Abstract
Infection with Leishmania major triggers several pathways in the host cell that are crucial to initial infection as well as those that are used by Leishmania to enhance its replication and virulence. To identify the molecular events of the host cell in response to Leishmania, the global gene expression of the human monocytic cell line THP-1 either infected with Leishmania major in the presence and absence of gamma interferon (IFN-γ) or in the presence of IFN-γ alone was analyzed using high-density human oligonucleotide microarrays, followed by statistical analysis. The persistence of the parasite despite an extensive response to IFN-γ, added 24 h after infection with L. major, suggests that L. major can survive in an IFN-γ-enriched environment in vitro. Results demonstrate that L. major counteracts the IFN-γ response in macrophages on a large scale. Expression of genes involved in the innate immune response, cell adhesion, proteasomal degradation, Toll-like receptor expression, a variety of signaling molecules, and matrix metalloproteinases was significantly modulated.
Leishmaniasis is caused by the protozoan parasite Leishmania. Depending on the species of the pathogen and the host's immune response, the disease presents a spectrum from self-healing cutaneous lesions to severe visceral disease and death. Leishmania is a digenetic organism, alternating between a flagellated promastigote in the gut of the phlebotomine sand fly and an intracellular amastigote residing within macrophages of the mammalian host, which ranges from desert rodents to humans. Infection with Leishmania initiates complex cascades of events in macrophages that influence the ensuing immune response. One of the most important initial signaling events is the release of interleukin-12 (IL-12) by the infected macrophage, leading to subsequent priming of the Th1 response and production of gamma interferon (IFN-γ) (24, 45). IFN-γ has a pivotal role in the activation of macrophages to kill pathogens and protect the host cell from infection (6). However, Leishmania has developed sophisticated immune evasion strategies that allow for “silent entry,” thus avoiding the initial activation of the infected macrophage (52). These strategies include entry via receptor-mediated phagocytosis involving a number of different receptors on the host macrophage, including CR1, CR3, C3b, and the fucose/mannose receptor (59). Thus, Leishmania promastigotes are taken up without triggering the oxidative burst (9). Further, Leishmania actively inhibits host defense mechanisms such as protein kinase C (PKC) activation (60) as well as the upregulation of inducible nitric oxide synthase following IFN-γ stimulation, a consequence of which is delayed antimicrobial activity in infected macrophages (44, 45). As a result, response to IFN-γ of infected macrophages is dampened. It has been reported that Leishmania donovani-infected U937 cells exhibit reduced phosphorylation of the IFN-γ receptor (46), and L. donovani amastigotes influence negatively the expression of molecules of the major histocompatibility complex class II (MHC-II) complex following stimulation with IFN-γ (47) and the B7-1 costimulatory molecule (28, 53). Most importantly, Leishmania parasites inhibit the initial IL-12 production by their host macrophage, thereby impairing the signaling cascade which, in a healthy immune-competent host, would induce production of IFN-γ by target T cells and NK cells (12).
Analysis of gene expression at the RNA level using microarray techniques has provided a global genetic perspective on biological processes important in parasite survival and host-parasite interactions (8, 13, 41) as it enables the investigation of entire biological pathways. Microarray technology can provide a molecular profile of virulence-associated responses as well as host defense mechanisms that occur following infection (5, 14, 16, 18, 50, 62). Since innate defenses have the potential to block infection, the mechanisms used for evasion are potential targets for vaccine and/or pharmacologic intervention (52).
DNA microarray studies have been conducted to identify differentially expressed genes associated with developmental stages of Leishmania (26, 30) and host cell responses to a variety of parasites (8, 13, 34, 51). The current study focuses on the analysis of global gene expression in the human macrophage cell line THP-1 following infection with Leishmania major in the presence or absence of IFN-γ. Differentially expressed genes are likely involved with parasite-induced modulation of the host response or host defense, and potential novel macrophage genes involved in the downregulation of the response to IFN-γ may contribute to the completion of our understanding of key regulatory pathways that can be targeted to restore this response, resulting in the control of intracellular pathogens. Infection of macrophages by L. major, followed by treatment with IFN-γ, represents an in vitro correlate of the ideal immune response in a healthy, Leishmania-naïve individual during macrophage activation and subsequent Th1-dominant immune response. To stratify the contributions of L. major infection and the effect of the IFN-γ-enriched environment, both effectors were also added to the macrophages separately. Global mRNA gene expression levels were determined 24 h after IFN-γ treatment, thus focusing on changes in gene expression that occur during Leishmania-macrophage interplay alone, isolated from further stimulating or attenuating factors that can contribute to the nonhealing state of leishmaniasis in an immune-privileged site (45). This study provides further information on both the effect of Leishmania infection on the modulation of macrophage gene expression (8, 13, 34, 51) in a defined IFN-γ-enriched environment that parallels the natural state of an infected individual and a global approach to understanding the complex biological response to intracellular infection.
MATERIALS AND METHODS
Cell culture.
The human mononuclear cell line THP-1 (202 TIB; American Type Culture Collection, Rockville, MD) was used as a model for human macrophage cells in infection studies due to its similarity to human monocyte-derived macrophages. THP-1 cells were maintained in RPMI 1640 medium (StemCell Technologies, Vancouver, BC, Canada), supplemented with 10% heat-inactivated fetal calf serum (HyClone, Logan, UT), 2 mM l-glutamine (Sigma, St. Louis, MO), 100 U/ml penicillin, 100 mg/ml streptomycin (Invitrogen, Burlington, ON, Canada), and 1× 10−5 M β-mercaptoethanol (Bio-Rad, Hercules, CA) at 37°C in a humidified atmosphere containing 5% CO2. THP-1 cells were cultured in flat-bottom tissue culture flasks (75 cm2) until the cell density reached 1 × 106 cells/ml in a volume of 50 ml. Cell viability was assessed by a trypan blue exclusion test (0.4% solution), followed by counting in a hemocytometer. Cells were allowed to adhere and differentiate for 18 h in the presence of 20 ng/ml phorbol-12-myristate-13-acetate (Sigma). Cells were then washed with Hanks balanced salt solution (HBSS) to remove nonadherent cells; phorbol-12-myristate-13-acetate and fresh medium were added, and adherent macrophages were incubated for a further 6 h prior to treatment.
L. major Friedlin strain (MHOM/IL/80/Friedlin) promastigotes were cultured at 26°C in M199 medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 mg/ml streptomycin and routinely subcultured every second to third day. Promastigotes were grown to stationary phase by seeding promastigotes at 1 × 106 cells/ml in M199 medium and allowing them to grow at 26°C for 4 to 5 days, when the cell density reached a plateau of 2 × 107 to 3 × 107 cells/ml. Cultures were then maintained for two additional days, at which time metacyclic cells were separated by adding 50 μl/ml peanut agglutinin (Sigma) followed by incubation for 30 min on rotary shaker. Nonmetacyclic cells binding to peanut agglutinin were sedimented by gravity at room temperature for 10 min, and metacyclics were removed from the top layer, washed by centrifugation at 2,700 × g for 6 min, and resuspended in M199 medium. Heat-killed L. major promastigotes were prepared by heating metacyclic promastigotes at 11 × 107 cells/ml in M199 medium at 62°C for 45 min.
Macrophage infection and treatment.
L. major metacyclic promastigotes or heat-killed promastigotes were added to THP-1 cells at an infection ratio of 20:1, resulting in infection rate of over 92% as determined by microscopic examination of cells stained with Hemacolor (Harleco; EM Sciences, Kansas City, MO). Latex beads (Sigma) were used at a ratio of 40:1, resulting in a rate of one to six beads per cell in greater than 90% of cells. After 24 h of coincubation of THP-1 cells with either live or heat-killed promastigotes, latex beads, or medium, cells were washed three times with HBSS to remove free parasites, and the medium was replenished. At this time point, THP-1 macrophage cultures were treated with either IFN-γ (100 U/ml) (Sigma) or medium, and cells were cultured for an additional 24 h. All cultures were then harvested at 48 h.
RNA isolation.
Treated or nontreated THP-1 cells were washed three times with HBSS and resuspended and scraped in Trizol reagent (2 ml per plate), and RNA was isolated as described by the manufacturer (Invitrogen). Total RNA was purified further using RNeasy as described by the manufacturer (QIAGEN Inc., Burlington, ON, Canada). Total macrophage RNA was analyzed by gel electrophoresis and stained with ethidium bromide. There were no detectable Leishmania rRNA bands that would have been clearly discernible from the Leishmania 28S and 18S bands, and the macrophage 28S and 18S rRNA bands were stained with equal intensities. By these criteria, there was no significant contamination of macrophage RNA by Leishmania RNA.
Microarray hybridization.
Total RNA (1 μg) from treated or nontreated THP-1 cells was first amplified using the Message Amp antisense RNA (aRNA) procedure as described by the manufacturer (Ambion, Inc. Austin, TX). A 1-μg sample of the resulting aRNA was then fluorescently labeled with either Cy3 or Cy5 using the CyScribe Direct mRNA labeling procedure as described by the manufacturer (Amersham Biosciences, Pittsburgh, PA). Control aRNA of nontreated THP-1 cells was labeled with Cy5, and aRNA of treated THP-1 cells was labeled with Cy3. Following labeling, unconjugated fluorescent reagents were removed by using an Amersham purification kit, and labeled probe was resuspended in 5 μl of RNase free water.
DNA oligonucleotide microarrays representing 22,225 unique human genes (Operon, version 2.0; Operon Biotechnologies, Inc. Huntsville, AL) were arrayed on glass slides as described previously (40). Slides were prehybridized in 0.2% bovine serum albumin, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and 0.1% sodium dodecyl sulfate (SDS) for 45 min at 42°C, washed twice in deionized H2O and once in 2-propanol, and spun dry in a centrifuge. A two-color hybridization strategy was used. Cy3 and Cy5 probes were combined, and 1 μl (1 μg/μl) of Cy3-Cy5-labeled green fluorescent protein cDNA was added as a positive control. Probes were denatured at 95°C for 3 min, followed by cooling on ice for 3 min. Denatured probes were added to hybridization buffer (Amersham), applied to a prehybridized microarray, and covered with a glass coverslip. Hybridizations were carried out in a humidified hybridization chamber (TeleChem International, Inc. Sunnyvale, CA) at 42°C for 12 to 16 h in the dark. Hybridized slides were washed at 55°C for 10 min in 1× SSC-0.2% SDS, two times in 0.1× SSC-0.1% SDS for 10 min, and for 2 min in deionized H2O. After a spin-drying step using a centrifuge, the hybridized slides were scanned using a GenePix Pro 4000 scanner (Axon Instruments, San Francisco, CA), and data from each fluorescence channel were collected and stored as separate 16-bit TIFF files.
Microarray data analyses.
Microarray images were processed using Imagene software, version 5.5 (BioDiscovery Inc., El Segundo, CA). Spots were visually examined, and those that exhibited poor quality, such as small or irregular shape, were flagged. Spot segmentation was performed using a histogram segmentation method that uses the distribution of pixel intensities to separate probable signal from background. Background per pixel is estimated as a median of the pixels in this area and is multiplied by the spot area to give an estimated spot background value. Data normalization and quantitation were then performed using GeneSpring, version 7.2, software (Agilent Technologies, Mississauga, ON, Canada). Values imported from Imagene were normalized using local weight regression to remove intensity-dependent effects. To identify differentially expressed genes, analysis of variance (ANOVA) and multiple testing procedures to control the false discovery rate (3) were applied to the microarray expression data. Two groups were analyzed using a cutoff of 0.05 on adjusted P values: THP-1 cells treated with live L. major, heat-killed L. major, or latex beads; and THP-1 cells treated with live L. major, L. major followed by IFN-γ, or IFN-γ alone. Ratios among treated versus nontreated THP-1 cells were then calculated, and the results were expressed as relative change. Genes that exhibited a difference of ≥4 in expression levels in either of the three treatment groups were selected for presentation in Tables 1 and 2. Table 2 represents genes that were filtered for those genes modulated by phagocytosis alone. See the supplemental material for complete data sets.
TABLE 1.
Comparison of macrophage gene expression in response to latex beads, heat-killed L. major, or viable L. major
| Biological significance of gene | Gene or protein description | Gene symbol | GenBank accession no. | P value | Change (n-fold) in expressiona
|
||
|---|---|---|---|---|---|---|---|
| Latex beads | Heat-killed L. major | Viable L. major | |||||
| Viable L. major-induced macrophage genes | |||||||
| Cell adhesion | Protocadherin beta 13 | PCDHB13 | AF217745 | 0.01 | −2.00 | −1.73 | 7.85 |
| Latrophilin 1 | LPHN1 | NM_024679 | 0.01 | 1.26 | 1.04 | 6.12 | |
| SNED1 | FLJ00133 | AL050143 | 0.02 | −1.77 | 1.68 | 16.74 | |
| Cell growth and/or | Potassium voltage-gated channel | KCNC2 | AF268897 | 0.05 | −3.40 | −2.55 | 6.39 |
| maintenance | Capping protein (actin filament) | CAPZA3 | NM_033328 | 0.01 | 1.77 | 1.51 | 7.40 |
| Glypican 5 | GPC5 | NM_004466 | 0.02 | −1.61 | 1.06 | 6.38 | |
| ATP-binding | KATNAL-2 Katanin p60 subunit A like 2 | MGC33211 | AL512748 | 0.00 | 1.43 | 2.31 | 12.05 |
| Replication factor C | RFC2 | NM_002914 | 0.01 | −2.57 | −1.69 | 12.85 | |
| Innate immune response | Proteoglycan 3 | PRG3 | NM_006093 | 0.01 | 1.16 | 1.28 | 13.71 |
| Metabolism of complex lipids | Dehydrogenase/reductase (SDR family) member 9 | DHRS9 | NM_005771 | 0.01 | −1.40 | 1.56 | 7.03 |
| ELOVL family member 6 | ELOVL6 | NM_024090 | 0.02 | 1.05 | −1.83 | 11.07 | |
| Metabolism (nitrogen) | Ureidopropionase, beta | UPB1 | AK024277 | 0.03 | 1.51 | −1.20 | 9.02 |
| Carbonic anhydrase VI | CA6 | NM_001215 | 0.00 | −1.74 | −1.29 | 9.19 | |
| Metalloproteinase | RCE1 homolog, prenyl protein protease | RCE1 | NM_005133 | 0.02 | 2.80 | 2.90 | 7.27 |
| Protein metabolism | Peptidyl arginine deiminase, type I | PADI1 | AK026652 | 0.00 | 1.11 | 1.70 | 7.12 |
| Ubiquitin-conjugating enzyme E2-like | UEV3 | NM_018314 | 0.04 | −1.33 | 1.41 | 7.43 | |
| Hypothetical protein FLJ12595 | FLJ12595 | NM_024994 | 0.05 | 1.02 | −1.26 | 9.69 | |
| Alpha kinase 1 | ALPK | NM_025144 | 0.02 | −1.51 | 2.53 | 13.04 | |
| Endoplasmic reticulum-to-nucleus signaling 1 | ERN1 | NM_001433 | 0.01 | −2.40 | −1.44 | 5.58 | |
| Signaling | Phosphoinositide-3-kinase | PIK3R3 | NM_003629 | 0.03 | −1.01 | 1.39 | 10.61 |
| Testis-specific protein TES101RP | MGC4766 | NM_031451 | 0.01 | −1.31 | −5.86 | 7.48 | |
| Artemin | ARTN | NM_057091 | 0.05 | −7.43 | −2.00 | 5.57 | |
| Transcription | Zinc finger protein 37a (KOX 21) | ZNF37A | BC015858 | 0.04 | −1.24 | 1.41 | 7.92 |
| Zinc finger protein 237 | ZNF237 | NM_014242 | 0.04 | 1.39 | −1.78 | 14.94 | |
| Transport | Potassium voltage-gated channel | KCNB1 | L02840 | 0.03 | −1.01 | −1.50 | 6.19 |
| Potassium voltage-gated channel | KCNAB3 | NM_004732 | 0.02 | −1.70 | −2.40 | 16.30 | |
| Chromosome 6 open reading frame 192 | C6orf192 | NM_052831 | 0.01 | 1.05 | 1.06 | 6.87 | |
| Viable L. major-repressed macrophage genes | |||||||
| Apoptosis | IL-6 signal transducer | IL6ST | NM_002184 | 0.04 | 1.53 | 1.07 | −5.26 |
| Growth arrest-specific | GAS1 | NM_002048 | 0.00 | 1.78 | −1.65 | −7.00 | |
| Cell adhesion | C-type lectin | CLECSF7 | AF293615 | 0.03 | 1.17 | −1.51 | −10.97 |
| Testis-specific kinase substrate | TSKS | NM_021733 | 0.03 | 1.68 | 2.81 | −45.00 | |
| Cell growth and/or | KIAA0882 protein | KIAA0882 | AB020689 | 0.02 | 2.66 | 1.54 | −4.16 |
| maintenance | Spectrin repeat-containing nuclear envelope | SYNE1 | AB033088 | 0.01 | −1.50 | 2.48 | −9.22 |
| Discs, large homolog 3 | DLG3 | NM_021120 | 0.02 | −1.16 | 4.63 | −37.22 | |
| Metabolism of complex lipids | Serine palmitoyltransferase | SPTLC1 | NM_006415 | 0.01 | 1.66 | 1.80 | −5.00 |
| Proteosome | Proteasome (prosome and macropain) subunit, alpha type, 3 | PSMA3 | AK021499 | 0.03 | 1.22 | 1.15 | −4.55 |
| Proteasome (prosome and macropain) subunit, beta type, 2 | PSMB2 | BC000268 | 0.00 | −1.44 | 1.15 | −11.11 | |
| Response to abiotic stimulus | Interphotoreceptor matrix proteoglycan 2 | IMPG2 | NM_016247 | 0.05 | 2.21 | 1.47 | −10.15 |
| Signal transduction | Protein tyrosine phosphatase, receptor type, A | PTPRA | NM_002836 | 0.05 | 1.18 | 2.51 | −4.55 |
| Transport | KIAA1613 protein | KIAA1613 | AB046833 | 0.02 | 3.40 | 2.13 | −6.67 |
| Solute carrier family 6 | SLC6A7 | NM_014228 | 0.03 | 1.05 | 1.88 | −7.67 | |
The value of the untreated control was set at 1.0.
TABLE 2.
Impact of L. major on macrophage gene expression modulated by IFN-γ
| Biological significance of gene | Gene or protein description | Gene symbol | GenBank accession no. | P value | Change (n-fold) in expressiona
|
||
|---|---|---|---|---|---|---|---|
| IFN-γ | L. major +IFN-γ | L. major | |||||
| IFN-γ-activated macrophage genes | |||||||
| Chemokines and cell adhesion | Protein phosphatase 1, regulatory subunit 12A | PPP1R12A | NM_002480 | 0.01 | 35.29 | 1.68 | −2.87 |
| Nuclear receptor subfamily 2, group C, member 2 | NR2C2 | NM_003298 | 0.01 | 12.81 | 2.92 | 1.36 | |
| Platelet endothelial cell adhesion molecule (CD31 antigen) | PECAM-1 | NM_000442 | 0.00 | 5.45 | −1.68 | −2.18 | |
| Myosin, light polypeptide 5, regulatory | MYL5 | NM_002477 | 0.00 | 8.03 | −1.02 | 1.10 | |
| C-type lectin, superfamily member 7 | CLECSF7 | AF293615 | 0.01 | 2.29 | 5.02 | −10.97 | |
| Complement and coagulation factors | Coagulation factor VII | F7 | NM_000131 | 0.02 | 5.11 | 3.01 | −1.28 |
| Immunity | Colony-stimulating factor 1 | CSF1 | M37435 | 0.01 | 6.72 | 2.61 | 1.25 |
| CD4 | CD4 | S79267 | 0.03 | 16.41 | 3.65 | 1.84 | |
| Metalloproteinase | Matrix metalloproteinase 15 (membrane inserted) | MMP15 | NM_002428 | 0.03 | 8.26 | 1.42 | −1.52 |
| Matrix metalloproteinase 7 | MMP7 | NM_002423 | 0.01 | 6.07 | 1.69 | 1.17 | |
| Metabolism | Carbamoyl-phosphate synthetase 1, mitochondrial | CPS1 | NM_001875 | 0.02 | 1.14 | −1.32 | 4.24 |
| Metabolism of lipids | Phosphatidylinositol glycan, class B | PIGB | NM_004855 | 0.03 | 9.70 | −1.17 | −3.45 |
| Degenerative spermatocyte homolog, lipid desaturase | DEGS | NM_003676 | 0.03 | 5.83 | −1.71 | −4.76 | |
| Myo-inositol 1-phosphate synthase A1 | ISYNA1 | NM_016368 | 0.01 | 11.68 | 1.27 | 2.25 | |
| Dihydroorotate dehydrogenase | DHODH | NM_001361 | 0.05 | 4.20 | −1.09 | 1.82 | |
| Leukotriene A4 hydrolase | LTA4H | NM_000895 | 0.04 | 8.73 | 1.89 | −1.19 | |
| Metabolism of nitrogen | Carbonic anhydrase VI | CA6 | NM_001215 | 0.04 | 1.27 | −1.88 | 9.19 |
| Pathogenesis of Parkinson disease | Synuclein, alpha (non-A4 component of amyloid precursor) | SNCA | NM_000345 | 0.02 | 4.70 | 1.59 | −1.66 |
| Protein synthesis | methionine-tRNA synthetase | MARS | NM_004990 | 0.04 | 13.30 | 2.34 | 1.46 |
| Proteasome | Proteasome (prosome and macropain) subunit, beta type, 5 | PSMB5 | NM_002797 | 0.03 | 8.47 | 2.51 | −1.05 |
| Anaphase-promoting complex subunit 5 | ANAPC5 | NM_016237 | 0.03 | 4.29 | −1.53 | 1.28 | |
| OTU domain, ubiquitin aldehyde binding 2 | OTUB2 | NM_023112 | 0.01 | 6.33 | 1.35 | −1.13 | |
| Respiration | NADH dehydrogenase | NDUFS4 | NM_002495 | 0.04 | 7.85 | 1.54 | −1.28 |
| Malate dehydrogenase 2, NAD | MDH2 | NM_005918 | 0.04 | 8.22 | 7.30 | 1.08 | |
| Signaling (calcium) | Calcium channel, voltage dependent | CACNG2 | NM_006078 | 0.02 | 13.81 | 2.00 | −1.16 |
| Signal transduction | Signal transducer and activator of transcription 5A | STAT5A | NM_003152 | 0.01 | 7.96 | 1.02 | 1.29 |
| G protein, beta polypeptide 1 | GNB1 | NM_002074 | 0.03 | 7.68 | −1.53 | 1.54 | |
| Phospholipase C, gamma 1 (formerly subtype 148) | PLCG1 | NM_002660 | 0.03 | 5.87 | −1.32 | −2.78 | |
| Protein tyrosine phosphatase, receptor type, H | PTPRH | NM_002842 | 0.05 | 5.02 | −1.23 | −2.33 | |
| Protein tyrosine phosphatase, receptor type, A | PTPRA | NM_002836 | 0.01 | 1.56 | −1.72 | −4.63 | |
| Guanylate cyclase 1 | GUCY1B3 | NM_000857 | 0.03 | 4.69 | −3.96 | −21.30 | |
| Signaling (G-protein) | G protein, gamma transducing activity polypeptide 2 | GNGT2 | NM_031498 | 0.02 | 15.44 | 1.70 | 1.23 |
| G protein, beta polypeptide 4 | GNB4 | AK001890 | 0.01 | 4.15 | 2.55 | −1.01 | |
| Signaling (PKC) | Protein kinase C, gamma | PRKCG | NM_002739 | 0.01 | 4.01 | 1.42 | −2.86 |
| Signaling (TGF-β) | SMAD, mothers against DPP homolog 5 | SMAD5 | AK055211 | 0.05 | 12.04 | 1.91 | 1.91 |
| Sp1 transcription factor | SP1 | BC012008 | 0.04 | 4.25 | 1.36 | 1.44 | |
| SKI-like | SKIL | NM_005414 | 0.02 | 13.38 | 1.80 | 1.28 | |
| Signaling (Toll-like receptor) | Toll-interacting protein | TOLLIP | AL136835 | 2.80 | −1.75 | −6.94 | |
| Transcription | Hypothetical protein MGC2705 | MGC2705 | NM_032701 | 0.03 | 1.64 | −4.54 | 4.31 |
| General transcription factor IIA | GTF2A1 | NM_015859 | 0.04 | 26.88 | 4.34 | 2.13 | |
| Pleomorphic adenoma gene-like 2 | PLAGL2 | NM_002657 | 0.02 | 5.63 | 2.84 | 1.71 | |
| Polymerase (RNA) II (DNA directed) polypeptide F | POLR2F | NM_021974 | 0.02 | 14.51 | 2.01 | −1.27 | |
| Transcription factor 7 (T-cell specific, HMG-box) | TCF7 | NM_003202 | 0.04 | 5.71 | 2.69 | −5.00 | |
| Polymerase (DNA directed), epsilon 3 (p17 subunit) | POLE3 | BC004170 | 0.02 | 4.75 | 1.75 | 1.10 | |
| Hairy and enhancer of split 1 | HES1 | NM_005524 | 0.05 | 3.20 | −1.71 | −20.00 | |
| Translation | Eukaryotic translation initiation factor 4B | EIF4B | NM_001417 | 0.03 | 20.89 | 1.69 | 1.17 |
| Eukaryotic translation initiation factor 2 | EIF2S2 | NM_003908 | 0.02 | 6.56 | −1.43 | −1.58 | |
| Histidyl-tRNA synthetase | HARS | AK055917 | 0.02 | 5.16 | −1.77 | 5.85 | |
| Ribosomal protein L26 | RPL26 | NM_000987 | 0.04 | 5.81 | 2.95 | 1.34 | |
| Ribosomal protein S9 | RPS9 | NM_001013 | 0.05 | 3.88 | 4.10 | 1.44 | |
| Ribosomal protein S24 | RPS24 | NM_033022 | 0.05 | 4.42 | 1.42 | −1.65 | |
| G1 to S phase transition 2 | GSPT2 | NM_018094 | 0.04 | 7.29 | 1.34 | −1.40 | |
| Transport | Nucleoporin 62kDa | NUP62 | NM_016553 | 0.05 | 12.87 | 3.38 | 1.36 |
| Ubiquinol-cytochrome c reductase core protein II | UQCRC2 | NM_003366 | 0.01 | 12.80 | 2.30 | 1.12 | |
| ATP-binding cassette, sub-family B, member 11 | ABCB11 | NM_003742 | 0.03 | 1.20 | 12.77 | 1.68 | |
| IFN-γ-repressed macrophage genes | |||||||
| DNA metabolism | Ureidopropionase, beta | UPB1 | AK024277 | 0.03 | −2.08 | 1.50 | 9.02 |
| Replication factor C | RFC2 | NM_002914 | 0.01 | −3.68 | −2.81 | 20.50 | |
| Metalloproteinase | Matrix metalloproteinase 16 | MMP16 | NM_005941 | 0.02 | −10.13 | 1.61 | 5.31 |
| Protein metabolism | KIAA0251 protein | KIAA0251 | D87438 | 0.03 | −2.39 | 6.01 | −1.38 |
| Proteosome | Rurin (paired basic amino acid cleaving enzyme) | FURIN | NM_002569 | 0.03 | −1.49 | 4.87 | 1.19 |
| Signaling (calcium) | Calcium channel, voltage dependent | CACNA1B | NM_000718 | 0.04 | −1.51 | 1.17 | 4.44 |
| Signal transduction | Par-6 partitioning defective 6 homolog alpha | PARD6A | NM_016948 | 0.04 | −5.00 | 1.49 | 5.88 |
| Phosphoinositide-3-kinase, regulatory subunit, polypeptide 3 (p55, gamma) | PIK3R3 | NM_003629 | 0.03 | −2.28 | 1.63 | 10.61 | |
| Tensin | TNS | NM_022648 | 0.03 | −4.13 | −8.47 | 2.58 | |
| Eendothelial cell growth factor 1 (platelet derived) | ECGF1 | NM_001953 | 0.04 | −3.68 | 5.99 | 1.21 | |
| Signaling G protein | Sorting nexin family member 27 | SNX27 | AB007957 | 0.03 | −9.78 | 5.56 | −4.71 |
| Melatonin receptor 1B | MTNR1B | NM_005959 | 0.03 | −1.36 | −2.22 | 5.41 | |
| Transport | Tyrosinase | TYR | NM_000372 | 0.02 | −8.33 | 1.01 | 6.84 |
| Phosphogluconate dehydrogenase | PGD | NM_002631 | 0.01 | −2.06 | 4.41 | 1.42 | |
The value of the untreated control was set at 1.0.
Quantitative real-time PCR.
Quantitative real-time PCR assays were performed on selected genes using a Superscript one-step reverse transcription-PCR (RT-PCR) kit (QIAGEN). Primers listed in Table 3 were designed using Primer Express software (Applied Biosystems, Foster City, CA). Total RNA (0.25 μg) was used for synthesis of the cDNA that was to be used as template DNA for PCR using Quantitet SYBR Green PCR master mix (QIAGEN). Reactions in triplicate were carried out in an ABI prism 5700 Sequence Detector (Applied Biosystems) for 15 min at 95°C and 40 cycles of 15 s at 94°C, 30 s at 60°C, and 30 s at 72°C. Results were obtained and analyzed using GeneAmp 5700 SDS software. The comparative cycle threshold method was used for the quantitation of gene expression, and expression values were normalized to the constitutive levels of β-actin in untreated THP-1 cells.
TABLE 3.
Primer sequences for real-time RT-PCR
| Gene no. | GeneBank accession no. | Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|---|---|
| 1 | NM_004994 | MMP9 | GGACGATGCCTGCAACGT | CAAATACAGCTGGTTCCCAATCT |
| 2 | NM_002569 | FURIN | GCAAAGCGACGGACTAAACG | CAGGTCCCGCTGAGTGACA |
| 3 | NM_002535 | OAS2 | GCAGGGAGTGGCCATAGGT | CCATCGGAGTTGCCTCTTAAGA |
| 4 | NM_001252 | TNFSF7 | CGCCTCCCGTAGCATCAG | CAATGGTACAACCTTGGTGGAA |
| 5 | NM_000120 | EPHX1 | AAGCATGAGCGGATGAAGGT | GCTCAAAAGGGAAGGCAGAGA |
| 6 | NM_000716 | C4BPb | GTGTGCGTGCTGTCTTATGGTT | CTGGACAGTGCTCTGCATCTG |
| 7 | NM_023945 | MS4A5 | TCTTAGTGCCCTGGGAGCAA | TTGATCTAGGATGAAACCAAATGTG |
| 8 | NM_002773 | PRSS8 | CCCAGGGCGACATTGC | GATGTAGCGGGAGAAGGTGATG |
| 9 | NM_014240 | LIMD1 | TTCATCGAGGACCTGAACATGT | CCCTTGTCCACTCGGAAGAG |
| 10 | NM_003682 | MADD | TGGTGTGAGCCTGACGTCTAGT | CTCACGCCGATGACAGAGTCT |
| 11 | NM_006359 | SLC9A6 | CCGCTTCCTGCACGAAA | GCACAAGGCCCACCAAAA |
| 12 | NM_005306 | GPR43 | CTGTGGTGACGCTGCTCAAT | CAGGTGGGACACGTTGTAAGG |
| 13 | NM_002739 | PKCG | AGAAGACCCGAACGGTGAAA | TTGAACACAAAGGTCTCATTCCA |
RESULTS
Data analysis and statistical validation of global changes in gene expression.
The transcriptional responses of macrophages infected with L. major in the presence or absence of IFN-γ were analyzed using DNA oligonucleotide microarrays. Three conditions were evaluated in duplicate:(i) human THP-1 macrophages were infected with L. major metacyclic promastigotes and at 48 h macrophage RNA was isolated; (ii) THP-1 macrophages were infected with L. major metacyclic promastigotes, and at 24 h IFN-γ was added for an additional 24 h, when RNA was isolated; and (iii) to evaluate the effect of IFN-γ alone, IFN-γ was added to uninfected macrophages for 24 h prior to RNA isolation (Table 2). To control for phagocytosis, controls were included in a separate experiment in which THP-1 macrophages were incubated with either latex beads or heat-killed L. major metacyclic promastigotes or with viable L. major (Table 1). Cy3- or Cy5-labeled probes were prepared from macrophage RNA and hybridized to DNA oligonucleotide microarrays representing 22,225 unique human genes. As discussed in Methods and Materials, no Leishmania RNA was detected in the total macrophage RNA preparations; therefore, no Leishmania-derived probes would have hybridized to the microarrays.
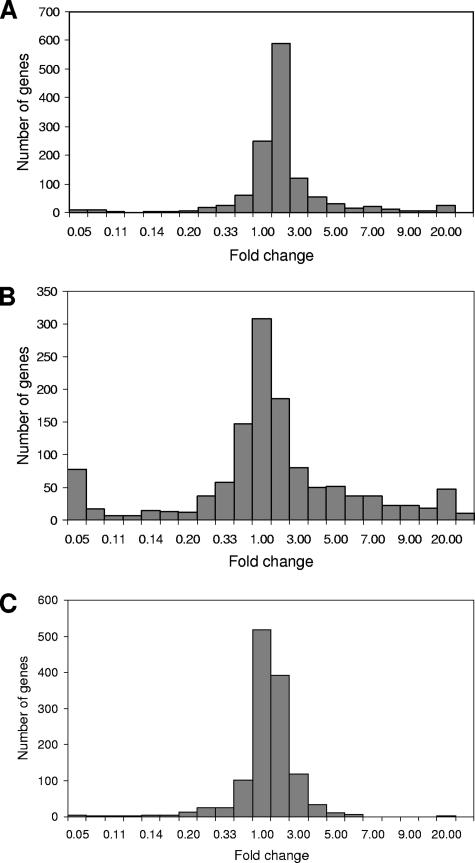
Differentially expressed genes were identified by ANOVA and multiple testing procedures to control the false discovery rate using a cutoff of 0.05 (3) in response to either L. major infection, phagocytosis, and/or IFN-γ. Following incubation with L. major, heat-inactivated L. major, and latex beads, 741 differentially expressed macrophage genes were identified. Following L. major infection in the presence or absence of IFN-γ or treatment of uninfected macrophages with IFN-γ alone (Fig. 1), 1,265 differentially expressed macrophage genes were identified. From the ANOVA-generated list of 741 differentially expressed genes in response to Leishmania infection or controls, those that showed a fourfold or higher induction or repression were selected (Table 1). From the ANOVA list of 1,265 differentially expressed genes in response to IFN-γ, those that were induced or repressed fourfold by either L. major, IFN-γ, or the combination of both were selected (Table 2). See the supplemental material for complete data sets. Selected genes were mapped to known biological pathways (from the Kyoto Encyclopedia of Genes and Genomes database, Gene Microarray Pathway Profiler, and the Biological Biochemical Image Database) by using Expression Analysis Systematic Explorer (http://david.niaid.nih.gov/david/ease.htm) and Metacore database (http://trials.genego.com/cgi/index.cgi) and found to be linked to known pathways including innate immune response, matrix metalloproteinases (MMPs), proteasome pathway, chemokines and cell adhesion, transport, and apoptosis as well as various signaling pathways.
FIG. 1.
Histograms showing differential gene regulation in macrophages: the number of induced and repressed genes in response to L. major infection (A), the number of induced and repressed genes by IFN-γ treatment alone (B), and the number of induced and repressed genes in response to L. major infection followed by IFN-γ treatment (C). Differentially expressed genes were identified by ANOVA and multiple testing procedures to control the false discovery rate using a cutoff of 0.05 (3).
Macrophage gene expression modulated by viable L. major, heat-inactivated L. major, or latex beads: a control for surface molecules and phagocytosis.
Table 1 identifies genes whose modulation, either up or down, in macrophages was shown to be specific to viable Leishmania and not induced by phagocytosis alone (latex beads) or the presence of antigen molecules on the surface of a phagocytosed object (heat-killed L. major). In addition to cell signaling molecules, viable L. major parasites were able to induce expression of a variety of genes involved in cell survival, energy metabolism, fatty acid metabolism (ELOVL6), and arginine-metabolism (PADI1; expressed in the living parts of the human epidermis [22]) and the gene PIK3R3, involved in growth factor signaling via insulin-like growth factor 1 receptor and insulin receptor (17).
Repression of apoptosis by Leishmania has been reported as one mechanism to ensure survival of the parasite within the host cell (35). In concordance with this finding, significant downregulation was observed by live L. major but not by phagocytosis/surface molecule controls of IL6ST, implicated in regulation of myocyte apoptosis in mice (Entrez Gene database), as well as GAS1, a mediator of cell death (11). Dramatic downregulation of the DLG3 gene, a negative regulator of cell proliferation (32), 37.22-fold suggests a positive influence of live L. major on the proliferation of infected macrophages.
Three genes involved in lipid metabolism were specifically regulated by viable L. major parasites but not controls. Indeed, infection with Leishmania leads to increased membrane fluidity, resulting in diminished clustering of MHC-II-peptide complexes and impairment of PKC translocation and phagosome fusion (45).
These rather consistent observations highlight the dominant role that viable Leishmania parasites play in eliciting a broad spectrum of cellular responses within the infected macrophage, as opposed to phagocytosis alone. These findings suggest the presence of several genes that are as yet unidentified or not connected with the disease process but that may play a role in Leishmania infection and persistence.
IFN-γ-induced macrophage gene expression is abrogated by L. major infection.
A global summary of the way that viable L. major parasites are able to influence expression of various genes in macrophages induced by IFN-γ is shown in Fig. 1 and Table 2. Treatment with L. major induced or repressed a number of THP-1 genes an average of 5- to 10-fold (Fig. 1A). In response to IFN-γ alone (Fig. 1B), a significant number of genes were either up- or downregulated >10-fold (Fig. 1B, outermost bars), whereas the presence of L. major actively inhibited this response (Fig. 1C). Abrogation of the IFN-γ response in macrophages by Leishmania is well established (8, 46, 47, 48).
In line with documented inhibition of the respiratory burst in Leishmania-infected macrophages, expression of a member of the PKC signaling pathway, PRKCG, was induced fourfold in response to IFN-γ and downregulated in response to L. major infection only 2.9-fold, leading to a net gene expression that was similar to expression in resting macrophages. A further early response gene, CSF-1, encoding the cytokine colony stimulating factor involved in the control of macrophage function, was activated by IFN-γ (57), but infection of the THP-1 cells with Leishmania followed by IFN-γ led to reduced gene expression compared to IFN-γ alone (Table 2).
Significant upregulation by IFN-γ was observed in many genes related to the chemokine/cell adhesion pathways. Following Leishmania infection alone, these IFN-γ-induced genes exhibited either very low expression or downregulation in THP-1 cells infected with L. major, and the combination of infection with IFN-γ treatment led to gene expression which was, for the most part, not significantly different from resting THP-1 cells. Among the genes upregulated in response to IFN-γ and repressed by L. major infection were members of the C-type lectins, platelet endothelial cell adhesion molecule 1 (PECAM-1 also known as CD31) and CLECF7. PECAM-1 has been shown to be responsible for detachment and protection from macrophage engulfment of viable cells, while on apoptotic leukocytes, PECAM-1 encouraged binding and subsequent phagocytosis by THP-1 cells (7). Whereas Leishmania infection prevents apoptosis in the host macrophage (25, 35), it is known that the process of ingestion by other macrophages is harnessed by Leishmania to promote dissemination (45). It is a possibility that downregulation of PECAM-1 expression on infected but viable THP-1 cells abolishes the “detachment” signal, leading to earlier phagocytosis by neighboring macrophages and thereby ensuring the continuity of infection.
MMPs are responsible for the removal of extracellular matrix during manifold biological and pathological process. Many MMP genes are inducible by a variety of stressors, and two MMP genes were shown to be activated by furin, a member of the proteasome pathway (37). Results revealed antagonistic regulation of MMP7 and MMP15 (both upregulated by IFN-γ; no change in gene expression in response to Leishmania or the combination of Leishmania and IFN-γ) versus MMP16 (downregulated by IFN-γ >10-fold and upregulated by Leishmania infection, resulting in no significant net change in gene expression compared to resting THP-1 cells). Gene expression of furin, the regulator of two other MMPs (MMP11 and MMP14) and a member of the proteasome pathway, did not show significant modulation by IFN-γ and L. major infection alone but showed 4.9-fold induction by L. major infection with IFN-γ. The complete abolition of the dramatic IFN-γ-induced overexpression (35.3-fold) of a member of the protein phosphatase 1 family by Leishmania infection highlights the powerful influence that the parasite can exert on virtually every aspect of cellular gene expression and function: protein phosphatase 1 dephosphorylates RNA polymerase II prior to its recruitment to the preinitiation complex (61). In addition, the analyses found an RNA polymerase II polypeptide (F) to be upregulated by IFN-γ, an effect that was counteracted by Leishmania infection.
The gene encoding myo-inositol 1-phosphate synthase (ISYNA1), while normally expressed at low levels in leukocytes (21), was upregulated >10-fold in response to treatment of THP-1 cells with IFN-γ, while IFN-γ treatment following L. major infection did not increase mRNA expression levels above those of resting macrophages. Guan et al. (21) have suggested that G-protein-mediated signal transduction is involved in the regulation of gene expression. In concordance, the expression of two members of the G-protein signaling family were upregulated in response to IFN-γ alone, which was abolished by infection with L. major (GNGT2 and GNB4), whereas one gene (SNX27) was downregulated with either IFN-γ or L. major infection alone but significantly upregulated in response to a combination of L. major infection followed by IFN-γ treatment.
NADH dehydrogenase (NDUSF4), encoding the 18-kDa subunit of respiratory complex I, necessary for cellular ATP production (55), was upregulated in response to IFN-γ, whereas cells infected with Leishmania showed no significant difference in gene expression from resting THP-1 cells, indicating a dampening of IFN-γ's effort to increase the metabolic rate in infected cells.
Increased membrane fluidity has been held responsible in part for the diminished presentation of MHC-associated antigens in Leishmania-infected macrophages, an effect which can be corrected by the addition of cholesterol (45). Two genes involved in the metabolism of complex lipids (PIGB and DEGS1) were upregulated by IFN-γ and significantly downregulated by Leishmania infection.
The proteasome pathway plays an important role in immunity as it is responsible for degradation of foreign proteins (44). Upregulation of several members of this pathway in response to IFN-γ is shown in Table 2, whereas infection with Leishmania alone has no effect on the infected macrophage. However, Leishmania infection abrogates the effect on the proteasome metabolism observed with IFN-γ alone.
A large number of genes encoding a variety of signaling proteins were differentially expressed in response to Leishmania infection, IFN-γ, or the combination of both (Table 2). These genes were mapped to calcium signaling (CACNG2 and CACNA1B), G-protein signaling (GNGT2, GNB4, SNX27, and MTNR1B), PKC signaling (PRKCG), general signaling pathways (STAT5A, GNB1, PLCG1, protein tyrosine phosphatase receptor type H [PTPRH], PTPRA, PARD6A, PIK3R3, TNS, ECGF1, and GUCY1B3), transforming growth factor β (TGF-β) signaling (SMAD5, SP1, and SKIL/SNON), and Toll-like receptor signaling (TOLLIP), as well as many transcription factors (Table 2). While the analysis of the interplay of all modulated members of signaling pathways extends beyond the scope of this paper, it is obvious that L. major infection prevents the normal signaling events elicited by IFN-γ alone from coming to fruition. Calcium signaling in host macrophages has been shown to be associated with Leishmania virulence (31), and it is interesting that L. major infection reduced the IFN-γ induction of CACNG2 that encodes a calcium channel subunit (Table 2). PTP is a key mechanism for signal transduction and participates in the JAK-STAT pathway, which mediates signaling from receptors for interferons. It seems probable that in immune cells the JAK-STAT pathway is regulated by PTPs, and previous studies have shown this pathway to be inhibited by infection with L. donovani (38). The PTPs also play an important role in the progression of Leishmania infection and pathogenesis (43, 44). Since cellular antimicrobial mechanisms including cytokine-induced nitric oxide generation, oxidative burst, and other functional responses are modulated by PTPs (39, 42, 44, 47, 58), it seems probable that microbial mechanisms may manipulate the activities of host cell PTPs, thus promoting pathogenesis. PTPRs (PTPR family) are involved in the regulation of integrin signaling, cell adhesion, and proliferation, and PTPRA has been implicated in the activation of tyrosine kinases of the Src family. PTPRH was upregulated by IFN-γ, and both PTPRH and PTPRA were significantly downregulated following Leishmania infection alone. The combination of Leishmania infection followed by IFN-γ treatment yielded no net change in gene expression compared to uninfected THP-1 cells.
A clear example of Leishmania-induced downregulation of cellular immunity via signaling molecules may be the expression of TOLLIP, an important player in the proinflammatory IL-1 receptor pathway (10); this gene is upregulated by IFN-γ but significantly downregulated with Leishmania infection.
TGF-β from antigen-presenting cells has an immunosuppressive effect (20). In response to IFN-γ, the gene coding for a SMAD-protein (SMAD5) and a regulator of SMAD proteins, SKIL (SNON), which can repress the growth inhibitor function of TGF-β (23), were upregulated >10-fold. Neither effect was observed with Leishmania infection alone nor with the combination of Leishmania infection followed by treatment with IFN-γ, thus demonstrating the extent to which Leishmania parasites protect the immunosuppressive function of the TGF-β pathway (19).
A number of genes were downregulated in response to IFN-γ, most notably two genes involved in DNA metabolism, whereas Leishmania infection alone induced these genes (UPB1 and RCF2) significantly. The RCF2 gene (replication factor C) has been shown to accelerate cell proliferation (27), thus suggesting an interest of Leishmania in acceleration of cell proliferation. Further, the tyrosinase gene (TYR) involved in pigmentation was drastically repressed in response to IFN-γ but significantly upregulated by Leishmania infection, resulting in no net change in gene expression. Melanocytes, Schwann cells, and melanoma cells normally express TYR mRNA; TYR mRNA expression in circulating melanoma cells has been used as a clinical marker for melanoma relapse (33). While IFN-γ treatment reduced the number of TYR-positive cells in the circulation of melanoma patients, this effect was attributed to cell death rather than downregulation of TYR mRNA following treatment with IFN-γ. The significance of tyrosinase in macrophages or the necessity for Leishmania to maintain stable tyrosinase levels in its host macrophage is unclear.
The expression of certain macrophage genes changed synergistically in response to L. major infection combined with IFN-γ treatment and did not modulate with the response of IFN-γ or L. major infection alone: KIAA0251, involved in protein metabolism, did not show significant modulation by IFN-γ or L. major infection alone but showed 6.01-fold induction by L. major infection with IFN-γ treatment. The expression of two genes related to transport did not show significant modulation by IFN-γ or L. major infection alone but showed significant induction (4.4- to 12.8-fold) by L. major infection with IFN-γ treatment.
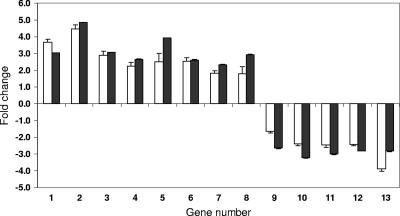
Verification of microarray hybridization by real-time RT-PCR.
For verification of the microarray results, levels of specific mRNAs from L. major infection with and without IFN-γ treatment were quantitated by PCR. Real-time RT-PCR assays were performed using gene-specific primers on a set of 13 genes that were found to be differentially expressed by microarray analysis of L. major infection with and without IFN-γ treatment (Table 3). As an internal control, the relative level of β-actin mRNA was analyzed using the same method. Results obtained by real-time RT-PCR for the genes examined were consistent with microarray-based observations (Fig. 2). With all 13 genes the relative change as measured by microarray quantitation was similar (upregulated or downregulated) to values obtained by real-time RT-PCR, thus validating the measurement of gene expression by oligonucleotide microarray hybridization.
FIG. 2.
Verification of microarray results by real-time RT-PCR analysis. Relative mRNA levels (y axis, expressed as relative [n-fold] change) of selected gene transcripts were measured by using real-time RT-PCR assays (open bars) in the same samples (x axis) as those used to prepare probes for the microarray hybridizations (black bars). Quantitative measurements of induced (genes 1 to 8) and suppressed (genes 9 to 13) genes (P < 0.05) from both assays produced similar expression patterns. Error bars represent standard deviations. Errors smaller than 0.01 are not shown. Gene numbers correspond in their respective order to the genes listed in Table 3.
DISCUSSION
The human monocytic THP-1 cell line was used in the present study as a model of human monocyte-derived macrophages in order to reduce the genetic heterogeneity and variability of donor-derived macrophages and to ensure continuity between individual experiments. THP-1 cells have been utilized as an in vitro model for Leishmania infection previously (1, 2, 36, 63). Studies using L. donovani and THP-1 cells showed a high degree of correlation to experiments carried out using peripheral blood-derived human mononuclear phagocytes (15). THP-1 cells have been used as a model for Mycobacterium tuberculosis-induced apoptosis in macrophages (29, 49, 56).
The present study shows that IFN-γ and L. major induce changes in macrophage gene expression in a large and diverse set of genes. The present results provide an overarching summary of gene expression flexibility and its contributory influences in macrophage activation that can serve as a biological context for mechanistic studies of individual genes. Four major conclusions were drawn from the present results: (i) viable L. major infection significantly modulates macrophage gene expression, (ii) viable L. major parasites counteract the effect of IFN-γ on macrophage gene expression, (iii) in isolated instances L. major and IFN-γ act synergistically on macrophage gene expression, and (iv) importantly, phagocytosis and surface antigen controls are inferior stimuli for altering gene expression in macrophages (Table 1).
Leishmania metacyclic promastigotes as well as intracellular amastigotes undermine host defenses at every step during infection and development, and their ability to delay the onset of macrophage activation, cause chronic infection, and persist for years in the immunocompetent host has been under investigation in many laboratories (4, 13, 46, 48) and extensively reviewed (22, 25, 45, 48, 53). Approximately four times as many macrophage genes were significantly (more than fourfold) induced by IFN-γ and downregulated by infection with L. major as were repressed by IFN-γ and upregulated as a consequence of L. major infection (Table 2). While Chaussabel et al. (13) reported an equal number of genes up- and downregulated following infection with both L. major and L. donovani for 16 h in both macrophages and dendritic cells, the mouse model of Buates and Matlashewski (8) demonstrated a general suppression of macrophage gene expression following infection with L. donovani. Rodriguez et al. (51) described the effects of Leishmania chagasi infection on gene expression in murine bone marrow-derived macrophages. In this study, proinflammatory cytokines, receptors, and Th1-type immune response genes were reported to be downregulated while some anti-inflammatory or Th2-like genes were upregulated (51). The expression of alternative (arginase) or type II activation macrophage genes, such as IL-10 and tumor necrosis factor alpha, was not affected, suggesting that macrophage infection by Leishmania results in a hybrid activation more characteristic of an alternative than classical activation pathway leading to parasite survival (51). The results from this study are consistent as type II activation macrophage genes were also not induced by infection with L. major.
Microarray studies have also identified the importance of vesicular protein transport processing in Leishmania-infected macrophages. Infection of murine macrophage cell lines with virulent, and not avirulent, species of Leishmania was shown to downregulate the expression of 7SL RNA, an essential component of the signal recognition particle, resulting in impaired protein transport (34). Overexpression of 7SL RNA resulted in macrophages’ becoming resistant to Leishmania infection while inhibition of 7SL RNA expression by small interfering RNA resulted in macrophages’ becoming susceptible to infection by avirulent strains of Leishmania (34).
The diverse ways in which Leishmania parasites exert their influence over gene expression in the host macrophage are by no means simple to interpret, and targeting specific genes or gene products for therapeutic purposes must be approached with caution. Three genes involved in proteasome function were found to be upregulated in response to IFN-γ (Table 2), whereas in response to Leishmania infection, five proteasome genes were significantly downregulated (Tables 1 and 2). This may be expected, since proteasome function is crucial for antigen presentation by the host macrophage. However, the ubiquitin-proteasome pathway also degrades STAT-1α, a pivotal transcription factor in the signaling cascade, following cell activation by IFN-γ (6). Furthermore, IFN-γ enhances the survival of the cell by activating NF-κB (6). Heussler et al. (25) reviewed the fact that NF-κB is harnessed by a number of intracellular protozoan parasites to avoid apoptosis and ensure survival of the host cell and continuity of infection. These two examples, in the context of a 48-h time frame of host cell gene regulation during early infection with Leishmania, demonstrate the complexity of a defined model system devoid of normal host innate and adaptive immune responses.
While therapies may target one gene or gene product that appears to confer vulnerability to the pathogen, Leishmania parasites exert a profound overall effect on their host (cell or organism) on every level of metabolism, survival, procreation, and even cell death. Without inserting their DNA into the host nucleus, they have managed to regulate the macrophage to their advantage. An important background strategy aimed at every parasitic disease is to stabilize the host's immune regulatory cycles that are prone to fail due to stress and malnutrition (54).
Supplementary Material
Acknowledgments
This study was supported by the Canadian Institutes for Health Research (grant number MOP 7399).
We thank Robert Bell for statistical advice.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 30 April 2007.
Supplemental material for this article is available at http://iai.asm.org/.
REFERENCES
- 1.Antilla, H. S. I., S. Reitamo, M. Ceska, and M. Hurme. 1992. Signal transduction pathways leading to the production of IL-8 by human monocytes are differentially regulated by dexamethasone. Clin. Exp. Immunol. 89:509-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 3.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57:289-300. [Google Scholar]
- 4.Beverley, S. M. 1996. Hijacking the cell: parasites in the driver's seat. Cell 87:787-789. [DOI] [PubMed] [Google Scholar]
- 5.Bigger, C. B., K. M. Brasky, and R. E. Lanford. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 75:7059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to IFN-γ. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 7.Brown, S., I. Heinisch, E. Ross, K. Shaw, C. D. Buckley, and J. Savill. 2002. Apoptosis disables CD-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418:200-203. [DOI] [PubMed] [Google Scholar]
- 8.Buates, S., and G. Matlashewski. 2001. General suppression of macrophage gene expression during Leishmania donovani infection. J. Immunol. 166:3416-3422. [DOI] [PubMed] [Google Scholar]
- 9.Buchmuller-Rouiller, Y., and J. Mauel. 1987. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect. Immun. 55:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns, K., J. Clatworthy, L. Martin, F. Martinon, C. Plumpton, B. Maschera, A. Lewis, K. Ray, J. Tschopp, and F. Volpe. 2000. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell. Biol. 2:346-351. [DOI] [PubMed] [Google Scholar]
- 11.Cabrera, J. R., L. Sanchez-Pulido, A. M. Rojas, A. Valencia, S. Manes, J. R. Naranjo, and B. Mellstrom. 2006. Gas1 is related to the glial cell-derived neurotrophic factor family receptors alpha and regulates Ret signaling. J. Biol. Chem. 281:14330-14339. [DOI] [PubMed] [Google Scholar]
- 12.Carrera, L., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaussabel, D., R. T. Semnani, M. A. McDowell, D. Sacks, A. Sher, and T. B. Nutman. 2003. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102:672-681. [DOI] [PubMed] [Google Scholar]
- 14.Cummings, C. A., and D. A. Relman. 2000. Using DNA microarrays to study host-microbe interactions. Emerg. Infect. Dis. 6:513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta, B., K. Roychoudhury, S. Ganguly, M. A. Akbar, P. Das, and S. Roy. 2003. Infection of human mononuclear phagocytes and macrophage-like THP1 cells with Leishmania donovani results in modulation of expression of a subset of chemokines and a chemokine receptor. Scand. J. Immunol. 57:366-374. [DOI] [PubMed] [Google Scholar]
- 16.de la Fuente, C., F. Santiago, L. Deng, C. Eadie, I. Zilberman, K. Kehn, A. Maddukuri, S. Baylor, K. Wu, C. G. Lee, A. Pumfery, and F. Kashanchi. 2002. Gene expression profile of HIV-1 Tat expressing cells: a close interplay between proliferative and differentiation signals. BMC Biochem. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey, B. R., R. W. Furlanetto, and S. P. Nissley. 1998. Cloning of human p55 gamma, a regulatory subunit of phosphatidylinositol 3-kinase, by a yeast two-hybrid library screen with the insulin-like growth factor-I receptor. Gene 209:175-183. [DOI] [PubMed] [Google Scholar]
- 18.Galey, D., K. Becker, N. Haughey, A. Kalehua, D. Taub, J. Woodward, M. P. Mattson, and A. Nath. 2003. Differential transcriptional regulation by human immunodeficiency virus type 1 and gp120 in human astrocytes. J. Neurovirol. 9:358-371. [DOI] [PubMed] [Google Scholar]
- 19.Gantt, K. R., S. Schultz-Cherry, N. Rodriguez, S. M. B. Jeronimo, E. T. Nascimento, T. L. Goldman, T. J. Recker, M. A. Miller, and M. E. Wilson. 2003. Activation of TGF-β by Leishmania chagasi: importance for parasite survival in macrophages. J. Immunol. 170:2613-2620. [DOI] [PubMed] [Google Scholar]
- 20.Goto, H., and J. A. L. Lindoso. 2004. Immunity and immunosuppression in experimental visceral leishmaniasis. Braz. J. Med. Biol. Res. 37:615-623. [DOI] [PubMed] [Google Scholar]
- 21.Guan, G., P. Dai, and I. Shechter. 2003. cDNA cloning and gene expression analysis of human myo-inositol 1-phosphate synthase. Arch. Biochem. Biophys. 417:251-259. [DOI] [PubMed] [Google Scholar]
- 22.Guerrin, M., A. Ishigami, M. C. Mechin, R. Nachat, S. Valmary, M. Sebbag, M. Simon, T. Senshu, and G. Serre. 2003. cDNA cloning, gene organization and expression analysis of human peptidylarginine deiminase type I. Biochem. J. 370:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, J., S. B. Tegen, A. R. Krawitz, G. S. Martin, and K. Luo. 2003. The transforming activity of Ski and SnoN is dependent on their ability to repress the activity of Smad proteins. J. Biol. Chem. 278:30540-30547. [DOI] [PubMed] [Google Scholar]
- 24.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heussler, V. T., P. Küenzi, and S. Rottenberg. 2001. Inhibition of apoptosis by intracellular protozoan parasites. Int. J. Parasitol. 31:1166-1176. [DOI] [PubMed] [Google Scholar]
- 26.Holzer, T. R., W. R. McMaster., and J. D. Forney. 2006. Expression profiling by whole-genome interspecies microarray hybridization reveals differential gene expression in procyclic promastigotes, lesion-derived amastigotes, and axenic amastigotes in Leishmania mexicana. Mol. Biochem. Parasitol. 146:198-218. [DOI] [PubMed] [Google Scholar]
- 27.Jaharul Hague, S., H. van der Kuip, A. Kumar, W. E. Aulitzky, M. N. Rutherford, C. Huber, T. Fischer, and B. R. Williams. 1996. Overexpression of mouse p140 subunit of replication factor C accelerates cellular proliferation. Cell Growth Differ. 7:319-326. [PubMed] [Google Scholar]
- 28.Kaye, P. M., N. J. Rogers, A. J. Curry, and J. C. Scott. 1994. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur. J. Immunol. 24:2850-2854. [DOI] [PubMed] [Google Scholar]
- 29.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leifso, K., G. Cohen-Freue, N. Dogra, A. Murray, and W. R. McMaster. 2007. Genomic and proteomic expression analysis of Leishmania promastigote and amastigote life stages: the Leishmania genome is constitutively expressed. Mol. Biochem. Parasitol. 152:35-46. [DOI] [PubMed] [Google Scholar]
- 31.Lu, H., L. Zhong, K. Chang, and R. Docampo. 1997. Intracellular Ca2+ pool content and signaling and expression of a calcium pump are linked to virulence in Leishmania mexicana amazonesis amastigotes. J. Biol. Chem. 272:9464-9473. [DOI] [PubMed] [Google Scholar]
- 32.Makino, K., H. Kuwahara, N. Masuko, Y. Nishiyama, T. Morisaki, J. Sasaki, M. Nakao, A. Kuwano, M. Nakata, Y. Ushio, and H. Saya. 1997. Cloning and characterization of NE-dlg: a novel human homolog of the Drosophila discs large (dlg) tumor suppressor protein interacts with the APC protein. Oncogene 14:2425-2433. [DOI] [PubMed] [Google Scholar]
- 33.Mellado, B., M. del Carmen Vela, D. Colomer, L. Gutierrez, T. Castel, L. Quinto, M. Fontanillas, N. Reguart, J. M. Domingo-Domenech, C. Montagut, J. Estape, and P. Gascon. 2002. Tyrosinase mRNA in blood of patients with melanoma treated with adjuvant interferon. J. Clin. Oncol. 20:4032-4039. [DOI] [PubMed] [Google Scholar]
- 34.Misra, S., M. K. Tripathy, and G. Chaudhuri. 2005. Down-regulation of 7SL RNA expression and impairment of vesicular protein transport pathways by Leishmania infection of macrophages. J. Biol. Chem. 280:29364-29373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore, K. J., and G. Matlashewski. 1994. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J. Immunol. 152:2930-2937. [PubMed] [Google Scholar]
- 36.Moser, B., L. Barella, S. Mattei, C. Schumacher, F. Boulay, M. P. Colombo, and M. Baggiolini. 1993. Expression of transcripts of two interleukin-8 receptors in human leukocytes, lymphocytes and melanoma cells. Biochem. J. 294:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagase, H., and J. F. Woessner. 1999. Matrix metalloproteinases. J. Biol. Chem. 274:21491-21494. [DOI] [PubMed] [Google Scholar]
- 38.Nandan, D., and N. E. Reiner. 1995. Attenuation of γ-interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: selective inhibition of signaling through Janus kinases and Stat1. Infect. Immun. 63:4495-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nandan, D., and N. E. Reiner. 2005. Leishmania donovani engages in regulatory interference by targeting macrophage protein tyrosine phosphatase SHP-1. Clin. Immunol. 114:266-277. [DOI] [PubMed] [Google Scholar]
- 40.Nelson, C. C., D. Hoffart, M. E. Gleave, and P. S. Rennie. 2003. Application of gene microarrays in the study of prostate cancer. Methods Mol. Med. 81:299-320. [DOI] [PubMed] [Google Scholar]
- 41.Ockenhouse, C. F., W.-C. Hu, K. E. Kester, J. F. Cummings, A. Stewart, D. G. Heppner, A. E. Jedlicka, A. L. Scott, N. D. Wolfe, M. Vahey, and D. S. Burke. 2006. Common and divergent immune response signaling pathways discovered in peripheral blood mononuclear cell gene expression patterns in presymptomatic and clinically apparent malaria. Infect. Immun. 74:5561-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver, M., R. W. Brownsey, and N. E. Reiner. 1992. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc. Natl. Acad. Sci. USA 89:7481-7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivier, M., B. J. Romero-Gallo, C. Matte, J. Blanchette, B. I. Posner, M. J. Tremblay, and R. Faure. 1998. Modulation of interferon-gamma-induced macrophage activation by phosphotyrosine phosphatase inhibition. Effect on murine leishmaniasis progression. J. Biol. Chem. 273:13944-13949. [DOI] [PubMed] [Google Scholar]
- 44.Olivier, M., D. J. Gregory, and G. Forget. 2005. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 18:293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters, N., and D. Sacks. 2006. Immune privilege in sites of chronic infection: Leishmania and regulatory T-cells. Immunol. Rev. 213:159-179. [DOI] [PubMed] [Google Scholar]
- 46.Ray, M., A. A. Gam, R. A. Boykins, and R. T. Kenney. 2000. Inhibition of IFN-γ signaling by Leishmania donovani. J. Infect. Dis. 181:1121-1128. [DOI] [PubMed] [Google Scholar]
- 47.Reiner, N. E., W. Ng, T. Ma, and W. R. McMaster. 1988. Kinetics of gamma-interferon binding and induction of major histocompatibility complex class II mRNA in Leishmania-infected macrophages. Proc. Natl. Acad. Sci. USA 85:4330-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiner, N. E. 1994. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol. Today 15:374-381. [DOI] [PubMed] [Google Scholar]
- 49.Riendeau, C. J., and H. Kornfeld. 2003. THP-1 cell apoptosis in response to mycobacterial infection. Infect. Immun. 71:254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts, E. S., M. A. Zandonatti, D. D. Watry, L. J. Madden, S. J. Henriksen, M. A. Taffe, and H. S. Fox. 2003. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am. J. Pathol. 162:2041-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez, N. E., H. K. Chang, and M. E. Wilson. 2004. Novel program of macrophage gene expression induced by phagocytosis of Leishmania chagasi. Infect. Immun. 72:2111-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacks, D., and A. Sher. 2002. Evasion of immunity by parasitic protozoa. Nature 3:1041-1047. [DOI] [PubMed] [Google Scholar]
- 53.Saha, B., G. Das, H. Vohra, N. K. Ganguly, and G. C. Mishra. 1995. Macrophage-T cell interaction in experimental visceral leishmaniasis: failure to express costimulatory molecules on Leishmania-infected macrophages and its implication in the suppression of cell-mediated immunity. Eur. J. Immunol. 25:2492-2598. [DOI] [PubMed] [Google Scholar]
- 54.Saha, S., S. Mondal, A. Banerjee, J. Ghose, S. Bhowmick, and N. Ali. 2006. Immune responses in kala-azar. Indian J. Med. Res. 123:245-266. [PubMed] [Google Scholar]
- 55.Scacco, S., V. Petruzzella, S. Budde, R. Vergari, R. Tamborra, D. Panelli, L. P. van den Heuvel, J. A. Smeitink, and S. Papa. 2003. Pathological mutations of the human NDUFS4 gene of the 18 kDa (AQDQ) subunit of complex I affect the expression of the protein and the assembly and function of the complex. J. Biol. Chem. 278:44161-44167. [DOI] [PubMed] [Google Scholar]
- 56.Sly, L. M., S. M. Hingley-Wilson, N. E. Reiner, and W. R. McMaster. 2003. Virulent Mycobacterium tuberculosis induces anti-apoptotic Bcl-2 family member Mcl-1 to prevent host macrophage apoptosis and promote its intracellular survival. J. Immunol. 170:430-437. [DOI] [PubMed] [Google Scholar]
- 57.Tsuchimoto, D., A. Tojo, and S. Asano. 2004. A mechanism of transcriptional regulation of the CSF-1 gene by interferon-gamma. Immunol. Investig. 33:397-405. [DOI] [PubMed] [Google Scholar]
- 58.Turco, S. J., and A. Descoteaux. 1992. The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 46:65-94. [DOI] [PubMed] [Google Scholar]
- 59.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]
- 60.Vannier-Santos, M. A., A. Martiny, and W. de Souza. 2002. Cell biology of Leishmania spp.: invading and evading. Curr. Phar. Des. 8:297-318. [DOI] [PubMed] [Google Scholar]
- 61.Washington, K., T. Ammosova, M. Beullens, M., Jerebtsova, A. Kumar, M. Bollen, and S. Nekhai. 2002. Protein phosphatase-1 dephosphorylates the C-terminal domain of RNA polymerase-II. J. Biol. Chem. 277:40442-40448. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, Y., M. Jamaluddin, S. Wang, B. Tian, R. P. Garofalo, A. Casola, and A. R. Brasier. 2003. Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J. Virol. 77:5933-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zieglar, S. F., T. W. Tough, V. Franklin, R. J. Armitage, and M. R. Alderson. 1991. Induction of macrophage inflammatory protein-1b gene expression in human monocytes by lipopolysaccharide and IL-7. J. Immunol. 147:2234-2239. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.