Abstract
The human pathogen group A Streptococcus (GAS) produces many secreted proteins that play important roles in GAS pathogenesis, including hydrolases that degrade proteins and nucleic acids. This study targets another kind of hydrolase, carboxylic esterase, with the objectives of identifying GAS esterase and determining whether it is a protective antigen. The putative esterase gene SPy1718 was cloned, and the recombinant protein (Sse) was prepared. Sse was detected in GAS culture supernatant, and patients with streptococcal pharyngitis seroconverted to Sse, indicating that Sse was produced in vivo and in vitro. Sse hydrolyzes p-nitrophenyl butyrate, and the residue 178Ser is critical for this esterase activity. There are two Sse variant complexes according to the available genome databases, consistent with the previous finding of two antigenic Sse variants. Complex I includes serotypes M1, M2, M3, M5, M6, M12, and M18, whereas M4, M28, and M49 belong to complex II. Sse variants share >98% identity in amino acid sequence within each complex but have about 37% variation between the two groups. Active immunization with M1 Sse significantly protects mice against lethal subcutaneous infection with virulent M1 and M3 strains and inhibits GAS invasion of mouse skin tissue. Passive immunization with anti-Sse antiserum also significantly protects mice against subcutaneous GAS infection, indicating that the protection is mediated by Sse-specific antibodies. The results suggest that Sse plays an important role in tissue invasion and is an antigen protective in subcutaneous infection against GAS strains of more than one serotype.
The human pathogen Streptococcus pyogenes, commonly referred to as group A Streptococcus (GAS), causes a variety of diseases, including pharyngitis, cellulitis, necrotizing fasciitis, streptococcal toxic shock syndrome, scarlet fever, acute rheumatic fever, rheumatic heart disease, and glomerulonephritis. The pathogenesis of GAS infection is mediated by an abundance of virulence factors (5), which make effective immunotherapeutic control of GAS diseases extremely difficult. Consequently, no licensed GAS vaccine is available, despite considerable efforts made in the last several decades (9).
Many studies conducted to develop a GAS vaccine have focused on M protein, a major surface protein and virulence factor (1, 3, 4, 7, 27). The M protein is highly variable in amino acid sequence, and more than 100 different M protein serotypes are known. Anti-M protein antibody made by patients after GAS infection is serotype specific (13, 30), and immunization of mice with peptides derived from the variable amino-terminal region confers serotype-specific protection (1). Multivalent M protein vaccines are being developed using fused recombinant amino-terminal peptides derived from commonly occurring M proteins to develop broader M protein amino-terminal-region-based vaccines (7). However, these multivalent M protein vaccines will not provide immunity against infections caused by strains of all M serotypes, due to the variable nature of this protein. Thus, non-M protein vaccine candidates have been studied. Several other GAS vaccine candidates have been described previously, including C5a peptidase (15), streptococcal pyrogenic exotoxin A (29), streptococcal pyrogenic exotoxin B (16), streptococcal pyrogenic exotoxin C (26), streptococcal protective antigen (6), fibronectin-binding proteins (10, 17), R28 protein (18), and carbohydrate (35). In addition, a streptococcal cell surface heme-binding protein (21) and several conserved lipoproteins (23) have been shown to induce production of antibodies with bactericidal activity in mice.
GAS produces many secreted proteins that play important roles in GAS pathogenesis. These proteins include hydrolases that degrade proteins and nucleic acids (25, 33). Another kind of hydrolase, carboxylic esterase, has been detected in the supernatant of GAS, and convalescent-phase sera from patients with streptococcal pharyngitis have esterase-specific antibodies (11, 12, 32). However, the esterase has not been fully characterized. The carboxylic ester hydrolases are a diverse group of enzymes that can split the carboxylic acid ester bond in carboxylic esters, triglycerides, phospholipids, and/or acetylcholine (34) and thus may play important roles in tissue invasion and nutrient utilization by bacteria.
We have been studying GAS extracellular proteins to identify novel virulence factors and vaccine candidates (20, 23). As a continued effort in this regard, this study identifies the secreted streptococcal esterase (designated Sse) and determines whether Sse is a protective antigen. The putative esterase gene, SPy1718, of the serotype M1 genome (8) was cloned, and recombinant Sse was prepared. Sse was found to be a serine esterase, and active and passive immunizations with Sse protect mice against subcutaneous GAS infection and inhibit the invasion of the skin tissue, suggesting that Sse is involved in tissue invasion and is a protective antigen against GAS subcutaneous infection.
MATERIALS AND METHODS
Materials.
Adjuvant aluminum hydroxide gel (ALUM) (catalogue no. A-8222) and p-nitrophenyl butyrate (PNPB) were purchased from Sigma (St. Louis, MO). Convalescent-phase serum samples were obtained from four patients with streptococcal pharyngitis, at about 21 days after diagnosis. These sera were opsonic for GAS strain MGAS5005 (serotype M1), suggesting that the patients had been infected with an M1 strain.
Bacterial strains and growth.
GAS strains MGAS5005 and MGAS315 (serotype M3) have been described previously (2, 14). The GAS strains were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, MI) supplemented with 0.2% yeast extract (THY). Tryptose agar with 5% sheep blood (Becton Dickinson, Cockeysville, MD) was used as the solid medium. GAS bacteria used for the challenge experiments were harvested at the exponential growth phase and washed three times with and resuspended in pyrogen-free Dulbecco's phosphate-buffered saline (DPBS). Inocula were determined by plating. Escherichia coli strains NovaBlue and BL21(DE3) (Novagen, Madison, WI) were used for gene cloning and protein expression, respectively.
Gene cloning.
The Sse (SPy1718) gene encoding the secreted esterase was PCR cloned from MGAS5005 using primers 5′-ACCATGGGTTCTCGTTCTTGGAAGAG-3′ and 5′-CGAATTCTTAAGGAGTTTTGTTGATGGC-3′. The PCR product was digested with NcoI and EcoRI and was ligated into pET-His2 (22) at the NcoI and EcoRI sites to yield plasmid pSSE. The cloned gene was sequenced to rule out spurious mutations. Recombinant Sse made from the construct had 12 amino acid residues, MHHHHHHLETMG, fused to the second amino acid residue, 28Ser, of mature Sse.
Expression and purification of recombinant Sse.
Recombinant Sse was expressed and purified from E. coli strain BL21(DE3) containing pSSE. The bacteria were grown in 6 liters of Luria-Bertani broth supplemented with 100 mg of ampicillin/liter at 37°C with shaking. When the optical density at 600 nm (OD600) of the culture was about 0.5, 0.5 mM isopropyl-β-d-thiogalactopyranoside was added to induce Sse production. After 10 h of induction, bacteria were harvested by centrifugation.
All solutions in Sse purification were buffered with 20 mM Tris-HCl. The bacterial paste obtained was sonicated for 20 min at 4°C in 80 ml Tris-HCl and centrifuged. The lysate was loaded onto a Ni-nitrilotriacetic acid agarose column (2.5 by 5 cm). The column was washed with 100 ml Tris-HCl, and Sse was eluted with a 100-ml gradient of 0 to 75 mM imidazole. Fractions containing Sse were pooled and dialyzed against 3 liters of Tris-HCl at 4°C for 20 h with two buffer changes. The dialyzed sample was loaded onto a DEAE Sepharose column (1.5 by 20 cm) and eluted with a 50-ml gradient of 0 to 50 mM NaCl. The Sse obtained was dialyzed against 3 liters of Tris-HCl and concentrated using a Centricon Plus 20 filtration device (Millipore, Bedford, MA). The protein concentration was determined using a modified Lowry protein assay kit from Pierce (Rockford, IL) with bovine serum albumin as a standard.
Site-directed mutagenesis.
The 178Ser residue of Sse was replaced with Ala by site-directed mutagenesis using a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and primers 5′-GTTTTCCTTATGGGAGATGCAGCAGGTGGAGGTTTAGCC-3′ and 5′-GGCTAAACCTCCACCTGCTGCATCTCCCATAAGGAAAAC-3′. The mutated codon for the Ser-to-Ala replacement is underlined. The mutated gene was verified by DNA sequencing. The procedures for the preparation of wild-type Sse were followed to express and purify the mutated protein SseS178A.
Esterase activity assay.
The esterase activity of Sse was determined using PNPB, a chromogenic substrate of carboxylic esterases, as described previously (31), with modifications. Briefly, 10 μl of PNPB solution at various concentrations in acetonitrile was added into 1 ml Tris-HCl containing Sse. The changes in absorbance at 410 nm (ΔA410) were monitored with time to quantify hydrolysis of PNPB. Since only the deprotonated form of p-nitrophenol has absorbance at 410 nm, the amount of the deprotonated form formed per min was calculated from ΔA410/min using the extinction coefficient ɛ410 = 18,400 M−1cm−1, and the rate of the formation of deprotonated and protonated p-nitrophenol was then determined according to pKa, 7.244, of p-nitrophenol and pH 8.0.
Detection of Sse by Western blotting.
To obtain proteins in culture supernatant, MGAS5005 was grown in protein-reduced THY (PR-THY) to an OD600 of 0.4, and the cultures were centrifuged to obtain the culture supernatant. Proteins in 8 ml of culture supernatant were precipitated with 24 ml of cold ethanol, and the precipitates were pelleted by centrifugation and dissolved in 200 μl of 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. PR-THY was prepared by passing THY through a membrane filter with a 10,000 molecular-weight cutoff as described previously (19). Samples of proteins in the cell wall and protoplast were prepared as follows. MGAS5005 grown to an OD600 of 0.4 in 50 ml THY was harvested by centrifugation, and the bacterial pellet was resuspended in 0.45 ml phosphate-buffered saline (PBS) containing 20% sucrose and treated with 100 units of mutanolysin at 37°C overnight. The sample was then centrifuged to obtain supernatant (the cell wall fraction) and a protoplast pellet. The protoplasts were resuspended in 0.45 ml of DPBS and sonicated briefly. Loading buffer was added to the cell wall and protoplast sample to a total volume of 0.6 ml. Western blotting analysis was performed as described previously (19).
Active immunization and challenge.
Two groups of eight outbred female CD-1 Swiss mice (4 to 5 weeks old) were immunized subcutaneously with 50 μg Sse and adjuvant ALUM or ALUM only (control) on days 1, 14, and 28. Sera were collected on day 40 for measurement of anti-Sse antibody titers, which were determined by enzyme-linked immunosorbent assay, as described previously (23). On day 42, the immunized mice were subcutaneously inoculated with 1 × 108 CFU of MGAS5005 or MGAS315 and monitored daily for 14 days to determine survival rate. The mice that could not reach for food and water were euthanized and counted as dead. These procedures and the following animal experiments were approved by the Institutional Animal Care and Use Committee at Montana State University.
Passive immunization and challenge.
Groups of 10 CD-1 mice were immunized with ALUM only, Sse/ALUM, and SPy0019/ALUM as described above, and control, anti-Sse, and anti-SPy0019 sera were collected on day 50. In passive immunization, groups of 10 outbred female CD-1 Swiss mice (5 weeks old) were intraperitoneally injected with 0.5 ml pooled control, anti-Sse, or anti-SPy0019 sera, and 3 h later, 9 × 107 CFU MGAS5005 were subcutaneously introduced into each of 8 mice in each group. The mice were monitored daily to determine the survival rate. Sera were collected from the other two mice from each group 24 h after the serum administration for titer determination.
Skin infection in hairless mice.
Groups of 10 outbred, immunocompetent, and hairless female mice (strain Crl:SKH1-hrBR; Charles River Laboratories) (5 weeks old) were immunized with 50 μg Sse/ALUM, 50 μg MtsA/ALUM (negative protein control), or ALUM (adjuvant control) on days 1, 14, and 28. The mice were inoculated subcutaneously with 50 μl of 2 × 108 CFU MGAS5005 on day 50. The infection area in the skin was measured on day 4 after the inoculation.
Statistical analyses.
The survival rates and lesion sizes were analyzed by the log rank test and two-tailed unpaired t test with Welch's correction, respectively, using GraphPad Prism (GraphPad Software, Inc.).
GAS growth in human blood.
The previously described procedures (24) were followed to monitor the growth of MGAS5005 in heparinized human blood in the presence of anti-Sse mouse antisera. Three CD-1 mice were immunized with Sse/ALUM or ALUM (control) as described above. Sera were prepared from these mice. Blood from nonimmune and immune healthy individuals was collected in accordance with a protocol approved by the Institutional Review Board for the Protection of Human Subjects at Montana State University, Bozeman, MT. MGAS5005 harvested at the exponential growth phase was washed with DPBS, and ∼1,000 CFU of MGAS5005 was incubated for 15 min with 100 μl of mouse control, mouse anti-Sse, nonimmune human, or immune human serum and then mixed with 0.9 ml of nonimmune human blood. The triplicate samples were rotated end-to-end at 37°C for 4 h, and numbers of viable GAS in the samples and actual inocula were determined by plating on THY agar. Growth factor is defined as the ratio of CFU of each sample after 4 h incubation to CFU in the inoculum.
RESULTS
Sse gene and protein.
The gene SPy1718 (designated sse) of the M1 GAS genome (8) encodes a putative esterase of 328 amino acid residues. The inferred amino acid sequence contains a putative secretion signal sequence (amino acids 1 to 26). There are two distinct genetic complexes for the Sse gene according to the sequenced GAS genomes (designated complex I and complex II). Complex I includes serotypes M1, M2, M3, M5, M6, M12, and M18, whereas M4, M28, and M49 belong to complex II. Sse variants in each of the two complexes are closely related, sharing >98% identity in amino acid sequence, but complex I and complex II variants share about 63% identify in amino acid sequence (Table 1).
TABLE 1.
Identity of amino acid sequences among Sse from GAS strains of different M protein serotypesa
| Serotype | % Identity
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M5 | M6 | M12 | M18 | M4 | M28 | M49 | |
| M1 | 100 | |||||||||
| M2 | 98.3 | 100 | ||||||||
| M3 | 99 | 98.7 | 100 | |||||||
| M5 | 99 | 98 | 99.3 | 100 | ||||||
| M6 | 99 | 98.3 | 99.7 | 99.7 | 100 | |||||
| M12 | 99 | 98 | 99.3 | 99.3 | 99.7 | 100 | ||||
| M18 | 99 | 98.3 | 99.7 | 99.7 | 100 | 99.7 | 100 | |||
| M4 | 63 | 63 | 63 | 63.6 | 63.3 | 63.3 | 63.3 | 100 | ||
| M28 | 63.2 | 60 | 63.3 | 63.3 | 63.2 | 63.2 | 63.2 | 99.7 | 100 | |
| M49 | 63.2 | 60 | 63.3 | 63.3 | 63.2 | 63.2 | 63.2 | 99.7 | 100 | 100 |
The deduced amino acid sequences of Sse were from GenBank or other databases, as follows: M1, accession no. NC_002737; M2, accession no. NC_008022; M3, accession no. NC_004070; M4, accession no. NC_008024; M6, accession no. NC_006086; M12, accession no. NC_008023; M18, accession no. NC_003485; M28, accession no. NC_007296; M49, accession no. ZP_00365884; and M5, available from http://www.sanger.ac.uk/Projects/S_pyogenes/.
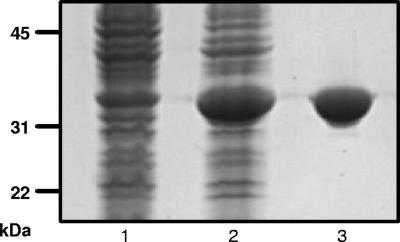
To obtain recombinant Sse, the sse gene was cloned to produce six-His-tagged mature Sse (amino acids 27 to 328) of serotype M1 GAS. The recombinant Sse protein was overexpressed in E. coli and purified to apparent homogeneity (Fig. 1).
FIG. 1.
SDS-PAGE analysis of recombinant Sse. The gel was stained with GelCode Blue. Lane 1, E. coli with empty vector (control); lane 2, E. coli lysate containing Sse; lane 3, purified recombinant Sse.
In vitro production.
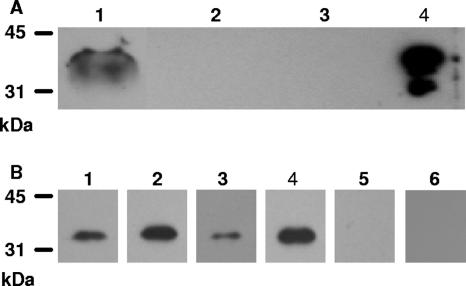
The production of Sse in vitro was assessed by Western blotting analysis. The proteins in the culture supernatant, cell wall fraction, and protoplast of GAS were probed by Western blotting analysis using anti-Sse mouse antisera. As shown in Fig. 2A, a protein band with the size of Sse in the culture supernatant reacted with anti-Sse antibody, but no Sse band was detected in the cell wall fraction and protoplast. These results indicate that Sse is produced in vitro and is a secreted protein.
FIG. 2.
In vitro and in vivo production of Sse. (A) Western blot demonstrating the presence of Sse in MGAS5005 culture supernatant. Proteins in culture supernatant (lane 1), cell wall fraction (lane 2), protoplast (lane 3), and recombinant Sse (lane 4) were resolved by SDS-PAGE and probed with anti-Sse mouse antiserum as described in Materials and Methods. (B) Western blots showing the presence of Sse-specific antibodies in convalescent-phase sera from pharyngitis patients. Recombinant Sse was resolved by SDS-PAGE and probed with convalescent-phase sera from four patients (lanes 1 to 4) and sera of two individuals without prior GAS exposure (lanes 5 and 6) at a serum dilution of 1:1,000.
In vivo production.
Whether Sse was produced in vivo during infection was indirectly assessed by the presence of Sse-specific antibody in convalescent-phase sera from patients with streptococcal pharyngitis. Recombinant Sse was probed with sera from four patients and two individuals without GAS exposure by Western blotting analysis. All four patients, but not the control persons, were seroconverted to Sse (Fig. 2B). These results indicate that Sse was produced in vivo during infection.
Esterase activity of Sse.
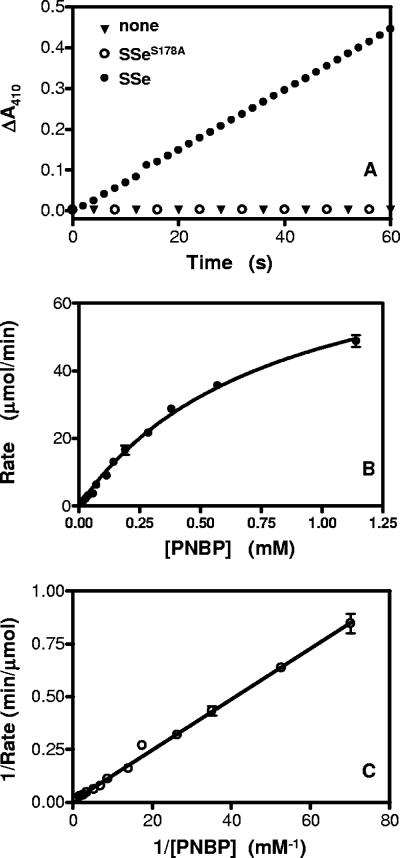
Sse is homologous to esterase in amino acid sequence. A chromogenic assay of esterase activity using PNPB was used to determine whether Sse has esterase activity. Esterase-catalyzed hydrolysis of PNPB produces p-nitrophenol with an A410 in basic solution. Sse protein was mixed with PNPB at various concentrations, and the A410 was monitored. The A410 increased linearly with time in the reaction containing Sse but not in the control reaction without Sse (Fig. 3A), indicating that Sse has esterase activity. The reaction rate increased hyperbolically with increasing PNPB (Fig. 3B). The double-reciprocal plotting analysis of the data in Fig. 3B indicates that the reaction catalyzed by Sse follows the Michaelis-Menten equation (equation 1) or its rearranged form (equation 2)
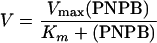 |
(1) |
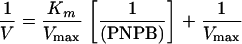 |
(2) |
where Km is the Michaelis constant and Vmax is the reaction rate when PNPB is saturated with Sse. According to the slope and intercept on the y axis in Fig. 3C and Sse concentration, the values of Km and specific enzyme activity of Sse were calculated to be 1.8 mM and 3.0 mmol s−1 mg−1, respectively.
FIG. 3.
Serine esterase activity of recombinant Sse. (A) Sse-catalyzed hydrolysis of PNPB. PNPB (0.1 mM) was mixed with 0.9 μg of Sse in 1 ml of 20 mM Tris-HCl (pH 8.0), and the ΔA410 of the sample was recorded as a measure of hydrolysis product p-nitrophenol. (B) Dependence of reaction rate on PNPB in the Sse-catalyzed PNPB hydrolysis. The rates at various PNPB concentrations were determined using the slopes of ΔA410 versus time plots as described in Materials and Methods. (C) The double reciprocal plotting of the data in panel B is shown.
Catalytic serine residue of Sse activity.
Sse contains a GXSXG motif present in serine esterases (28), and the Ser residue in the motif is a catalytic residue in known serine esterases, suggesting that Sse is a serine esterase. To test this idea, the Ser residue (178Ser) of the GXSXG motif in Sse was replaced with Ala by using site-directed mutagenesis. The mutant recombinant protein SseS178A was purified and tested for esterase activity using the PNPB assay. The A410 of the reaction containing SseS178A did not change with time (Fig. 3A), indicating that the S178A replacement abolished the ability of Sse to catalyze the hydrolysis of PNPB. These results suggest that 178Ser is a catalytic residue of Sse and that Sse is a serine esterase.
Immunization with Sse protects mice against M1 and M3 GAS infection.
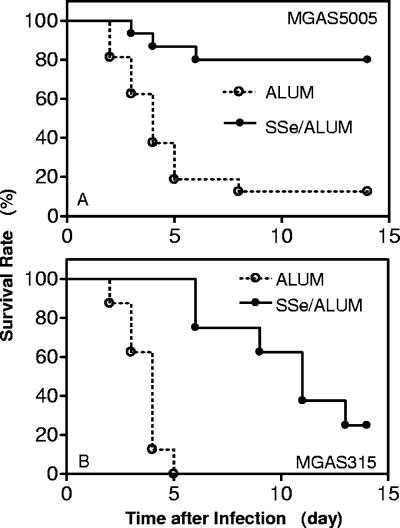
Sse is a secreted antigen and may be a protective antigen if its function is important for GAS pathogenesis. To test this possibility, groups of eight mice were immunized with 50 μg Sse with ALUM or ALUM only and challenged subcutaneously with MGAS5005. The average ± standard deviation geometric titer for the immunized mice was 15,300 ± 5,600. Six of the 8 mice immunized survived, while 7 of the 8 control mice died (P = 0.0171). The experiment was repeated, and 1 of the 8 immunized mice died, whereas 7 of the 8 control mice died (P = 0.0055). The combined survival rates are shown in Fig. 4A. These results indicate that immunization with Sse significantly protected mice against the infection of soft tissue with the serotype M1 strain.
FIG. 4.
Active immunization with M1 Sse protects mice against infections with M1 and M3 GAS strains. Eight CD-1 Swiss mice were subcutaneously immunized with 50 μg of Sse absorbed to 40 μl of ALUM on days 1, 14, and 28, and control mice were similarly treated with 40 μl of ALUM. The mice were inoculated subcutaneously with 1 ×108 CFU of MGAS5005 (serotype M1) or MGAS315 (serotype M3) and monitored daily to determine survival rate. (A) Combined survival rates of the 16 immunized (solid circles) or control (open circles) mice infected with MGAS5005 from two experiments (experiment 1, P = 0.0171; experiment 2, P = 0.0055). (B) Survival rates are shown for the eight immunized (solid circles) or control (open circles) mice infected with MGAS315 (P < 0.0001).
M1 Sse is highly conserved in 7 of the 10 different serotypes with known genome sequences (Table 1). To test whether immunization with M1 Sse protects mice against infection with a heterologous GAS strain, groups of eight mice were treated with Sse/ALUM or ALUM only and inoculated subcutaneously with the virulent M3 strain MGAS315. All control mice died, with the mean time to death of 3.5 days. Although 6 of the 8 immunized mice also died, the mean time to death of these mice was 9.3 days. Thus, immunization with M1 Sse also significantly protected mice against MGAS315 infection (P < 0.0001). These data suggest that Sse may be a protective antigen in subcutaneous GAS infection against strains of more than one serotype.
Passive immunization protects mice against subcutaneous GAS infection.
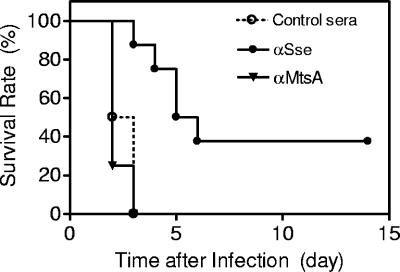
The observed protection could be due to nonspecific stimulation of innate immune responses. Passive immunization was used to examine this possibility. Groups of 10 CD-1 mice were administered 0.5 ml anti-Sse, anti-SPy0019, or control sera intraperitoneally and challenged subcutaneously with 2 × 108 CFU MGAS5005. The geometric titers of anti-Sse and anti-SPy0019 in the specific antisera were 17,030 and 28,100, respectively. The average anti-Sse and anti-SPy0019 titers of two checked mice of each group passively immunized with anti-Sse and anti-SPy0019 antisera were 3,400 and 6,200, respectively, 1 day after the passive immunization. While all the mice treated with the control and anti-SPy19 sera died 3 days after the infection, 3 of the 8 mice treated with anti-Sse antisera survived, and the dead mice of the Sse group survived longer than the control groups (Fig. 5). Passive immunization with anti-Sse antisera significantly protected mice against the subcutaneous GAS infection (P value versus the ALUM group, 0.0004; P value versus the SPy0019 group, 0.0002). The results indicate that the protection was mediated at least partially by specific antibodies to Sse.
FIG. 5.
Passive immunization with Sse immune serum protects mice against subcutaneous GAS infection. Eight 5-week-old female CD-1 mice were injected intraperitoneally with 0.5 ml of Sse-specific, SPy0019-specific, or control mouse serum, inoculated subcutaneously with 9 × 107 CFU of MGAS5005 3 h after the serum administration, and monitored daily to determine survival rates, which are presented.
Anti-Sse antisera are not opsonic.
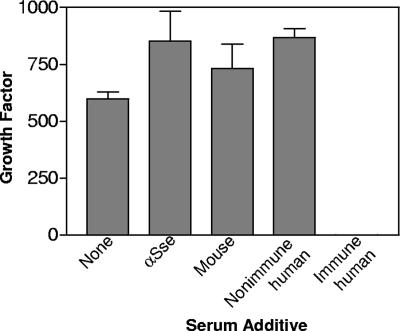
To determine whether Sse elicits antibody that is bactericidal and/or opsonic, the growth of MGAS5005 in nonimmune human blood containing anti-Sse or control mouse antiserum was assessed. While GAS treated with nonimmune human serum grew well in nonimmune human blood, GAS treated with immune human serum could not survive in human blood, indicating that the assay worked well in evaluating the bactericidal or opsonic activity of GAS antibodies. MGAS5005 grew slightly better in the presence of both anti-Sse and control sera than in the absence of the sera (Fig. 6). Thus, anti-Sse antibody could not inhibit GAS growth in blood. These results indicate that the basis for the observed protection is not anti-Sse antibody-mediated opsonization or bactericidal activity.
FIG. 6.
The lack of a detrimental effect of anti-Sse antibody on GAS growth in nonimmune human blood. Approximately 1,000 CFU of MGAS5005 was incubated with 100 μl of DPBS buffer, anti-Sse antiserum, control mouse serum, immune human serum, or nonimmune human serum at room temperature for 15 min and then combined with 0.9 ml of nonimmune human blood in triplicate. CFU of inocula and viable GAS in the samples after end-to-end rotation at 37°C for 4 h were determined by plating. The average growth factor (viable CFU/inoculum CFU) ± SD of a representative one out of three experiments is presented.
Inhibition of skin tissue invasion by Sse immunization.
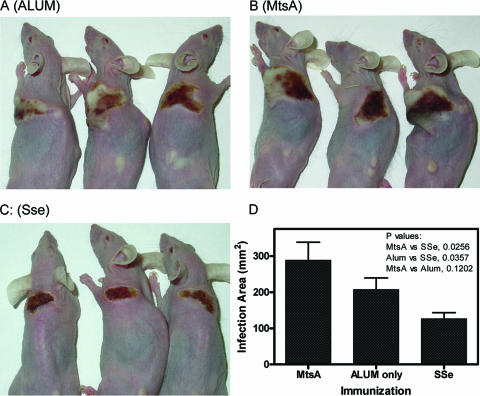
The results of the assessment of protection and growth in human blood suggest that Sse may play a role in skin invasion by GAS. To test the idea, immunocompetent Crl:SKH1-hrBR hairless mice were immunized with Sse, negative control MtsA, or adjuvant only and were challenged with 2 × 108 CFU MGAS5005. The geometric titer averages ± standard deviations of anti-Sse and anti-MtsA of mice checked were 18,600 ± 3,200 and 25,400 ± 6,400, respectively. On day 4 after the infection, 8 of 8, 7 of 8, and 6 of 8 mice treated with Sse/ALUM, MtsA/ALUM, and ALUM were still living. On this day, the Sse/ALUM-treated mice were in the process of resolving the infection, but the mice of the other two groups had bigger spreading lesions with pus-like edges (Fig. 7A, B, and C). The areas of the lesions in the Sse/ALUM-treated mice were significantly smaller than those in the other two groups (P value versus the ALUM group, 0.0357; P value versus the MtsA group, 0.0256), while the mice of these two groups did not show significant differences in their lesion sizes (P = 0.1202) (Fig. 7D). These results suggest that Sse plays a role in invasion of the skin tissue.
FIG. 7.
Active immunization with Sse inhibits the invasion of the skin tissue by GAS. Eight immunocompetent hairless female mice were immunized with 50 μg Sse/ALUM, 50 μg MtsA/ALUM, or ALUM on days 1, 14, and 28 and were inoculated subcutaneously with 50 μl of 2 × 108 CFU of MGAS5005 on day 50. Panels A (ALUM control), B (the MtsA negative control), and C (the Sse group) show the infection lesions of three representative mice in each group. (D) The average area ± SD of the lesions in each of the groups is shown. The data were from 8, 6, and 7 mice of the Sse, the MtsA (2 mice died), and the ALUM group (1 mouse died), respectively. The pictures and data were obtained on day 4 after the infection.
DISCUSSION
We have identified a GAS-secreted antigen, Sse, as a serine esterase and found that active and passive immunizations with Sse protect mice against subcutaneous GAS infection and that the active immunization also protects mice against subcutaneous infection with a heterologous GAS strain and decreases the size of GAS-caused lesions of the skin. These results suggest that Sse is involved in the invasion of the skin tissue by GAS and is a novel antigen protective in subcutaneous infection against GAS strains of more than one serotype.
Sse catalyzes the hydrolysis of PNPB, a chromogenic substrate of esterases and lipases. The GXSXG motif of esterases is present in Sse, and the residue 178Ser of the motif is essential for the esterase activity of Sse. Thereby, Sse is most likely a serine esterase that uses 178Ser as a catalytic residue. The protein is present in the culture supernatant but not in the proteins released from the cell wall or protoplast, and patients with streptococcal pharyngitis produced Sse-specific antibodies. Thus, Sse is a secreted antigen, which is produced both in vitro and in vivo.
Extracellular esterase was detected in the supernatant of GAS culture several decades ago, which had two antigenic variants (11, 12, 32). The Sse characterized in this study also has two variant complexes, which differ by about 37% in amino acid sequence. It appears that our Sse is the esterase detected previously. A BLAST search found that Sse has homologs in group B Streptococcus, Streptococcus equi, and Staphylococcus aureus. These homologs share 35 to 76% identity in amino acid sequences (Table 2) and have putative secretion signal sequences. Thus, these pathogens appear to secrete esterases that are similar to Sse.
TABLE 2.
Identity of amino acid sequences among M1 Sse and its homologs in group B Streptococcus (GBS), Streptococcus equi, and Staphylococcus aureusa
| Organism | % Identity of Sse
|
||||
|---|---|---|---|---|---|
| M1 GAS | GBS (Sal_0446) | S. equi | S. aureus | GBS (Sal_0839) | |
| M1 GAS | 100 | ||||
| GBS (Sal_0446) | 76 | 100 | |||
| S. equi | 62 | 62 | 100 | ||
| S. aureus | 33 | 32 | 32 | 100 | |
| GBS (Sal_0839) | 39 | 41 | 45 | 34 | 100 |
The deduced amino acid sequences of Sse and its homologs were from GenBank or other databases, as follows: M1 GAS, accession no. NC_002737; GBS (Sal_0446), accession no. NZ_AAJP01000032; S. equi, available from http://www.sanger.ac.uk/Projects/S_equi/; S. aureus, accession no. CP000046; and GBS (Sal_0839), accession no. NZ_AAJP01000019.
One difficulty in the development of M protein-based GAS vaccines is the type-specific protection due to the variation in the N-terminal region of the M protein. Identification of a conserved protective antigen would be critical to the development of a vaccine with broad protection. Immunization of mice with Sse of serotype M1 provides significant protection against subcutaneous infection with virulent strains M1 and M3, in which Sse is highly conserved. Sse has only two variant complexes in the strains, representing 10 different serotypes. Inclusion of the two variants in a vaccine might provide protection against strains of various serotypes. We are in the process of testing this idea.
The protection conferred by the Sse immunization could be due to nonspecific stimulation of innate immune responses in the Sse/ALUM-inoculated group. The Sse immune serum, but not the sera of the adjuvant and negative protein controls, protected mice in the passive immunization and challenge experiment. These results strongly suggest that the protection was at least partially mediated by specific antibodies to Sse, instead of innate immune responses.
Our data also suggest that Sse plays a role in the invasion of the skin tissue. Anti-Sse antisera did not inhibit the growth of GAS in nonimmune human blood. This result suggests that anti-Sse antibodies are not opsonic, which is expected since Sse is a secreted protein. In addition, it has been suggested that only the M protein is able to induce antibodies that override the resistance of GAS to phagocytosis by polymorphonuclear leukocytes (9). Second, the result suggests that anti-Sse antibodies cannot control systemic GAS infection. GAS disseminates from the subcutis in the subcutaneous infection, resulting in systemic infection and then death. Immunization with Sse protects mice in the subcutaneous infection, supporting the idea that Sse is important for GAS to cross the skin barrier. The immunization with Sse significantly inhibits the spreading of GAS in the skin, as evidenced by the smaller lesion size in the Sse/ALUM group of the hairless mice than in the adjuvant and negative control groups, further supporting this idea.
Sse possesses esterase activity. However, the in vivo substrate(s) of Sse is(are) not known yet. The elucidation of its native target(s) would unveil the basis for the involvement of Sse in the invasion of the skin tissue and the mechanism of the anti-Sse antibodies-mediated protection.
Acknowledgments
This work was supported in part by National Institutes of Health grants P20 RR-020185 and K22 AI057347, U.S. Department of Agriculture National Research Initiative/competitive grants programs grant 2004-35204-14637 and Formula Funds, and the Montana State University Agricultural Experimental Station.
We thank Maki Fukumura and Tracey Hanks for technical support.
Editor: F. C. Fang
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Beachey, E. H., J. M. Seyer, J. B. Dale, W. A. Simpson, and A. H. Kang. 1981. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature 292:457-459. [DOI] [PubMed] [Google Scholar]
- 2.Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, J. K. McCormick, D. Y. M. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessen, D., and V. A. Fischetti. 1990. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J. Immunol. 145:1251-1256. [PubMed] [Google Scholar]
- 4.Brandt, E. R., K. S. Sriprakash, R. I. Hobb, W. A. Hayman, W. Zeng, M. R. Batzloff, D. C. Jackson, and M. F. Good. 2000. New multi-determinant strategy for a group A streptococcal vaccine designed for the Australian Aboriginal population. Nat. Med. 6:455-459. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale, J. B., E. Y. Chiang, S. Liu, H. S. Courtney, and D. L. Hasty. 1999. New protective antigen of group A streptococci. J. Clin. Investig. 103:1261-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale, J. B., T. Penfound, E. Y. Chiang, V. Long, S. T. Shulman, and B. Beall. 2005. Multivalent group A streptococcal vaccine elicits bactericidal antibodies against variant M subtypes. Clin. Diagn. Lab. Immunol. 12:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischetti, V. A. 2006. Vaccine approaches to protect against group A streptococcal pharyngitis, p. 113-122. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens, 2nd ed. ASM Press, Washington, DC.
- 10.Guzman, C. A., S. R. Talay, G. Molinari, E. Medina, and G. S. Chhatwal. 1999. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J. Infect. Dis. 179:901-906. [DOI] [PubMed] [Google Scholar]
- 11.Hayano, S., and A. Tanaka. 1973. Extracellular esterases of group A streptococci. Infect. Immun. 7:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayano, S., and A. Tanaka. 1973. Distribution of antibodies to streptococcal esterases in patients with scarlet fever. Extracellular esterases of group A streptococci. Infect. Immun. 15:300-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirst, G. K., and R. C. Lancefield. 1939. Antigenic properties of the type-specific substances derived from group A hemolytic streptococci. J. Exp. Med. 69:425-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoe, N. P., K. Nakashima, D. Grigsby, X. Pan, S. J. Dou, S. Naidich, M. Garcia, E. Kahn, D. Bergmire-Sweat, and J. M. Musser. 1999. Rapid molecular genetic subtyping of serotype M1 group A Streptococcus strains. Emerg. Infect. Dis. 5:254-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji, Y., B. Carlson, A. Kondagunta, and P. P. Cleary. 1997. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect. Immun. 65:2080-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapur, V., J. T. Maffei, R. S. Greer, L. L. Li, G. J. Adams, and J. M. Musser. 1994. Vaccination with streptococcal extracellular cysteine protease (interleukin-1β convertase) protects mice against challenge with heterologous group A streptococci. Microb. Pathog. 16:443-450. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata, S., E. Kunitomo, Y. Terao, I. Nakagawa, K. Kikuchi, K. Totsuka, and S. Hamada. 2001. Systemic and mucosal immunizations with fibronectin-binding protein FBP54 induce protective immune responses against Streptococcus pyogenes challenge in mice. Infect. Immun. 69:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancefield, R. C., and G. E. Perlmann. 1952. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J. Exp. Med. 96:83-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei, B., S. Mackie, S. Lukomski, and J. M. Musser. 2000. Identification and immunogenicity of group A Streptococcus culture supernatant proteins. Infect. Immun. 68:6807-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and acquired immunity by a bacterial homologue of CD11b that inhibits opsonophagocytosis. Nat. Med. 7:1298-1305. [DOI] [PubMed] [Google Scholar]
- 21.Lei, B., L. M. Laura, H. M. Menning, J. M. Voyich, S. V. Kala, F. R. DeLeo, S. D. Reid, and J. M. Musser. 2002. Identification and characterization of a novel heme-associated cell surface protein made by Streptococcus pyogenes. Infect. Immun. 70:4494-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei, B., M. Liu, J. M. Voyich, C. I. Prater, S. V. Kala, F. R. DeLeo, and J. M. Musser. 2003. Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes. Infect. Immun. 71:5962-5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei, B., M. Liu, G. Chesney, and J. M. Musser. 2004. Extracellular putative lipoproteins made by Streptococcus pyogenes: identification of new potential vaccine candidate antigens. J. Infect. Dis. 189:79-89. [DOI] [PubMed] [Google Scholar]
- 24.Liu, M., T. S. Hanks, J. Zhang, M. J. McClure, D. W. Siemsen, J. L. Elser, M. T. Quinn, and B. Lei. 2006. Defects in ex vivo and in vivo growth and sensitivity to osmotic stress of group A Streptococcus caused by interruption of response regulator gene vicR. Microbiology 152:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukomski, S., C. A. Montgomery, J. Rurangirwa, R. S. Geske, J. P. Barrish, G. J. Adams, and J. M. Musser. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick, J. K., T. J. Tripp, S. B. Olmsted, Y. V. Matsuka, P. J. Gahr, D. H. Ohlendorf, and P. M. Schlievert. 2000. Development of streptococcal pyrogenic exotoxin C vaccine toxoids that are protective in the rabbit model of toxic shock syndrome. J. Immunol. 165:2306-2312. [DOI] [PubMed] [Google Scholar]
- 27.McNeil, S. A., S. A. Halperin, J. M. Langley, B. Smith, A. Warren, G. P. Sharratt, D. M. Baxendale, M. A. Reddish, M. C. Hu, S. D. Stroop, J. Linden, L. F. Fries, P. E. Vink, and J. B. Dale. 2005. Safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114-1122. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki, H., M. Igarashi, M. Nishi, M. Tajima, M. Sekiya, S. Okazaki, N. Yahagi, K. Ohashi, K. Tsukamoto, M. Amemiya-Kudo, T. Matsuzaka, H. Shimano, N. Yamada, J. Aoki, R. Morikawa, Y. Takanezawa, H. Arai, R. Nagai, T. Kadowaki, J. Osuga, and S. Ishibashi. 2006. Identification of a novel member of the carboxylesterase family that hydrolyzes triacylglycerol: a potential role in adipocyte lipolysis. Diabetes 55:2091-2097. [DOI] [PubMed] [Google Scholar]
- 29.Roggiani, M., J. A. Stoehr, S. B. Olmsted, Y. V. Matsuka, S. Pillai, D. H. Ohlendorf, and P. M. Schlievert. 2000. Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect. Immun. 68:5011-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt, W. C. 1960. Type-specific antibody formation in man following injection of streptococcal M protein. J. Infect. Dis. 106:250-255. [DOI] [PubMed] [Google Scholar]
- 31.Shirai, K., and R. L. Jackson. 1982. Lipoprotein lipase-catalyzed hydrolysis of p-nitrophenyl butyrate. J. Biol. Chem. 257:1253-1258. [PubMed] [Google Scholar]
- 32.Stock, A. H., J. Uriel, and P. Grabar. 1961. Esterase in extracellular concentrates of group A streptococci and the homologous antibody. Nature 192:434-435. [DOI] [PubMed] [Google Scholar]
- 33.Sumby, P., K. D. Barbian, D. J. Gardner, A. R. Whitney, D. M. Welty, R. D. Long, J. R. Bailey, M. J. Parnell, N. P. Hoe, G. G. Adams, F. R. Deleo, and J. M. Musser. 2005. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. USA 102:1679-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitaker, J. R. 1972. The esterases, p. 481-501. In Principles of enzymology for the food sciences. Marcel Dekker, Inc., New York, NY.
- 35.Zabriskie, J. B., T. Poon-King, M. S. Blake, F. Michon, and M. Yoshinaga. 1997. Phagocytic, serological, and protective properties of streptococcal group A carbohydrate antibodies. Adv. Exp. Med. Biol. 418:917-919. [DOI] [PubMed] [Google Scholar]