Abstract
Chronic lung infection by opportunistic pathogens, such as Pseudomonas aeruginosa and members of the Burkholderia cepacia complex, is a major cause of morbidity and mortality in patients with cystic fibrosis. Outer membrane proteins (OMPs) of gram-negative bacteria are promising vaccine antigen candidates. In this study, we evaluated the immunogenicity, protection, and cross-protection conferred by intranasal vaccination of mice with OMPs from B. multivorans plus the mucosal adjuvant adamantylamide dipeptide (AdDP). Robust mucosal and systemic immune responses were stimulated by vaccination of naive animals with OMPs from B. multivorans and B. cenocepacia plus AdDP. Using a mouse model of chronic pulmonary infection, we observed enhanced clearance of B. multivorans from the lungs of vaccinated animals, which correlated with OMP-specific secretory immunoglobulin A responses. Furthermore, OMP-immunized mice showed rapid resolution of the pulmonary infection with virtually no lung pathology after bacterial challenge with B. multivorans. In addition, we demonstrated that administration of B. multivorans OMP vaccine conferred protection against B. cenocepacia challenge in this mouse infection model, suggesting that OMPs provide cross-protection against the B. cepacia complex. Therefore, we concluded that mucosal immunity to B. multivorans elicited by intranasal vaccination with OMPs plus AdDP could prevent early steps of colonization and infection with B. multivorans and also ameliorate lung tissue damage, while eliciting cross-protection against B. cenocepacia. These results support the notion that therapies leading to increased mucosal immunity in the airways may help patients with cystic fibrosis.
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the CF transmembrane regulator gene, which encodes the CFTR chloride channel (35, 45). Chronic lung infection by opportunistic pathogens, such as Pseudomonas aeruginosa and the Burkholderia cepacia complex (Bcc) (12), causes significant morbidity and mortality in CF patients. The Bcc currently consists of at least nine species: B. cepacia, B. multivorans, B. cenocepacia, B. stabilis, B. vietnamiensis, B. dolosa, B. ambifaria, B. anthina, and B. pyrrocinia (13). Isolates of all Bcc species have been recovered from the sputum of patients with CF (12). B. cenocepacia and B. multivorans comprise about 83 and 10% of all Bcc isolates from CF patients in Canada, respectively (43). In the United States, B. cenocepacia and B. multivorans account for about 45 and 39% of all isolates recovered from CF patients, respectively (41). B. cenocepacia isolates are also prevalent in pediatric CF patients admitted to the Children's Hospital of Buenos Aires (L. Galanternik and M. A. Valvano, unpublished).
Bcc bacteria are not usually part of the normal flora of humans, and they do not commonly pose a risk to healthy individuals. However, a proportion of CF patients infected with Bcc can develop “cepacia syndrome,” a devastating illness characterized by a fatal acute necrotizing pneumonia that causes rapid and progressive respiratory failure, often leading to the patient's death (22). Intrinsic resistance of Bcc bacteria to many commonly used antibiotics (1) and induction of cross-resistance to unrelated antimicrobial agents (40) make it difficult to eradicate these bacteria from CF patients.
The specific mechanisms by which Bcc bacteria can subvert host defenses, invade deeper tissues of the lung, and ultimately become blood borne are poorly understood (26, 33). Chronic airway infection and exacerbated inflammation are significant clinical problems for CF patients, since ultimately these processes lead to destruction of the lung tissue. Given the morbidity, mortality, and health care costs associated with Bcc infection in CF patients and the growing concerns about increased antimicrobial resistance, it would be desirable to have therapeutic alternatives for protecting patients against early infection of the lungs. Strategies that prevent colonization or reduce bacterial transmission among CF patients while minimizing lung inflammation would help control the progression of CF lung disease.
Little is known about the humoral immune response to Bcc infection in CF patients. Immunoglobulin G (IgG) antibodies to B. cepacia outer membrane proteins (OMPs) have been detected in sera of CF patients colonized with both B. cepacia and P. aeruginosa (3, 4), suggesting cross-reactivity between the OMPs of these organisms. Another study showed that the antibody response was specific to B. cepacia antigens (29). Furthermore, serum IgG and sputum IgA titers against B. cepacia lipopolysaccharide (LPS) were significantly greater in CF patients colonized with B. cepacia than in age- and sex-matched CF patients colonized with P. aeruginosa or in healthy individuals without CF harboring neither organism (36).
To our knowledge, the protective value of anti-Bcc immune responses has not been explored. Since Bcc bacteria cause mucosal infections, a vaccine generating a mucosal immune response would be an effective approach for preventing bacterial colonization. The mucosal immune system is the first line of defense against invading pathogens. Nasopharynx-associated lymphoid tissue (NALT) and Peyer's patches are important inductive sites for the initiation of antigen-specific mucosal IgA and serum IgG responses, as well as cytotoxic T-lymphocyte immune responses, at both mucosal and systemic sites. Thus, both NALT and Peyer's patches maximize the two-tiered immunological barrier of the host. Intranasal (i.n.) delivery of vaccines is an attractive mode of immunization. The nose, like the mouth, is a practical site for vaccine administration, and NALT stimulation efficiently induces antigen-specific immune responses in both mucosal and systemic compartments (15, 28). In the past decade, several clinical studies have confirmed that local immunity and systemic immunity are generated after nasal immunization of humans against diphtheria and tetanus (2), influenza (21), and infection with Streptococcus mutans (32). A large number of studies performed with mice, pigs, and monkeys have also confirmed the effectiveness of nasal immunization with a variety of vaccines (15). We have previously reported that the adjuvant adamantylamide dipeptide (AdDP) can enhance protective immune responses against antigens administered by a mucosal route (5, 6).
We hypothesize that generating a mucosal specific immune response in the respiratory tract could prevent early steps of colonization and infection by Bcc bacteria and thus could prevent or ameliorate lung damage due to inflammation during subsequent infection. Using a murine model of chronic pulmonary infection with B. multivorans, we show here that i.n. immunization with a B. multivorans OMP preparation can induce specific mucosal immune responses in the respiratory tract, which in turn enhance the clearance of B. multivorans and minimize lung inflammatory damage after bacterial challenge. In addition, we demonstrated that administration of the B. multivorans OMP vaccine conferred protection against B. cenocepacia challenge in this mouse infection model, suggesting that OMPs may provide cross-protection against other Bcc members.
MATERIALS AND METHODS
Bacterial strains and media.
The Bcc strains used in this study included B. multivorans ATCC 17616, B. vietnamiensis LMG16232, and clinical isolates of B. cenocepacia, B. stabilis, and B. ambifaria obtained from sputum of CF patients at the Ricardo Gutiérrez Children's Hospital, Buenos Aires, Argentina. Bacteria were maintained in Luria-Bertani (LB) broth containing 20% (vol/vol) glycerol at −80°C until they were used, and they were streaked onto LB agar or grown in LB broth and incubated at 37°C overnight, as required.
Adjuvant.
AdDP was synthesized by Bachem, Switzerland, using a previously described procedure (18).
Animals.
Female BALB/c mice that were 8 to 12 weeks old were obtained from Gador S.A. Laboratory (Buenos Aires, Argentina). Mice were housed in groups of five or six, and food and water were provided ad libitum. All procedures were in compliance with U.S. National Institutes of Health guidelines for handling laboratory animals.
Preparation of OMPs.
OMPs were prepared by using a previously described method (7). In brief, bacteria were grown overnight in 10 ml of LB broth, harvested by centrifugation at 5,000 × g for 20 min at room temperature, and washed twice with saline. The bacterial pellet was suspended in 3 ml of 10 mM Tris-HCl (pH 8.0) and sonicated six times for 20 s at 40 W. The suspension was centrifuged at 10,000 × g for 1 min to remove debris and unbroken bacteria, and the supernatant was centrifuged at 40,000 × g for 30 min at 4°C. The pellet containing total membranes was resuspended in distilled H2O, and an equal volume of a 20 mM Tris solution containing 1.5% Sarkosyl was added. The suspension was incubated for 20 min at room temperature to solubilize the inner membranes and then centrifuged at 40,000 × g for 30 min at 4°C. The resulting pellet was highly enriched for OMPs. The protein concentration was determined by using a protein assay kit (Bio-Rad Laboratories, Richmond, CA). OMP preparations were tested for the presence of endotoxin by chromogenic Limulus amebocyte lysate (LAL Endochrome; Charles River Endosafe, Charleston, SC) according to the manufacturer's instructions. Endotoxin levels were less than 30,000 endotoxin units/mg in OMP preparations, which corresponded to approximately 70 μg of LPS per mg of protein.
LPS extraction.
LPS was extracted from B. multivorans by the method described by Darveau and Hancock (14). LPS samples were resuspended in pyrogen-free water, and the activity was determined by the Limulus amebocyte lysate method. The protein concentration was determined using a protein assay kit (Bio-Rad Laboratories). Protein in LPS samples accounted for less than 0.1% of the total weight of the LPS. LPS was characterized by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) as described below, and silver staining was performed as described by Tsai and Frasch (44).
SDS-PAGE and Western blot analysis.
SDS-PAGE was performed as described by Laemmli (30). Proteins present on the gels were detected by using Coomassie blue stain. For immunoblotting, B. multivorans OMPs, B. cenocepacia OMPs, or B. multivorans LPS samples were separated by SDS-PAGE, electroblotted onto nitrocellulose membranes, and then reacted with mouse antiserum raised against B. multivorans OMPs by use of standard protocols (9).
Immunization and sample collection.
Groups of five or six mice were immunized by i.n. inoculation (10 μl/nostril) of OMPs purified from B. multivorans, B. cenocepacia, B. vietnamiensis, B. stabilis, or B. ambifaria (30 μg/dose) together with AdDP (200 μg/dose) as a mucosal adjuvant diluted in sterile phosphate-buffered saline (PBS) on days 0, 7, and 14. A control group received only AdDP (200 μg/dose). On day 21, serum, saliva, bronchoalveolar lavage (BAL), and nasal wash (NAL) samples were obtained as previously described (6) and examined for the presence of OMP-specific antibodies. Briefly, saliva samples were obtained following intraperitoneal injection of 100 μl of pilocarpine (1 mg/ml; Sigma) diluted in sterile PBS to induce salivary secretion. Blood samples were collected by cardiac puncture immediately after sacrifice. NAL specimens were obtained by gently flushing the nasal cavities from the posterior opening of the nose with 200 μl of PBS after the mandible was removed. BAL samples were obtained by irrigation with 400 μl of PBS, using a blunted needle inserted into the trachea after a tracheotomy. The wash samples recovered were centrifuged at 3,000 × g for 5 min to remove cellular debris, and the supernatants were examined by using an enzyme-linked immunosorbent assay (ELISA) (see below).
Assessment of the effects of LPS.
Groups of eight mice were immunized i.n. on days 0, 7, and 14 with either LPS (2 or 20 μg/dose), LPS (2 μg/dose) plus AdDP (200 μg/dose), or B. multivorans OMPs plus AdDP. A control group received only saline. The effects of LPS were assessed by determining the body temperature, the total cell count and percentage of polymorphonuclear leukocytes in BAL samples, and lung histopathology and by detecting LPS-specific antibodies in serum samples. The body temperature was measured by determining the rectal temperature using a digital thermometer. For each measurement, after 1 h of adaptation two values were averaged to determine the baseline. The rectal temperature was determined 1 day before immunization and 1, 2, 4, 6, 8, 24, and 48 h after immunization. Twenty-four hours after immunization, three mice in each group were sacrificed to obtain BAL samples and lungs for histopathological examination (see below). The total cell counts in BAL samples were determined with an autoanalyzer hemacytometer (Cell-Dyn 1600; ABBOT). A cytospin analysis of BAL cells was performed on standard microscope slides using cytobucket carriers (Fisher Scientific). A 200-μl BAL sample was centrifuged at 700 rpm for 10 min. Cells were air dried, fixed directly with methanol, and stained with Giemsa stain. Differential polymorphonuclear leukocyte counts were obtained using stained cells, and averages were determined for at least 200 cells. On day 21, serum samples were obtained from the remainder of the mice, and the presence of LPS-specific antibodies was determined by ELISA as described below.
Detection of OMP- and LPS-specific antibodies by ELISA.
OMP-specific antibody titers in mucosal secretions and sera and LPS-specific antibody titers in sera were determined by ELISA. Briefly, 96-well Nunc-Immuno MaxiSorp assay plates (Nunc, Roskilde, Denmark) were coated with 0.5 μg/well of the OMPs purified from each Bcc strain or LPS in coating buffer (sodium bicarbonate, pH 9.4), as indicated in the experiment design. After overnight incubation at 4°C, the plates were blocked with 0.2% Tween 20 in PBS for 2 h at 37°C. Serial twofold dilutions of samples in PBS-0.05% Tween 20 were added (100 μl/well), and the plates were incubated for 2 h at 37°C. After four washes with PBS-0.05% Tween 20, horseradish peroxidase-conjugated γ-chain-specific goat anti-mouse IgG (Chemicon) or phosphatase alkaline-conjugated α-chain-specific rabbit anti-mouse IgA (ICN) was added as a secondary antibody. The plates were incubated for 2 h at 37°C, and after four washes, the reactions were developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) in 0.1 M citrate-phosphate buffer (pH 4.3) containing 0.01% H2O2 or with p-nitrophenyl phosphate in 10 mM diethanolamine (pH 9.5) containing 0.5 mM MgCl2. The absorbance was determined at a wavelength of 405 nm. Endpoint titers were expressed as the reciprocal log2 of the last dilution that gave an optical density of ≥0.1 U for negative control samples obtained from nonimmunized animals.
Bacterial challenge.
The bacterial challenge studies were performed using a chronic pulmonary model of B. cepacia infection described previously (10, 11), with some modifications. Briefly, cyclophosphamide (150 mg/kg of body weight; Filaxis Laboratories) was administered intraperitoneally on days −1, 4, 9, and 13 of challenge. On days −2, 0, 5, and 13 a sample of blood was obtained from the tail vein. Total peripheral leukocyte counts were determined with an autoanalyzer hemacytometer (Cell-Dyn 1600), and differential counts were determined microscopically using Giemsa-stained blood smears. A pulmonary challenge with live bacteria was performed on day 21 after the first i.n. immunization. B. multivorans or B. cenocepacia was prepared from overnight cultures as described above and resuspended in PBS. The concentration of the inoculum was estimated by determining the optical density at 630 nm and was confirmed by counting the CFU in serial dilutions of the inoculum. Mice were challenged i.n. with 2.8 × 107 CFU (11) in a 20-μl dose. For B. multivorans challenge, five animals were sacrificed at 4 h (day 0) and on days 5 and 15 after pulmonary infection, whereas for B. cenocepacia challenge mice were sacrificed at 4 h (day 0) and on day 5 after pulmonary infection. Lungs were excised, weighed, and homogenized with a pestle, and serial dilutions in PBS of the homogenate were plated on LB agar. Viable counts were determined after 24 to 48 h of incubation at 37°C and were expressed as the log10 CFU/g of lungs (mean ± standard error of the mean [SEM]). Blood samples and NAL samples were collected to monitor the presence of OMP-specific antibodies.
Changes in weight and clinical illness scores following challenge were determined by weighing the mice with a digital scale and by determining the appearance of the mice, respectively. Clinical illness scores were assigned by a blinded examiner using an index derived by assigning numbers to a set of clinical features seen in mice with different degrees of illness, as follows: 0, healthy; 1, barely ruffled fur; 2, ruffled fur and active; 3, ruffled fur and inactive; 4, ruffled fur, inactive, hunched posture, and gaunt; 5, dead.
Histopathology.
Lungs were infused with 10% (vol/vol) neutral buffered formalin, carefully removed from the chest cavity, placed for 48 h in 10% neutral buffered formalin, and then processed for routine histological examination using paraffin-embedded sections stained with hematoxylin and eosin.
Statistical analyses.
In the immunogenicity and protection studies the significance of the differences between two groups was determined by Student's unpaired two-tailed t test with transformed data (log10 or log2), and the significance of the differences between three or more groups was determined by one-way analysis of variance (ANOVA) with the Tukey-Kramer multiple-comparison test. The linear correlation between two variables was determined using the Pearson correlation coefficient. Total peripheral leukocyte and polymorphonuclear leukocyte counts were compared by the nonparametric Mann-Whitney U test for two groups and by the Krustal-Wallis test with Dunn's multiple-comparison test for three or more groups. For parametric or log-transformed data the results were expressed as means ± SEM, whereas for nonparametric data the results were expressed as medians and ranges. Differences were considered significant at a P value of <0.05.
RESULTS
Specific antibody responses in serum and mucosal secretions are elicited by i.n. immunization with OMPs from Bcc species coadministered with AdDP.
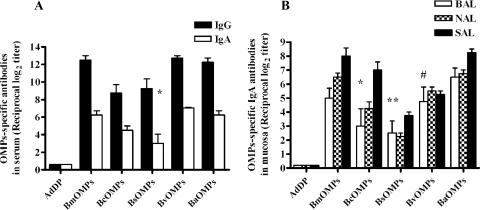
We examined the ability of OMPs to elicit serum and mucosal immune responses after i.n. vaccination, using a vaccination protocol that was successful in previous studies (6). Although Bcc OMPs themselves could have immunogenic properties, we included AdDP as an adjuvant in the immunization protocol. AdDP is a mucosal adjuvant that promotes a robust T helper 2 response when it is coadministered with native and recombinant antigens by intraperitoneal, oral, or i.n. routes (5, 6). High titers of OMP-specific IgG and IgA serum antibodies were elicited by i.n. immunization with OMPs from isolates of various Bcc species plus AdDP (Fig. 1A). The titers were significantly higher than those in AdDP-immunized controls (P < 0.001) in all cases except the serum IgA titers induced by OMPs from B. stabilis (P < 0.03). OMPs from B. multivorans, B. vietnamiensis, and B. ambifaria elicited higher endpoint titers of serum IgG antibodies than OMPs from B. cenocepacia and B. stabilis (P < 0.002). Since secretory antibodies are the main effectors in protection against pathogens at mucosal sites, we evaluated the efficacy of OMPs plus AdDP to elicit specific antibody production in the respiratory mucosa. Significant increases in OMP-specific IgA titers were observed in BAL, NAL, and saliva samples from mice immunized with OMPs plus AdDP (Fig. 1B), and these titers were significantly higher than all the titers obtained for mice immunized with AdDP (P < 0.0001) except the titers in BAL samples for OMPs derived from B. cenocepacia (P < 0.05), B. stabilis (P < 0.03), and B. vietnamiensis (P < 0.005). No statistically significant differences among the endpoint titers were observed for BAL samples, but, as we observed for serum IgA, OMPs from B. multivorans, B. ambifaria, B. cenocepacia, and B. vietnamiensis elicited greater specific secretory IgA responses than OMPs from B. stabilis elicited (P < 0.0001) in NAL and saliva samples. Furthermore, we detected OMP-specific IgG antibody responses in BAL samples from OMPs-vaccinated mice (data not shown). Together, these results demonstrated that the Bcc OMPs plus AdDP can elicit serum and mucosal antibody responses under our experimental conditions.
FIG. 1.
Systemic and mucosal humoral immune responses directed against OMPs in mice vaccinated i.n. with OMPs purified from B. multivorans (BmOMPs), B. cenocepacia (BcOMPs), B. stabilis (BsOMPs), B. ambifaria (BaOMPs), and B. vietnamiensis (BvOMPs) coadministered with AdDP. The results are expressed as the reciprocal log2 of the mean endpoint titer; the error bars indicate SEM. The AdDP data show the AdDP-immunized mouse IgG and IgA serum or mucosal IgA antibody response against OMPs, determined separately for each strain. (A) Specific IgG and IgA titers in the sera of control mice (AdDP) and mice vaccinated with each OMP preparation from a Bcc species plus AdDP. Most differences were statistically significant at a P value of < 0.0001 when values were compared with the value for the AdDP-vaccinated mice, as determined by Student's unpaired two-tailed t test); the only exception is indicated by an asterisk (P < 0.03). For differences in the endpoint titers for IgG between B. multivorans OMPs, B. vietnamiensis OMPs, and B. ambifaria OMPs and between B. cenocepacia OMPs and B. stabilis OMPs, the P value was <0.002, as determined by one-way ANOVA with the Tukey posttest. For differences in the endpoint titers for IgA between B. stabilis OMPs and all other Bcc OMPs, the P value was <0.002, as determined by one-way ANOVA with the Tukey posttest. (B) Specific IgA antibodies in BAL, NAL, and saliva (SAL) samples from control mice (AdDP) and mice vaccinated with Bcc OMPs plus AdDP. Most differences were statistically significant at a P value of <0.0001 when values were compared with the values for the AdDP-vaccinated mice; the exceptions are indicated by one asterisk (P < 0.05), two asterisks (P < 0.03), and a number sign (P < 0.005). For differences in the endpoint titers for secretory IgA in NAL and saliva samples between B. stabilis OMPs and all other Bcc OMPs, the P value was <0.0001, as determined by a one-way ANOVA with the Tukey posttest.
OMP-mediated immune response is independent of endotoxin.
To investigate the possible role of endotoxin in OMP preparations, we examined the potential proinflammatory effect of B. multivorans LPS after i.n. immunization by evaluating the body temperature response, the polymorphonuclear leukocyte counts in BAL samples, and the lung histopathology of mice immunized with B. multivorans LPS. There were no significant differences in the baseline body temperature among the groups. For animals inoculated i.n. with B. multivorans LPS (at the same concentration that was present as a contaminant in B. multivorans OPM preparations) or with B. multivorans OMPs plus AdDP we did not observe any change in the baseline body temperature compared to the baseline body temperature of the control group (Fig. 2). We observed an approximately 2°C increase in the baseline body temperature at 6 h postinoculation, which remained elevated throughout the 24-h recording period, when mice were inoculated with a 10-fold-higher dose of B. multivorans LPS (Fig. 2). No histological changes indicating inflammation or membrane barrier disruption were observed in any of the mice immunized with B. multivorans LPS (data not shown). Also, a 10-fold increase in the i.n. LPS dose did not have adverse effects on the mice. Consistent with these results, we observed no differences in the percentage of polymorphonuclear leukocytes present in the BAL samples for any of the immunized mouse groups (Table 1). Therefore, when administrated by the i.n. route, the OMP preparation appeared to be safe and nontoxic and did not trigger any significant inflammatory response.
FIG. 2.
Effects of i.n. immunization with B. multivorans LPS (BmLPS) on the body temperature response in mice. Mice were immunized i.n. with either B. multivorans LPS (2 or 20 μg/dose), B. multivorans LPS (2 μg/dose) plus AdDP (200 μg/dose), or B. multivorans OMPs (BmOMPs) plus AdDP. A control group received only saline. The data are the means ± SEM. ΔTb, change in body temperature from the baseline (time zero).
TABLE 1.
Total leukocytes and polymorphonuclear cells in BAL samples from LPS-immunized mice
| Group | Median total leukocyte concn (range) (109/liter) | Polymorphonuclear leukocytes
|
|
|---|---|---|---|
| Median concn (range) (109/liter) | % | ||
| Saline | 0.9 (0.8-1.0) | 0.102 (0.02-0.135) | 11.3 |
| B. multivorans LPS (2 μg) | 0.9 (0.8-1.0) | 0.092 (0.04-0.145) | 10.2 |
| B. multivorans LPS (20 μg) | 0.65 (0.4-0.9) | 0.067 (0.02-0.113) | 10.3 |
| B. multivorans OMPs + AdDP | 1.2 (1.1-1.3) | 0.051 (0.011-0.09) | 4.3 |
| B. multivorans LPS (2 μg) + AdDP | 1.15 (1.1-1.2) | 0.042 (0.011-0.012) | 4.5 |
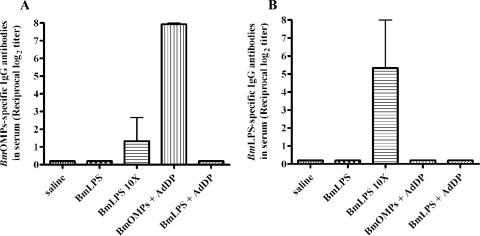
We also analyzed whether endotoxin contamination could have been responsible for the specificity of the immune response against OMPs. B. multivorans LPS with endotoxin activity comparable to the activity of the contaminating endotoxin in the B. multivorans OMP preparation did not induce serum anti-LPS immune responses (Fig. 3A). LPS doses that were at least 10-fold higher were required to detect a weak effect on serum anti-LPS responses (Fig. 3A). The presence of LPS as a contaminant in B. multivorans OMP preparations raised the question of whether the antibodies induced by i.n. immunization with B. multivorans OMPs plus AdDP were directed at or cross-reacted with the LPS core oligosaccharide region. We demonstrated that this immune response was specific against B. multivorans OMPs and not against contaminating endotoxin (Fig. 3B). No immunoreactive bands were detected on immunoblots when purified B. multivorans LPS was used as the antigen and sera from B. multivorans OMP-immunized mice were used as the staining antibody (data not shown). Collectively, these results confirmed that the presence of LPS has no effect on the immunogenicity of OMPs.
FIG. 3.
Effects of i.n. immunization with B. multivorans LPS (BmLPS) on the immunogenicity of OMPs. Mice were immunized i.n. on days 0, 7, and 14 with either B. multivorans LPS (2 or 20 μg/dose), B. multivorans LPS (2 μg/dose) plus AdDP (200 μg/dose), or B. multivorans OMPs (BmOMPs) plus AdDP. A control group received only saline. The results are expressed as the reciprocal log2 of the mean endpoint titer; the error bars indicate SEM. (A) B. multivorans LPS-specific IgG antibodies present in the serum of control (saline) and vaccinated mice. The differences were not statistically significant, as determined by a one-way ANOVA with the Tukey-Kramer posttest. (B) OMP-specific IgG antibodies in serum of control (saline) and vaccinated mice. The differences were statistically significant at a P value of <0.0005, as determined by a one-way ANOVA with the Tukey-Kramer posttest.
i.n. immunization with OMPs plus AdDP enhances the clearance of B. multivorans from the lungs.
We used the immunocompromised mice model of lung infection (10, 11) to investigate the kinetics of infection by B. multivorans in mice that were immunized with AdDP and mice that were immunized with B. multivorans OMPs plus AdDP. The two groups exhibited different rates of bacterial clearance, as shown by the number of B. multivorans CFU cultured from the lungs (Fig. 4A). The difference was significant throughout the experiment (P < 0.001 at days 5 and 15 after challenge with B. multivorans). The initial pulmonary bacterial load was 4.10 ± 0.12 log10 CFU/g of lungs (mean ± SEM), and the AdDP-vaccinated mice maintained a pulmonary bacterial load of 3.43 ± 0.19 log10 CFU/g of lungs for up to 15 days. In contrast, mice vaccinated with OMPs plus AdDP and infected with B. multivorans exhibited rapid and almost complete clearance of the infection after 2 weeks, and the bacterial load was 1.39 ± 0.461 log10 CFU/g of lungs. On day 15 postinfection, there was a nearly 3-log reduction in the bacterial load in OMP-vaccinated animals, compared with a 1-log reduction in the bacterial load in the AdDP-immunized mice.
FIG. 4.
Kinetics of B. multivorans clearance from lungs of infected mice. (A) Mice immunized with AdDP and B. multivorans OMPs (BmOMPs) were challenged i.n. with 2.8 × 107 CFU of B. multivorans. Pulmonary bacterial clearance was assessed after 0 (4 h), 5, and 15 days by plating dilutions of lung homogenates on LB agar. The results are expressed as the mean log10-transformed CFU/g of lungs; the error bars indicate SEM. The differences were statistically significant when values were compared with the values for the AdDP-immunized mice (P < 0.001, as determined by Student's unpaired two-tailed t test) (asterisks), and with the values for B. multivorans OMP-immunized mice on day 0 (P < 0.003, as determined by a one-way ANOVA with the Tukey-Kramer posttest) (number sign). (B) OMP-specific NAL IgA antibodies of infected mice. The results are expressed as the mean reciprocal log2 of the endpoint titer; the error bars indicate SEM. The differences were statistically significant when values were compared with the values for the AdDP-immunized mice, as determined by Student's unpaired two-tailed t test (one asterisk, P < 0.004; two asterisks, P < 0.0002; three asterisks, P < 0.002), and with the values for mice immunized with OMPs and AdDP on day 0 (P < 0.05, as determined by one-way ANOVA with the Tukey-Kramer posttest) (number sign). (C) OMP-specific serum IgG antibodies of infected mice. The results are expressed as the mean reciprocal log2 endpoint titer; the error bars indicate SEM. The differences were statistically significant when values were compared with the values for the control group, as determined by Student's unpaired two-tailed t test (one asterisk, P < 0.0002; two asterisks, P < 0.01).
Protection was directly correlated with the magnitude of the immune response after vaccination (Fig. 4B and C). Prior to infection (day 0), NAL samples from mice immunized with B. multivorans OMPs plus AdDP had a statistically significant high titer of IgA OMP-specific antibodies (P < 0.004), and the level of this response was maintained throughout the experiment (P < 0.0002 at day 5 and P < 0.002 at day 15). Moreover, the titers of OMP-specific IgA antibodies in NAL samples from mice immunized with OMPs plus AdDP were higher on day 5 postinfection than on day 0 (P < 0.05) (Fig. 4B). There was also a statistically significant negative correlation between the titers of OMP-specific IgA in mucosa and the number of B. multivorans CFU in lungs (r = −0.9428; P < 0.04).
As observed for i.n. immunization with B. multivorans OMPs and AdDP, infection of AdDP-immunized mice with B. multivorans induced increased OMP-specific IgG serum antibodies on day 15, but this immune response did not correlate with prevention of bacterial infection (Fig. 4C). These results indicate that i.n. immunization with B. multivorans OMPs plus AdDP enhanced the clearance of B. multivorans from the lungs, and the protective effect was associated with OMP-specific IgA antibody titers in mucosal secretions.
i.n. immunization with OMPs plus AdDP is associated with reduced disease signs after B. multivorans infection.
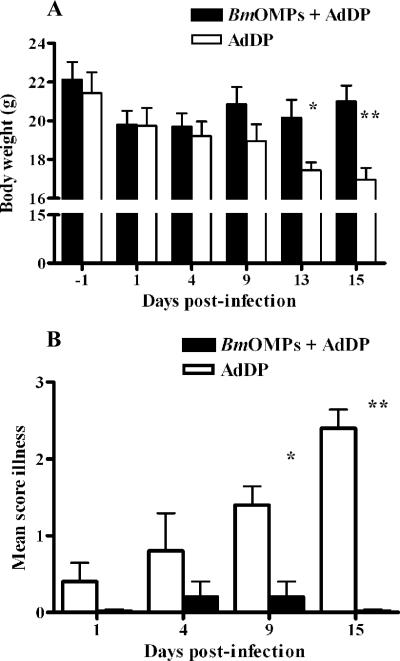
B. multivorans infection caused more weight loss in AdDP-immunized mice than in mice immunized with B. multivorans OMPs plus AdDP (Fig. 5A). The mean body weight of mice infected with B. multivorans was significantly lower for the former group than for the latter group on days 13 (P < 0.03) and 15 (P < 0.005). The median (minimum, maximum) percentages of weight loss for AdDP-vaccinated mice were 17.7% (10.6%, 23.3%) and 20.4% (10%, 31%) for days 13 and 15, respectively.
FIG. 5.
Effects of i.n. immunization with B. multivorans OMPs (BmOMPs) plus AdDP and B. multivorans infection on animal health over time. Mice immunized with AdDP and B. multivorans OMPs were challenged with 2.8 × 107 CFU of B. multivorans. Animals were monitored for the duration of the experiment. (A) Body weight. The results are expressed as the mean body weight; the error bars indicate SEM. The differences were statistically significant when values were compared with the values for the AdDP-immunized mice, as determined by Student's unpaired two-tailed t test (one asterisk, P < 0.03; two asterisks, P < 0.005). (B) Clinical illness score (0, healthy; 1, barely ruffled fur; 2, ruffled fur and active; 3, ruffled fur and inactive; 4, ruffled fur, inactive, hunched posture, and gaunt; 5, dead). The results are expressed as the means of the illness score; the error bars indicate SEM. The differences were statistically significant when values were compared with the values for the AdDP-immunized mice, as determined by Student's unpaired two-tailed t test (one asterisk, P < 0.005; two asterisks, P < 0.0001).
The illness score was also significantly higher for AdDP-immunized mice than for mice immunized with B. multivorans OMPs plus AdDP (Fig. 5B). The difference was significant throughout the experiment (P < 0.005 at day 9 and P < 0.0001 at day 15 after challenge with B. multivorans). On day 4 postinfection, 40% of AdDP-immunized mice and 10% of mice immunized with OMPs plus AdDP (n = 10 for each group) exhibited signs of illness. This was more evident on day 15 postinfection, when all five mice that received AdDP were ill, while no disease signs were observed in the animals that received B. multivorans OMPs plus AdDP (illness scores, 2.4 ± 0.24 and 0, respectively; P < 0.0001).
OMP-vaccinated mice infected with B. multivorans exhibit minimal evidence of lung pathology.
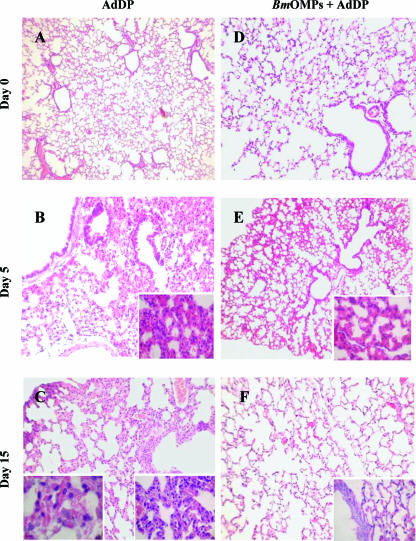
We hypothesized that the rapid clearance of bacteria from the lungs and the absence of disease signs in OMP-immunized mice were associated with mild changes in lung histology. The lungs of AdDP- and B. multivorans OMP-immunized mice were examined 0, 5, and 15 days after challenge with B. multivorans. On day 5, in lung sections from control mice infected with B. multivorans there were focal cellular infiltrates of inflammatory cells (mainly macrophages with limited lymphocytes and polymorphonuclear leukocytes) in alveolar, peribronchial, and perivascular areas (Fig. 6B). On day 15, these areas showed a more intense compromise characterized by diffuse and extended cellular infiltrates (mainly macrophages, lymphocytes, and polymorphonuclear leukocytes), along with disruption of the normal architecture of the parenchyma (Fig. 6C). In contrast, the histological changes in lung sections from B. multivorans OMP-immunized mice infected with B. multivorans were less pronounced. These animals exhibited reduced and limited parenchymal involvement and inflammatory cell infiltrates, and the overall architecture of the respiratory areas was conserved (Fig. 6E). Furthermore, on day 15 the lungs of B. multivorans OMP-immunized mice showed minimal evidence of pathology (Fig. 6F).
FIG. 6.
Lung histopathology in mice immunized with B. multivorans OMPs (BmOMPs) following infection with B. multivorans. (A, B, and C) Representative hematoxylin- and eosin-stained sections of mouse lung samples from AdDP-immunized mice at 0, 5, and 15 days postinfection, respectively. (D, E, and F) Representative hematoxylin- and eosin-stained sections of mouse lung samples from B. multivorans OMP-immunized mice at 0, 5, and 15 days postinfection, respectively. Magnifications: ×5 (A to F), ×40 (insets in panels B, E, and F and right inset in panel C), and ×100 (left inset in panel C).
Supporting these results, the total peripheral leukocyte counts determined on days 0, 5, and 13 after challenge for B. multivorans OMP- and AdDP-immunized mice were not significantly different from the values observed for preinfection animals (day −2) except for day 13, when the number of total leukocytes in control mice was higher than the number in B. multivorans OMP-immunized mice (Table 2). Differential counts for blood smears showed that on day 5 postinfection, the numbers of polymorphonuclear leukocytes were reduced to the same extent in AdDP-immunized mice and mice immunized with B. multivorans OMPs plus AdDP, as expected from the cyclophosphamide treatment. On day 13, the polymorphonuclear leukocytes counts for the AdDP-immunized mice were significantly higher than the polymorphonuclear leukocyte counts for the B. multivorans OMP-immunized mice (Table 2). The observation of an extended cellular infiltrate on day 15 in the lungs of infected control mice is consistent with the increased number of polymorphonuclear leukocytes in these mice.
TABLE 2.
Total peripheral leukocytes and polymorphonuclear blood cells in B. multivorans OMP-immunized mice following infection with B. multivorans
| Day postinfection | Median total peripheral leukocyte concn (range) (109/liter)
|
Median polymorphonuclear leukocyte concn (range) (109/liter)
|
||
|---|---|---|---|---|
| AdDP | B. multivorans OMPs + AdDP | AdDP | B. multivorans OMPs + AdDP | |
| −2 | 8.9 (6.8-17.0) | 9.4 (6.2-12.8) | 3.39 (1.77-7.34) | 2.98 (2.15-3.34) |
| 0 | 9.4 (6.5-15.9) | 7.9 (6.1-11.4) | 3.40 (1.76-7.08) | 2.35 (1.77-2.99) |
| 5 | 7.0 (3.9-8.0) | 7.3 (5.4-8.0) | 1.90 (1.33-2.32) | 1.46 (1.40-2.30) |
| 13 | 19.1 (14.5-30.1)a | 8.1 (6.5-11.8) | 11.50 (10.36-15.0)b | 2.43 (1.18-4.03) |
P < 0.01 for a comparison with the mice immunized with B. multivorans OMPs plus AdDP, as determined by the Mann-Whitney U test.
P < 0.0001 for a comparison with the mice immunized with B. multivorans OMPs plus AdDP, as determined by the Mann-Whitney U test.
i.n. immunization with B. multivorans OMPs plus AdDP induces cross-reactivity and confers protection against B. cenocepacia lung infection.
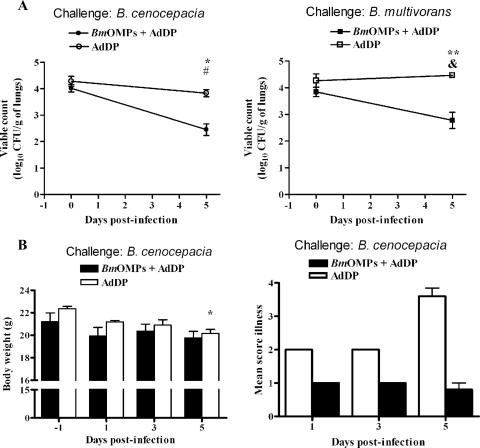
As immunization with B. multivorans OMPs resulted in a strong immune response and protection of vaccinated mice against pulmonary infection with B. multivorans, we assessed whether immunization with this preparation could cross-protect mice against challenge with B. cenocepacia. In these experiments, mice in the AdDP-vaccinated control group exhibited more severe illness than the animals immunized with B. multivorans OMPs plus AdDP exhibited after B. cenocepacia challenge. Therefore, animals were sacrificed on day 5 postinfection, and samples were collected to evaluate cross-protection. Based on clinical illness scores, lung histopathology, and clearance of the bacterial load, mice immunized with B. multivorans OMPs were protected against pulmonary challenge with B. cenocepacia. As we observed in the experiment described above (Fig. 4A) and confirmed in this experiment (Fig. 7A, right panel) for B. multivorans pulmonary infection, the control group and the group treated with B. multivorans OMPs plus AdDP exhibited different rates of bacterial clearance after challenge with B. cenocepacia, as shown by the number of bacteria recovered from the lungs (Fig. 7A, left panel). The initial pulmonary bacterial load was 4.2 ± 0.2 log10 CFU/g of lungs (mean ± SEM), and by day 5 the load in the AdDP-vaccinated mice was 3.83 ± 0.13 log10 CFU/g of lungs (P < 0.03). In contrast, mice vaccinated with B. multivorans OMPs plus AdDP and infected with B. cenocepacia exhibited more rapid clearance of the infection, and the load was 2.28 ± 0.3 log10 CFU/g of lungs, which corresponded to a nearly 2-log reduction in the bacterial load for this group (P < 0.001 for a comparison with day 0 data and P < 0.006 for a comparison with the AdDP-immunized group).
FIG. 7.
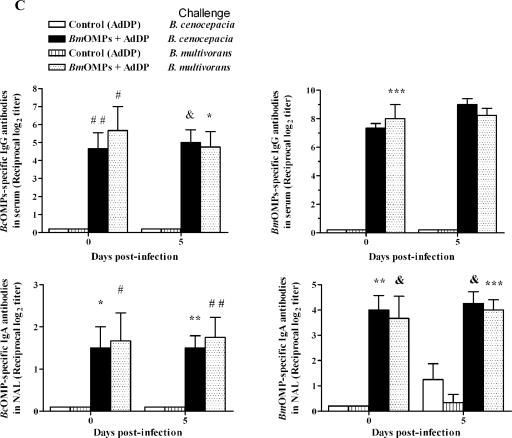
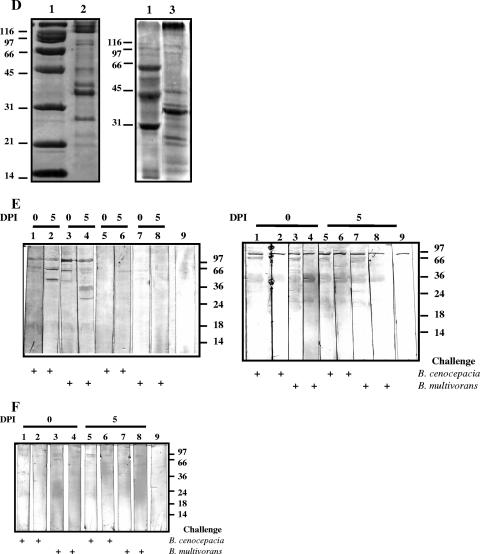
i.n. immunization with B. multivorans OMPs (BmOMPs) plus AdDP induces cross-reactivity and confers protection against B. cenocepacia lung infection. Mice immunized with AdDP and B. multivorans OMPs were challenged i.n. with 2.8 × 107 CFU of B. multivorans or B. cenocepacia. (A) Kinetics of B. cenocepacia (left panel) or B. multivorans (right panel) clearance from lungs of infected mice. Pulmonary bacterial clearance was assessed after 0 (4 h) and 5 days by plating dilutions of lung homogenate on LB agar. The results are expressed as the mean log10-transformed CFU/g of lungs; the error bars indicate SEM. One asterisk indicates that the P value is <0.0066 and two asterisks indicate that the P value is <0.009 for a comparison with the AdDP-immunized group, as determined by Student's unpaired two-tailed t test; a number sign indicates that the P value is <0.001 and an ampersand indicates that the P value is <0.04 for comparison with the B. multivorans OMP-immunized group on day 0, as determined by Student's unpaired two-tailed t test. (B) Changes in body weight (left panel) and the clinical illness score (right panel) of B. cenocepacia-infected mice. The body weight results are expressed as the mean body weight; the error bars indicate SEM. The asterisk indicates that the P value is <0.005 for a comparison with the AdDP-immunized group, as determined by a Student's unpaired two-tailed t test. The following clinical illness scores were used: 0, healthy; 1, barely ruffled fur; 2, ruffled fur and active; 3, ruffled fur and inactive; 4, ruffled, inactive, hunched posture, and gaunt; 5, dead. The results are expressed as the means of the illness score; the error bars indicate SEM. The differences were statistically significant when values were compared with the values for the AdDP-immunized group at P < 0.0001, as determined by Student's unpaired two-tailed t test. (C) Comparison of the reactivities of OMP-specific antibodies of infected mice as determined by ELISA using B. multivorans OMPs (right panels) or B. cenocepacia OMPs (BcOMP) (left panels) as the coating antigens. The titers of OMP-specific serum IgG antibodies (upper panels) and OMP-specific NAL IgA antibodies (lower panels) were determined. The results are expressed as the mean reciprocal log2 of the endpoint titer; the error bars indicate SEM. Mice vaccinated with AdDP and with B. multivorans OMPs plus AdDP were challenged with B. cenocepacia and with B. multivorans. Most differences were statistically significant at a P value of <0.0001 when values were compared with the values for the AdDP-immunized group, as determined by Student's unpaired two-tailed t test; the exceptions are indicated by one asterisk (P < 0.02), two asterisks (P < 0.002), three asterisks (P < 0.001), one number sign (P < 0.05), two number signs (P < 0.03), and an ampersand (P < 0.01). (D) Coomassie blue staining of B. multivorans OMPs and B. cenocepacia OMPs. OMPs were separated by 12% SDS-PAGE and analyzed by Coomassie blue staining. Lane 1, molecular weight markers; lane 2, B. multivorans OMPs; lane 3, B. cenocepacia OMPs. (E) Comparison of the reactivities of B. multivorans OMP-specific serum IgG antibodies (left panel) and B. multivorans OMP-specific NAL IgA antibodies (right panel) of infected mice by Western blotting using B. multivorans OMPs as the immobilized antigens. Lanes 1, 2, 5, and 6, mice infected with B. cenocepacia; lanes 1 and 5, B. multivorans OMP-immunized mice; lanes 2 and 6, AdDP immunized mice; lanes 3, 4, 7, and 8, mice infected with B. multivorans; lanes 3 and 7, B. multivorans OMP-immunized mice; lanes 4 and 8, AdDP-immunized mice; lane 9, conjugate control. (F) Comparison of the cross-reactivities of B. multivorans OMP NAL IgA antibodies of infected mice by Western blotting using B. cenocepacia OMPs as the immobilized antigens. Lanes 1, 2, 5, and 6, mice infected with B. cenocepacia; lanes 1 and 5, B. multivorans OMP-immunized mice; lanes 2 and 6, AdDP-immunized mice; lanes 3, 4, 7, and 8, mice infected with B. multivorans; lanes 3 and 7, B. multivorans OMP-immunized mice; lanes 4 and 8, AdDP-immunized mice; lane 9, conjugate control. The positions of the molecular mass standards are indicated on the right (in kDa). DPI, days postinfection.
On day 5 postinfection, examination of lung sections from control mice infected with B. cenocepacia revealed a focal cellular infiltrate of inflammatory cells that were mainly mononuclear cells, which also included a limited number of polymorphonuclear leukocytes in alveolar, peribronchial, and perivascular areas. In contrast, the histological changes in lung sections from B. multivorans OMPs-immunized mice infected with B. cenocepacia were less pronounced. These animals exhibited reduced and limited parenchymal involvement and inflammatory cell infiltrates, and the overall architecture of the respiratory areas was conserved. The histological changes were similar to those shown in Fig. 6B and E.
The mean body weight of AdDP-immunized mice infected with B. cenocepacia was significantly lower than the mean body weight of the B. multivorans OMP-immunized group on day 5 (P < 0.005) (Fig. 7B, left panel). On day 5 the median (minimum, maximum) percentage of weight loss for AdDP-vaccinated mice was 12% (9%, 13%). The illness score was also significantly higher for the AdDP-immunized mice than for the mice immunized with B. multivorans OMPs plus AdDP (Fig. 7B, right panel). The difference was significant throughout the experiment for AdDP-immunized mice (P < 0.0001, as determined by ANOVA). This was more evident on day 5 postinfection, when all five mice that received AdDP had signs of severe disease, while no signs of disease were observed in the mice immunized with B. multivorans OMPs plus AdDP (illness scores, 3.6 ± 0.24 and 0.8 ± 0.2, respectively; P < 0.0001).
Cross-protection directly correlated with the immune response elicited by vaccination cross-reactivity (Fig. 7C), as indicated by a comparison of the reactivities of OMP-specific antibodies of B. multivorans OMP-vaccinated mice challenged with B. multivorans or B. cenocepacia against homologous antigens (Fig. 7C, right panel) and nonhomologous antigens (Fig. 7C, left panel). The B. multivorans OMP serum IgG (Fig. 7C, upper right panel) and NAL B. multivorans OMP IgA (Fig. 7C, lower right panel) antibodies induced by vaccination with B. multivorans OMPs plus AdDP in both B. multivorans- and B. cenocepacia-challenged mice also cross-reacted with the nonhomologous antigens of B. cenocepacia OMPs (Fig. 7C, upper left and lower left panels). There were no statistically significant differences between the endpoint titers obtained for mice challenged with B. multivorans and the endpoint titers obtained for mice challenged with B. cenocepacia. Moreover, this level of response was maintained in both conditions throughout the experiment.
We observed similar patterns of B. multivorans OMPs and B. cenocepacia OMPs using SDS-PAGE with Coomassie blue staining (Fig. 7D). There were polypeptide bands at apparent molecular masses of 97, 91, 72, 45, 42, 37, 26, 22, and 20 kDa and some faint bands at 60 to 66 kDa. IgG serum antibodies from B. multivorans OMP-vaccinated mice after challenge with the homologous and nonhomologous species against B. multivorans OMPs showed similar patterns of specific reactivity to the 97-, 91-, 72-, and 45-kDa polypeptides throughout the experiment and bands between 60 and 66 kDa on day 5 after the challenge (Fig. 7E, left panel). On the other hand, the reactivity patterns of antibodies present in NAL samples indicated that secretory IgA antibodies recognized the 90-, 72-, 66- to 60-, and 45-kDa polypeptides and some other faint bands with molecular masses ranging from 26 to 18 kDa on day 5 postchallenge in both B. multivorans- and B. cenocepacia-infected mice (Fig. 7E, right panel). A comparison of the results of the immunoblotting analysis of the reactivity patterns for cross-reactive NAL antibodies of B. multivorans OMP-vaccinated mice after challenge with nonhomologous OMPs showed that the antibodies directed against B. multivorans OMP antigens also recognized several OMP antigens from B. cenocepacia. The cross-reactivity was observed mainly with the 90-, 72-, and 60- to 66-kDa antigens for NAL secretory IgA antibodies (Fig. 7F).
These results indicate that i.n. immunization with B. multivorans OMPs plus AdDP enhanced the clearance of the nonhomologous species B. cenocepacia from the lungs, and the protective effect was associated with cross-reactivity of OMP-specific IgA antibody titers in NAL samples that recognized 90-, 72-, and 60- to 66-kDa antigens.
DISCUSSION
We demonstrate here that i.n. immunization with OMPs from Bcc species plus AdDP, a mucosal adjuvant with an adequate safety profile (8, 25), elicited significant Bcc-specific serum and mucosal immune responses. All titers were significantly higher in OMP-immunized animals than in AdDP-immunized controls. Given its pharmacokinetics and safety profiles, AdDP is a promising candidate for incorporation in mucosal vaccine formulations. The inclusion of AdDP in this immunization protocol should be relevant for future studies on the use of potential candidate antigens for developing recombinant subunit protein-based vaccines, since soluble antigens generally must be administered in combination with suitable adjuvants to evoke effective immune responses (42).
Although the level of endotoxin contamination was low in OMP preparations, we wanted to be sure that the amount of endotoxin present did not have a toxic effect or influence the specificity of the immune response. Our results demonstrated that the level of LPS present in the B. multivorans OMP preparation did not have a pyrogenic effect and that the OMP preparation not only was safe and nontoxic but also did not trigger any inflammatory responses when it was administrated by the i.n. route. Furthermore, our results confirmed that LPS has no effect on the immunogenicity of OMPs. These results are consistent with the results of previous i.n. immunization studies of humans with OMP vesicle preparations containing LPS, which revealed no problems with LPS in the nasal vaccine formulation (16, 27). Moreover, LPS is used as the protective antigen component of anti-Shigella vaccines that have advanced to clinical trials, and when given i.n. to laboratory rodents or nonhuman primates, the vaccine proved to be safe, nontoxic, well tolerated, and highly immunogenic and protected against Shigella challenge in various models of Shigella infection (34, 38). Similarly, the vaccine was safe, nontoxic, and well tolerated in phase I and II clinical trials in which more than 100 volunteers received doses of up to 1.5 mg of LPS (20).
The mouse model of chronic lung infection allowed us to evaluate the protective effect of i.n. immunization after challenge with B. multivorans. This species was chosen since previous studies have shown that, unlike B. cenocepacia, B. multivorans can establish a chronic infection in the mildly neutropenic mouse model (10, 11). Also, B. multivorans is regularly isolated from CF patients (41). Although this model does not exactly mimic CF disease, it provides a cost-effective screening tool for selecting the most promising vaccine formulation for further development and determining a correlation with protection. Our results clearly demonstrated that immunization with B. multivorans OMPs plus AdDP, followed by challenge with B. multivorans, dramatically decreased the lung pathology compared to that in AdDP-immunized mice. The reduction in the severity of lung disease correlated with significantly enhanced and almost complete bacterial clearance in the lungs of B. multivorans OMP-vaccinated mice compared to the control groups. Also, all control animals exhibited signs of illness and weight loss at the end of the experiment, whereas no evidence of clinical disease was observed in B. multivorans OMP-immunized mice.
Specific IgA antibodies elicited by mucosal immunization are likely to play an important role in the adaptive immune system, inhibiting adhesion and colonization of bacterial pathogens (48). This indicates that IgA is the first line of defense in the mucosal compartment (31). Although serum IgA exhibits both pro- and anti-inflammatory activities (17, 19, 37, 39, 49, 50), secretory IgA is generally considered a noninflammatory antibody because it does not elicit inflammatory processes after binding to antigens (23, 24, 47). Our data suggest that local immunity in the respiratory tract before exposure to Bcc bacteria would be beneficial for the host. Indeed, the enhanced bacterial clearance in the lungs of OMP-vaccinated animals correlated with the anti-OMP secretory IgA response observed in NAL samples. Although we demonstrated that the 97-, 91-, 72-, 66- to 60-, and 45-kDa OMPs are recognized by serum of B. multivorans OMP-vaccinated mice, we found that the principal targets of secretory IgA were the 90-, 72-, 66- to 60-, and 45-kDa antigens.
We cannot rule out the possibility that specific IgG detected in respiratory secretions also contributes to bacterial clearance. Like IgA, IgG may limit the entry of mucosal pathogens into the host and their multiplication in the host, thereby preventing systemic infection (46). Although in the present study we did not examine these mechanisms directly, we propose that the more rapid resolution of pulmonary infection and the absence of disease signs in OMP-vaccinated animals were probably due to the inhibition of adhesion or colonization by bacterial pathogens and to the anti-inflammatory role of secretory IgA that may have limited lung damage caused by inflammation during infection. This is supported by the mild disease process observed to occur in OMP-vaccinated mice during the first few days after infection, which finally progressed to complete resolution of lung inflammation and no signs of lung pathology.
The range of cross-species protection that may be achieved has particular relevance since Bcc species show a great deal of diversity at the subspecies level. We demonstrated that administration of the B. multivorans OMP vaccine enhanced the clearance of B. cenocepacia from the lungs, and the protective effect was associated with cross-reactivity of OMP-specific IgA antibody titers in NAL samples that recognized the 90-, 72-, and 66- to 60-kDa antigens. Therefore, our results suggest that B. multivorans OMPs have determinants that are exposed on the surface of bacterium and are antigenically conserved in B. multivorans and B. cenocepacia, two species belonging to the Bcc.
In conclusion, our data demonstrate the important role of mucosal antibodies as a defense mechanism against infection with B. multivorans or B. cenocepacia, suggesting that increased mucosal immunity in the airways may help patients with CF. Moreover, the 90-, 72-, and 66- to 60-kDa OMPs targeted by secretory IgA and conserved in B. multivorans and B. cenocepacia may be promising candidates for formulations of recombinant subunit protein-based vaccines, providing a basis for rational vaccine design. Studies to identify these proteins and individually investigate their antigenicities and protective effects are under way in our laboratories.
Acknowledgments
We thank Nélida Mondelo of the Gador Laboratory, Buenos Aires, Argentina, for kindly providing the animals used in this work and María Fernanda Repetto of the Richet Laboratory, Buenos Aires, Argentina, for kindly performing the endotoxin test analysis.
This work was supported in part by the Special Program Grant Initiative “In Memory of Michael O'Reilly” funded by the Canadian Cystic Fibrosis Foundation and by the Cardiovascular and Respiratory Health Institute of the Canadian Institutes of Health Research (to M.A.V.). M.A.V. holds a Canada Research Chair in Infectious Disease and Microbial Pathogenesis.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Aaron, S. D., W. Ferris, D. A. Henry, D. P. Speert, and N. E. Macdonald. 2000. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 161:1206-1212. [DOI] [PubMed] [Google Scholar]
- 2.Aggerbeck, H., S. Gizurarson, J. Wantzin, and I. Heron. 1997. Intranasal booster vaccination against diphtheria and tetanus in man. Vaccine 15:307-316. [DOI] [PubMed] [Google Scholar]
- 3.Aronoff, S. C., F. J. Quiner, and R. C. Stern. 1991. Longitudinal serum IgG response to Pseudomonas cepacia surface antigens in cystic fibrosis. Paediatr. Pulmonol. 1:289-293. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff, S. C., and R. C. Stern. 1988. Serum IgG antibody to the outer membrane antigens of Pseudomonas cepacia and Pseudomonas aeruginosa in cystic fibrosis. J. Infect. Dis. 157:934-940. [DOI] [PubMed] [Google Scholar]
- 5.Becker, P. D., R. S. Corral, C. A. Guzmán, and S. Grinstein. 2001. Adamantylamide dipeptide as effective immunoadjuvant in rabbits and mice. Vaccine 19:4603-4609. [DOI] [PubMed] [Google Scholar]
- 6.Bertot, G. M., P. D. Becker, C. A. Guzmán, and S. Grinstein. 2004. Intranasal vaccination with recombinant P6 protein and adamantylamide dipeptide as mucosal adjuvant confers efficient protection against otitis media and lung infection by nontypeable Haemophilus influenzae. J. Infect. Dis. 189:1304-1312. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, M. J., J. A. Hopkins, R. M. Berka, M. L. Vasil, and W. L. Wang. 1983. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect. Immun. 42:276-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchar, E., I. Janku, H. Farghali, and K. Masek. 1991. The effect of some immunomodulators administration to rats on palmitic and oleic acids incorporation into the lipids of liver cells organelles. Methods Find. Exp. Clin. Pharmacol. 131:269-271. [PubMed] [Google Scholar]
- 9.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 10.Chu, K. K., D. J. Davidson, T. K. Halsey, J. W. Chung, and D. P. Speert. 2002. Differential persistence among genomovars of the Burkholderia cepacia complex in a murine model of pulmonary infection. Infect. Immun. 70:2715-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu, K. K., K. L. MacDonald, D. J. Davidson, and D. P. Speert. 2004. Persistence of Burkholderia multivorans within the pulmonary macrophage in the murine lung. Infect. Immun. 72:6142-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coenye, T., and P. Vandamme. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5:719-729. [DOI] [PubMed] [Google Scholar]
- 13.Coenye, T., P. Vandamme, J. R. W. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darveau, R., and R. Hancock. 1983. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 155:831-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis, S. S. 2001. Nasal vaccines. Adv. Drug Deliv. Rev. 51:21-42. [DOI] [PubMed] [Google Scholar]
- 16.Drabick, J. J., B. L. Brandt, E. E. Moran, N. B. Saunders, D. R. Shoemaker, and W. D. Zollinger. 1999. Safety and immunogenicity testing of an intranasal group B meningococcal native outer membrane vesicle vaccine in healthy volunteers. Vaccine 18:160-172. [DOI] [PubMed] [Google Scholar]
- 17.Ferreri, N. R., W. C. Howland, and H. L. Spiegelberg. 1986. Release of leukotrienes C4 and B4 and prostaglandin E2 from human monocytes stimulated with aggregated IgG, IgA, and IgE. J. Immunol. 136:4188-4193. [PubMed] [Google Scholar]
- 18.Flegel, M., J. Seifert, H. Farghali, K. Masek, and M. Krojidlo. 1986. Synthesis and pharmacological properties of adamantylamide analogs of muramyl-dipeptide, p. 561-564. In D. Theodoropoulos (ed.), Peptides. Walter de Gruyter and Co., Berlin, Germany.
- 19.Foreback, J. L., D. G. Remick, E. Crockett-Torabi, and P. A. Ward. 1997. Cytokine responses of human blood monocytes stimulated with Igs. Inflammation 21:501-517. [DOI] [PubMed] [Google Scholar]
- 20.Fries, L. F., A. D. Montemarano, C. P. Mallett, D. N. Taylor, T. L. Hale, and G. H. Lowell. 2001. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect. Immun. 69:4545-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluck, U., J. O. Gebbers, and R. Gluck. 1999. Phase 1 evaluation of intranasal virosomal influenza vaccine with and without Escherichia coli heat-labile toxin in adult volunteers. J. Virol. 73:7780-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govan, J. R. W., and P. Vandamme. 1998. Agricultural and medical microbiology: a time for bridging gaps. Microbiology 144:2373-2375. [DOI] [PubMed] [Google Scholar]
- 23.Heystek, H. C., C. Moulon, A. M. Woltman, P. Garonne, and C. van Kooten. 2002. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J. Immunol. 168:102-107. [DOI] [PubMed] [Google Scholar]
- 24.Honorio-Franca, A. C., P. Launay, M. M. Carneiro-Sampaio, and R. C. Monteiro. 2001. Colostral neutrophils express Fc alpha receptors (CD89) lacking gamma chain association and mediate noninflammatory properties of secretory IgA. J. Leukoc. Biol. 69:289-296. [PubMed] [Google Scholar]
- 25.Horak, P., and K. Masek. 1988. Analgesic activity of two synthetic immunomodulators, muramyl dipeptide and adamantylamide dipeptide, in mice and rats. Meth. Find. Exp. Clin. Pharmacol. 10:569-574. [PubMed] [Google Scholar]
- 26.Hunt, T. A., C. Kooi, P. A. Sokol, and M. A. Valvano. 2004. Identification of Burkholderia cenocepacia genes required for bacterial survival in vivo. Infect. Immun. 72:4010-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katial, R. K., B. L. Brandt, E. E. Moran, S. Marks, V. Agnello, and W. D. Zollinger. 2002. Immunogenicity and safety testing of a group B intranasal meningococcal native outer membrane vesicle vaccine. Infect. Immun. 70:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyono, H., and S. Fukuyama. 2004. NALT- versus Peyer's patch-mediated mucosal immunity. Nat. Rev. Immunol. 4:699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacy, D., A. Smith, D. Stableforth, G. Smith, P. Weller, and M. Brown. 1995. Serum IgG response to B. cepacia outer membrane antigens in cystic fibrosis: assessment of cross-reactivity with P. aeruginosa. FEMS Immunol. Med. Microbiol. 10:253-262. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311-340. [DOI] [PubMed] [Google Scholar]
- 32.Li, F., S. M. Michalek, A. P. Dasanayake, Y. Li, K. Kirk, and N. K. Childers. 2003. Intranasal immunization of humans with Streptococcus mutans antigens. Oral Microbiol. Immunol. 18:271-277. [DOI] [PubMed] [Google Scholar]
- 33.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-156. [DOI] [PubMed] [Google Scholar]
- 34.Mallett, C. P., T. L. Hale, R. W. Kaminski, T. Larsen, N. Orr, D. Cohen, and G. H. Lowell. 1995. Intranasal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infections. Infect. Immun. 63:2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta, A., and A. Bush. 2005. Beyond chloride transport: CFTR in the 21st century—introductory remarks to a new state of the art series. Pediatr. Pulmonol. 39:289-291. [DOI] [PubMed] [Google Scholar]
- 36.Nelson, J. W., S. L. Butler, P. H. Brown, A. P. Greening, and J. R. Govan. 1993. Serum IgG and sputum IgA antibody to core lipopolysaccharide antigen from Pseudomonas cepacia in patients with cystic fibrosis. J. Med. Microbiol. 39:39-47. [DOI] [PubMed] [Google Scholar]
- 37.Olas, K., H. Butterbeck, W. Teschner, H. P. Schwarz, and B. M. Reipert. 2005. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin. Exp. Immunol. 140:478-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orr, N., G. Robin, D. Cohen, R. Arnon, and G. H. Lowell. 1993. Immunogenicity and efficacy of oral or intranasal Shigella flexneri 2a and Shigella sonnei proteosome-lipopolysaccharide vaccines in animal models. Infect. Immun. 61:2390-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patry, C., A. Herbelin, A. Lehuen, J. F. Bach, and R. C. Monteiro. 1995. Fc alpha receptors mediate release of tumour necrosis factor-alpha and interleukin-6 by human monocytes following receptor aggregation. Immunology 86:1-5. [PMC free article] [PubMed] [Google Scholar]
- 40.Rajyaguru, J. M., and M. J. Muszynski. 1997. Association of resistance to trimethoprim/sulphamethoxazole, chloramphenicol and quinolones with changes in major outer membrane proteins and lipopolysaccharide in Burkholderia cepacia. J. Antimicrob. Chemother. 40:803-809. [DOI] [PubMed] [Google Scholar]
- 41.Reik, R., T. Spilker, and J. J. LiPuma. 2005. Distribution of Burkholderia cepacia complex species among isolates recovered from persons with or without cystic fibrosis. J. Clin. Microbiol. 43:2926-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan, E. J., L. M. Daly, and H. G. Mills. 2001. Immunoregulators and delivery systems for vaccination by mucosal route. Trends Biotechnol. 19:293-304. [DOI] [PubMed] [Google Scholar]
- 43.Speert, D. P., D. Henry, P. Vandamme, M. Corey, and E. Mahenthiralingam. 2002. Epidemiology of Burkholderia cepacia in patients with cystic fibrosis. Emerg. Infect. Dis. 8:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 45.Turcios, N. L. 2005. Cystic fibrosis: an overview. J. Clin. Gastroenterol. 39:307-317. [DOI] [PubMed] [Google Scholar]
- 46.Underdown, B. J., and S. A. Plotkin. 1999. The induction of mucosal protection by parenteral immunization, p. 719-728. In P. L. Olgra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, Inc., San Diego, CA.
- 47.van Egmond, M., C. A. Damen, A. B. van Spriel, G. Vidarsson, E. van Garderen, and J. G. van de Winkel. 2001. IgA and the IgA Fc receptor. Trends Immunol. 22:205-211. [DOI] [PubMed] [Google Scholar]
- 48.Williams, R. C., and R. J. Gibbons. 1972. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science 177:697-699. [DOI] [PubMed] [Google Scholar]
- 49.Wolf, H. M., M. B. Fischer, H. Puhringer, A. Samstag, E. Vogel, and M. M. Eibl. 1994. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 83:1278-1288. [PubMed] [Google Scholar]
- 50.Wolf, H. M., I. Hauber, H. Gulle, A. Samstag, M. B. Fischer, R. U. Ahmad, and M. M. Eibl. 1996. Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin. Exp. Immunol. 105:537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]