroN-negative E. coli strains [TH2(pJS13) and TH2(pJS14)] exhibited comparable levels of adherence (Fig. 1B). Therefore, we excluded the possibility that the increased-invasion phenotype of IroN-expressing strains is the result of enhanced attachment to HCV29 cells. This is in agreement with results obtained in a recent study (13).
In order to confirm the IroN-mediated effect on invasion of urothelial cells in an ExPEC background, we performed invasion assays with the iroN-deficient ExPEC strain Do768. This strain is a clinical isolate harboring several typical ExPEC virulence genes (e.g., fyuA, chuA, usp, iha, pap, and auf) but not the iroN gene. Interestingly, strain Do768 was about 10-fold less invasive than iroN-positive ExPEC strain CFT073 (Fig. 1C). The introduction of iroN-carrying plasmids (pJS12, pJS332, and pRF11) into strain Do768 resulted in a significant increase in the invasion rate and intracellular bacterial counts comparable to those obtained with strain CFT073 (Fig. 1C). These data corroborate our finding that IroN contributes to the invasion of urothelial cells by ExPEC.
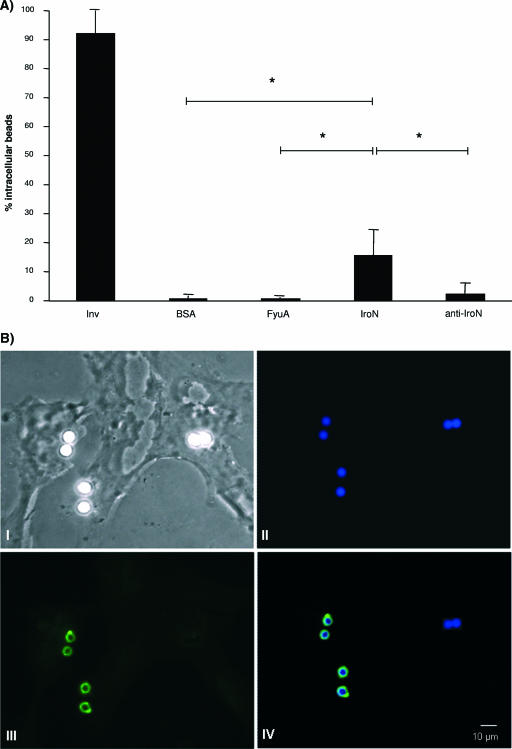
Furthermore, we performed invasion studies with latex beads coated with recombinant proteins in order to exclude the possibility that other E. coli virulence traits, such as fimbriae or adhesins, affect the phenotype observed. The latex beads (4-μm blue fluorescent sulfate microspheres; Molecular Probes, Invitrogen, Karlsruhe, Germany) were coated with purified recombinant proteins FyuA, IroN, and Inv (26) or with bovine serum albumin according to the manufacturer's recommendations. Coated beads were centrifuged (200 × g for 2 min) onto HCV29 cells grown on glass slides, and this was followed by incubation at 37°C for 1 h. Immunofluorescence microscopy was performed as described elsewhere (26). Ten areas per slide were examined to determine the amounts of intracellular beads. The number of intracellular beads was expressed as a percentage of all blue fluorescent latex beads present in the sample. All experiments were repeated at least three times, and the data were expressed as the means and standard deviations of 10 samples. The data for the uptake of the protein-coated latex beads by urothelial cells are shown in Fig. 2. Latex beads coated with recombinant IroN were internalized by the HCV29 cells at a frequency that was between 10- and 20-fold higher than the frequency with which FyuA- or bovine serum albumin-coated beads were internalized (Fig. 2). In addition, the uptake of IroN-coated beads could be inhibited by preincubation with a polyclonal antibody raised against IroN (Fig. 2).
FIG. 2.
Internalization of protein-coated latex beads by HCV29 urothelial cells. (A) Number of intracellular beads expressed as a percentage of all beads present in the sample. The data are means and standard deviations (error bars). An asterisk indicates that values are statistically significantly different at a P value of <0.05. (B) Fluorescence microscopy of IroN-coated latex beads placed on HCV29 cells. Image I is a phase-contrast micrograph. Corresponding fluorescent images show the total number of IroN-coated latex beads (blue) (image II) and the fraction of extracellular beads (green) (image III). Images II and III were merged to obtain image IV, in which the intracellular beads are blue and the extracellular beads are turquoise.
Together, these results demonstrate that the salmochelin receptor IroN contributes to the invasion by ExPEC strains in vitro. In contrast, the yersiniabactin receptor FyuA did not appear to have any direct effect on the invasion by ExPEC strains in vitro. As shown in Fig. 1, no increase in adherence was conferred by the presence of IroN, suggesting that IroN-mediated invasion is not due to enhanced adherence. Instead, IroN may directly trigger the uptake of ExPEC by host cells. Furthermore, we observed that the IroN-mediated invasion was not unique to HCV29 cells, as similar invasion results were obtained using different urothelial cell lines (data not shown).
The functional redundancy of five or more iron uptake systems is a unique feature of ExPEC strains, which may indicate that siderophore receptors play additional roles in the pathogenicity of these strains. In this regard, the recently identified siderophore receptor IreA (iron-responsive element) has been demonstrated to function both as a siderophore receptor and as an adhesin in UTIs (18). A recent study characterized the outer membrane protein Iha as both a catecholate siderophore receptor and an adherence factor in ExPEC strains (13). Previous epidemiological studies revealed the prevalence of FyuA and IroN in ExPEC strains (5, 8, 10). There is also accumulating experimental evidence indicating that the IroN protein is involved in infection by ExPEC strains (16, 20, 21). However, the previous studies did not provide evidence concerning the direct involvement of IroN in invasion of urothelial cells. Here we demonstrate for the first time that the catecholate receptor IroN contributes to the invasion of urothelial cells by ExPEC strains. The findings presented here corroborate the hypothesis that siderophore receptors may have a dual role in the pathogenesis of ExPEC, as they facilitate both metabolic functions and host cell interactions. Studies are under way to characterize a putative host receptor responsible for the IroN-mediated invasion and to determine the exact mechanism by which IroN contributes to urovirulence.
Acknowledgments
This study was supported by the Bundesministerium für Bildung und Forschung (BMBF) and the Deutsche Forschungsgemeinschaft (DFG) (grant SCHU1494/2-1 to S.S.). We gratefully acknowledge the support of F.F. by grants from the DFG-Graduiertenkolleg “Infektion und Immunität” and from the European Graduate Academy of the Network of Excellence EuroPathoGenomics (NoE-EPG).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iron (E. coli). J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 4.Carniel, E. 2001. The Yersinia high-pathogenicity island: an iron-uptake island. Microbes Infect. 3:561-569. [DOI] [PubMed] [Google Scholar]
- 5.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clermont, O., S. Bonacorsi, and E. Bingen. 2001. The Yersinia high-pathogenicity island is highly predominant in virulence-associated phylogenetic groups of Escherichia coli. FEMS Microbiol. Lett. 196:153-157. [DOI] [PubMed] [Google Scholar]
- 7.Crosa, J. H. 1989. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol. Rev. 53:517-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duriez, P., O. Clermont, S. Bonacorsi, E. Bingen, A. Chaventre, J. Elion, B. Picard, and E. Denamur. 2001. Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology 147:1671-1676. [DOI] [PubMed] [Google Scholar]
- 9.Hantke, K., G. Nicholson, W. Rabsch, and G. Winkelmann. 2003. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 100:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J. R., M. A. Kuskowski, A. Gajewski, S. Soto, J. P. Horcajada, M. T. Jimenez de Anta, and J. Vila. 2005. Extended virulence genotypes and phylogenetic background of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J. Infect. Dis. 191:46-50. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, J. R., F. Scheutz, P. Ulleryd, M. A. Kuskowski, T. T. O'Bryan, and T. Sandberg. 2005. Phylogenetic and pathotypic comparison of concurrent urine and rectal Escherichia coli isolates from men with febrile urinary tract infection. J. Clin. Microbiol. 43:3895-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 13.Leveille, S., M. Caza, J. R. Johnson, C. Clabots, M. Sabri, and C. M. Dozois. 2006. Iha from an Escherichia coli urinary tract infection outbreak clonal group A strain is expressed in vivo in the mouse urinary tract and functions as a catecholate siderophore receptor. Infect. Immun. 74:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masters, J. R., P. J. Hepburn, L. Walker, W. J. Highman, L. K. Trejdosiewicz, S. Povey, M. Parkar, B. T. Hill, P. R. Riddle, and L. M. Franks. 1986. Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines. Cancer Res. 46:3630-3636. [PubMed] [Google Scholar]
- 15.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negre, V. L., S. Bonacorsi, S. Schubert, P. Bidet, X. Nassif, and E. Bingen. 2004. The siderophore receptor IroN, but not the high-pathogenicity island or the hemin receptor ChuA, contributes to the bacteremic step of Escherichia coli neonatal meningitis. Infect. Immun. 72:1216-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard, B., J. S. Garcia, S. Gouriou, P. Duriez, N. Brahimi, E. Bingen, J. Elion, and E. Denamur. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo, T. A., U. B. Carlino, and J. R. Johnson. 2001. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 69:6209-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo, T. A., U. B. Carlino, A. Mong, and S. T. Jodush. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, R. Olson, and G. E. Wilding. 2003. The siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect. Immun. 71:7164-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert, S., B. Picard, S. Gouriou, J. Heesemann, and E. Denamur. 2002. Yersinia high-pathogenicity island contributes to virulence in Escherichia coli causing extraintestinal infections. Infect. Immun. 70:5335-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schubert, S., A. Rakin, H. Karch, E. Carniel, and J. Heesemann. 1998. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect. Immun. 66:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulte, R., R. Zumbihl, D. Kampik, A. Fauconnier, and I. B. Autenrieth. 1998. Wortmannin blocks Yersinia invasin-triggered internalization, but not interleukin-8 production by epithelial cells. Med. Microbiol. Immunol. 187:53-60. [DOI] [PubMed] [Google Scholar]
- 25.Sorsa, L. J., S. Dufke, J. Heesemann, and S. Schubert. 2003. Characterization of an iroBCDEN gene cluster on a transmissible plasmid of uropathogenic Escherichia coli: evidence for horizontal transfer of a chromosomal virulence factor. Infect. Immun. 71:3285-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiedemann, A., S. Linder, G. Grassl, M. Albert, I. Autenrieth, and M. Aepfelbacher. 2001. Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages. Cell. Microbiol. 3:693-702. [DOI] [PubMed] [Google Scholar]