Abstract
The ability to induce long-term immunity to Helicobacter pylori is necessary for an effective vaccine. This study was designed to establish the most efficient route(s) (systemic, mucosal, or a combination) of immunization for induction of long-term immunity and to define correlates of protection. Mice were immunized orally alone (oral group), intramuscularly (i.m.) alone (i.m. group), orally followed by i.m. (oral/i.m. group), or i.m. followed by orally (i.m./oral group). Long-term protective immunity to oral H. pylori challenge was observed 3 months after immunization through the i.m. or oral/i.m. route. Protection correlated with an increase in H. pylori-specific interleukin-12 and both immunoglobulin G1 (IgG1) and IgG2a serum titers following challenge. Mice that were not protected (oral or i.m./oral) had increased levels of IgA in both sera and Peyer's patches. This study demonstrates the ability to induce long-term immunity against H. pylori, provides correlates of protection, and illustrates the crucial role of the immunization route(s).
Helicobacter pylori is a gram-negative spiral bacterium that colonizes the gastric epithelium in nearly 50% of the world's population. Infection is typically acquired early in childhood and continues throughout the life of the host, leading to chronic gastritis and in some cases peptic ulcer disease or gastric cancer (32, 37). In spite of the development of H. pylori-specific immune responses, the bacteria are rarely eliminated from the gastric epithelium (6). Treatment for H. pylori infection includes a combination of a proton pump inhibitor and two antibiotics. However, antibiotic resistance, high cost, and recurrence of infection make world-wide eradication through drug therapy problematic. While development of a vaccine is ideal, the challenge lies in inducing long-lasting immunity. Various routes of immunization have been used to demonstrate protection against H. pylori in mice: oral, intranasal, intrarectal, intradermal, and subcutaneous (11, 13, 15, 20, 24, 34, 35, 43). Most H. pylori vaccine studies challenge animals 2 to 4 weeks after immunization, when the immune response is still in the acute effector phase (13, 15, 18, 20-22, 35, 36), and this does not test generation and maintenance of immunologic memory. Thus, results from previous studies cannot be used to predict long-term protection.
Mucosal immunization is a route commonly used to induce mucosal immunity. While immunizing through a mucosal route seems optimal for eliciting mucosal immunity, no studies have reported its success in the generation of H. pylori immunologic memory. Unlike for Helicobacter felis, against which long-term protection can be achieved through immunization, a long-term-protection model for H. pylori has yet to be reported (27, 33). Recent studies have demonstrated that immunization through a combination of mucosal and systemic routes may increase mucosal immunity (22, 25, 39, 40).
The mechanisms by which protection against H. pylori occurs are still unknown. In general, immune responses, such as local and systemic antibody and cytokine production, may be used as immunological surrogate markers for protection. For H. pylori, recent literature shows that, except for the interleukin-12 (IL-12) response, most cytokine and antibody responses do not directly correlate with short-term protection. After immunization, IL-12 knockout mice maintained bacterial levels equivalent to those of unimmunized controls when challenged with H. pylori, while wild-type animals had a decrease in bacterial load in the stomach (2). In addition to the IL-12 response, a strong adaptive Th1 immune response has been shown to aid in protection (3, 5, 9, 44). However, these data were generated during the acute effector phase; thus, identifying correlates of protection following a long-term resting period is of great interest.
Herein, we compared immunization methods that combined both mucosal and systemic routes in order to determine which prime/boost regimen would be most effective in eliciting long-term mucosal immunity and protection from H. pylori challenge. Mice were immunized orally alone (oral group), intramuscularly (i.m.) alone (i.m. group), orally followed by i.m. (oral/i.m. group), or i.m. followed by orally (i.m./oral group) and then challenged orally with live H. pylori 3 months following the final immunization. Our goal was to establish the most efficient route(s) of immunization for induction and maintenance of long-term immunity and to more accurately identify immunologic correlates of protection.
MATERIALS AND METHODS
Animals.
Specific-pathogen (Helicobacter)-free, female BALB/c mice were purchased from Taconic (Germantown, NY) and used between the ages of 8 and 12 weeks. Mice were housed in microisolator cages and provided with autoclaved food, water, and bedding to reduce opportunistic infections. Mice were fed and watered ad libitum. All animals were housed under protocols approved by the ALAAC and the Institutional Animal Care and Use Committee of the University of California, Davis.
H. pylori culturing and vaccine preparation.
H. pylori mouse-adapted Sydney strain 1 (SS1) was subcultured on brucella agar for 48 h prior to passage into brucella broth, both supplemented with 5% newborn calf serum. For H. pylori challenge, the liquid culture was harvested, with the final concentration adjusted to 109 bacteria/ml for each single dose of 0.1 ml in brucella broth (108). For vaccine preparation, the liquid culture was harvested at mid-log phase (optical density at 600 nm of 0.4 to 0.6) and pelleted by centrifugation. Pellets were resuspended in sterile phosphate-buffered saline (PBS) and sonicated (Sonic Dismembrator 550; Fisher Scientific, Pittsburgh, PA) on ice with five 10-s pulses at an amplitude between 7 and 9. Protein was measured via a protein concentration measurement assay (Bio-Rad, Hercules, CA) at an optical density of 595 nm. H. pylori SS1 sonicate was used for both vaccination and immunoassays (enzyme-linked immunosorbent assay [ELISA], Luminex assay, and enzyme-linked immunospot [ELISPOT] assay).
Immunizations, challenge, and experimental design.
Five groups of mice were immunized five times at 10-day intervals. Oral immunizations consisted of 100 μg of H. pylori sonicate and 10 μg cholera toxin (CT) (Sigma) suspended in 0.5 ml of 3% sodium bicarbonate, administered by gavage, whereas i.m. immunizations contained 10 μg H. pylori sonicate and 1 μg CT injected into the right thigh muscle. The groups were structured as follows. The i.m. group (n = 12) received five immunizations i.m., the oral group (n = 12) received five immunizations orally, the oral/i.m. group (n = 9) received three oral followed by two i.m. immunizations, the i.m./oral group (n = 10) received two i.m. followed by three oral immunizations, and the mock-infected (mock) control group (n = 10) received three oral immunizations and two i.m. immunizations of 3% sodium bicarbonate (oral) or PBS (i.m.) (Table 1). Sera were collected 7 days after the final immunization, 3 months after the final immunization prechallenge, and 7 days postchallenge. Animals were challenged 3 months after the final immunization and sacrificed 8 days later. Mice were challenged with three doses of 108 CFU of H. pylori SS1, suspended in 0.1 ml brucella broth, at 2-day intervals. Doses were administered by oral gavage using a ball-end feeding needle. Mice were euthanized with an overdose of pentobarbital sodium solution (Nembutal; Abbott Laboratories, North Chicago, IL). Peyer's patches, spleens, and stomachs were sterilely collected from all mice at the time of sacrifice.
TABLE 1.
Schedule of immunization, challenge, and sacrifice
| Group | Immunization type | Immunization solution and method
|
H. pylori challenge (day 132 [3 mo]) | Sacrifice (day 144) | ||
|---|---|---|---|---|---|---|
| First/second immunizations (days 0 and 10) | Third immunization (day 20) | Fourth/fifth immunizations (days 30 and 40) | ||||
| i.m. (n = 12) | Parenteral | 10 μg sonicate + 1 μg CT, i.m. | 10 μg sonicate + 1 μg CT, i.m. | 10 μg sonicate + 1 μg CT, i.m. | Three doses of 108 CFU | Serum/tissue collection and bacterial culture |
| Oral (n = 12) | Mucosal | 100 μg sonicate + 10 μg CT, oral | 100 μg sonicate + 10 μg CT, oral | 100 μg sonicate + 10 μg CT, oral | Three doses of 108 CFU | Serum/tissue collection and bacterial culture |
| Oral/i.m. (n = 9) | Mucosal prime/parenteral boost | 100 μg sonicate + 10 μg CT, oral | 100 μg sonicate + 10 μg CT, oral | 10 μg sonicate + 1 μg CT, i.m. | Three doses of 108 CFU | Serum/tissue collection and bacterial culture |
| i.m./oral (n = 10) | Parenteral prime/mucosal boost | 10 μg sonicate + 1 μg CT, i.m. | 100 μg sonicate + 10 μg CT, oral | 100 μg sonicate + 10 μg CT, oral | Three doses of 108 CFU | Serum/tissue collection and bacterial culture |
| Mock (n = 10) | Sham | PBS, oral | PBS, oral | PBS, i.m. | Three doses of 108 CFU | Serum/tissue collection and bacterial culture |
Lymphocyte isolation.
Spleens were processed individually whereas Peyer's patches were pooled from groups of three mice. Tissues were ground through a screen mesh, and red blood cells were lysed with ACK lysis buffer (Biosource, Camarillo, CA). Lymphocytes from gastric tissues were isolated by collagenase type II (Sigma Chemical Co., St. Louis, MO) digestion, as previously published (12). Stomachs, pooled for each group, were homogenized and incubated in 20 ml of 440 U/ml collagenase II in complete RPMI 1640 (Invitrogen/Gibco BRL, Grand Island, NY) containing 5% fetal bovine serum (FBS) (Gemini, Woodland, CA), 2 mM glutamine, penicillin, streptomycin, nonessential amino acids, sodium pyruvate, 10 mM HEPES (all from Gibco), and 5 × 10−5 M 2-ME (Sigma) with shaking (200 rpm) for 20 min at 37°C, for a total of three incubations (New Brunswick, NJ). Cell suspensions from all mice in each group were washed, centrifuged, combined, and passed through a 45 μM filter. All cells were then resuspended at 2 × 107 cells/ml in complete RPMI with 10% FBS.
Antigen-specific cytokine assays.
Antigen-specific IL-4 responses in lymphocytes from immunized mice were measured using an ELISPOT assay as previously described (40). Briefly, 2 × 106 lymphocytes were added to polyvinylidene difluoride plates (Millipore) precoated with rat anti-mouse IL-4 (Endogen, Woburn, MA) and blocked with complete RPMI-10% FBS. Cells were then incubated with 50 μg/ml of H. pylori sonicate for 13 h at 37°C. Following incubation, supernatants from all ELISPOT assay plates were collected and frozen at −80°C for use in Luminex assays. Plates were washed with PBS-0.02% Tween 20 and incubated at room temperature for 2 h with biotinylated rat anti-mouse IL-4 (Endogen) in PBS-0.02% Tween 20-0.1% bovine serum albumin. Plates were washed and incubated with avidin-peroxidase (1 h at 37°C), followed by diaminobenzidine substrate in Tris-HCl (pH 7.5) buffer for 15 min. Spots were counted by use of a Zeiss KS automatic ELISPOT reader. Luminex technology was used to detect the remaining cytokines from 50 μl of previously frozen cell culture supernatant. IL-1β, IL-2, IL-5, IL-6, IL-10, IL-12, granulocyte-macrophage colony-stimulating factor, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) levels were measured according to the manufacturer's instructions by using a Beadlyte mouse multicytokine detection system 2 (Upstate, Lake Placid, NY) and Luminex 100 (Luminex, Austin, TX). A minimum of 100 beads were read, and data were analyzed by MasterPlexQT software (MiraiBio, Alameda, CA) using five-parameter logistics.
Antigen-specific antibody assays.
H. pylori-specific IgG1, IgG2a, and IgA titers were measured from both serum and cell culture supernatant from lymphocytes isolated as described above and cultured overnight in complete RPMI-10% FBS at 2 × 105 cells/ml. U-bottomed, 96-well ELISA plates (Nunc Maxisorp, Denmark) were coated with 5 μg/well H. pylori sonicate in PBS overnight at 4°C. Plates were washed with PBS-0.3% Tween 20 and then blocked with PBS-2% goat serum (Gibco, Carlsbad, CA) for 1 h at 37°C. Serum samples were added at an initial dilution of 1:200 in duplicate, with 1:3 serial dilutions performed in PBS-2% goat serum. Cell culture supernatant was serially diluted 1:3 in PBS-2% goat serum. Plates were incubated for 1 h at 37°C and then washed in PBS-0.3% Tween 20. A 1:10,000 dilution of biotinylated goat anti-mouse IgG1, IgG2a, or IgA (Southern Biotech, Birmingham, AL) was added to the plates for 1 h at 37°C. Plates were washed and then incubated with a 1:1,000 dilution of streptavidin-horseradish peroxidase (BD/Pharmingen) for 1 h at 37°C. Plates were again washed, developed with tetramethylbenzidine (Kirkegaard and Perry, Gaithersburg, MD) for 10 min, and stopped with 2 M HCl. The optical density of each well was measured at 450 nm on a VMax plate reader (Molecular Devices, Sunnyvale, CA).
Quantitative H. pylori culture from gastric tissue.
Stomachs were divided in half longitudinally for quantitative analysis of H. pylori infection. Briefly, samples were placed in 300 μl of brucella broth, weighed, and homogenized using a sterile ground-glass pestle. Tenfold serial dilutions were plated on brucella agar plates and incubated in a 5% CO2 incubator for 5 to 7 days. H. pylori isolates were identified by colony morphology, microscopy, and biochemistry. The CFU per gram of gastric mucosa was calculated by enumerating colonies, adjusting for the dilution, and dividing by the tissue weight.
Statistical analysis.
The primary analysis was a comparison between immunized groups and mock controls. Secondary comparisons among immunized groups were performed selectively as described in Results. Statistical significance was assessed by a nonparametric Mann-Whitney test for multiple comparisons. Differences between groups were considered statistically significant at a P value of <0.005 based on a Bonferroni correction for multiple comparisons. SPSS (Statistical Product and Service Solutions) comprehensive statistical software was utilized for all analyses.
RESULTS
Acute effector and prechallenge serum IgG1, IgG2a, and IgA antibody titers.
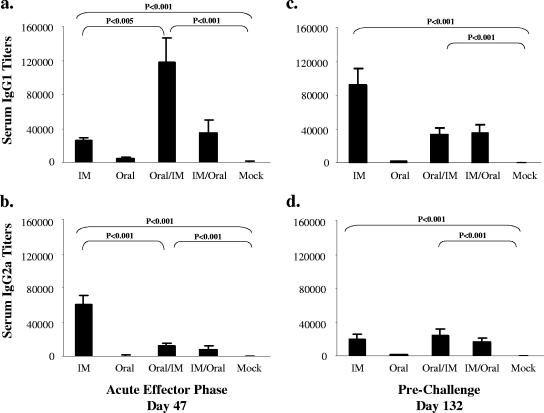
Mice were immunized five times using various single immunizations or combinations of systemic and mucosal routes of immunization and rested 3 months before oral challenge with H. pylori (Table 1). To determine how combinations of mucosal/systemic immunizations influence humoral immune responses over time, sera were taken 7 days after the last immunization (acute effector phase) and 120 days after the last immunization (prechallenge). At the acute effector phase, the i.m., oral/i.m., and i.m./oral groups all had significantly higher H. pylori-specific IgG1 and IgG2a serum antibody titers than the mock group (P < 0.001) (Fig. 1a and b and Table 2). The oral/i.m. group had the highest IgG1 antibody titers, while the i.m. group had the highest IgG2a antibody titers. The i.m., oral/i.m., and i.m./oral groups also had high IgG1 and IgG2a antibody titers compared to those of the oral or mock groups in sera collected prechallenge (P < 0.001) (Fig. 1c and d). However, at this phase the i.m. group had the highest IgG1 antibody titers and there were no significant differences in IgG2a antibody titers between the i.m., the oral/i.m., and the i.m./oral groups. Both IgG1 and IgG2a antibody titers were consistently low at all time points for mice immunized orally only, indicating that oral immunizations not combined with a systemic route were poor at stimulating serum IgG antibodies. During the acute effector phase, H. pylori-specific serum IgA was high in the oral and the i.m./oral mice (compared to levels for the mock controls [P = 0.07 and P < 0.005, respectively]) but not in the i.m. and oral/i.m. mice. Three months later (prechallenge), all groups had higher H. pylori-specific IgA titers than the mock controls (P < 0.005) (data not shown). These results show that in the acute effector phase, i.m. and oral/i.m. immunizations stimulated the highest IgG2a and IgG1 antibody responses, respectively. However, 3 months after the final immunization, before challenge, serum IgG1 antibody responses were the highest following i.m. immunizations, whereas IgG2a responses were comparable following the i.m. and oral/i.m. immunizations.
FIG. 1.
H. pylori-specific IgG1 and IgG2a titers measured by ELISA during the acute effector phase (after the fifth immunization) and prechallenge. Sera were collected 7 days after the fifth immunization, during the acute effector phase (a and b), and prior to challenge, 3 months later (c and d) (means ± standard errors of the means). IM, i.m.
TABLE 2.
Serum IgG1/IgG2a titer ratios
| Time point | Mean (±SEM) serum IgG1/IgG2a titer ratioa
|
|||
|---|---|---|---|---|
| i.m. | Oral | Oral/i.m. | i.m./oral | |
| Acute effector phase | 0.48 ± 0.08b | 4.26 ± 2.77 | 124.78 ± 113.39c | 6.39 ± 2.19 |
| Prechallenge | 8.71 ± 2.23 | 1.93 ± 1.77 | 1.32 ± 0.26 | 2.25 ± 0.62 |
| Postchallenge | 5.02 ± 2.42 | 24.09 ± 13.99 | 4.41 ± 1.50 | 13.18 ± 4.98 |
H. pylori-specific serum titer ratios, measured by ELISA (see Materials and Methods).
Lowest IgG1/IgG2a ratio during the acute effector phase.
Highest IgG1/IgG2a ratio during the acute effector phase.
Long-term protection in stomach against infection with H. pylori.
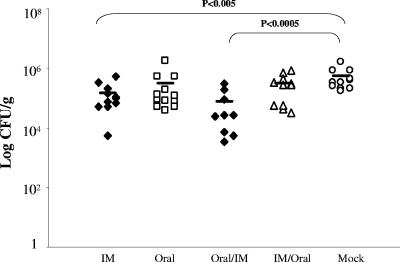
We next examined which route(s) of immunization best conferred protection against oral H. pylori challenge. Protection, as defined by a statistically significant decrease in bacterial load in vaccinated groups compared to the level for mock controls, was found to be dependent upon the route of vaccination. Only the i.m. and oral/i.m. groups were protected against challenge in comparison to the mock control group, as determined by quantitative culturing of H. pylori from gastric tissue (P < 0.005) (Fig. 2). While the oral and i.m./oral groups appeared to have slightly decreased bacterial loads, there were no significant differences found in comparison to the mock control group. The oral/i.m. group (protected) and the i.m./oral group (not protected) differed in gastric bacterial load by nearly 1 log (P < 0.01), which emphasizes that not only the route but also the order of immunization was important.
FIG. 2.
Bacterial loads in stomach after oral challenge. Mean log10 CFU/gram of tissue obtained 8 days after challenge. Open symbols denote unprotected groups, and filled symbols denote protected groups. IM, i.m.
Peripheral and local antibody responses following challenge.
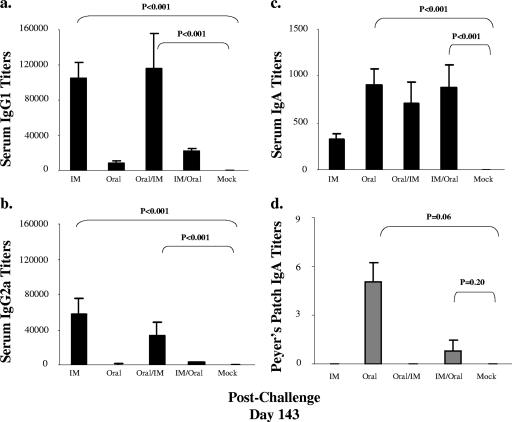
Sera and tissues (splenocytes, Peyer's patches, and stomachs) were collected at 7 and 8 days postchallenge, respectively, to determine which immunization routes induced the highest local and systemic antibody responses. All groups, except oral alone, had higher H. pylori-specific serum IgG1 and IgG2a titers than the mock controls (P < 0.001) (Fig. 3a and b). IgG1 and IgG2a were undetectable in Peyer's patches and gastric tissues. The protected groups (i.m. alone and oral/i.m.) had significantly higher IgG1 and IgG2a serum antibody titers than either the oral or the i.m./oral group (P < 0.005). The oral and i.m./oral groups had lower IgG1 and IgG2a serum antibody titers than the i.m. and oral/i.m. groups; however, except for IgG2a titers in the oral group, all values were significantly higher than those for the mock controls (P < 0.001). While the i.m. and oral/i.m. groups maintained IgG1/IgG2a ratios of approximately 5.00, the oral and i.m./oral groups had ratios that increased to 24.09 and 13.18, respectively. These data suggest that a lower IgG1/IgG2a ratio, which reflects a Th1 response, may be related to increased protection against H. pylori challenge.
FIG. 3.
Serum and Peyer's patch antibody responses following oral challenge. H. pylori-specific antibody titers in sera were measured by ELISA 7 days after challenge (a, b, and c) or in culture supernatant from Peyer's patches 8 days after challenge (d) (means ± standard errors of the means). IM, i.m.
As IgA antibody responses are generally thought to be associated with mucosal responses, we next determined local and serum IgA responses. Postchallenge, all groups except for the mock control had detectable serum IgA titers (P < 0.001) (Fig. 3c). The oral and i.m./oral groups induced higher serum IgA antibody titers than the i.m. or oral/i.m. groups. Although H. pylori-specific IgA was detected only in Peyer's patches of mice immunized through the oral or the i.m./oral route (Fig. 3d), these values did not reach statistical significance compared to values for mock controls. IgA was not detected in gastric tissue from any of the groups. Together, these data show that following oral challenge mucosal IgA responses, as well as serum IgA responses significantly higher than those for the mock control group, were detected in the two groups that were not protected, namely, the oral and i.m./oral groups (Fig. 3d).
Innate-type cytokine responses following challenge.
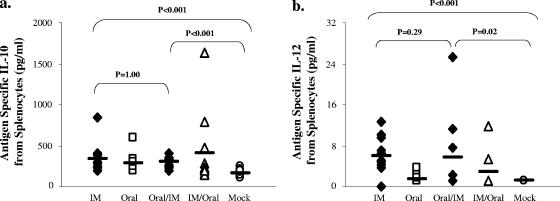
It is unclear how immunization through different routes may affect innate immune responses following H. pylori challenge. Although innate responses are induced early after immunization or challenge, we reasoned that some innate-type cytokine responses may persist postchallenge. Therefore, we next measured several innate-type cytokines thought to be involved in H. pylori infection. Innate-type cytokines were found only in splenocytes and were not detected from Peyer's patches or gastric lymphocytes. All immunized groups secreted significantly more granulocyte-macrophage colony-stimulating factor, IL-1β, and TNF-α than the mock control group. However, no significant differences were seen among the groups (data not shown). The i.m., oral, and oral/i.m. groups all produced more IL-10 than the mock control group, while the i.m./oral group did not (P < 0.005) (Fig. 4a). Only i.m.-immunized mice secreted increased levels of IL-12 that were significantly higher than those for the mock controls (P < 0.005) (Fig. 4b). While oral/i.m.-immunized mice also secreted increased levels of IL-12, these levels did not reach statistical significance (P = 0.02). Overall, these data show that while all routes of immunization produced significant innate-type cytokine responses, enhanced IL-12 secretion was detected in the protected groups.
FIG. 4.
IL-10 and IL-12 responses following oral challenge. Splenocytes were cultured with H. pylori sonicate and assayed for IL-10 (a) and IL-12 (b) production by Luminex assay. Open symbols denote unprotected groups, and filled symbols denote protected groups. IM, i.m.
Adaptive Th1- and Th2-type cytokine responses following challenge.
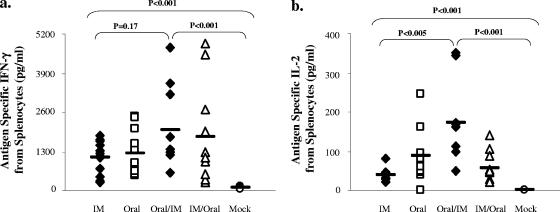
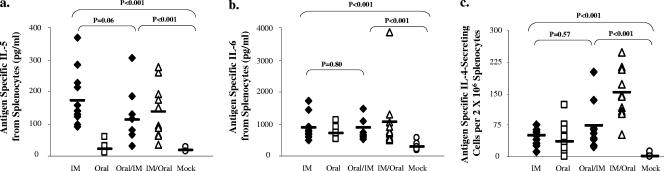
We next determined the effects of the route of immunization on Th1 or Th2 cytokine responses in splenocytes after antigen restimulation postchallenge. All groups produced significantly more IFN-γ and IL-2 than the mock controls (P < 0.005) (Fig. 5a and b). There was no significant difference among the experimental groups when measuring IFN-γ. However, the oral and the oral/i.m. groups produced more IL-2 than the i.m. group (P < 0.005). Mice receiving systemic immunizations (i.m., oral/i.m., and i.m./oral) produced significantly more IL-5 (P < 0.005) than the mock group, while the oral group did not (Fig. 6a). Although there was no difference in IL-6 production among the groups, these levels were all significantly higher than that for the mock controls (P < 0.005) (Fig. 6b). All groups secreted significantly more IL-4 than the mock group (P < 0.005), with the i.m./oral group secreting the largest amount (Fig. 6c). Together, these data suggest that the combinations or single routes of mucosal/systemic immunizations stimulated both Th1 and Th2 adaptive responses following challenge.
FIG. 5.
IFN-γ and IL-2 antigen-specific responses following oral challenge. Splenocytes were cultured with H. pylori sonicate and assayed for IFN-γ (a) and IL-2 (b) production by Luminex assay. Open symbols denote unprotected groups, and filled symbols denote protected groups. IM, i.m.
FIG. 6.
IL-5, IL-6, and IL-4 antigen-specific responses following oral challenge. Splenocytes were cultured with H. pylori sonicate and assayed for IL-5 (a) and IL-6 (b) production by Luminex assay. IL-4 (c) secretion was measured by ELISPOT assay. Open symbols denote unprotected groups, and filled symbols denote protected groups. IM, i.m.
DISCUSSION
The aim of this study was to determine which single route or combination of systemic and mucosal routes of immunizations would generate long-term protection against H. pylori and to define the immune correlates of protection. We found that induction of long-term protection against H. pylori can be achieved by immunizing either through the systemic route alone (i.m. group) or through a combination of mucosal followed by systemic routes (oral/i.m.). Mice challenged 3 months after i.m. and oral/i.m. immunizations had a significant reduction in bacterial load as well as increased levels of H. pylori-specific IL-12 and serum IgG1 and IgG2a responses, with no detectible intestinal IgA production. Mice immunized orally alone or through a combination of i.m./oral routes were not protected and, following oral challenge, had decreased IL-12 responses, decreased IgG1 and IgG2a antibody responses, and increased IgA antibody responses compared to levels for protected mice.
Many studies have shown that protection against H. pylori can be achieved through vaccination by either the mucosal or the systemic route (11, 13, 15, 20, 24, 34, 35, 43). Immunogenicity studies have also shown that the combination of mucosal priming followed by systemic boosting results in high antigen-specific antibody responses (25, 39, 40). However, in most H. pylori vaccine/protection studies immunized animals were challenged within a month after immunization, whereas we challenged the animals 3 months after immunization (11, 13, 15, 20, 24, 34, 35, 43). Challenging animals while still in the acute phase after vaccination (1 to 6 weeks) does not predict immunity during the resting memory phase, when the host would most likely be exposed to H. pylori.
A study by Garhart et al. focusing on H. pylori eradication postchallenge reported that there were no significant differences in H. pylori bacterial load between vaccinated and unvaccinated mice 1 week postchallenge and that there was only a slight difference at 2 weeks postchallenge (10). However, a significant reduction in bacterial load was observed by week 4 and remained up to a year. To observe peak humoral and cellular immune responses, mice in our study were sacrificed 8 days after challenge, which, according to the Garhart study, was not optimal for measuring decreased colonization. This observation likely explains why our study showed only a modest (less than 2 log), yet still significant, decrease in bacterial load. It is likely that the decreases would have been greater if the mice had been sacrificed at a later time point. Therefore, in this study we have defined protection as a statistically significant decrease in bacterial load in vaccinated groups compared to that for mock controls.
It is commonly accepted that systemic immunization (specifically i.m.) is not optimal for inducing long-term mucosal immunity (7, 17, 30, 42). However, we found that 3 months after i.m. or oral/i.m. immunization mice were protected from oral H. pylori challenge, whereas after oral or i.m./oral immunization mice were not protected. These data suggest that i.m. immunizations induce immune effector functions that can reach the stomach mucosa and may confer protection following a long-term resting period.
After clearance, H. pylori reinfection can occur readily even in the face of a robust antibody response (1, 26, 31, 41). It has been shown that vaccination through a mucosal route can provide long-term protection against other pathogens (19, 23, 27, 33). However, unlike immunity against H. felis, long-term protective immunity against H. pylori has yet to be achieved (27, 33, 38). Our study is the first of its kind to demonstrate that protection against H. pylori can occur 3 months after immunization, when responses are beyond the acute effector phase, and that protection can occur in mice immunized either systemically alone or through mucosal followed by systemic routes. It is of interest to note that the two routes leading to protection ended with systemic (i.m.) boosts.
The correlates of protection for H. pylori immunity are not clearly defined. However, recent studies have suggested the importance of IL-12 as a correlate of protection against H. pylori challenge. Secreted factors from H. pylori have been shown to inhibit IL-12 production (16), and IL-12 genetic polymorphisms in H. pylori-infected patients may play a role in cancer acquisition (28). Additionally, Akhiani et al. determined that IL-12 is necessary for protection against H. pylori infection in studies where IL-12 knockout mice were unable to mount a protective immune response upon challenge (2). The increase in IL-12 that we report for protected groups (i.m. and oral/i.m. groups) is consistent with these results, indicating that IL-12 may be a correlate of protection. Many other cytokines, such as IL-2, IL-4, IFN-γ, and TNF-α, have been suggested to correlate with protection (3, 29, 35, 40). However, we were unable to detect a direct correlation between protection and these cytokines (Fig. 4 to 6). Since the mechanisms of H. pylori clearance in protected animals are still not well established, more-detailed studies that focus on antigen-specific cytokine and cellular responses in local tissues, comparing before and after challenge (i.e., vaccinated, uninfected controls), as well as gastritis following immunizations and a long-term resting phase would shed more light on this issue.
In contrast to results with cytokine production, several studies have suggested that H. pylori-specific antibodies do not appear to play a role in protection (9). Specifically, Garhart et al. showed that immunized, antibody-deficient mice were protected from H. pylori challenge similarly to their wild-type counterparts (9). Our results, however, demonstrate that IgG1 and IgG2a titers may serve as a correlate of protective immunity, with an increase in both IgG1 and IgG2a after challenge in the protected groups (i.m. alone and oral/i.m.). These results are consistent with a previous study in which mucosal followed by systemic immunizations with H. pylori NAP and CagA yielded higher IgG1 serum responses than either the mucosal or the systemic route alone (40).
IgA, on the other hand, is considered the primary mucosal antibody and is suggested in some studies to be necessary for protection against H. pylori (11, 29). However, a study by Akhiani et al. recently reported that production of IgA and IL-10 is disadvantageous because they can suppress the protective inflammatory Th1 response at the site of infection (4). Our study supports these findings in that mice immunized through the oral route alone or the i.m./oral routes had high H. pylori-specific IgA titers from both splenocytes and Peyer's patches and were not protected from challenge. Similarly, IL-10 production appeared to be slightly higher (though not statistically significant) in the oral and i.m./oral groups.
Immunization solely through a mucosal route is commonly employed to induce mucosal immunity. However, our study showed that the systemic route alone or the mucosal followed by the systemic route was protective against oral challenge 3 months after immunization, while the mucosal route (oral) or the combination of i.m./oral routes was not. These studies were performed with CT as the adjuvant for all groups, indicating that the sole factor of protection is the route of immunization. This concept is made even clearer when considering the differences between the oral/i.m. and the i.m./oral groups. These two groups differed only in the order in which the vaccine was given, but one was protective (oral/i.m.) and the other was not (i.m./oral). The processes regulating these differing responses are unknown yet have been described for other systems. Studies of vaccines against polio (14) and influenza (8) have shown enhanced immunity from i.m. boost only in individuals that were preexposed mucosally. Thus, there appears to be a protective benefit when the final boost is given systemically.
In this study, the only two routes of immunization tested were i.m. and oral. Evaluations of combinations of other systemic and mucosal routes, such as subcutaneous, intraperitoneal, and intranasal immunizations, would be of great interest. Intranasal immunizations against H. pylori have been shown to be effective against challenge (20, 35). While the oral and i.m. routes of immunization may be clinically the most practical, exploring other routes will be of scientific interest.
Acknowledgments
This research was supported by Public Health Service grant A142081 from the National Institutes of Health.
We thank Giuseppe del Giudice and Paolo Ruggiero (helpful discussion), Paul Luciw and Imran Khan (technical support and assay development), Jennifer Huff (animal handling, technical support, and helpful discussion), and Jerome Braun (statistical support).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Akhiani, A. A. 2005. The role of type-specific antibodies in colonization and infection by Helicobacter pylori. Curr. Opin. Infect. Dis. 18:223-227. [DOI] [PubMed] [Google Scholar]
- 2.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 3.Akhiani, A. A., K. Schon, L. E. Franzen, J. Pappo, and N. Lycke. 2004. Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J. Immunol. 172:5024-5033. [DOI] [PubMed] [Google Scholar]
- 4.Akhiani, A. A., A. Stensson, K. Schon, and N. Y. Lycke. 2005. IgA antibodies impair resistance against Helicobacter pylori infection: studies on immune evasion in IL-10-deficient mice. J. Immunol. 174:8144-8153. [DOI] [PubMed] [Google Scholar]
- 5.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 6.Del Giudice, G., and P. Michetti. 2004. Inflammation, immunity and vaccines for Helicobacter pylori. Helicobacter 9(Suppl. 1):23-28. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich, G., M. Griot-Wenk, I. C. Metcalfe, A. B. Lang, and J. F. Viret. 2003. Experience with registered mucosal vaccines. Vaccine 21:678-683. [DOI] [PubMed] [Google Scholar]
- 8.el-Madhun, A. S., R. J. Cox, A. Soreide, J. Olofsson, and L. R. Haaheim. 1998. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J. Infect. Dis. 178:933-939. [DOI] [PubMed] [Google Scholar]
- 9.Garhart, C. A., J. G. Nedrud, F. P. Heinzel, N. E. Sigmund, and S. J. Czinn. 2003. Vaccine-induced protection against Helicobacter pylori in mice lacking both antibodies and interleukin-4. Infect. Immun. 71:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto, T., A. Nishizono, T. Fujioka, J. Ikewaki, K. Mifune, and M. Nasu. 1999. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy, B., C. Hessler, S. Fourage, J. Haensler, E. Vialon-Lafay, B. Rokbi, and M. J. Millet. 1998. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine 16:850-856. [DOI] [PubMed] [Google Scholar]
- 14.Herremans, T. M., J. H. Reimerink, A. M. Buisman, T. G. Kimman, and M. P. Koopmans. 1999. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 162:5011-5018. [PubMed] [Google Scholar]
- 15.Jeremy, A. H., Y. Du, M. F. Dixon, P. A. Robinson, and J. E. Crabtree. 2006. Protection against Helicobacter pylori infection in the Mongolian gerbil after prophylactic vaccination. Microbes Infect. 8:340-346. [DOI] [PubMed] [Google Scholar]
- 16.Kao, J. Y., S. Rathinavelu, K. A. Eaton, L. Bai, Y. Zavros, M. Takami, A. Pierzchala, and J. L. Merchant. 2006. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am. J. Physiol. Gastrointest. Liver Physiol. 291:G73-G81. [DOI] [PubMed] [Google Scholar]
- 17.Kaul, D., and P. L. Ogra. 1998. Mucosal responses to parenteral and mucosal vaccines. Dev. Biol. Stand. 95:141-146. [PubMed] [Google Scholar]
- 18.Keenan, J. I., S. G. Rijpkema, Z. Durrani, and J. A. Roake. 2003. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol. Med. Microbiol. 36:199-205. [DOI] [PubMed] [Google Scholar]
- 19.Kew, O. M., R. W. Sutter, E. M. de Gourville, W. R. Dowdle, and M. A. Pallansch. 2005. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59:587-635. [DOI] [PubMed] [Google Scholar]
- 20.Kleanthous, H., G. A. Myers, K. M. Georgakopoulos, T. J. Tibbitts, J. W. Ingrassia, H. L. Gray, R. Ding, Z. Z. Zhang, W. Lei, R. Nichols, C. K. Lee, T. H. Ermak, and T. P. Monath. 1998. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect. Immun. 66:2879-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleanthous, H., T. J. Tibbitts, H. L. Gray, G. A. Myers, C. K. Lee, T. H. Ermak, and T. P. Monath. 2001. Sterilizing immunity against experimental Helicobacter pylori infection is challenge-strain dependent. Vaccine 19:4883-4895. [DOI] [PubMed] [Google Scholar]
- 22.Lee, C. K., K. Soike, P. Giannasca, J. Hill, R. Weltzin, H. Kleanthous, J. Blanchard, and T. P. Monath. 1999. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine 17:3072-3082. [DOI] [PubMed] [Google Scholar]
- 23.Lycke, N., and J. Holmgren. 1986. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand. J. Immunol. 23:611-616. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti, M., M. Rossi, V. Giannelli, M. M. Giuliani, M. Pizza, S. Censini, A. Covacci, P. Massari, C. Pagliaccia, R. Manetti, J. L. Telford, G. Douce, G. Dougan, R. Rappuoli, and P. Ghiara. 1998. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine 16:33-37. [DOI] [PubMed] [Google Scholar]
- 25.McCluskie, M. J., R. D. Weeratna, P. J. Payette, and H. L. Davis. 2002. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol. Med. Microbiol. 32:179-185. [DOI] [PubMed] [Google Scholar]
- 26.Michetti, P., and A. M. Svennerholm. 2003. Helicobacter pylori-inflammation, immunity and vaccines. Helicobacter 8(Suppl. 1):31-35. [DOI] [PubMed] [Google Scholar]
- 27.Myers, G. A., T. H. Ermak, K. Georgakopoulos, T. Tibbitts, J. Ingrassia, H. Gray, H. Kleanthous, C. K. Lee, and T. P. Monath. 1999. Oral immunization with recombinant Helicobacter pylori urease confers long-lasting immunity against Helicobacter felis infection. Vaccine 17:1394-1403. [DOI] [PubMed] [Google Scholar]
- 28.Navaglia, F., D. Basso, C. F. Zambon, E. Ponzano, L. Caenazzo, N. Gallo, A. Falda, C. Belluco, P. Fogar, E. Greco, F. Di Mario, M. Rugge, and M. Plebani. 2005. Interleukin 12 gene polymorphisms enhance gastric cancer risk in H pylori infected individuals. J. Med. Genet. 42:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nystrom, J., S. Raghavan, and A. M. Svennerholm. 2006. Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice. Microbes Infect. 8:442-449. [DOI] [PubMed] [Google Scholar]
- 30.Oien, N. L., R. J. Brideau, E. E. Walsh, and M. W. Wathen. 1994. Induction of local and systemic immunity against human respiratory syncytial virus using a chimeric FG glycoprotein and cholera toxin B subunit. Vaccine 12:731-735. [DOI] [PubMed] [Google Scholar]
- 31.Parsonnet, J. 2003. What is the Helicobacter pylori global reinfection rate? Can. J. Gastroenterol. 17(Suppl. B):46B-48B. [DOI] [PubMed] [Google Scholar]
- 32.Peek, R. M., Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 33.Radcliff, F. J., M. Chen, and A. Lee. 1996. Protective immunization against Helicobacter stimulates long-term immunity. Vaccine 14:780-784. [DOI] [PubMed] [Google Scholar]
- 34.Radcliff, F. J., S. L. Hazell, T. Kolesnikow, C. Doidge, and A. Lee. 1997. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect. Immun. 65:4668-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi, T., W. Z. Liu, F. Gao, G. Y. Shi, and S. D. Xiao. 2005. Intranasal CpG-oligodeoxynucleotide is a potent adjuvant of vaccine against Helicobacter pylori, and T helper 1 type response and interferon-gamma correlate with the protection. Helicobacter 10:71-79. [DOI] [PubMed] [Google Scholar]
- 36.Solnick, J. V., D. R. Canfield, L. M. Hansen, and S. Z. Torabian. 2000. Immunization with recombinant Helicobacter pylori urease in specific-pathogen-free rhesus monkeys (Macaca mulatta). Infect. Immun. 68:2560-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 38.Sutton, P., S. J. Danon, M. Walker, L. J. Thompson, J. Wilson, T. Kosaka, and A. Lee. 2001. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut 49:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vajdy, M., M. Singh, J. Kazzaz, E. Soenawan, M. Ugozzoli, F. Zhou, I. Srivastava, Q. Bin, S. Barnett, J. Donnelly, P. Luciw, L. Adamson, D. Montefiori, and D. T. O'Hagan. 2004. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intranasal and parenteral immunizations. AIDS Res. Hum. Retrovir. 20:1269-1281. [DOI] [PubMed] [Google Scholar]
- 40.Vajdy, M., M. Singh, M. Ugozzoli, M. Briones, E. Soenawan, L. Cuadra, J. Kazzaz, P. Ruggiero, S. Peppoloni, F. Norelli, G. del Giudice, and D. O'Hagan. 2003. Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations. Immunology 110:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Ende, A., R. W. van der Hulst, J. Dankert, and G. N. Tytgat. 1997. Reinfection versus recrudescence in Helicobacter pylori infection. Aliment. Pharmacol. Ther. 11(Suppl. 1):55-61. [DOI] [PubMed] [Google Scholar]
- 42.Walker, R. I. 1994. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine 12:387-400. [DOI] [PubMed] [Google Scholar]
- 43.Weltzin, R., B. Guy, W. D. Thomas, Jr., P. J. Giannasca, and T. P. Monath. 2000. Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect. Immun. 68:2775-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto, T., M. Kita, T. Ohno, Y. Iwakura, K. Sekikawa, and J. Imanishi. 2004. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol. Immunol. 48:647-654. [DOI] [PubMed] [Google Scholar]