Abstract
Streptococcus pyogenes is a major cause of pharyngitis in humans and encodes several fibronectin-binding proteins. M protein and protein F1 (PrtF1/SfbI) are differentially regulated by CO2 and O2, respectively, and both mediate the invasion of epithelial cells. This study examined whether PrtF1/SfbI shares other properties with M protein. Expression of the PrtF1/SfbI protein by an M-negative mutant conferred resistance to phagocytosis and partial inhibition of C3 deposition on the S. pyogenes surface.
Streptococcus pyogenes (group A streptococcus) is an important human pathogen that causes pharyngitis, skin infections, invasive disease, sepsis, and toxic shock-like syndrome (7). S. pyogenes can persist at the pharyngeal mucosa and in tonsils and can be shed in saliva, despite antibiotic therapy and the absence of clinical disease. Tonsils of 13 of 14 children who were treated with antibiotics and subjected to tonsillectomies retained intracellular S. pyogenes, indicating that tonsils are an important reservoir (20, 23). S. pyogenes expresses several fibronectin (Fn)-binding proteins, which allow binding to extracellular-matrix components at mucosal surfaces and subsequent invasion of several cell lines and tonsillar epithelial cells (5, 15). The mga regulon encodes one or more M-like proteins, which vary functionally in their abilities to bind immunoglobulins, Fn, fibrinogen, and albumin (7, 9). Protein F1 (PrtF1/SfbI), which is not part of the mga regulon, also binds Fn and promotes adhesion and internalization by epithelial cells (11, 17, 24, 26). M protein and PrtF1/SfbI are genetically unlinked, and their expression is differentially regulated by oxygen and carbon dioxide, respectively (10).
M protein expression allows S. pyogenes to resist phagocytosis by two mechanisms. M protein-expressing bacteria bind factor H, a regulator of the complement system, which inhibits C3b deposition (9). M protein can also bind fibrinogen, which inhibits the alternative complement pathway (12). The opsonization of S. pyogenes with neutralizing antibodies against M protein or other surface proteins enhances C3 fixation and subsequent phagocytosis by neutrophils (7). Recently, we have demonstrated that both M protein-mediated and PrtF1/SfbI-mediated streptococcal invasions of epithelial cells activate a common signaling pathway (28). The functional similarities between M protein and PrtF1/SfbI prompted us to investigate whether PrtF1/SfbI, like M protein, permits S. pyogenes to resist phagocytosis. In this study, we demonstrate that PrtF1/SfbI expression confers both increased invasion of epithelial cells and resistance to phagocytosis when it is expressed in an S. pyogenes strain with an M1 background.
Bacterial strains and growth.
Streptococci were grown in THY (Todd-Hewitt broth supplemented with 0.5% yeast extract), in THB-Neo (Todd-Hewitt broth supplemented with 2% Neopeptone [Difco Laboratories, Detroit, MI]), or on solid media containing Difco blood agar base and 5% sheep blood. Strain 90-226 (serotype M1) was originally isolated from the blood of a septic patient (8), and its isogenic mutant 90-226Δemm1 has been described previously (30). The pPTF8 plasmid was constructed by inserting a prtF1-containing fragment of the JRS75 chromosome into the Escherichia coli-streptococcus shuttle vector pLZ12 (11). Plasmid pKH3, which contains the prtF1 promoter but not the prtF1 gene, was constructed from pPTF8 by a long PCR amplification of the plasmid using PfuTurbo (Stratagene) with the forward primer 5′-GTTTAAACCTGTCAGGCGCGCCTGACGTAAAAGTGTTCCATA-3′ and the reverse primer 5′-GGCGCGCCTGACAGGTTTAAACTCTCCTCTCACAAACATATA-3′ (AscI and PmeI restriction sites are underlined). The circular PCR product was digested with DpnI and used to transform E. coli DH5α, and purified plasmids were electroporated into strain 90-226Δemm1. Strains JSR4 and SAM1 were kindly provided by M. G. Caparon (11).
Protein analysis.
Extracts of cell wall-attached proteins were prepared from bacteria grown to logarithmic phase (A600 ≈ 0.5). Bacterial cell walls were digested by incubation in 10 mM Tris, 1 mM EDTA, and 20% sucrose buffer with lysozyme and mutanolysin (Sigma) in the presence of protease inhibitors for 2 h at 37°C. The soluble fraction was concentrated with a Centricon YM-10 centrifugal filter device (nominal molecular weight limit, 10,000) (Millipore), and the higher-molecular-weight fractions were quantitated by bicinchoninic acid assay (Pierce). Fifteen micrograms of total protein was analyzed by probing a Western blot with anti-SfbI rabbit serum, a kind gift from Gursharan S. Chhatwal. The blot was scanned, and the color and brightness of the resulting image were adjusted by Adobe Photoshop Elements, version 2.0.
Invasion assay.
Invasion assays of the HEp-2 (human larynx epithelial) cell line were performed as described previously (28). Data are presented as percentages of the number of CFU in the initial inoculum.
Phagocytosis assay.
S. pyogenes strains grown in THB-Neo with 500 μg/ml kanamycin overnight were diluted in THB-Neo and incubated at 37°C with 5% CO2 until early logarithmic phase (A600 ≈ 0.3). A sample (100 μl) containing ∼1,500 CFU was mixed with 0.9 ml of freshly drawn heparinized human blood from nonimmune human donors. The tubes were rotated at 37°C for 3 h, and the contents were enumerated by spread plating on THY plates (14). Plates containing 20 to 200 CFU were counted. Bacterial growth (multiplication factor) was calculated as the mean number of CFU after 3 h divided by the initial mean number of CFU.
Analysis of C3 deposition by S. pyogenes flow cytometry.
Fluorescence-activated cell sorter analysis was performed as described previously (4). Briefly, streptococcal strains were grown as described for the phagocytosis assay to early log phase (A600 ≈ 0.3). Washed bacteria (∼5 × 106 CFU) were mixed with freshly drawn heparinized human blood and incubated at 37°C for 10 min in 5% CO2. After being washed with phosphate-buffered saline (PBS), bacteria were incubated with 20 μg/ml of anti-human C3d and incubated for 10 min at room temperature. Bacteria were washed with PBS and incubated with fluorescein isothiocyanate-conjugated goat F(ab′)2 anti-mouse immunoglobulin G for 10 min at room temperature. Washed bacteria were resuspended in PBS and analyzed with a FACSCalibur, with 10,000 events collected.
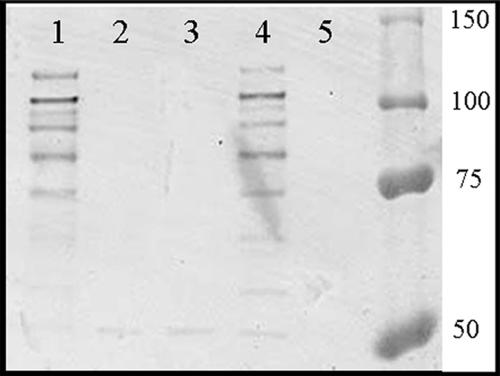
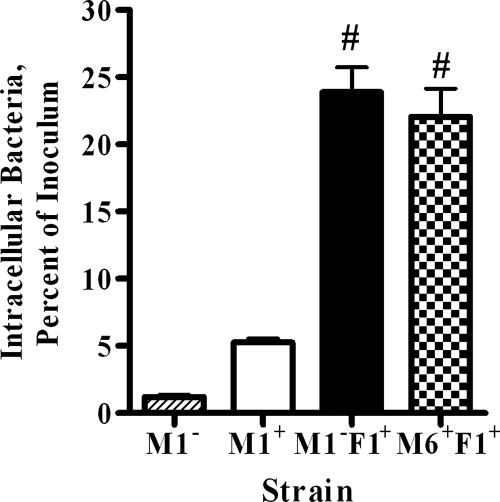
In order to test whether PrtF1/SfbI can block phagocytosis, recombinant 90-226 M1− F1+ was constructed. Plasmid pPT8, which carries the prtF1 gene and its native promoter, was used to transform 90-226Δemm1. The activities of PrtF1/SfbI protein could then be compared to those of M1 protein in the same genetic background. Proteins attached to the cell wall were extracted and analyzed by Western blotting with anti-SfbI serum (Fig. 1) to ensure that PrtF1/SfbI was expressed by the recombinant 90-226 M1− F1+ strain. Protein bands of the appropriate sizes reacted with the anti-PrtF1/SfbI antibody, which demonstrated that 90-226 M1− F1+ expresses PrtF1/SfbI at levels similar to those of JSR4 (M6+ F1+), the original source of the prtF1 gene. To ensure that the expressed PrtF1/SfbI protein was functional, intracellular-invasion assays were performed with the HEp-2 epithelial cell line. Strain 90-226 M1+ was ingested by epithelial cells efficiently, while strain 90-226 M1−, which is unable to bind Fn, lost this ability. As expected, when PrtF1/SfbI was expressed in 90-226Δemm1, strain 90-226 M1− (M1− F1+) streptococci regained the ability to invade epithelial cells as efficiently as M6+ F1+ strain JRS4 and more efficiently than a strain in which invasion is mediated by M1 alone (Fig. 2). These results indicate that PrtF1/SfbI functions well in the 90-226 genetic background.
FIG. 1.
90-226 M1− F1+ expresses PrtF1/SfbI at levels similar to those of JSR4 (M6+ F1+). Equivalent amounts of surface protein extracts were analyzed by Western blotting using anti-SfbI rabbit serum. Lane 1, 90-226 M1− F1+; lane 2, 90-226 M1− pKH3; lane 3, 90-226 M1−; lane 4, JSR4 (M6+ F1+); lane 5, SAM1 (M6+ F1−). Numbers on the right are molecular masses (in kDa).
FIG. 2.
PrtF1/SfbI significantly enhances S. pyogenes strain 90-226 M1− invasion of the HEp-2 epithelial cell line to levels achieved by an M6+ F1+ strain. Invasion assays were performed with 90-226 M1−, 90-226 M1+, 90-226 M1− F1+, and JSR4 (M6+ F1+). Data are presented as percentages of the number of CFU in the inoculum (means ± standard deviations from triplicate experiments). #, P < 0.001, compared to the value obtained with M1− or M1+ invasion, using one-way analysis of variance.
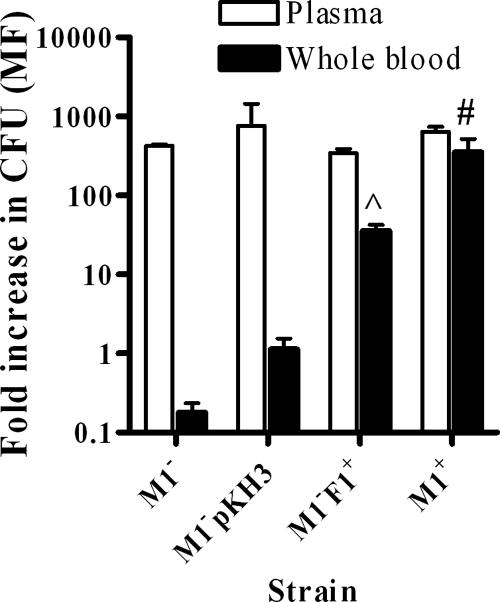
Strain 90-226 M1− F1+ was used to investigate the ability of PrtF1/SfbI to confer phagocytosis resistance, using the traditional Lancefield whole-blood assay (12). After incubation in nonimmune human blood, strain 90-226 M1− F1+ multiplied ∼30-fold while the M1− strain (90-226Δemm1) and the strain containing the empty vector (90-226 M1− pKH3) did not grow in the whole blood (Fig. 3). The level of growth of streptococci in the presence of plasma over 3 h was the same for all strains (Fig. 3), indicating that the above-mentioned difference in survival was not due to an effect on streptococcal growth.
FIG. 3.
PrtF1/SfbI promotes phagocytosis resistance in whole blood but not in plasma. The bacterial strains were analyzed for their ability to multiply in nonimmune human blood. Results are from three to five experiments with blood or plasma from two different blood donors. ^, P < 0.01; #, P < 0.001, compared to the value obtained with 90-226 M1− pKH3, using one-way analysis of variance. The multiplication factor (MF) (n-fold increase) was calculated as the mean number of CFU after 3 h divided by the initial mean number of CFU. The isogenic strains analyzed were 90-226 M1+ (M1+), its M-negative mutant (M1−), 90-226 M1− containing the empty expression vector (90-226 M1− pKH3), and 90-226 M1− containing the PrtF1/SfbI vector (M1− F1+).
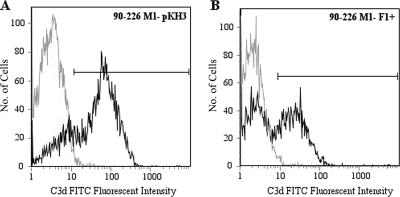
Since M protein can inhibit C3b deposition on the bacterial surface (13), we assessed the ability of PrtF1/SfbI to function similarly. An antibody specific for C3d was used to quantitate C3 deposition, as C3d is present in all surface-deposited C3 fragments. The expression of PrtF1/SfbI decreased C3d deposition on the surface of the M1− F1+ strain. In the control strain 90-226 M1− pKH3, 80% of the population fixed C3d on their surfaces (Fig. 4A) compared to 50% of M1− F1+ organisms (Fig. 4B). The bacterial cells from the 90-226 M1− F1+ population that have decreased C3d deposition (50%) are able to resist phagocytosis, while the cells which fix C3d are susceptible. A small percentage of strain 90-226 M1− pKH3 cells exhibited reduced C3d deposition, which reflects nonuniform binding of the anti-C3d antibody. In the presence of 10 mM EDTA, which blocks both the classical and alternative complement pathways, C3d deposition on 90-226 M1− was completely abolished (data not shown).
FIG. 4.
The expression of PrtF1/SfbI partially decreases C3d deposition on the surface of 90-226 M1−. In strain 90-226 M1− pKH3 (A), 80% of the bacteria fix C3d on their surfaces, compared to 50% of the strain 90-226 M1− F1+ population (B). The 90-226 strains were incubated with human heparinized plasma, and C3d deposition was quantitated by flow cytometry. Gray line, isotype control antibody; black line, mouse anti-human C3d antibody. Representative data from two experiments are shown. FITC, fluorescein isothiocyanate.
PrtF1/SfbI and M proteins are structurally different, yet both contain Fn binding domains which confer similar functions, namely, adherence to and invasion of epithelial cells. PrtF1/SfbI can contain between one and five tandem Fn binding repeats, while emm1 contains two Fn binding sites (6, 25). Both intergenic recombination and horizontal gene transfer have played a role in generating variability in the prtF1 or sfbI gene and the emm gene (25, 29). While the distribution of the prtF1 gene within M serotypes is fairly consistent, there is variability in the number of Fn binding repeats in PrtF1/SfbI from different clinical isolates of M8 and M28 strains (18). Not all S. pyogenes strains express both proteins; M1 strains do not encode PrtF1/SfbI protein (2, 19). The prtF1 gene is present in 30% to 77% of streptococcal clinical isolates, depending on the population examined (2, 16, 19). In addition, in patients with antibiotic treatment failure or asymptomatic S. pyogenes carriage, a significant proportion of the streptococcal isolates carry the prtF1 gene (19). Both M protein and PrtF1/SfbI mediate invasion of epithelial cells. M1-mediated epithelial-cell invasion requires the streptococcus-bound Fn engagement of α5β1 integrin (6), while PrtF1/SfbI-mediated epithelial-cell invasion occurs in an integrin α5β1- or αvβ3-dependent manner (21). When the emm1 gene was deleted from strain 90-226, this strain lost the potential to bind Fn (6) and exhibited reduced invasion of epithelial cell lines (Fig. 2) (28). Both M1- and PrtF1/SfbI-mediated invasions of epithelial cells require activation of the integrin-linked kinase signaling pathway (28).
The expression of PrtF1/SfbI in some strains is controlled transcriptionally in response to high O2 concentration and the presence of superoxide, while the expression of M protein is increased under high-CO2 conditions (3, 10, 27). The differential regulation of these two Fn-binding proteins in high O2 or high CO2 may allow S. pyogenes to adapt to several different in vivo environments, such as those on the skin, on mucosal surfaces, and within the tonsils. For example, the expression of PrtF1/SfbI while the streptococci are in the nasopharynx would allow more-efficient invasion of epithelial cells covering the tonsils, while the expression of M protein at systemic sites would allow the streptococci to avoid phagocytosis by neutrophils in the blood and invade cells within tissues. This model is supported by a recent epidemiological study which demonstrated that PrtF1/SfbI was not encoded by clinical streptococcal isolates causing invasive disease but was detected in 35% of isolates from pharyngotonsillitis cases (1).
Our experiments demonstrate that PrtF1/SfbI expression imparts S. pyogenes resistance to phagocytosis in the M1 background and partially inhibits C3b deposition on the S. pyogenes surface, properties which were previously attributed to M protein. The 90-226 M− F1+ culture proved to be a mixture of cells, some of which were resistant to C3d deposition and others of which had significant amounts of this opsonin on their surfaces. Similar results were observed when the impact of M protein on C3b deposition was investigated. M+ chains were occasionally observed to contain M− cells with significant amounts of C3b on their surfaces. Moreover, M+ bacteria had foci of bound C3b at their septa of division (13). PrtF1/SfbI has not previously been described as mediating resistance to phagocytosis, and PrtF1/SfbI is not known to bind factor H or CD46, regulators of activation of the alternate complement pathway and characteristics of M protein, which contributes to the decay of bound C3b opsonin (13). Our experiments do not address the mechanism by which PrtF1/SfbI restricts C3 deposition in this M1 strain. These data are consistent with the possibility that PrtF1/SfbI either sterically interferes with covalent bonding to C3b or promotes the decay of C3b by binding factor H or some other regulator of the complement system. The enormous diversity in genetic composition between serotypes and even within a serotype precludes us from making the general statement that PrtF1/SfbI functions to block phagocytosis in all S. pyogenes strains. In studies of the role of M6 protein in phagocytosis resistance, an M6 deletion mutant which presumably expresses PrtF1/SfbI did not grow in human blood, while reintroduction of the emm6 gene into that deletion strain revived its capacity to resist phagocytosis (22). We cannot explain why this M6 deletion strain was not at least partially resistant to phagocytosis, since the expression of PrtF1/SfbI by streptococci in those experiments was not addressed. Moreover, as shown here, antibody against PrtF1/SfbI promotes phagocytosis; therefore, it is also possible that the blood used by Perez-Casal et al. contained antibody directed against PrtF1/SfbI.
Acknowledgments
The pPTF8 plasmid was a gift from Michael G. Caparon, and the anti-SfbI rabbit serum was from Gursharan S. Chhatwal. Thanks go to Fredric Carlsson and Charlotta Sandin for their assistance with the C3 deposition assay.
Experiments were financially supported by a grant from the NIAID (AI059533).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Bianco, S., T. Allice, M. Zucca, and D. Savoia. 2006. Survey of phenotypic and genetic features of Streptococcus pyogenes strains isolated in Northwest Italy. Curr. Microbiol. 52:33-39. [DOI] [PubMed] [Google Scholar]
- 2.Brandt, C. M., F. Allerberger, B. Spellerberg, R. Holland, R. Lutticken, and G. Haase. 2001. Characterization of consecutive Streptococcus pyogenes isolates from patients with pharyngitis and bacteriological treatment failure: special reference to prtF1 and sic/drs. J. Infect. Dis. 183:670-674. [DOI] [PubMed] [Google Scholar]
- 3.Caparon, M. G., R. T. Geist, J. Perez-Casal, and J. R. Scott. 1992. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J. Bacteriol. 174:5693-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson, F., C. Sandin, and G. Lindahl. 2005. Human fibrinogen bound to Streptococcus pyogenes M protein inhibits complement deposition via the classical pathway. Mol. Microbiol. 56:28-39. [DOI] [PubMed] [Google Scholar]
- 5.Cue, D., P. E. Dombek, and P. Cleary. 2000. Intracellular invasion by Streptococcus pyogenes: invasins, host receptors, and relevance to human disease, p. 27-33. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, DC. [DOI] [PMC free article] [PubMed]
- 6.Cue, D., H. Lam, and P. P. Cleary. 2001. Genetic dissection of the Streptococcus pyogenes M1 protein: regions involved in fibronectin binding and intracellular invasion. Microb. Pathog. 31:231-242. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombek, P. E., D. Cue, J. Sedgewick, H. Lam, S. Ruschkowski, B. B. Finlay, and P. P. Cleary. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31:859-870. [DOI] [PubMed] [Google Scholar]
- 9.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2:285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson, C., G. Fogg, N. Okada, R. T. Geist, E. Hanski, and M. Caparon. 1995. Regulation of host cell recognition in Streptococcus pyogenes. Dev. Biol. Stand. 85:137-144. [PubMed] [Google Scholar]
- 11.Hanski, E., P. A. Horwitz, and M. G. Caparon. 1992. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect. Immun. 60:5119-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horstmann, R. D., H. J. Sievertsen, M. Leippe, and V. A. Fischetti. 1992. Role of fibrinogen in complement inhibition by streptococcal M protein. Infect. Immun. 60:5036-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacks-Weis, J., Y. Kim, and P. P. Cleary. 1982. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J. Immunol. 128:1897-1902. [PubMed] [Google Scholar]
- 14.Lancefield, R. C. 1957. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J. Exp. Med. 106:525-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaPenta, D., C. Rubens, E. Chi, and P. P. Cleary. 1994. Group A streptococci efficiently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. USA 91:12115-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma, X., H. Kikuta, N. Ishiguro, M. Yoshioka, T. Ebihara, T. Murai, I. Kobayashi, and K. Kobayashi. 2002. Association of the prtF1 gene (encoding fibronectin-binding protein F1) and the sic gene (encoding the streptococcal inhibitor of complement) with emm types of group A streptococci isolated from Japanese children with pharyngitis. J. Clin. Microbiol. 40:3835-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molinari, G., S. R. Talay, P. Valentin-Weigand, M. Rohde, and G. S. Chhatwal. 1997. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect. Immun. 65:1357-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natanson, S., S. Sela, A. E. Moses, J. M. Musser, M. G. Caparon, and E. Hanski. 1995. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J. Infect. Dis. 171:871-878. [DOI] [PubMed] [Google Scholar]
- 19.Neeman, R., N. Keller, A. Barzilai, Z. Korenman, and S. Sela. 1998. Prevalence of internalisation-associated gene, prtF1, among persisting group-A streptococcus strains isolated from asymptomatic carriers. Lancet 352:1974-1977. [DOI] [PubMed] [Google Scholar]
- 20.Osterlund, A., R. Popa, T. Nikkila, A. Scheynius, and L. Engstrand. 1997. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107:640-647. [DOI] [PubMed] [Google Scholar]
- 21.Ozeri, V., I. Rosenshine, D. F. Mosher, R. Fässler, and E. Hanski. 1998. Role of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 30:625-637. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Casal, J., M. G. Caparon, and J. R. Scott. 1992. Introduction of the emm6 gene into an emm-deleted strain of Streptococcus pyogenes restores its ability to resist phagocytosis. Res. Microbiol. 143:549-558. [DOI] [PubMed] [Google Scholar]
- 23.Podbielski, A., S. Beckert, R. Schattke, F. Leithauser, F. Lestin, B. Gossler, and B. Kreikemeyer. 2003. Epidemiology and virulence gene expression of intracellular group A streptococci in tonsils of recurrently infected adults. Int. J. Med. Microbiol. 293:179-190. [DOI] [PubMed] [Google Scholar]
- 24.Talay, S. R., P. Valentin-Weigand, P. G. Jerlström, K. N. Timmis, and G. S. Chhatwal. 1992. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 60:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Towers, R. J., P. K. Fagan, S. R. Talay, B. J. Currie, K. S. Sriprakash, M. J. Walker, and G. S. Chhatwal. 2003. Evolution of sfbI encoding streptococcal fibronectin-binding protein I: horizontal genetic transfer and gene mosaic structure. J. Clin. Microbiol. 41:5398-5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valentin-Weigand, P., S. R. Talay, A. Kaufhold, K. N. Timmis, and G. S. Chhatwal. 1994. The fibronectin binding domain of the Sfb protein adhesin of Streptococcus pyogenes occurs in many group A streptococci and does not cross-react with heart myosin. Microb. Pathog. 17:111-120. [DOI] [PubMed] [Google Scholar]
- 27.VanHeyningen, T., G. Fogg, D. Yates, E. Hanski, and M. Caparon. 1993. Adherence and fibronectin binding are environmentally regulated in the group A streptococci. Mol. Microbiol. 9:1213-1222. [DOI] [PubMed] [Google Scholar]
- 28.Wang, B., R. S. Yurecko, S. Dedhar, and P. P. Cleary. 2006. Integrin-linked kinase is an essential link between integrins and uptake of bacterial pathogens by epithelial cells. Cell. Microbiol. 8:257-266. [DOI] [PubMed] [Google Scholar]
- 29.Whatmore, A. M., V. Kapur, J. M. Musser, and M. A. Kehoe. 1995. Molecular population genetic analysis of the enn subdivision of group A streptococcal emm-like genes: horizontal gene transfer and restricted variation among enn genes. Mol. Microbiol. 15:1039-1048. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerlein, B., H. S. Park, S. Li, A. Podbielski, and P. P. Cleary. 2005. The M protein is dispensable for maturation of streptococcal cysteine protease SpeB. Infect. Immun. 73:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]