Abstract
Cholera toxin (CT) is one of the most effective and widely studied mucosal adjuvants. Although the ADP-ribosylating A subunit has been implicated in augmenting immune responses, the receptor-binding B subunit (CT-B) has greater immunogenicity and may be a repository of adjuvant activity without potential toxicity. In order to elucidate mechanisms of immune modulation by CT-B alone, primary B cells and macrophages were assessed for responses to CT-B in vitro, as measured by the expression of cell surface markers, cellular signaling events, and cytokine secretion. Increased phosphorylation of multiple signaling molecules, including Erk1/2 and p38, was detected. CT-B also induced transactivation of the transcription elements cyclic AMP-responsive element and NF-κB, the latter of which was inhibited by phosphotyrosine inhibition. While specific inhibition of MEK1/2 did not reduce CT-B induction of cell surface marker expression, it did attenuate CT-B-mediated interleukin-6 secretion. These data show that CT-B induces a set of signaling events related to cellular activation, surface molecule expression, and cytokine production that has potential implications for elucidating CT-B adjuvant activity in the absence of enzymatically active holotoxin.
The development of effective mucosally delivered vaccines has been hampered by the paucity of useful adjuvants and limited knowledge of their modes of action. Currently, the most powerful mucosal adjuvants known are cholera toxin (CT) and the closely related Escherichia coli heat-labile toxin (LT), which cause the often fatal “rice-water” diarrhea of cholera and traveler's diarrhea, respectively. The CT holotoxin is composed of a single, enzymatically active A subunit noncovalently linked to a pentamer of receptor-binding B subunits. The toxin ADP ribosylates the α subunit of the GTP-binding regulatory protein Gs, thereby inducing permanent adenylate cyclase activation (8). This results in the accumulation of cyclic AMP (cAMP) that is related to the induction of the profuse diarrhea seen in cholera (15). Oral administration of CT with antigen to mice elicits long-term immunological memory (48) characterized by antigen-specific responses involving both CD4+ and CD8+ T cells, secretory immunoglobulin A (IgA) (10), high IgG1 and IgE serum titers (26, 47), and the predominantly Th2 cytokines interleukin-4 (IL-4), IL-5, IL-6, and IL-10 (31, 52). It is likely that enhancement of antigen presentation is a major mechanism of CT adjuvanticity. Indeed, antigen-presenting cells (APCs) treated with CT are able to augment T-cell proliferative responses to anti-CD3 (55). CT holotoxin has been shown to up-regulate expression levels of the costimulatory molecules CD80 (B7.1) and CD86 (B7.2) on B cells, macrophages, and dendritic cells (17). The finding that the administration of anti-CD86 antibody inhibits antigen-specific secretory IgA and serum IgG1 responses in mice fed keyhole limpet hemocyanin plus CT (12) further suggests that a direct effect on APCs may be an important mechanism of CT adjuvanticity.
Although the current data suggest that the CT holotoxin harbors the toxin's most potent adjuvant activity (35), the use of this molecule in vaccination poses risks, such as the increased incidence of Bell's palsy in recipients in a recent clinical trial testing the efficacy of a nasal vaccine for influenza using LT as an adjuvant (13). Successful use of these molecules to elicit immune responses through vaccination in humans requires separation of toxicity from adjuvanticity. Accumulating data indicate there are multiple immune modulating pathways triggered by CT, including mechanisms independent of ADP ribosyltransferase activity (14, 16, 56). For example, oral vaccines utilizing CT's nontoxic B subunit (CT-B) as an adjuvant to protect against E. coli and cholera itself have been safe and immunogenic in clinical trials (40, 46). Nonetheless, little is known about the mechanisms of CT-induced cellular activation of APCs. Numerous studies have suggested that engagement of the ganglioside GM1, the major receptor for CT and LT, is required for the ability of these molecules to alter immune responses (21, 30). Therefore, we surveyed various APCs for responses to CT-B alone, focusing on signaling events commonly associated with lipid rafts and cellular activation. We generated data demonstrating that in the absence of the toxic A subunit, CT-B induces intracellular signaling associated with the development of immunity in murine B cells and macrophages.
MATERIALS AND METHODS
Primary cell isolation and cell culture.
All primary cells were isolated from 6- to 12-week-old female C57BL/6 mice or from the lipopolysaccharide (LPS)-nonresponsive strain C3H/HeJ (Jackson Laboratory, Bar Harbor, ME). Naïve splenic B cells were isolated as previously described (51). Briefly, red blood cells in a splenocyte suspension were lysed with 0.15 M Tris-buffered NH4Cl. T cells were depleted via complement-mediated lysis, and depletion of other nonspecific cells was achieved by passage over a Sephadex G10 column. Ficoll gradient centrifugation was used in the final purification steps to remove remaining dead cells. The purity of the B-cell population (typically 88 to 92%) was determined by flow cytometric analysis of B220-expressing cells as described below. Depletion of Mac-1- and CD3-expressing populations was also verified by flow cytometry. Alternatively, B cells were isolated after red blood cell lysis by negative selection using a MACS magnetic bead kit as described by the manufacturer (Miltenyi Biotec, Auburn, CA). The typical purity when this method was used was 98 to 99%. Freshly isolated B cells were incubated in 24-well tissue culture plates at a concentration of 5 × 106 cells/ml R10 medium (RPMI 1640 with 10% fetal bovine serum [FBS], 10 mM HEPES, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine). Primary B cells were allowed to rest at 37°C for 1 h before treatment.
Murine macrophages were obtained by two methods. (i) Macrophages were elicited by intraperitoneal injection of 2.5 ml thioglycolate (Remel, Lenexa, KS). Peritoneal washouts were collected 4 days postinjection and plated in 12-well plates at concentrations of 0.5 × 106 to 1 × 106 cells/well in 1 ml R5 medium (RPMI 1640 with 5% FBS, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine). Nonadherent cells were removed after overnight culture. The macrophages were allowed to rest for 2 to 3 days before treatment. (ii) Bone marrow-derived macrophage cultures were established from tibia and femur bone marrow. Marrow suspensions were centrifuged over Lympholyte M (Cedar Lane, Hornby, Canada) and then cultured overnight in RPMI (supplemented with 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine) with 25% L-cell conditioned medium (clone 929). Nonadherent cells were aspirated, replated in the same medium, and cultured for 5 days before treatment with CT-B. Macrophage maturation was assessed by flow cytometric analysis of CD11c and CD11b expression. The murine macrophage cell line RAW 264.7 (36) was cultured in R5 medium and plated at a concentration of 5 × 104 cells/ml in 24-well plates the night before treatment.
Toxin and inhibitor treatments.
CT-B preparations were obtained from List Biologicals (Campbell, CA) or were purified from Vibrio cholerae strain 0395-NT transformed with the pGEM-3 plasmid containing the CT gene under control of the CT promoter (kindly provided by Mariagrazia Pizza, Novartis Vaccines, Siena, Italy) as previously described (16). Recombinant CT-B (rB) was obtained from Molecular Probes (Eugene, OR). The CT A subunit was not detectable in CT-B preparations by matrix-assisted laser desorption ionization mass spectrometry (Voyager-DE matrix-assisted laser desorption ionization—time of flight mass spectrometer [Applied Biosystems, Inc.] used according to the manufacturer's recommendations) or on a Coomassie blue-stained sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel. Increases in cAMP levels were not detected by a cAMP enzyme immunoassay (Amersham Biosciences) performed according to the manufacturer's protocol in splenic C3H/HeJ B cells treated with CT-B preparations. Possible endotoxin contamination was evaluated by a Limulus lysate assay (Cell Sciences, Canton, MA). The concentration of endotoxin in the CT-B preparations was always less than 10 pg/ml, which is lower than the concentration of LPS that we found to be necessary to augment mitogen-activated protein (MAP) kinase phosphorylation or expression of CD86 and major histocompatibility complex class II (MHC-II) in splenic B cells. Cells were incubated at 37°C with 10 μg/ml CT-B or rB for the times indicated below prior to whole-cell lysis or nuclear extraction. For time course experiments, treatments were performed in a staggered manner, so that all samples were in culture for the same amount of time and were harvested together at the end of the experiment. Cells to be analyzed by flow cytometry were treated for 20 h.
The kinase inhibitors PD98059 (Upstate, Lake Placid, NY), U0126 (Sigma), SB202190 (Upstate), genistein (Sigma), and herbimycin A (Sigma) were added as indicated below directly to the cell cultures 30 min prior to the addition of CT-B. The activity of the kinase inhibitors was confirmed by immunoblot analysis of the following downstream targets of the inhibited kinases: phospho-Erk1/2 for PD98059 and U0216, phospho-p38 for SB202190, and phosphotyrosine for genistein and herbimycin A. The LPS inhibitors polymyxin B and compound E5564 (provided by Eisai Research Institute, Andover, MA) were added to cultured cells 30 min prior to treatment at concentrations of 20 and 10 μg/ml, respectively. The activities of the LPS inhibitors were established by their abilities to inhibit endotoxin-induced activation markers.
Flow cytometry.
The fluorescently labeled antibodies fluorescein isothiocyanate (FITC)-conjugated anti-B220, -CD3, -Mac1, and -CD11c (Caltag, Burlingame, CA) were used to assess the purity of the freshly isolated and cultured cell populations. FITC-conjugated anti-CD86, -I-Ak, -I-Ab, -CD14, and rat anti-IgG2a (Caltag), as well as FITC-conjugated anti-CD86, -CD80, -CD69, -CD40, -CD95, -CD14, -CD54, and rat anti-IgG1 and phycoerythrin-conjugated anti-CD69, -I-Ab, -CD40, -CD25, and rat anti-IgG1 (BD, Franklin Lakes, NJ), were used to evaluate the expression of cell surface markers following 20 h of cell culture. A total of 1 × 106 cells in phosphate-buffered saline (PBS) supplemented with 0.2% bovine serum albumin (BSA) were incubated with antibodies to cell surface markers on ice for 30 min. Cells were then washed and resuspended in PBS with 0.2% BSA and 2% paraformaldehyde prior to analysis with a FACScan (BD), for which the settings were optimized based on the fluorescence of isotype control stained cells. Ten thousand events were collected in the forward/side scatter viable cell gate. Data were acquired and analyzed using the CellQuest software (BD) or the WinMDI 2.8 software (Joseph Trotter, The Scripps Institute).
Immunoblotting.
Whole-cell lysates were generated by direct lysis of 5 × 106 cells in 2× SDS-PAGE loading dye. Five microliters per sample was loaded onto a 10% PAGE gel (4% stacking gel) and run at 150 V. Proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA) in Tris-buffered methanol-glycine transfer buffer at 200 mA for 60 min. All washing, blocking, and antibody binding steps were done at room temperature in PBS with 0.1% Tween 20. Blots were developed with the ECL Plus Western blotting detection reagent (Amersham Biosciences, Uppsala, Sweden) and exposed to Hyperfilm ECL (Amersham) for 30 s to 5 min. Anti-phosphotyrosine 4G10 was obtained from Upstate. All other immunoblot antibodies and horseradish peroxidase-linked anti-rabbit or anti-mouse IgG were obtained from Cell Signaling, Beverly, MA. Antibodies specific for the following phosphorylated proteins were used: MEK1/2 (Ser217/221), Erk1/2 (Thr202/Tyr204), p38 (Thr180/Tyr182), p90rsk (Ser380), Btk (Tyr223), PLCγ2 (Tyr1217), and cAMP response element binding protein (CREB) (Ser133). Exposed and scanned films were quantified by densitometry using the Kodak 1D image analysis software with automatic band detection and background correction.
Nuclear extraction and electrophoretic mobility shift assay (EMSA).
Nuclear extracts were obtained by allowing the cells to swell on ice for 30 min in HB buffer (10 mM HEPES [pH 8.0], 10 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 125 μM dithiothreitol, 1 μg/ml aprotinin, 1 μg/ml antipain, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml chymostatin). Nuclei were spun down for 5 min at 6,500 × g, and this was followed by extraction of nuclear proteins in a solution containing 20 mM HEPES (pH 8.0), 0.2 mM EDTA, 1.5 mM MgCL2, 430 mM NaCl, and 10% glycerol supplemented with 1 mM phenylmethylsulfonyl fluoride, 625 μM dithiothreitol, and 10 μg/ml of each of the protease inhibitors listed above. The extracts were stored, and the protein concentrations were determined by using the Bio-Rad (Hercules, CA) protein assay dye reagent at an optical density at 595 nm. For all samples equal amounts of protein were added to DNA fragments labeled at the 5′ end with [α-32P]deoxynucleotide triphosphate and containing the NF-κB consensus sequence (GGGACTTTCC; a gift from M. J. Fenton, University of Maryland, Baltimore [29]) and incubated for 30 min at room temperature. Binding reactions were run at 100 V on a acrylamide gel that was subsequently dried and exposed to radiography film (Kodak) overnight for band shift detection. The band intensity on exposed, scanned film was quantified with the Kodak 1D image analysis software using automatic band detection and background correction.
Transient transfection with luciferase reporter constructs.
RAW 264.7 macrophages were plated in 24-well plates 1 day before transfection at a concentration of 5 × 104 cells per well. Cells were transfected with 1 μg of the BD Mercury pathway profiling luciferase system plasmids (BD Biosciences) per well with Superfect transfection reagent (QIAGEN, Germantown, MD) by following the manufacturer's instructions. After transfection, cells were rested overnight prior to CT, LPS, or tumor necrosis factor alpha treatment in triplicate for 5 h or as indicated below. Cells were washed and lysed in reporter lysis buffer (Promega, Madison, WI) by snap freezing them on dry ice, and luciferase activities were determined using the luciferase reporter assay system (Promega) with a Monolight 3010 luminometer (BD). Standard deviations are indicated below. Luminescence was expressed in relative light units.
Immunocytochemistry.
Thioglycolate-elicited macrophages were isolated and cultured as described above on poly-l-lysine-coated glass coverslips. Following treatment, cells were rinsed with PBS, fixed with 4% paraformaldehyde for 15 min at room temperature, and permeabilized with 0.2% Triton X-100 in 10% FBS-PBS for 20 min. Cells were stained with anti-NF-κB p65 (Rockland, Gilbertsville, PA) in 1% saponin-10% FBS-PBS for 2 h at room temperature, washed, and stained with anti-rabbit IgG-Texas Red (Rockland) for 1 h. Cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI) prior to mounting with VectaShield (Vector Laboratories, Burlingame, CA). The p65 shift to the nucleus was quantified by averaging the red channel mean intensity for each nucleus in the untreated (n = 20) or treated (n = 7) images. The P value was calculated by using Student's two-tailed t test.
ELISA.
A sandwich enzyme-linked immunosorbent assay (ELISA) was performed using BD's mouse IL-6 kit with the following modifications: 96-well plates were coated with anti-IL-6 capture antibody in 0.1 M Na carbonate buffer (pH 9.5) overnight at 4°C. The plates were blocked with 1% BSA in PBS for 4 h at room temperature. Cell culture supernatant was added at a 1:10 dilution in blocking buffer overnight at 4°C. The ELISA plates were further processed according to the manufacturer's protocol and read with a SpectraMax 340 ELISA plate reader from Molecular Devices. Unknown concentrations were calculated based on an IL-6 standard curve.
RESULTS
CT-B induces expression of cell surface markers.
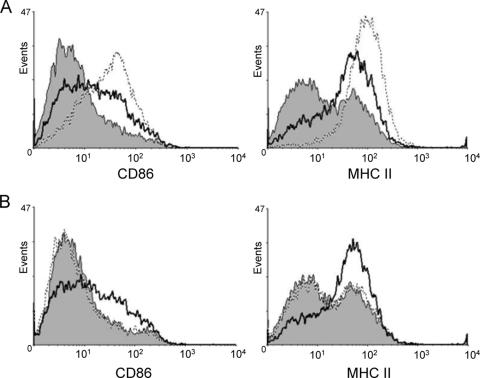
To confirm the ability of CT-B to induce cell surface activation marker expression on APCs, as previously reported (17), splenic B cells were isolated from C57BL/6 mice, treated in vitro with CT-B, and stained with fluorescent conjugated antibodies prior to analysis by flow cytometry. Culturing B cells for 20 h with CT-B resulted in increased surface expression of CD86 and MHC-II (Fig. 1A). The LPS inhibitor E5564 was unable to prevent CT-B-induced increases in activation marker expression while abolishing LPS-induced marker expression, verifying that the preparations were not contaminated with activating levels of endotoxin (Fig. 1B). Moreover, CT-B treatment also augmented CD86 and MHC-II levels on splenic B cells from the LPS-nonresponsive C3H/HeJ strain (data not shown). The increase in surface expression, detected as early as 10 h after addition of CT-B, was consistently reproduced and served as a control for subsequent signaling experiments. Modest increases in CD80, CD69, CD40, CD25, and CD95 levels were also observed on splenic B cells after 20 h of treatment with CT-B (data not shown).
FIG. 1.
CT-B increases expression of molecules involved in antigen presentation. Splenic B cells from C57BL/6 mice were preincubated in medium alone (A) or in medium with the LPS inhibitor E5564 (B) for 30 min. Cells were treated with 10 μg/ml CT-B (solid line) or 100 ng/ml LPS (dotted line) or left untreated (shaded area) for 20 h before analysis of CD86 and MHC-II expression by flow cytometry.
Specific phosphorylation events induced by CT-B in B cells.
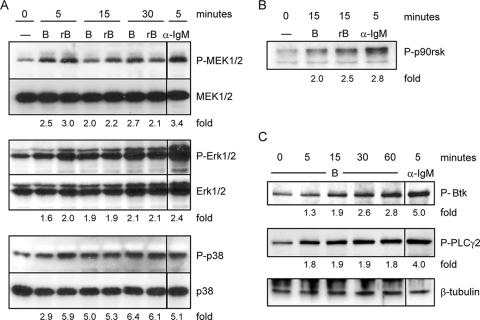
CT-B bound to Jurkat T cells has been shown to induce tyrosine kinase events only when anti-CT-B antibody is added to induce lipid raft patching (23). However, we have found that CT-B alone elevates cell surface marker expression on B cells, as shown in Fig. 1. We therefore hypothesized that CT-B can induce signaling events which lead to cellular activation. Although GM1 is not considered a typical pattern recognition receptor, responses to CT exhibit similarities to responses to innate immune receptors, such as the Toll-like receptors. Toll-like receptors have been shown to activate MAP kinases that are involved in a variety of developmental, stress, and immune responses, usually leading to changes in gene expression (3). Furthermore, E. coli LT, which binds to gangliosides, including GM1, has been shown to induce phosphorylation of the Erk1/2 MAP kinases in B cells (6). We therefore examined the Erk1/2 MAP kinase pathway in CT-treated cells. Splenic B cells were treated with 10 μg/ml of CT-B as described above. The B cells were also treated with a recombinant preparation of CT-B (rB) at the same concentration to rule out the possibility that signaling events were a result of undetectable subunit A contamination. Both preparations induced increased levels of phosphorylated MEK1/2 and its target Erk1/2 within 15 min (Fig. 2A). To corroborate the activation of Erk1/2, we measured the impact of CT-B on the levels of phosphorylated p90rsk, a protein kinase that is a direct target of Erk1/2 and can activate transcription factors, including CREB, serum response factor, and c-Fos (5, 23, 54). We found that both CT-B and rB induced phosphorylation of this kinase (Fig. 2B). Phosphorylation of c-Jun N-terminal kinase and p38, which are stress-activated MAP kinases with roles in inflammatory responses and apoptosis, was also evaluated. While the changes in phospho-c-Jun N-terminal kinase upon exposure to CT-B and rB were equivocal, the phospho-p38 levels increased (Fig. 2A).
FIG. 2.
CT-B induces specific phosphorylation events in B cells. Primary splenic B cells from C3H/HeJ mice were either left untreated (0 min) or stimulated with 10 μg/ml CT-B or rB for the times indicated and were harvested together at the end of the experiment. Cells were stimulated with 10 μg/ml anti-IgM F(ab′)2 as a positive control. Whole-cell lysates were generated, and this was followed by separation of cellular proteins by SDS-PAGE and transfer to a polyvinylidene difluoride membrane for immunoblotting. Phospho-MAP kinases (A), phospho-p90rsk (B), and phospho-Btk and phospho-PLCγ2 (C) were detected. Equal sample loading was confirmed by reprobing for total MAP kinase (A), a lower-molecular-weight nonspecific band (B), or β-tubulin (C). The mean intensity of each phosphoprotein band was normalized to the corresponding control, and the fold increases for phosphoproteins from treated samples compared to proteins from untreated samples are indicated.
In an effort to further characterize the cellular mechanisms of CT-B activation of APCs, we examined signaling events upstream from the MAP kinases. Btk and phospholipase Cγ (PLCγ) participate in receptor signaling in several immune cells; for example, both are recruited to the membrane upon triggering of the BCR, where activated Btk aids in the activation of PLCγ (27). We observed increases in the phosphorylation levels of both these molecules in B cells treated with CT-B (Fig. 2C).
CT-B induces activation of the transcription factors CREB and NF-κB.
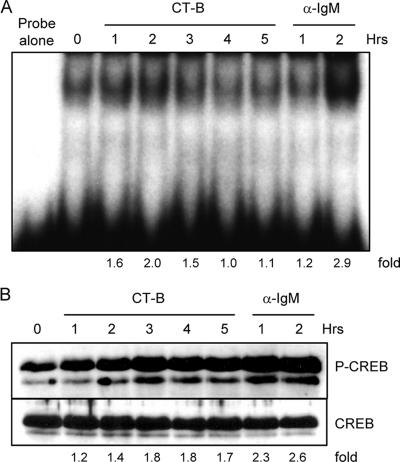
An important function of the MAP kinases is to alter gene transcription in response to extracellular signals. Translocation of transcription factors to the nucleus is essential in the activation of cells of the immune system (18) and is likely linked to the immune activation seen with CT-B. LT B-subunit induction of apoptosis in CD8+ T cells has recently been shown to be dependent on NF-κB and caspase 3 (39). To examine transcription factor translocation, splenic B cells were incubated with CT-B for the times indicated below and then subjected to extraction of nuclear proteins. Translocation of NF-κB upon toxin stimulation was assessed using a 32P-labeled NF-κB consensus sequence for EMSA. The nuclear levels of NF-κB were elevated compared to the levels in medium-incubated cells 2 to 3 h after treatment with CT-B (Fig. 3A). We also observed CT-B-induced phosphorylation of nuclear CREB by immunoblotting over a 5-h treatment period (Fig. 3B).
FIG. 3.
NF-κB and CREB activation in primary B cells. Splenic B cells from C3H/HeJ mice were either left untreated (0 h) or incubated with 10 μg/ml CT-B or anti-IgM for the times indicated prior to nuclear extraction, as described in Materials and Methods. (A) An EMSA was performed with nuclear extracts by incubation with an NF-κB consensus sequence, followed by separation by PAGE to detect a gel shift. The fold changes in shifted bands for treated cells compared to untreated cells are indicated. (B) Nuclear extracts were also examined by immunoblotting for phosphorylation of CREB. The lower band in the phospho-CREB (P-CREB) blot is phospho-ATF-1. The fold increases in phospho-CREB (normalized to total CREB) from treated samples compared to phospho-CREB from untreated samples are indicated.
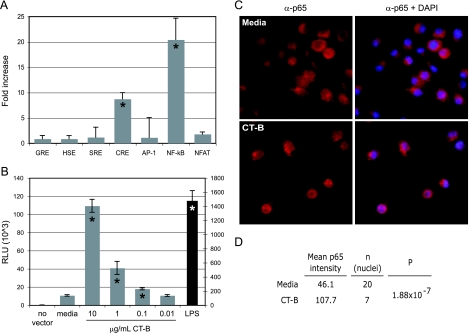
We expanded our studies to include macrophages in order to examine transcription factors commonly involved in immunological responses via a reporter construct transfection assay. The murine macrophage cell line RAW 264.7 was transiently transfected to examine CT-B's ability to transactivate various cis elements (Fig. 4A). Interestingly, only NF-κB and CRE were significantly and reproducibly activated in cells treated with CT-B. NFAT was minimally activated, although not significantly, while glucocorticoid response element, heat shock element, serum response element, and AP-1 were not affected. We further confirmed the CT-B activity in this system by showing transactivation of NF-κB in a dose-dependent manner (Fig. 4B).
FIG. 4.
CT-B induces CRE and NF-κB p65 transactivation in macrophages. (A and B) RAW macrophages were transiently transfected with luciferase reporter constructs containing the cis elements indicated prior to treatment with 2 μg/ml CT-B (A) or were transfected with an NF-κB reporter construct prior to treatment with CT-B as indicated (B). Cell lysate luciferase activity was measured by luminescence with a substrate assay. The values for the LPS control in panel B are reported on the y axis on the right. An asterisk indicates that the P value is <0.01, as determined by Student's two-tailed t test. GRE, glucocorticoid response element; HSE, heat shock element; SRE, serum response element; RLU, relative light units. (C) Peritoneal macrophages from C3H/HeJ mice were treated with 10 μg/ml CT-B for 2 h and stained with anti-NF-κB p65 (Texas Red secondary antibody) and blue DAPI to visualize nuclei. The left panels show p65 alone, while the right panels show p65 and DAPI-stained overlaid images. (D) Mean nuclear intensities of p65 quantified as described in Materials and Methods.
While it has recently been shown that the CT holotoxin induces NF-κB translocation to the nucleus in dendritic cells (21), this is the first demonstration that the enzymatically active A subunit is not required for this attribute of CT. We also demonstrated that the B subunit alone specifically induces the translocation of the NF-κB p65 subunit. Thioglycolate-elicited macrophages were treated with 10 μg/ml of CT-B or left untreated for 2 h, followed by immunocytostaining with an anti-NF-κB (p65) antibody. In untreated macrophages, most of the red stained p65 resides in the cytoplasm, leaving the DAPI-stained nuclei blue when the two images were merged (Fig. 4C, upper panels). However, upon addition of CT-B p65 was concentrated in the nucleus, resulting in the magenta nuclei in the merged images (Fig. 4C, lower panels). This CT-B-induced p65 shift to the nucleus was found to be highly significant (P = 1.88 × 10−7) (Fig. 4D).
Inhibition of phospho-Tyr decreases CT-B-induced NF-κB and CD14 in macrophages.
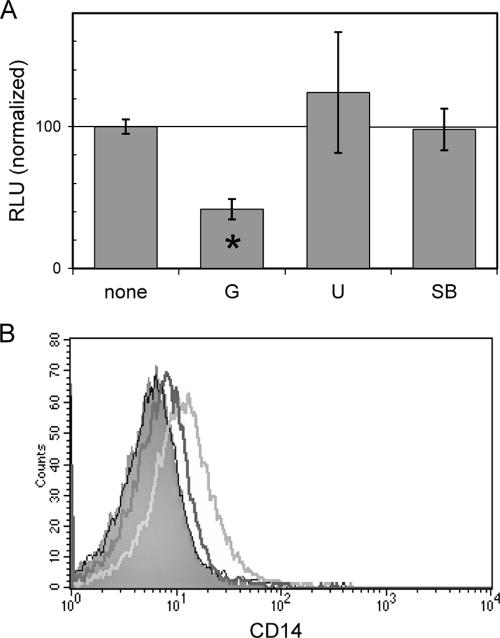
Many signals from lipid raft-associated components involve tyrosine phosphorylation, such as those in the Src family of tyrosine kinases (43). To determine if tyrosine phosphorylation plays a role in CT-B signal transduction, we pretreated RAW macrophages that were transiently transfected with a NF-κB reporter construct with genistein. Figure 5A demonstrates that the CT-B-induced transactivation of the NF-κB binding element was impaired when tyrosine kinases were inhibited by genistein. U0126 and SB202190, inhibitors of MEK1/2 and p38, respectively, had no significant effect on luciferase levels in this system.
FIG. 5.
Phosphotyrosine inhibition blocks CT-B-mediated events in macrophages. (A) RAW macrophages transiently transfected with an NF-κB luciferase reporter construct were pretreated with 25 μg/ml genistein (G) (a pan-tyrosine kinase inhibitor), 25 μM U0126 (U) (a MEK1/2 inhibitor), or 20 μM SB202190 (SB) (a p38 inhibitor) for 30 min prior to addition of 10 μg/ml CT-B for 5 h. The inhibitor function was confirmed by immunoblotting for the appropriate phosphoproteins (see Materials and Methods). The results are expressed as the percent luminescence in cells treated with CT-B alone. The asterisk indicates that the P value is 0.04, as determined by Student's two-tailed t test. RLU, relative light units. (B) Thioglycolate-elicited macrophages from C3H/HeJ mice were either pretreated with 25 μg/ml genistein for 30 min (dark gray line) or left alone (light gray line) prior to treatment with 10 μg/ml CT-B. Cells were analyzed by flow cytometry the next day to examine activation marker expression. The results for untreated cells are indicated by shading, and the results for the inhibitor alone are indicated by the thin black line.
The expression of membrane-bound CD14 is regulated by a number of factors, including cytokines present in the microenvironment and cellular activation via innate immune receptors. Genistein was able to block CT-B-induced up-regulation of CD14 without altering the levels on resting, primary macrophages (Fig. 5B). Similar results were obtained with a mechanistically unrelated tyrosine kinase inhibitor, herbimycin A (data not shown). These data suggest that one or more tyrosine kinases are involved in CT-B-induced cellular activation.
MEK1/2 inhibition affects CT-B-induced surface activation marker expression and production of IL-6.
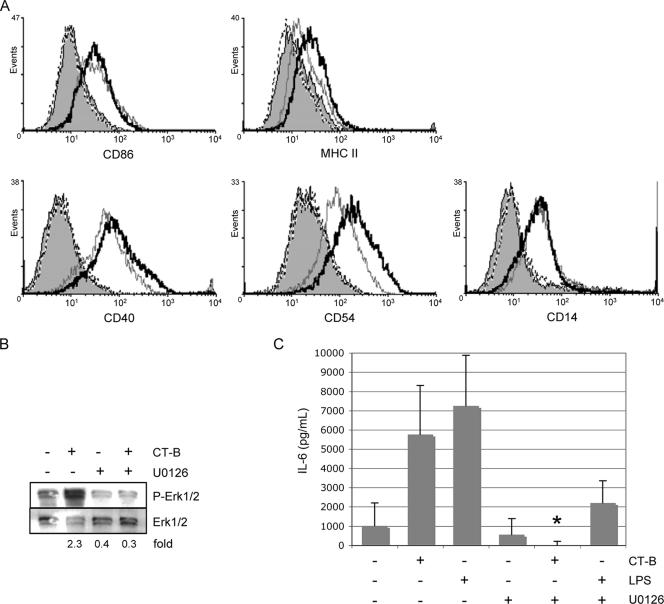
Although inhibition of MEK1/2 was not sufficient to inhibit CT-B-induced NF-κB transactivation, we asked whether CT-B-induced MEK1/2 signaling is necessary for APC functions, such as costimulatory marker expression and cytokine secretion. Bone marrow-derived macrophages were exposed to the MEK1/2 inhibitor U0126 prior to overnight treatment with CT-B and subsequent analysis by flow cytometry. CT-B, as well as LPS (data not shown), induced increases in the expression of CD86, MHC-II, CD54, CD14, and CD40 (Fig. 6A). Surprisingly, pretreatment with U0126 had little effect on the expression of CD86, CD14, and CD40, while it augmented MHC-II and CD54 expression (Fig. 6A). This suggests that MEK1/2 has a role in negative regulation of MHC-II and CD54. U0126 inhibition of CT-B-induced Erk1/2 phosphorylation was confirmed by immunoblotting (Fig. 6B).
FIG. 6.
MEK1/2 inhibition reduces CT-B-induced IL-6 expression but not surface marker expression. Bone marrow-derived macrophages were pretreated with 25 μM U0126 or the inhibitor's solvent (0.1% dimethyl sulfoxide) for 30 min prior to the addition of CT-B (2 μg/ml) or LPS (100 ng/ml) or no treatment and cultured for 24 h. (A) Expression of the cell surface markers indicated was assessed by fluorescence-activated cell sorting. Treatments are indicated as follows: shading, solvent; dashed line, U0216; gray line, solvent plus CT-B; black line, U0126 plus CT-B. (B) MEK1/2 inhibition by U0126 (30-min pretreatment) in cells treated with CT-B for 30 min or left untreated was confirmed by phospho-Erk1/2 immunoblotting. Fold changes in phospho-Erk1/2 (normalized to total Erk1/2) from treated cells compared to phospho-Erk1/2 from untreated cells are indicated. (C) IL-6 levels in supernatants were determined by ELISA. Samples from two independent experiments were used, and the asterisk indicates that there was a significant (P = 0.003, as determined by Student's two-tailed t test) reduction in CT-B-induced IL-6 in the presence of U0126.
The proinflammatory cytokine IL-6 has diverse immunological functions in inflammatory reactions, antigen-specific responses, and induction of acute-phase proteins. Because the production of IL-6 by macrophages aids in the induction of inflammation and immune cell recruitment, we examined IL-6 production in response to CT-B in vitro. IL-6 secretion occurred at very low levels in resting bone marrow-derived macrophages. However, IL-6 secretion by macrophages was greatly increased after 24 h of treatment with CT-B (Fig. 6C). While polymyxin B abolished the effect of LPS on IL-6 production, it had little effect on CT-B-induced IL-6 (concentration with LPS, 2,000 pg/ml; concentration with LPS plus polymyxin B, below the level of detection; concentration with CT-B, 3,000 pg/ml; concentration with CT-B plus polymyxin B, 2,500 pg/ml), indicating that the effect of CT-B on IL-6 production in bone marrow-derived macrophages is not due to synergy with contaminating LPS. Interestingly, inhibition of MEK1/2 decreased CT-B's induction of IL-6 (Fig. 6C), indicating that this MAP kinase pathway is involved in CT-B-stimulated IL-6 production.
DISCUSSION
CT's adjuvanticity was initially attributed to its enzymatic activity (25); however, our data add to the mounting evidence that this activity is not an absolute requirement for all immune modulating effects of the toxin. Catalytically inactive mutants that have the native structure and recombinant CT-B have been shown to exhibit different levels of ability to elicit specific responses to unrelated antigens (14, 16, 56). Whether the B subunit alone can act as a useful adjuvant has also been controversial because early preparations of purified B subunit were later found to be contaminated with the enzymatically active holotoxin (4, 45). We took great pains to ensure that this was not the case with the preparations that we used. The A subunit was not detectable in multiple assays (see Materials and Methods). This is noteworthy as cAMP has been shown to induce activation of APCs and augment surface expression of costimulatory molecules (44). Although the major mechanism for activation of CRE is via cAMP-induced protein kinase A phosphorylation of CREB at Ser133, numerous reports indicate that other kinases, including Ca2+/calmodulin-dependent kinases (41), a Ras-dependent kinase (19), and p90rsk (5), can phosphorylate CREB. Our results add to the accumulating data demonstrating that CT's enzymatic activity is not solely responsible for the molecule's ability to elicit cellular responses. This is highly significant as a major goal for the use of CT as a vaccine adjuvant is to separate its toxicity from immune enhancement.
We have shown that CT-B can increase MEK1/2, Erk1/2, and p38 phosphorylation, which directly correlates with the enzymatic activity of these kinases (38). In addition, CT-B induces phosphorylation of p90rsk, a direct target of Erk1/2. However, inhibition of MEK1/2 had little effect on CT-B-induced increases in CD86, CD14, CD69, and CD40 expression in macrophages, indicating that CT-B works via other pathways to generate these responses. Interestingly, MEK1/2 inhibition augmented MHC-II and CD54 expression, suggesting that this pathway is involved in the negative regulation of the surface expression of these proteins. Recent work has established a precedent for this negative regulatory function of the MEK1/2 pathway. For example, Erk1/2 dampens induction of CD54 by the protein kinase C activator 12-O-tetradecanoyl-phorbol-13-acetate in an epithelial cell line, but not IFN-γ-induced CD54 expression (42). Erk1/2 and p38 can also negatively regulate gene expression of CIITA, the master regulator of MHC-II (33, 57). Moreover, Erk1-deficient mice have a greater Th1 bias than wild-type mice and develop experimental autoimmune encephalomyelitis with increased susceptibility and severity (2), highlighting the importance of this pathway in immune regulation. Our observation that MEK1/2 inhibition blocks CT-B-mediated production of IL-6 is consistent with this paradigm, as IL-6 drives Th2 cell skewing via induction of early IL-4 from naïve CD4+ T cells (37). These data indicate that in the context of CT-B stimulation Erk1/2 regulates the surface expression of molecules involved in antigen presentation and mediates the augmentation of IL-6 release.
IL-6 is a pleiotropic cytokine produced by many cell types, such as macrophages, dendritic cells, T and B lymphocytes, fibroblasts, and hepatocytes, in response to an array of exogenous stimuli. Macrophages participate in the initial responses to pathogens by producing IL-6 among other potent proinflammatory cytokines. IL-6 is also involved in the terminal differentiation of B cells to antibody-secreting plasma cells and stimulates proliferation of T cells. Thus, induction of IL-6 would be beneficial in an immunization context. Other workers have shown that CT synergizes with LPS to produce IL-6 in immature dendritic cells and macrophages (11, 22), while CT-B represses LPS-induced IL-6 secretion in a monocyte cell line and has no effect alone (7). Conversely, we showed that CT-B alone induces IL-6 in bone marrow-derived macrophages. These studies and others show the distinctive effects of the holotoxin and the B subunit on different cell types.
We demonstrated that CT-B alone can induce transactivation of NF-κB and CRE in RAW macrophages and can increase nuclear NF-κB and phospho-CREB levels in splenic B cells. Both transcription factors have been implicated in the regulation of innate immune responses, including secretion of proinflammatory cytokines and cell survival (20, 28). They have also been shown to work together, for example, in the induction of Pai-2 gene transcription via p38 in macrophages to promote cell survival (32). The murine CD86 promoter has been shown to contain, among other sites, putative AP-1, NF-κB, and CREB binding sites (49), while the rat CD14 promoter has been shown to be regulated by AP-1 and SP-1 and contains putative CREB and STAT-1 binding sites (24). Our observation that total cellular protein levels of CD86 increase upon treatment with CT-B (unpublished results) suggests that the elevated expression of cell surface CD86 is due to de novo protein synthesis, potentially due to transcriptional regulation by NF-κB and/or CREB. The fact that inhibiting MEK1/2 activity did not block CT-B-induced increases in CD86 and CD14 could indicate that p90rsk does not mediate the CT-B-induced phosphorylation of CREB. Indeed, studies in other cellular contexts have suggested that there is an Erk1/2-independent p90rsk activation mechanism (1).
There have been no reports demonstrating CT or CT-B activity in the absence of binding to its receptor, GM1. GM1 is composed of a pentasaccharide bound to the outer leaflet of the cell membrane by a lipophilic ceramide tail, which does not extend into the cytoplasmic side of the cell. How is it possible for a membrane molecule with no intracellular domain to send a signal to the cell? GM1 localizes to lipid rafts, cholesterol-rich domains of the plasma membrane that are resistant to detergent solubilization. Labeled CT-B is most commonly used to visualize and identify lipid rafts. It is becoming apparent that rafts play a role in organizing signal transduction to the cell from the extracellular environment. For example, the B-cell receptor is excluded from rafts in resting B cells but quickly moves into rafts upon cross-linking (9, 50). Several signaling molecules, including the Src kinases Lck, Fyn, and Lyn, preferentially associate with rafts on lymphocytes, while other molecules, such as CD45 and CD22, are excluded (34). Significantly, disruption of lipid rafts by chelation of cholesterol inhibits endocytosis and trafficking of CT through the cell, with a delay in toxicity (53). It is important to investigate the influence that CT-B binding to GM1 has on the organization of signaling molecules within rafts. With increasing knowledge of the mechanism by which CT's receptor binding unit exerts its effect on cellular activity, we can take advantage of this unique innate response to induce immunity through vaccination.
Acknowledgments
We thank Carol King for purification of CT-B preparations, Lisa Ganley-Leal (Section of Infectious Diseases, Department of Medicine, Boston Medical Center) for a critical review of the manuscript, and Heather MacLeod for constructive discussions of this work.
A.C.S. and J.M.B. were supported by the Immunology Training Program of BUSM.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Abe, J., M. Okuda, Q. Huang, M. Yoshizumi, and B. C. Berk. 2000. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J. Biol. Chem. 275:1739-1748. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal, A., S. Dillon, T. L. Denning, and B. Pulendran. 2006. ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 176:5788-5796. [DOI] [PubMed] [Google Scholar]
- 3.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard, T. G., N. Lycke, S. J. Czinn, and J. G. Nedrud. 1998. Recombinant cholera toxin B subunit is not an effective mucosal adjuvant for oral immunization of mice against Helicobacter felis. Immunology 94:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohm, M., G. Moellmann, E. Cheng, M. Alvarez-Franco, S. Wagner, P. Sassone-Corsi, and R. Halaban. 1995. Identification of p90RSK as the probable CREB-Ser133 kinase in human melanocytes. Cell Growth Differ. 6:291-302. [PubMed] [Google Scholar]
- 6.Bone, H., S. Eckholdt, and N. A. Williams. 2002. Modulation of B lymphocyte signalling by the B subunit of Escherichia coli heat-labile enterotoxin. Int. Immunol. 14:647-658. [DOI] [PubMed] [Google Scholar]
- 7.Burkart, V., Y. E. Kim, B. Hartmann, I. Ghiea, U. Syldath, M. Kauer, W. Fingberg, P. Hanifi-Moghaddam, S. Muller, and H. Kolb. 2002. Cholera toxin B pretreatment of macrophages and monocytes diminishes their proinflammatory responsiveness to lipopolysaccharide. J. Immunol. 168:1730-1737. [DOI] [PubMed] [Google Scholar]
- 8.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, P. C., M. L. Dykstra, R. N. Mitchell, and S. K. Pierce. 1999. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, C. J., A. D. Wilson, N. A. Williams, and C. R. Stokes. 1991. Mucosal priming of T-lymphocyte responses to fed protein antigens using cholera toxin as an adjuvant. Immunology 72:323-328. [PMC free article] [PubMed] [Google Scholar]
- 11.Cong, Y., A. O. Oliver, and C. O. Elson. 2001. Effects of cholera toxin on macrophage production of co-stimulatory cytokines. Eur. J. Immunol. 31:64-71. [DOI] [PubMed] [Google Scholar]
- 12.Cong, Y., C. T. Weaver, and C. O. Elson. 1997. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J. Immunol. 159:5301-5308. [PubMed] [Google Scholar]
- 13.Couch, R. B. 2004. Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N. Engl. J. Med. 350:860-861. [DOI] [PubMed] [Google Scholar]
- 14.Douce, G., M. Fontana, M. Pizza, R. Rappuoli, and G. Dougan. 1997. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect. Immun. 65:2821-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field, M. 2003. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Investig. 111:931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana, M. R., R. Manetti, V. Giannelli, C. Magagnoli, A. Marchini, R. Olivieri, M. Domenighini, R. Rappuoli, and M. Pizza. 1995. Construction of nontoxic derivatives of cholera toxin and characterization of the immunological response against the A subunit. Infect. Immun. 63:2356-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George-Chandy, A., K. Eriksson, M. Lebens, I. Nordstrom, E. Schon, and J. Holmgren. 2001. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect. Immun. 69:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 19.Ginty, D. D., A. Bonni, and M. E. Greenberg. 1994. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell 77:713-725. [DOI] [PubMed] [Google Scholar]
- 20.Hanada, T., and A. Yoshimura. 2002. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 13:413-421. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura, Y. I., R. Kawashima, Y. Shirai, R. Kato, T. Hamabata, M. Yamamoto, K. Furukawa, K. Fujihashi, J. R. McGhee, H. Hayashi, and T. Dohi. 2003. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-kappaB translocation. Eur. J. Immunol. 33:3205-3212. [DOI] [PubMed] [Google Scholar]
- 22.Lavelle, E. C., E. McNeela, M. E. Armstrong, O. Leavy, S. C. Higgins, and K. H. Mills. 2003. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J. Immunol. 171:2384-2392. [DOI] [PubMed] [Google Scholar]
- 23.Lian, J. P., R. Huang, D. Robinson, and J. A. Badwey. 1999. Activation of p90RSK and cAMP response element binding protein in stimulated neutrophils: novel effects of the pyridinyl imidazole SB 203580 on activation of the extracellular signal-regulated kinase cascade. J. Immunol. 163:4527-4536. [PubMed] [Google Scholar]
- 24.Liu, S., R. A. Shapiro, S. Nie, D. Zhu, Y. Vodovotz, and T. R. Billiar. 2000. Characterization of rat CD14 promoter and its regulation by transcription factors AP1 and Sp family proteins in hepatocytes. Gene 250:137-147. [DOI] [PubMed] [Google Scholar]
- 25.Lycke, N., T. Tsuji, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277-2281. [DOI] [PubMed] [Google Scholar]
- 26.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, and H. Bluethmann. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 27.Marshall, A. J., H. Niiro, T. J. Yun, and E. A. Clark. 2000. Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cgamma pathway. Immunol. Rev. 176:30-46. [DOI] [PubMed] [Google Scholar]
- 28.Matt, T. 2002. Transcriptional control of the inflammatory response: a role for the CREB-binding protein (CBP). Acta Med. Austriaca 29:77-79. [DOI] [PubMed] [Google Scholar]
- 29.Monks, B. G., B. A. Martell, J. A. Buras, and M. J. Fenton. 1994. An upstream protein interacts with a distinct protein that binds to the cap site of the human interleukin 1 beta gene. Mol. Immunol. 31:139-151. [DOI] [PubMed] [Google Scholar]
- 30.Nashar, T. O., H. M. Webb, S. Eaglestone, N. A. Williams, and T. R. Hirst. 1996. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc. Natl. Acad. Sci. USA 93:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okahashi, N., M. Yamamoto, J. L. Vancott, S. N. Chatfield, M. Roberts, H. Bluethmann, T. Hiroi, H. Kiyono, and J. R. McGhee. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park, J. M., F. R. Greten, A. Wong, R. J. Westrick, J. S. Arthur, K. Otsu, A. Hoffmann, M. Montminy, and M. Karin. 2005. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis—CREB and NF-kappaB as key regulators. Immunity 23:319-329. [DOI] [PubMed] [Google Scholar]
- 33.Pennini, M. E., R. K. Pai, D. C. Schultz, W. H. Boom, and C. V. Harding. 2006. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J. Immunol. 176:4323-4330. [DOI] [PubMed] [Google Scholar]
- 34.Pierce, S. K. 2002. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2:96-105. [DOI] [PubMed] [Google Scholar]
- 35.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 36.Raschke, W. C., S. Baird, P. Ralph, and I. Nakoinz. 1978. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 15:261-267. [DOI] [PubMed] [Google Scholar]
- 37.Rincon, M., J. Anguita, T. Nakamura, E. Fikrig, and R. A. Flavell. 1997. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 39.Salmond, R. J., R. S. Pitman, E. Jimi, M. Soriani, T. R. Hirst, S. Ghosh, M. Rincon, and N. A. Williams. 2002. CD8+ T cell apoptosis induced by Escherichia coli heat-labile enterotoxin B subunit occurs via a novel pathway involving NF-kappaB-dependent caspase activation. Eur. J. Immunol. 32:1737-1747. [DOI] [PubMed] [Google Scholar]
- 40.Savarino, S. J., E. R. Hall, S. Bassily, T. F. Wierzba, F. G. Youssef, L. F. Peruski, Jr., R. Abu-Elyazeed, M. Rao, W. M. Francis, H. El Mohamady, M. Safwat, A. B. Naficy, A. M. Svennerholm, M. Jertborn, Y. J. Lee, and J. D. Clemens. 2002. Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. Pediatr. Infect. Dis. J. 21:322-330. [DOI] [PubMed] [Google Scholar]
- 41.Sheng, M., M. A. Thompson, and M. E. Greenberg. 1991. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252:1427-1430. [DOI] [PubMed] [Google Scholar]
- 42.Shibuya, Y., N. Hirasawa, T. Sakai, Y. Togashi, R. Muramatsu, K.-I. Ishii, M. Yamashita, M. Takayanagi, and K. Ohuchi. 2004. Negative regulation of the protein kinase C activator-induced ICAM-1 expression in the human bronchial epithelial cell line NCI-H292 by p44/42 mitogen-activated protein kinase. Life Sci. 75:435. [DOI] [PubMed] [Google Scholar]
- 43.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, M., F. Shinohara, K. Sato, T. Taniguchi, H. Takada, and H. Rikiishi. 2003. Interleukin-1beta converting enzyme subfamily inhibitors prevent induction of CD86 molecules by butyrate through a CREB-dependent mechanism in HL60 cells. Immunology 108:375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura, S., A. Yamanaka, M. Shimohara, T. Tomita, K. Komase, Y. Tsuda, Y. Suzuki, T. Nagamine, K. Kawahara, and H. Danbara. 1994. Synergistic action of cholera toxin B subunit (and Escherichia coli heat-labile toxin B subunit) and a trace amount of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine 12:419-426. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, D. N., V. Cardenas, J. L. Sanchez, R. E. Begue, R. Gilman, C. Bautista, J. Perez, R. Puga, A. Gaillour, R. Meza, P. Echeverria, and J. Sadoff. 2000. Two-year study of the protective efficacy of the oral whole cell plus recombinant B subunit cholera vaccine in Peru. J. Infect. Dis. 181:1667-1673. [DOI] [PubMed] [Google Scholar]
- 47.Vajdy, M., M. H. Kosco-Vilbois, M. Kopf, G. Kohler, and N. Lycke. 1995. Impaired mucosal immune responses in interleukin 4-targeted mice. J. Exp. Med. 181:41-53. [DOI] [PubMed] [Google Scholar]
- 48.Vajdy, M., and N. Y. Lycke. 1992. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 75:488-492. [PMC free article] [PubMed] [Google Scholar]
- 49.Weatherill, A. R., J. Y. Lee, L. Zhao, D. G. Lemay, H. S. Youn, and D. H. Hwang. 2005. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J. Immunol. 174:5390-5397. [DOI] [PubMed] [Google Scholar]
- 50.Weintraub, B. C., J. E. Jun, A. C. Bishop, K. M. Shokat, M. L. Thomas, and C. C. Goodnow. 2000. Entry of B cell receptor into signaling domains is inhibited in tolerant B cells. J. Exp. Med. 191:1443-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wetzler, L. M., Y. Ho, H. Reiser, and L. W. Wetzler. 1996. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J. Exp. Med. 183:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson, A. D., M. Bailey, N. A. Williams, and C. R. Stokes. 1991. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur. J. Immunol. 21:2333-2339. [DOI] [PubMed] [Google Scholar]
- 53.Wolf, A. A., Y. Fujinaga, and W. I. Lencer. 2002. Uncoupling of the cholera toxin-G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of membrane cholesterol. J. Biol. Chem. 277:16249-16256. [DOI] [PubMed] [Google Scholar]
- 54.Xing, J., D. D. Ginty, and M. E. Greenberg. 1996. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273:959-963. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto, M., H. Kiyono, M. N. Kweon, S. Yamamoto, K. Fujihashi, H. Kurazono, K. Imaoka, H. Bluethmann, I. Takahashi, Y. Takeda, M. Azuma, and J. R. McGhee. 2000. Enterotoxin adjuvants have direct effects on T cells and antigen-presenting cells that result in either interleukin-4-dependent or -independent immune responses. J. Infect. Dis. 182:180-190. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, M. Yamamoto, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao, Y., Q. Xu, M. J. Kwon, R. Matta, Y. Liu, S. C. Hong, and C. H. Chang. 2006. ERK and p38 MAPK signaling pathways negatively regulate CIITA gene expression in dendritic cells and macrophages. J. Immunol. 177:70-76. [DOI] [PubMed] [Google Scholar]