Abstract
The cytokine interplay during the development of protective immunity to the radiation-attenuated (RA) schistosome vaccine has been extensively characterized over recent years, yet the role of costimulatory molecules in the development of cell-mediated immunity is much less well understood. Here we demonstrate the importance of CD40/CD154 in vaccine-induced immunity, as CD154−/− mice exposed to RA schistosomes develop no protection to challenge infection. We showed that vaccinated CD154−/− mice have defective Th1-associated immune responses in the skin-draining lymph nodes and the lungs, with reduced or absent levels of interleukin-12p40 (IL-12p40), gamma interferon, and nitric oxide, but elevated levels of lung IL-4 and IL-5. The expression of major histocompatibility complex II (MHC-II) on antigen-presenting cells recovered from the lungs of vaccinated CD154−/− mice was also severely compromised. The administration of anti-CD40 monoclonal antibody (MAb) to CD154−/− mice did not reconstitute sustained Th1 responses in the lymph nodes or the lungs, nor did the MAb restore anti-parasite immunoglobulin G production or protective immunity. On the other hand, the administration of recombinant IL-12 (rIL-12) to CD154−/− mice shortly after vaccination caused elevated and sustained levels of Th1-associated cytokines, rescued MHC-II expression by lung CD11c+ cells, and restored the appearance of inflammatory effector foci in the lungs. However, the treatment of CD154−/− mice with rIL-12 did not restore protection. We conclude that protective immunity to the RA schistosome vaccine is CD154 dependent but is independent of IL-12-orchestrated cellular immune mechanisms in the lungs.
Schistosomiasis is a parasitic infection of humans in many parts of the developing world, and it is estimated that 779 million people, largely in sub-Saharan Africa, are at risk of infection (51). Serious pathology results from granulomatous lesions that form in the liver, intestines, or bladder wall. These lesions are the result of the host's immune response to ova produced by adult worms (61). Over recent decades, attention has focused upon the development of a vaccine against schistosomiasis. The use of radiation-attenuated (RA) schistosome larvae has provided the most consistent way of inducing high levels of protection specifically against Schistosoma mansoni in a number of different mammalian hosts, including experimental mice (14) and nonhuman primates (21).
Studies of RA vaccine-induced protective immunity in mice have revealed that resistance to challenge infection is dependent on CD4+ Th1-type effector responses (56). For example, mice deficient in IL-12p40 (2, 16) or interferon receptor gamma (IFN-γR) (57) develop significantly reduced Th1-type responses during the induction and effector phases and exhibit low levels of protection. However, it is also clear that antibodies play a partial but important role in the protective effector mechanism, particularly in mice exposed to multiple doses of RA larvae (1, 20), and the current consensus view is that both antibody and IFN-γ-mediated immune responses are required for effective vaccine-induced protection (20, 59). Studies show that the transfer of serum from wild-type (WT) mice into vaccinated B-cell-deficient (μMT) mice can increase protection from ∼40% to upwards of 70% (20), while the transfer of serum, even from singly vaccinated mice, into IL-4Rα−/− mice also confers a significant reduction in the challenge worm burden (35). Protection appears to rely on the involvement of strong immunoglobulin G (IgG) responses, particularly of the IgG1 isotype (9, 35). On the other hand, the immunological basis of the cell-mediated immune response in the absence of antibody production remains unclear.
Recent studies have demonstrated that immune priming events in the skin and the balance of cytokine production, especially IL-12 versus IL-10, are important in determining the magnitude of the Th1 response in the skin-draining lymph nodes (sdLN) (17, 18, 36, 42). In turn, these events are integral steps in the ultimate priming of the lungs with IFN-γ-secreting CD4+ cells (34) and the development of a cellular foci capable of eliminating challenge parasites (58). While the cytokine interplay after exposure to RA schistosome larvae has become clearer (14), the role of costimulatory molecules in the interaction between potential antigen-presenting cells (APCs) and T lymphocytes is much less well understood. In this context, CD154 present on CD4+ cells is a key molecule in the maturation of CD40-expressing APCs (41, 49). APCs activated via the CD154/CD40 pathway upregulate other costimulatory molecules, such as CD80 and CD86, and inflammatory cytokines, such as IL-12, which together favor the development of Th1-type responses (6, 7, 13, 23, 40). This pathway is critical for the development of protective Th1-mediated immunity to the intracellular parasites Leishmania major and Toxoplasma gondii (5, 43), although several other reports show that CD154 is not required for the development of protective immunity against pathogens, such as Mycobacterium tuberculosis, L. major, and Histoplasma histolytica (26, 38, 39, 64). Its role in protective immunity against schistosomes is not known. Nevertheless, we identified CD154 as an important molecule in the induction of Th1-associated cytokine production following exposure to schistosome larvae (15). In this situation, cells from mice deficient in CD154 failed to release optimum levels of IL-12p40 and failed to produce antigen-driven IFN-γ. Furthermore, Th1-biased immune responses could be recreated in vaccinated CD154−/− mice following the administration of agonist anti-CD40 monoclonal antibody (MAb) or recombinant IL-12 (rIL-12) (15), and others have shown that rIL-12 restores Th1 responses and protective immunity in CD154−/− mice exposed to L. major (5).
In the current study, we sought to determine the role of CD154 in the development of protective immunity induced by the RA schistosome vaccine. Since CD154 is essential for IgG class switching (28, 48), the use of CD154−/− mice allows us to examine and manipulate the development of Th responses in the absence of antibody production. We show that without CD154, protective immunity induced by schistosome larvae was totally absent, and this was associated with the abrogation of Th1 immune responses in the lungs and the lack of anti-parasite IgG antibodies. While agonist anti-CD40 MAb administered to groups of vaccinated CD154−/− mice only transiently restored Th1-mediated immune responses, the administration of rIL-12 in the absence of CD154 had long-term restorative effects on the Th1-associated cellular immune response in the lungs. However, neither treatment was able to restore protective immunity. We conclude that endogenous CD154 is an obligatory component in the development of protective immune responses against schistosomes, but Th1-associated inflammatory responses in the lungs, induced following the administration of rIL-12, are not required for protection.
MATERIALS AND METHODS
Parasite and host.
Female wild-type (WT) C57BL/6 mice and CD154−/− mice on a C57BL/6 background (62) were immunized with 500 irradiated (20-kilorad 60Co source) S. mansoni cercariae via the abdomen (2). In some experiments, mice were treated with 100 μg anti-CD40 MAb clone FGK45.5 (46) or control rat IgG (Sigma-Aldrich, Poole, United Kingdom) via the tail vein on days 1 and 3 postvaccination (p.v.) and on days 6 and 10 (intraperitoneally). Alternatively, mice were treated with 1 μg murine rIL-12 (Genetics Institute, Cambridge, MA) or endotoxin-free 0.9% NaCl (Sigma-Aldrich), over the sternum (intradermally) on days 1 and 3 p.v. and on days 6 and 10 (intraperitoneally). All animal work was carried out in accordance with the guidelines of the United Kingdom Animals (Scientific Procedures) Act 1986.
Measurement of protection to challenge infection.
Vaccinated WT and CD154−/− mice, alongside naïve cohort mice (n = 5/group), were exposed to 200 cercariae via the tail 35 days p.v. Five weeks later, the adult worm burden in vaccinated (VC) and challenge control (CC) animals was enumerated following perfusion of the portal system. Resistance (R) to challenge infection was calculated [% R = (CC − VC/CC) × 100] in WT and CD154−/− mice.
Cell preparations and in vitro culture.
Cell suspensions were prepared from the axillary LN that drain the abdomen and cultured as described previously (18). Bronchoalveolar lavage (BAL) cells were recovered from the lungs (3) and cultured at 2.5 × 105 cells/well in 96-well, flat-bottomed plates (Nunclon surface; Nalge Nunc, Hereford, United Kingdom) in a total volume of 200 μl, in the absence or presence of 40 μg/ml soluble schistosomular antigen preparation (SSAP) (18) for 48 h at 37°C/5% CO2. Culture supernatants were removed and stored at −20°C until cytokine levels were assessed by enzyme-linked immunosorbent assay (ELISA).
Cytokine detection.
For the detection of secreted cytokines, antibody ELISAs were used to quantify IL-12p40, IFN-γ, IL-4, and IL-5 in the culture supernatants as described previously (17, 18). The lower limits of detection were 10 (IL-4), 40 (IL-12p40), and 50 (IFN-γ and IL-5) pg/ml. Nitric oxide (NO) production by cultured BAL cells was measured by Greiss assay as described previously (8).
Flow cytometric analysis of labeled cells.
BAL cells (1 × 105 to 2 × 105) were blocked with 4 μl normal rabbit serum and then labeled with the following antibodies: fluorescein isothiocyanate anti-CD11c (clone HL3; Pharmingen), biotin anti-CD4 (clone H129.19; Pharmingen), and biotin anti-MHC-II (I-Ab,d clone 28-16-8S; Caltag). Streptavidin-APC was used as a detection probe for biotin-conjugated antibodies. Irrelevant isotype-matched antibodies were used to determine levels of nonspecific binding. Analysis was performed using a CyAn flow cytometer (DakoCytomation, United Kingdom).
Tissue processing and histology of lung tissue.
Lung tissues were fixed in 10% neutral buffered formalin, embedded in wax, and then sectioned at 10 μm. Tissue sections were stained with Harris hematoxylin (BDH, Poole, United Kingdom), followed by 0.5% eosin (in 90% ethanol; BDH). At least 15 cellular foci were identified from each mouse.
Antibody detection.
Serum obtained via tail bleeds at times postvaccination and challenge was used to probe immunoplates (MaxiSorp; Nunc) coated with 10 μg/ml soluble worm antigen preparation (2). After washing with phosphate-buffered saline-0.05% Tween 20, bound antibodies were probed with goat anti-mouse IgG conjugated to horseradish peroxidase and revealed following the addition of SureBlue TMB substrate (Insight Biotechnology, Wembley, United Kingdom).
Statistics.
Comparisons of data were tested for significance with Student's t test and presented as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; and nonsignificant, P > 0.05. Arithmetic means ± the standard error of the means (SEM) are shown. Data shown are representative of two to four experimental repeats.
RESULTS
CD40/CD154 interactions are essential for vaccine-induced protection.
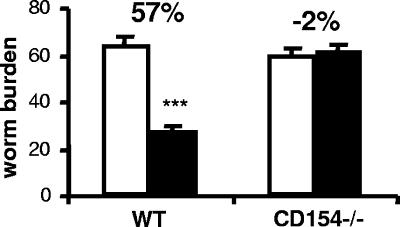
Protective immunity to S. mansoni induced by the RA vaccine was compared for CD154−/− mice and the WT cohorts. In WT mice, an average of 57% protection against challenge infection was induced (P < 0.001 [the P value was determined on the basis of a comparison with challenge control mice]) (Fig. 1), whereas vaccination of CD154−/− mice conferred no protection against challenge (−2% reduction in worm burden).
FIG. 1.
CD154 is essential for protective immunity. Worm burdens of challenge control (open bars) and vaccinated and challenged (closed bars) mice 5 weeks after infection with 200 normal cercariae. Bars show the means + SEM (error bars) for each group of mice. The percentage value is the calculated level of resistance in vaccinated mice as a percentage of the level for challenge control mice within each group. Significance values refer to the difference between worm burdens in vaccinated mice and the relevant challenge control group. ***, P < 0.001.
Defective lung stage Th1 responses and protective immunity in CD154−/− mice are not rescued by administration of anti-CD40 MAb.
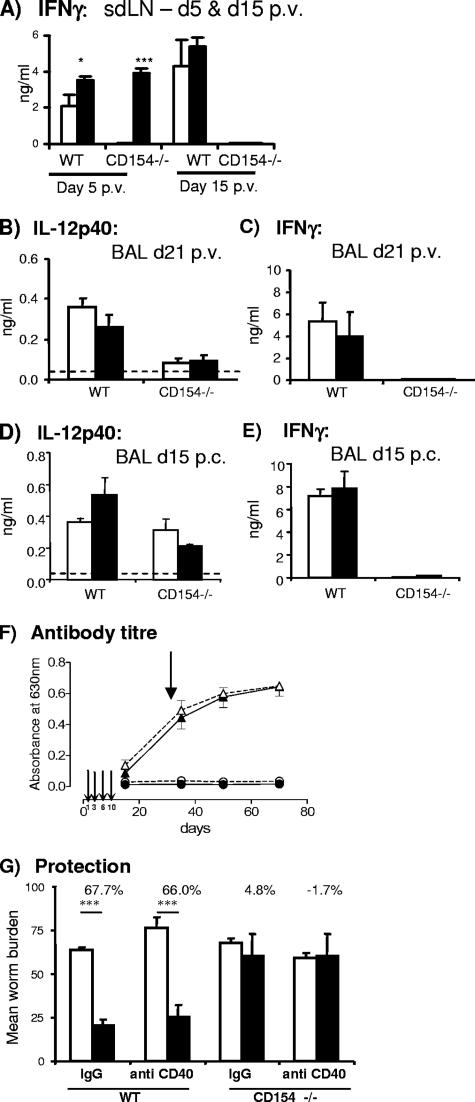
The lack of protection in CD154−/− mice suggested that CD40/CD154 interactions are critical for the generation of antiparasite cellular immunity. Indeed, we previously showed that in vivo ligation of CD40 using an agonistic anti-CD40 MAb (to mimic the missing CD154) was sufficient to restore Th1 responses in the skin and sdLN of vaccinated CD154−/− mice (15). This result was confirmed in the current study, as sdLN cells from CD154−/− mice given anti-CD40 MAb produced substantial amounts of IFN-γ at day 5, whereas cells from CD154−/− mice receiving control IgG did not (Fig. 2A). However, the effects of anti-CD40 MAb treatment (given on days 1, 3, 6, and 10 p.v.) were transient only, since at day 15, sdLN cells from CD154−/− mice failed to produce IFN-γ when cultured with SSAP, regardless of prior antibody treatment (Fig. 2A). This result contrasts with the sustained levels of SSAP-dependent IFN-γ production by WT sdLN cells at the same time point. The failure to restore Th1-associated responses following anti-CD40 MAb treatment was also seen in the lungs of mice 21 days p.v., as the production of spontaneous IL-12p40 and antigen-driven IFN-γ was not significantly improved in treated mice compared to in control CD154−/− mice (Fig. 2B and C). Although anti-CD40 MAb failed to restore Th1-associated cytokine production in the lungs of CD154−/− mice after vaccination, it was possible that a change would operate during the pulmonary phase of challenge parasite migration. Significant levels of IL-12p40 were secreted by BAL cells from vaccinated CD154−/− recovered on day 15 postchallenge (p.c.), but anti-CD40 MAb again failed to elevate the secretion of this cytokine (Fig. 2D). Anti-CD40 MAb also failed to restore antigen-driven IFN-γ (Fig. 2E).
FIG. 2.
Administration of anti-CD40 MAb does not restore long-term Th1-type responses, antibody production, or protective immunity in vaccinated CD154−/− mice. (A) Production of IFN-γ by antigen-stimulated sdLN cells from WT and CD154−/− mice on days 5 and 15 (d5 and d15) p.v. Significance values are for anti-CD40 MAb-treated mice compared to control IgG-treated WT and CD154−/− mice. *, P < 0.05; ***, P < 0.001. (B and D) The production of spontaneous IL-12p40 by unstimulated BAL cells and (C and E) production of IFN-γ by antigen-stimulated BAL cells on day 21 p.v. (d21) (B and C) and day 15 p.c. (D and E). Values are means + SEM (error bars) for groups of mice (n = 3 to 5 mice/group) given control rat IgG (open bars) or anti-CD40 MAb (closed bars). The horizontal dotted line denotes the minimum detection level for IL-12p40. (F) Level of anti- parasite IgG levels in WT (triangles) and CD154−/− mice (circles) given control rat IgG (solid lines) or anti-CD40 MAb (dashed lines). Anti-CD40 MAb or control IgG was administered on days 1, 3, 6, and 10 p.v. Thin arrows indicate the administration of anti-CD40 MAb, and the single thicker arrow denotes the time of challenge infection. (G) Worm burdens of challenge control (open bars) and vaccinated and challenged (closed bars) mice 5 weeks p.c. Bars are means + SEM (error bars) in each group. The percentage values are the calculated levels of resistance in vaccinated mice as a percentage of the challenge control level. Significance values refer to the difference in worm burdens in vaccinated mice relative to their respective challenge control group. ***, P < 0.001.
Anti-CD40 MAb treatment neither boosted anti-worm IgG antibody titers in WT mice nor restored any detectable level of class switching in CD154−/− mice (Fig. 2F); moreover, anti-CD40 MAb treatment had no effect on the induction of protective immunity (Fig. 2G). Although vaccinated WT mice harbored significantly lower worm burdens than did challenge control mice whether they had received rat IgG or anti-CD40 MAb (68 and 66%, respectively), neither vaccinated CD154−/− mice given IgG nor mice treated with anti-CD40 MAb were significantly protected against challenge infection (4.8 and −1.7%, respectively).
Exogenous IL-12 restores cellular immune responses in the lungs of CD154−/− mice.
To investigate why the absence of CD154 led to the failure to induce immune responses, even in the presence of a surrogate CD154 (i.e., anti-CD40 MAb), vaccinated CD154−/− mice were treated with rIL-12, as IL-12 production is one of the main functional consequences of CD40/CD154 signaling (23). We have previously shown that rIL-12 restores long-term Th1 responses in IL-12p40−/− mice, even when delivered only within the first few days after vaccination (2), and rIL-12 administered to CD154−/− mice completely restored IFN-γ production by sdLN cells on day 5 p.v. (15). Here we show that the inflammatory foci that formed in the lungs after challenge infection of CD154−/− mice were significantly smaller than those present in the lungs of WT mice (Fig. 3A, B, and D). However, treatment with rIL-12 restored foci size in CD154−/− mice back to WT levels (Fig. 3C and D). There was no significant difference in the number of foci present in the lungs of each group of mice (data not shown).
FIG. 3.
IL-12 restores the development of inflammatory foci in the lungs of CD154−/− mice after challenge. Representative inflammatory foci in the lungs of vaccinated and challenged (A) WT mice, (B) CD154−/− mice given saline, and (C) CD154−/− mice given rIL-12 at 15 days p.c. Tissue sections stained with hematoxylin and eosin; scale bars are 200 μm. (D) Diameter in micrometers (means + SEM [error bars]) of pulmonary foci (n = 15 to 20) at day 15 p.c.; significance values are for CD154−/− mice given saline connected to either WT mice or CD154−/− mice given rIL-12. **, P < 0.01; ***, P < 0.001.
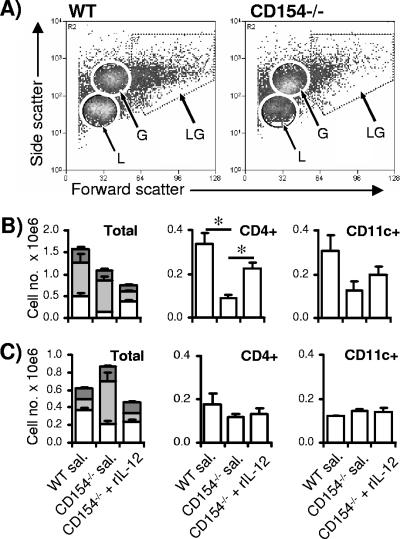
The cellular composition of the pulmonary immune response of rIL-12-treated CD154−/− mice recovered by BAL on day 21 p.v. and on day 15 p.c. (i.e., 12 and 40 days after final rIL-12 injection, respectively) was markedly changed compared to that of CD154−/− mice treated only with saline. Based upon their size and granularity, the BAL cell population was divided into one of three cell groupings (Fig. 4A), small nongranular cells (lymphocytes), small granular cells (granulocytes), and large granular cells (macrophages and dendritic cells [DCs]). After vaccination (day 21 p.v.), the lungs of control CD154−/− mice given saline contained fewer lymphocytes than did the lungs of WT mice, with a corresponding fall in the number of CD4+ cells (P < 0.05) (Fig. 4B). Importantly, rIL-12 treatment of CD154−/− mice restored the number of lymphocytes and CD4+ cells back to WT levels (P was >0.05 [the P value was determined on the basis of a comparison with the WT]). WT and CD154−/− lungs contained similar numbers of granulocytes (predominantly eosinophils; data not shown), but treatment with rIL-12 substantially reduced their numbers. In contrast, there was no significant difference in the number of CD11c+ cells, representing a mixture of macrophages and DCs (25), recovered from the three groups of mice. A slightly different pattern in BAL cell populations was observed after challenge infection (day 15 p.c.). At this later time point, comparable numbers of lymphocytes and CD4+ cells were detected in the lungs of WT and control CD154−/− mice, and this was unaffected by rIL-12 treatment (Fig. 4C). In contrast, lungs of control CD154−/− mice contained significantly more granulocytes than did lungs of WT mice (P < 0.01), but this was inhibited by rIL-12 treatment. Again, there was no difference in the number of CD11c+ cells.
FIG. 4.
IL-12 restores the BAL cell composition after vaccination and after challenge. (A) Representative scatter plots of BAL cells recovered from the lungs of WT (left) and CD154−/− mice (right) after vaccination showing size (forward scatter) and granularity (side scatter). (B) Numbers of BAL cell types in WT mice, CD154−/− mice given saline, and CD154−/− mice given rIL-12 on day 21 p.v. and (C) on day 15 p.c.. IL-12 was given on days 1, 3, 6, and 10 p.v. Small nongranular lymphocytes (L; open), granulocytes (G; light gray), and large granular macrophages and DCs (LG; dark gray), as determined by flow cytometric analysis, are shown as histogram bars + SEM (error bars) (n = 4 mice/group). Numbers of CD4+ and CD11c+ cells in BAL are shown as bars + SEM (error bars). Significance values are for CD154−/− mice given saline (sal.) compared to WT or to CD154−/− mice given rIL-12.
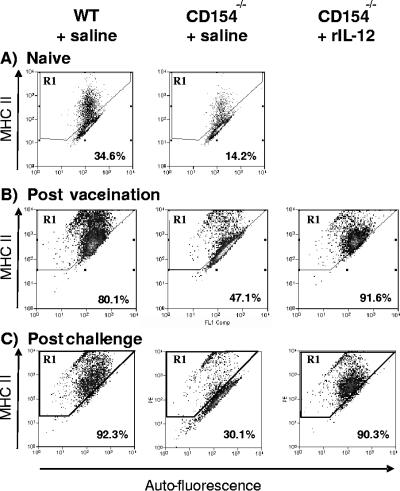
Although WT and CD154−/− lungs contained similar numbers of CD11c+ cells, substantial differences in the level of activation (as judged by MHC-II expression) of these cells were noted (Fig. 5). Compared to cells from naïve mice, the percentage of CD11c+ cells from WT and CD154−/− mice given saline that expressed MHC-II increased following vaccination and after challenge (Fig. 5A to C). However, the expression of MHC-II by cells from CD154−/− mice given saline was lower than that of their WT cohorts both after vaccination and after challenge. Remarkably, the treatment of CD154−/− mice with rIL-12 significantly increased the proportion of MHC-II+ cells to over 90% (P < 0.01 [the P value was determined on the basis of a comparison with CD154−/− mice given saline]) to the extent that it was not significantly different from that of WT mice (P was >0.05 [the P value was determined on the basis of a comparison with the WT]).
FIG. 5.
Expression of MHC-II on CD11c+ BAL cells is restored by administration of rIL-12. Flow cytometric analysis of MHC-II+ cells versus autofluorescence of gated CD11c+ BAL cells recovered on (A) day 0 from WT and CD154−/− mice; (B) day 21 p.v. from WT mice, CD154−/− mice given saline, and CD154−/− mice given rIL-12; and (C) day 15 p.c. from the same three groups. Percent values given are the mean values of MHC-II+ cells in region R1 from four mice in each group.
Exogenous IL-12 restores sustained pulmonary Th1 responses in CD154−/− mice and suppresses Th2 responses.
CD154−/− mice of the BAL cell population treated with saline were deficient in their production of antigen-specific IFN-γ and NO compared to WT mice at day 21 p.v. (Fig. 6A and B). However, in each case, the administration of rIL-12 to CD154−/−mice completely restored the production of these mediators to levels equivalent to or above those seen in vaccinated WT mice. BAL cells from saline-treated CD154−/− mice recovered after challenge also failed to produce IFN-γ and NO (Fig. 6C and D). Importantly however, the administration of rIL-12 given more than 5 weeks previously restored strong antigen-dependent IFN-γ and NO production by CD154−/− mice, equivalent to the levels produced by their WT counterparts (Fig. 6C and D). Compared to WT cells, CD154−/− lung cells produced significantly more IL-4 and IL-5 following stimulation with parasite antigen (both P values were less than 0.01) (Fig. 6E and F), and as expected, the production of Th2 cytokines by CD154−/− mice was significantly inhibited by rIL-12 treatment.
FIG. 6.
Th1-associated cytokines are restored and Th2 cytokines are ablated in CD154−/− mice treated with rIL-12. Production of (A) IFN-γ and (B) NO by SSAP-stimulated BAL cells recovered from WT mice, CD154−/− mice given saline, and CD154−/− mice treated with rIL-12, on day 21 (d21) p.v. Production of (C) IFN-γ, (D) NO, (E) IL-4, and (F) IL-5 by SSAP-stimulated BAL cells recovered from the same groups of mice on day 15 (d15) p.c. Values are means + SEM for groups of mice (n = 3 to 4 mice/group); significance values are for CD154−/− mice given saline connected to either WT mice or CD154−/− mice given rIL-12. Horizontal dotted lines denote minimum detection levels. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
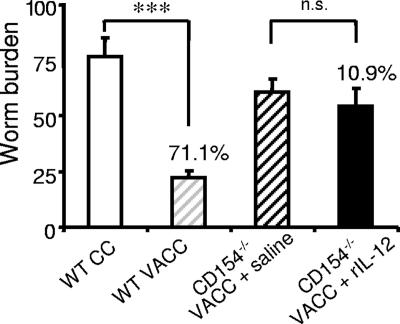
Exogenous IL-12 does not restore protection in CD154−/− mice.
Challenge parasite worm burdens were assessed in vaccinated CD154−/− mice treated with either saline or rIL-12 and compared to those of WT mice. The vaccination of WT mice induced 71% protection (Fig. 7). Unexpectedly, given their virtually identical pulmonary Th1 responses to those of WT mice, vaccinated CD154−/− mice given rIL-12 did not have worm burdens significantly lower than those of vaccinated saline-treated CD154−/− mice (P > 0.05; 10.9% reduction).
FIG. 7.
IL-12 does not restore protective immunity in CD154−/− mice. Worm burdens of CC (open bar) and vaccinated and challenged (VACC) (light gray hatched bar) WT mice 5 weeks after challenge infection with 200 normal cercariae. Worm burdens in VACC CD154−/− mice given saline (dark gray hatched bar) and CD154−/− mice treated with rIL-12 (closed bar). Bars are the means + SEM (error bars) in each group. ***, P < 0.001; n.s., nonsignificant (P > 0.05).
DISCUSSION
Our studies show that CD40/CD154 signaling has an essential role in the development of a protective immune response against schistosomes as CD154−/− mice exposed to the RA vaccine are not resistant to challenge infection. The complete absence of protective immunity in vaccinated CD154−/− mice is significant because the absence of many other immune components (e.g., IFN-γ [20], IFN-γR [57], IL-4Rα [35], and IL-12p40 [2, 16]) only leads to the partial abrogation of resistance. Our studies using CD154−/− mice that do not produce anti-parasite IgG antibodies have allowed dissection of the Th1-mediated cellular component of the effector response.
CD154 is important for optimal expression of MHC-II and CD86 by APCs in the skin and their migration from the skin to the sdLN (32), which are important in immune priming against schistosomes (24). CD154 is also essential for CD4+ cell proliferation in the sdLN and for the production of Th1 but not Th2 cytokines (15). Here, we show that Th1-associated cytokine production in the lungs is also CD154 dependent, while the secretion of IL-4 and IL-5 by BAL cells from CD154−/− mice after challenge is CD154 independent (15). In contrast to that in the sdLN, the lack of Th1 differentiation in the lungs of CD154−/− mice cannot be fully explained by the absence of CD40-dependent IL-12 production, because CD154−/− mouse lung cells produce measurable levels of IL-12p40 after challenge with normal cercariae. Furthermore, the absence of IFN-γ is not due to a lack of CD11c+ APC or responder CD4+ cells, as both are present (albeit in reduced numbers after vaccination in the case of CD4+ cells) in the lungs of CD154−/− mice. Instead, a more likely explanation is the immature phenotype (MHC-IIlo) of lung CD11c+ cells in CD154−/−mice, which leads to reduced T-cell activation. This is unlikely to be a direct consequence of the absence of CD40-dependent signaling into the APC, since full maturation (i.e., MHC-IIHI) can be restored by treating CD154−/− mice with rIL-12, suggesting that IL-12, IFN-γ, or an alternative costimulatory pathway, drives APC maturation. BAL cells from the lungs of vaccinated CD154−/− mice not only lack optimum expression of MHC-II but also do not express NO. This is consistent with other studies showing that macrophages produce NO via a CD40/CD154-dependent mechanism (37, 52, 55) (unfortunately, we were unable to measure detectable levels of tumor necrosis factor alpha by ELISA [data not shown]). Although NO may have only a limited direct role in protection against schistosomes (8, 19), its impaired production is further evidence of poor macrophage activation in CD154−/− mice.
The restoration of Th1-associated immune responses in the sdLN of CD154−/− mice by the administration of anti-CD40 MAb (15) raised the possibility that this treatment may induce protective immunity in vaccinated CD154−/− mice. Indeed, anti-CD40 MAb treatment of WT mice can induce strong Th1 responses in a variety of experimental models through both IL-12-dependent and -independent pathways (11, 13, 30, 47). An alternative explanation was that the defective cellular response and the absence of protection in CD154−/− mice was due to a failure of signaling through CD154 into the CD4+ cell (7). Anti-CD40 MAb treatment of vaccinated CD154−/− mice restored antigen-induced IFN-γ by sdLN cells at day 5, showing that signals transduced through CD154 directly into CD4+ cells are not required for initial Th1 differentiation. This agrees with other studies showing that Th1 responses can be restored in CD154−/− mice through anti-CD40 MAb treatment (63). However, the effect in the present study was transient only and the restorative effect on IFN-γ production in the sdLN was not detected by day 15. In addition, no increase was detected in the production of IL-12p40 and IFN-γ by BAL cells p.v. or p.c. following treatment with anti-CD40 MAb.
In the context of all the results described above, it was perhaps not surprising that anti-CD40 MAb failed to restore protection in CD154−/− mice; it also failed to boost immunity in WT mice. Nevertheless, the result was still disappointing because similar treatment regimens lead to sustained protective effects in other disease models (10-12, 47). The transient nature of the response to anti-CD40 MAb is unlikely to be caused by immune-mediated clearance, as CD154−/− mice do not produce detectable IgG antibody titers against the anti-CD40 MAb molecule itself (data not shown). It is also unlikely that the treatment causes the death/inactivation of CD40+ cells (31), since anti-CD40 MAb treatment does not impede vaccine-induced immune responses in WT mice. One interpretation is that CD40 stimulation alone, while sufficient for inducing short-term Th1 differentiation, cannot support the development of long-term Th1 clones and that CD154 is required for the generation of memory Th1 clones after vaccination (45). While manipulation of the CD40/CD154 pathway cannot increase protection against schistosomes, therapeutic targeting of alternative costimulatory pathways, for example, those associated with immune regulation, may prove more successful.
An important consequence of CD40/CD154 interactions is CD40-dependent IL-12 production, which we showed is defective in vaccinated CD154−/− mice, and in order to circumvent this deficiency, rIL-12 was administered to CD154−/− mice. The administration of rIL-12 stimulated elevated levels of antigen-specific IFN-γ production by sdLN cells in CD154−/− mice, consistent with the known ability of rIL-12 to promote Th1 differentiation in the RA vaccine model (2, 60). The effects of rIL-12 are long lasting, as vaccinated CD154−/− mice made strong pulmonary Th1 responses both after vaccination and after challenge (12 and 40 days, respectively, after the cessation of rIL-12 treatment). This indicates that the generation of memory Th1 cells does not require signaling through CD154 and implies that the main functional consequence of CD40/CD154 interaction is the production of IL-12 which supports Th1 memory cell development. In support of our conclusion, the treatment of mice with rIL-12 reverses the inhibitory effects of a CD40/CD154 blockade on intestinal inflammation (54) and protection to L. major (5). Since one of the main consequences of CD40 ligation is the release of IL-12p40, it is surprising that rIL-12 is a much more potent stimulus of Th1 responses than anti-CD40 MAb. The differential effects can be most likely explained in terms of the amount of rIL-12 used, approximating to the quantities used in other studies (2, 33, 60), compared to treatment of mice with anti-CD40 MAb, which induced the production of up to only 2 ng IL-12p40 by skin cells (15).
The potent and prolonged effects of rIL-12 are illustrated by its influence on the cell types present in the lungs after vaccination and challenge. For example, the number of CD4+ cells recovered from the lungs of CD154−/−mice was reduced compared to that recovered from WT mice following vaccination, but was restored to WT levels by rIL-12 treatment. This implies that rIL-12 either increases the efficiency of recruitment or delays the death of CD4+ cells in the lungs. The lungs of CD154−/− mice also contain an enhanced proportion of small granular cells which are thought to be eosinophils. Substantial pulmonary eosinophilia develops in the lungs of vaccinated IFN-γR−/− (57) and IL-12p40−/− (2, 3) mice that mount parasite-specific Th2 responses (16) in contrast to the Th1 bias seen in WT mice (14). In fact, BAL cells from vaccinated CD154−/− mice also produce elevated levels of IL-4 and IL-5 after challenge, contradicting several studies which show that CD40/CD154 interactions are essential for the Th2 development caused by helminth infection (22, 28, 29, 44) but supporting others (27).
Despite the apparent complete restoration of the pulmonary immune response akin to that in protected WT mice, the treatment of CD154−/− mice with rIL-12 does not restore protection against subsequent challenge. This was surprising since rIL-12 restored high levels of protection against schistosomes in IL-12p40−/− mice (2), and the treatment of CD154−/− mice with rIL-12 restored immunity against L. major (5). Thus, protection induced by the RA schistosome vaccine is completely dependent on the CD40/CD154 pathway but not upon the presence of IL-12-driven Th1-type immune responses. An IL-12-independent role for CD40/CD154 interactions is also evident in the development of crescentic glomerulonephritis caused by the presence of pathogenic Th1 cells in the kidneys (48). In this example, the treatment of CD40−/− mice with rIL-12 restores Th1 responses but still fails to induce pathology, coincident with a lack of activation of kidney macrophages. This differs from the current work in that an additional (CD40/CD154 independent) pathway of APC activation is evident in the vaccinated lung, as shown by the restoration of NO production and MHC-II expression in CD154−/− mice following rIL-12 treatment. Nevertheless, both our study and that of Ruth et al. (48) demonstrate that rIL-12 alone cannot compensate for all immune deficiencies that result from the disruption of the CD40/CD154 pathway. Our results also show that CD154−/− mice given rIL-12 are another example, in addition to IL-4Rα−/− and TNFR−/− mice (35, 53), of hosts that generate normal pulmonary inflammatory response to challenge infection in schistosome-vaccinated mice but exhibit little or no protection.
An important observation of our studies is that the treatment of vaccinated CD154−/− mice with antiCD40 MAb did not restore anti-parasite antibody production, in agreement with a study on allograft immunity (50), although other studies demonstrate that anti-CD40 MAb can restore antibody isotype class switching (4, 63). The administration of rIL-12 also did not restore antibody production in vaccinated CD154−/− mice (data not shown), and these mice were not protected against challenge infection. Combined, these results indicate that anti-parasite antibodies are essential for a protective effector mechanism. However, when we adoptively transferred serum from vaccinated WT mice into vaccinated CD154−/− mice, we failed to transfer even a limited extent of protective immunity, despite the presence of high levels of circulating antibody in the recipient mice (J. P. Hewitson, unpublished observations). This was surprising because we have previously been able to transfer protection to IL-4Rα−/− mice with serum from WT mice exposed to a single vaccination (35).
In conclusion, we show that a CD154-dependent effector mechanism(s) is responsible for challenge parasite elimination. The absence of antibodies in CD154−/− mice has allowed us to examine the nature of the cell-mediated effector mechanism that was previously thought to be at least partially responsible for conferring protection (1, 2, 14, 16, 20, 33, 53, 57, 59, 60). However, despite recreating the Th1-mediated immune responses in the lungs following the administration of IL-12, we were unable to recreate a protective response against challenge parasites, even to a limited extent. This challenges established dogma that Th1-mediated immune responses are either wholly or partially protective against challenge infection. We therefore have to conclude that the presence of anti-parasite antibodies generated following the ligation of CD154 is important to the generation of protective immunity, despite other studies (including our own) showing that antibodies are not fully responsible for effecting protection after a single vaccination (1, 20, 59). The effector mechanism may rely upon the interaction of coincident IgG humoral responses with an as-yet-unidentified CD154-mediated cellular reaction.
Acknowledgments
J.P.H. was supported by a Ph.D. studentship from the Biotechnology and Biological Sciences Research Council of the United Kingdom and a CASE studentship from GSK. This work was also supported by a Wellcome Trust University Fellowship to A.P.M. (no. 056213) and a Wellcome Trust project grant (no. 071762).
We thank the staff of the University of York BSF, Ann Bamford (University of York) for maintenance of the parasite life cycle, Paul Hissey (GSK, Stevenage, Hertfordshire, England) for providing the anti-CD40 MAb, and Gavin Jenkins and Marika Kullberg for providing helpful comments on the manuscript.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Anderson, S., P. S. Coulson, S. Ljubojevic, A. P. Mountford, and R. A. Wilson. 1999. The radiation-attenuated schistosome vaccine induces high levels of protective immunity in the absence of B cells. Immunology 96:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, S., V. L. Shires, R. A. Wilson, and A. P. Mountford. 1998. In the absence of IL-12, the induction of Th1-mediated protective immunity by the attenuated schistosome vaccine is impaired, revealing an alternative pathway with Th2-type characteristics. Eur. J. Immunol. 28:2827-2838. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, S., V. L. Shires, R. A. Wilson, and A. P. Mountford. 1999. Formation of multinucleated giant cells in the mouse lung is promoted in the absence of interleukin-12. Am. J. Respir. Cell Mol. Biol. 20:371-378. [DOI] [PubMed] [Google Scholar]
- 4.Barr, T. A., J. Carlring, and A. W. Heath. 2005. CD40 antibody as a potent immunological adjuvant: CD40 antibody provides the CD40 signal to B cells, but does not substitute for T cell help in responses to TD antigens. Vaccine 23:3477-3482. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, K. A., P. J. Ovendale, M. K. Kennedy, W. C. Fanslow, S. G. Reed, and C. R. Maliszewski. 1996. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4:283-289. [DOI] [PubMed] [Google Scholar]
- 6.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cayabyab, M., J. H. Phillips, and L. L. Lanier. 1994. CD40 preferentially costimulates activation of CD4+ T lymphocytes. J. Immunol. 152:1523-1531. [PubMed] [Google Scholar]
- 8.Coulson, P. S., L. E. Smythies, C. Betts, N. A. Mabbott, J. M. Sternberg, X. G. Wei, F. Y. Liew, and R. A. Wilson. 1998. Nitric oxide produced in the lungs of mice immunized with the radiation-attenuated schistosome vaccine is not the major agent causing challenge parasite elimination. Immunology 93:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado, V., and D. J. McLaren. 1990. Evidence for enhancement of IgG1 subclass expression in mice polyvaccinated with radiation-attenuated cercariae of Schistosoma mansoni and the role of this isotype in serum-transferred immunity. Parasite Immunol. 12:15-32. [DOI] [PubMed] [Google Scholar]
- 10.Diehl, L., A. T. den Boer, S. P. Schoenberger, E. I. van der Voort, T. N. Schumacher, C. J. Melief, R. Offringa, and R. E. Toes. 1999. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 5:774-779. [DOI] [PubMed] [Google Scholar]
- 11.Ferlin, W. G., T. von der Weid, F. Cottrez, D. A. Ferrick, R. L. Coffman, and M. C. Howard. 1998. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 MAb. Eur. J. Immunol. 28:525-531. [DOI] [PubMed] [Google Scholar]
- 12.French, R. R., H. T. Chan, A. L. Tutt, and M. J. Glennie. 1999. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 5:548-553. [DOI] [PubMed] [Google Scholar]
- 13.Gorbachev, A. V., and R. L. Fairchild. 2004. CD40 engagement enhances antigen-presenting langerhans cell priming of IFN-gamma-producing CD4+ and CD8+ T cells independently of IL-12. J. Immunol. 173:2443-2452. [DOI] [PubMed] [Google Scholar]
- 14.Hewitson, J. P., P. A. Hamblin, and A. P. Mountford. 2005. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol. 27:271-280. [DOI] [PubMed] [Google Scholar]
- 15.Hewitson, J. P., G. R. Jenkins, P. A. Hamblin, and A. P. Mountford. 2006. CD40/CD154 interactions are required for the optimal maturation of skin-derived APCs and the induction of helminth-specific IFN-gamma but not IL-4. J. Immunol. 177:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann, K. F., S. L. James, A. W. Cheever, and T. A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163:927-938. [PubMed] [Google Scholar]
- 17.Hogg, K. G., S. Kumkate, S. Anderson, and A. P. Mountford. 2003. Interleukin-12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1-mediated protective immunity to Schistosoma mansoni. Infect. Immun. 71:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogg, K. G., S. Kumkate, and A. P. Mountford. 2003. IL-10 regulates early IL-12-mediated immune responses induced by the radiation-attenuated schistosome vaccine. Int. Immunol. 15:1451-1459. [DOI] [PubMed] [Google Scholar]
- 19.James, S. L., A. W. Cheever, P. Caspar, and T. A. Wynn. 1998. Inducible nitric oxide synthase-deficient mice develop enhanced type 1 cytokine-associated cellular and humoral immune responses after vaccination with attenuated Schistosoma mansoni cercariae but display partially reduced resistance. Infect. Immun. 66:3510-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankovic, D., T. A. Wynn, M. C. Kullberg, S. Hieny, P. Caspar, S. James, A. W. Cheever, and A. Sher. 1999. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. J. Immunol. 162:345-351. [PubMed] [Google Scholar]
- 21.Kariuki, T. M., and I. O. Farah. 2005. Resistance to re-infection after exposure to normal and attenuated schistosome parasites in the baboon model. Parasite Immunol. 27:281-288. [DOI] [PubMed] [Google Scholar]
- 22.Khan, W. I., Y. Motomura, P. A. Blennerhassett, H. Kanbayashi, A. K. Varghese, R. T. El-Sharkawy, J. Gauldie, and S. M. Collins. 2005. Disruption of CD40-CD40 ligand pathway inhibits the development of intestinal muscle hypercontractility and protective immunity in nematode infection. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G15-G22. [DOI] [PubMed] [Google Scholar]
- 23.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumkate, S., G. R. Jenkins, R. A. Paveley, K. G. Hogg, and A. P. Mountford. 2007. CD207(+) Langerhans cells constitute a minor population of skin-derived antigen-presenting cells in the draining lymph node following exposure to Schistosoma mansoni. Int. J. Parasitol. 37:209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagranderie, M., M. A. Nahori, A. M. Balazuc, H. Kiefer-Biasizzo, J. R. Lapa e Silva, G. Milon, G. Marchal, and B. B. Vargaftig. 2003. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology 108:352-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarevic, V., A. J. Myers, C. A. Scanga, and J. L. Flynn. 2003. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity 19:823-835. [DOI] [PubMed] [Google Scholar]
- 27.Lu, P., J. F. Urban, X. D. Zhou, S. J. Chen, K. Madden, M. Moorman, H. Nguyen, S. C. Morris, F. D. Finkelman, and W. C. Gause. 1996. CD40-mediated stimulation contributes to lymphocyte proliferation, antibody production, eosinophilia, and mastocytosis during an in vivo type 2 response, but is not required for T cell IL-4 production. J. Immunol. 156:3327-3333. [PubMed] [Google Scholar]
- 28.MacDonald, A. S., E. A. Patton, A. C. La Flamme, M. I. Araujo, C. R. Huxtable, B. Bauman, and E. J. Pearce. 2002. Impaired Th2 development and increased mortality during Schistosoma mansoni infection in the absence of CD40/CD154 interaction. J. Immunol. 168:4643-4649. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald, A. S., A. D. Straw, N. M. Dalton, and E. J. Pearce. 2002. Cutting edge: Th2 response induction by dendritic cells: a role for CD40. J. Immunol. 168:537-540. [DOI] [PubMed] [Google Scholar]
- 30.Martin, D. L., C. L. King, E. Pearlman, E. Strine, and F. P. Heinzel. 2000. IFN-gamma is necessary but not sufficient for anti-CD40 antibody-mediated inhibition of the Th2 response to Schistosoma mansoni eggs. J. Immunol. 164:779-785. [DOI] [PubMed] [Google Scholar]
- 31.Mauri, C., L. T. Mars, and M. Londei. 2000. Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process. Nat. Med. 6:673-679. [DOI] [PubMed] [Google Scholar]
- 32.Moodycliffe, A. M., V. Shreedhar, S. E. Ullrich, J. Walterscheid, C. Bucana, M. L. Kripke, and L. Flores-Romo. 2000. CD40-CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J. Exp. Med. 191:2011-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mountford, A. P., S. Anderson, and R. A. Wilson. 1996. Induction of Th1 cell-mediated protective immunity to Schistosoma mansoni by co-administration of larval antigens and IL-12 as an adjuvant. J. Immunol. 156:4739-4745. [PubMed] [Google Scholar]
- 34.Mountford, A. P., P. S. Coulson, R. M. Pemberton, L. E. Smythies, and R. A. Wilson. 1992. The generation of interferon-gamma-producing T lymphocytes in skin-draining lymph nodes, and their recruitment to the lungs, is associated with protective immunity to Schistosoma mansoni. Immunology 75:250-256. [PMC free article] [PubMed] [Google Scholar]
- 35.Mountford, A. P., K. G. Hogg, P. S. Coulson, and F. Brombacher. 2001. Signaling via interleukin-4 receptor alpha chain is required for successful vaccination against schistosomiasis in BALB/c mice. Infect. Immun. 69:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mountford, A. P., and F. Trottein. 2004. Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol. 20:221-226. [DOI] [PubMed] [Google Scholar]
- 37.Netea, M. G., J. W. Meer, I. Verschueren, and B. J. Kullberg. 2002. CD40/CD40 ligand interactions in the host defense against disseminated Candida albicans infection: the role of macrophage-derived nitric oxide. Eur. J. Immunol. 32:1455-1463. [DOI] [PubMed] [Google Scholar]
- 38.Padigel, U. M., and J. P. Farrell. 2003. CD40-CD40 ligand costimulation is not required for initiation and maintenance of a Th1-type response to Leishmania major infection. Infect. Immun. 71:1389-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padigel, U. M., P. J. Perrin, and J. P. Farrell. 2001. The development of a Th1-type response and resistance to Leishmania major infection in the absence of CD40-CD40L costimulation. J. Immunol. 167:5874-5879. [DOI] [PubMed] [Google Scholar]
- 40.Peng, X., A. Kasran, P. A. Warmerdam, M. de Boer, and J. L. Ceuppens. 1996. Accessory signaling by CD40 for T cell activation: induction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 26:1621-1627. [DOI] [PubMed] [Google Scholar]
- 41.Quezada, S. A., L. Z. Jarvinen, E. F. Lind, and R. J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307-328. [DOI] [PubMed] [Google Scholar]
- 42.Ramaswamy, K., P. Kumar, and Y. X. He. 2000. A role for parasite-induced PGE2 in IL-10-mediated host immunoregulation by skin stage schistosomula of Schistosoma mansoni. J. Immunol. 165:4567-4574. [DOI] [PubMed] [Google Scholar]
- 43.Reichmann, G., W. Walker, E. N. Villegas, L. Craig, G. Cai, J. Alexander, and C. A. Hunter. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 68:1312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Sosa, M., A. R. Satoskar, J. R. David, and L. I. Terrazas. 2003. Altered T helper responses in CD40 and interleukin-12-deficient mice reveal a critical role for Th1 responses in eliminating the helminth parasite Taenia crassiceps. Int. J. Parasitol. 33:703-711. [DOI] [PubMed] [Google Scholar]
- 45.Rogers, N. J., I. M. Jackson, W. J. Jordan, G. Lombardi, A. Delikouras, and R. I. Lechler. 2003. CD40 can costimulate human memory T cells and favors IL-10 secretion. Eur. J. Immunol. 33:1094-1104. [DOI] [PubMed] [Google Scholar]
- 46.Rolink, A., F. Melchers, and J. Andersson. 1996. The SCID but not the RAG-2 gene product is required for Sμ-Sɛ heavy chain class switching. Immunity 5:319-330. [DOI] [PubMed] [Google Scholar]
- 47.Rolph, M. S., and S. H. Kaufmann. 2001. CD40 signaling converts a minimally immunogenic antigen into a potent vaccine against the intracellular pathogen Listeria monocytogenes. J. Immunol. 166:5115-5121. [DOI] [PubMed] [Google Scholar]
- 48.Ruth, A. J., A. R. Kitching, M. Li, T. J. Semple, J. R. Timoshanko, P. G. Tipping, and S. R. Holdsworth. 2004. An IL-12-independent role for CD40-CD154 in mediating effector responses: studies in cell-mediated glomerulonephritis and dermal delayed-type hypersensitivity. J. Immunol. 173:136-144. [DOI] [PubMed] [Google Scholar]
- 49.Schonbeck, U., and P. Libby. 2001. The CD40/CD154 receptor/ligand dyad. Cell. Mol. Life Sci. 58:4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shepherd, D. M., and N. I. Kerkvliet. 1999. Disruption of CD154:CD40 blocks generation of allograft immunity without affecting APC activation. J. Immunol. 163:2470-2477. [PubMed] [Google Scholar]
- 51.Steinmann, P., J. Keiser, R. Bos, M. Tanner, and J. Utzinger. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411-425. [DOI] [PubMed] [Google Scholar]
- 52.Stout, R. D., J. Suttles, J. Xu, I. S. Grewal, and R. A. Flavell. 1996. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J. Immunol. 156:8-11. [PubMed] [Google Scholar]
- 53.Street, M., P. S. Coulson, C. Sadler, L. J. Warnock, D. McLaughlin, H. Bluethmann, and R. A. Wilson. 1999. TNF is essential for the cell-mediated protective immunity induced by the radiation-attenuated schistosome vaccine. J. Immunol. 163:4489-4494. [PubMed] [Google Scholar]
- 54.Stuber, E., W. Strober, and M. Neurath. 1996. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J. Exp. Med. 183:693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian, L., R. J. Noelle, and D. A. Lawrence. 1995. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur. J. Immunol. 25:306-309. [DOI] [PubMed] [Google Scholar]
- 56.Vignali, D. A., P. Crocker, Q. D. Bickle, S. Cobbold, H. Waldmann, and M. G. Taylor. 1989. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology 67:466-472. [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson, R. A., P. S. Coulson, C. Betts, M. A. Dowling, and L. E. Smythies. 1996. Impaired immunity and altered pulmonary responses in mice with a disrupted interferon-gamma receptor gene exposed to the irradiated Schistosoma mansoni vaccine. Immunology 87:275-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson, R. A., P. S. Coulson, and A. P. Mountford. 1999. Immune responses to the radiation-attenuated schistosome vaccine: what can we learn from knock-out mice? Immunol. Lett. 65:117-123. [DOI] [PubMed] [Google Scholar]
- 59.Wynn, T. A., and K. F. Hoffmann. 2000. Defining a schistosomiasis vaccination strategy—is it really Th1 versus Th2? Parasitol. Today 16:497-501. [DOI] [PubMed] [Google Scholar]
- 60.Wynn, T. A., D. Jankovic, S. Hieny, A. W. Cheever, and A. Sher. 1995. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J. Immunol. 154:4701-4709. [PubMed] [Google Scholar]
- 61.Wynn, T. A., R. W. Thompson, A. W. Cheever, and M. M. Mentink-Kane. 2004. Immunopathogenesis of schistosomiasis. Immunol. Rev. 201:156-167. [DOI] [PubMed] [Google Scholar]
- 62.Xu, J., T. M. Foy, J. D. Laman, E. A. Elliott, J. J. Dunn, T. J. Waldschmidt, J. Elsemore, R. J. Noelle, and R. A. Flavell. 1994. Mice deficient for the CD40 ligand. Immunity 1:423-431. [DOI] [PubMed] [Google Scholar]
- 63.Yang, Y., and J. M. Wilson. 1996. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 273:1862-1864. [DOI] [PubMed] [Google Scholar]
- 64.Zhou, P., and R. A. Seder. 1998. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with histoplasma capsulatum. J. Exp. Med. 187:1315-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]