Abstract
Adherence of uropathogenic Escherichia coli to host tissue is required for infection and is mediated by fimbriae, such as pyelonephritis-associated pili (Pap). Expression of P fimbriae is regulated by phase variation, and to date, phase transition frequencies have been measured only for pap regulatory region constructs integrated into the E. coli K-12 chromosome. The aim of this work was to measure P phase transition frequencies in clinical isolates for the first time, including frequencies for the sequenced strain E. coli CFT073. P fimbriation and associated phase transition frequencies were measured for two E. coli clinical isolates and compared with levels for homologous pap constructs in E. coli K-12. Fimbriation and off-to-on transition frequencies were always higher in the clinical isolate. It was concluded that the regulatory inputs controlling papI expression are likely to be different in E. coli CFT073 and E. coli K-12 as (i) phase variation could be stimulated in E. coli K-12 by induction of papI and (ii) the level of expression of a papI::gfp+ fusion was higher in E. coli CFT073 than in E. coli K-12. Furthermore, phase transition frequencies for the two E. coli CFT073 pap clusters were shown to be different depending on the culture conditions, indicating that there is a hierarchy of expression depending on signal inputs.
Urinary tract infections (UTIs) are some of the most common human bacterial infections and are estimated to affect 150 million people worldwide and to cost more than $6 billion annually in direct health care expenditures. These infections include asymptomatic bacteriuria, cystitis, and acute pyelonephritis. The latter presents with severe clinical symptoms and can result in renal scarring and renal failure in children (53). Uropathogenic Escherichia coli (UPEC) is the leading cause of UTIs and is responsible for up to 80% of uncomplicated cases and more than 30% of nosocomial infections (8). The initial colonization and persistence of E. coli in the urinary tract require coordinated expression of multiple virulence factors, including fimbriae, iron acquisition systems, and toxins. Fimbrial adhesins promote colonization by binding to specific receptors on the surface of host cells. The type 1 fimbrial adhesin, FimH, binds α-d-mannose-containing receptors abundant in the bladder (11), and PapG, the P fimbria tip adhesin, recognizes kidney glycosphingolipids containing the Gal-α(1-4)β-Gal moiety (26-28). Epidemiological studies have established that there is a strong link between P fimbriae and pyelonephritis (3, 37), and recent studies have demonstrated that P fimbriae can trigger proinflammatory cascades in the human urinary tract (42, 51).
P fimbriae are encoded by the pyelonephritis-associated pilus (pap) operon that contains the structural genes papA, -E, -F, -G, and -H, the usher gene papC, the periplasmic chaperone gene papD, and genes encoding two regulators, papB and papI (4, 45). UPEC isolates associated with symptomatic disease are more likely to contain multiple P fimbria operons (20). Expression of P fimbriae is regulated in response to growth and environmental conditions (2, 7, 16, 50) and is subject to phase variation. Phase variation is dependent on a reversible epigenetic switch that controls the initiation of transcription of the pap operon genes, resulting in variable (on/off) expression of the structural subunits (6, 7). The switch involves the formation of protein complexes on one of two GATC methylation sites present in the pap regulatory region (papI-papB intergenic region). These GATC sites are distal (GATCdist) and proximal (GATCprox) to the main operon promoter (PBA), are methylation targets for DNA adenine methylase (Dam), and overlap two binding sites for the global regulator leucine-responsive regulatory protein (Lrp) (9, 10). Competition between Dam and Lrp for access to these sites results in two different methylation patterns that determine whether the pap operon is transcriptionally on or off (48, 49). Formation of the phase-on state also requires the pap-encoded regulator PapI, which is transcribed by a separate divergent promoter (PI) upstream of the main operon promoter (PBA). PapI has been shown to favor Lrp binding to promoter distal sites when GATCprox is methylated by Dam, promoting formation of the on phase (18, 24). A second pap-encoded protein, PapB, has been shown to indirectly regulate pap phase variation by activating papI transcription and to act as a transcriptional repressor at higher concentrations by binding to sites overlapping the PBA promoter and the papB coding sequence (14, 16).
Phase variation frequencies are important as they determine the proportion of the population expressing fimbriae and also govern expression of other surface factors at the single-cell level through positive and negative cross talk (19-21, 52). P fimbria phase variation frequencies have been studied extensively using lysogenic reporter constructs in an E. coli K-12 background (6, 7, 50), but little is known about pap phase variation frequencies in clinical isolates. What is clear is that the measured frequencies in E. coli K-12 are not predictive of the high P fimbriation levels reported for several UPEC clinical isolates (29, 34, 36).
In the current study, pap phase variation frequencies were measured for the first time in a clinical isolate, the sequenced strain E. coli CFT073, and were shown to be markedly higher than the frequencies measured for the same operons in E. coli K-12. The isolate background provides a regulatory context that allows pap phase variation to occur at frequencies high enough to account for the level of P fimbriation determined in this study for E. coli CFT073. Phase variation frequencies were determined for different environmental conditions, including human urine, and were shown to be equivalent to those exhibited in other bacterial phase-variable systems. Homologous pap clusters in E. coli CFT073 were shown to be differentially regulated, with cross talk between operons dependent on the culture conditions.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains, plasmids, and primer sequences are listed in Table 1. All genetic cloning steps were carried out with E. coli AAEC185A. E. coli CFT073 contains two Pap clusters, P1 and P2 (31), that show 98% sequence identity across the complete regions (papI-papG) and 96.2% identity across their regulatory regions (papI-papB). The F13 Pap cluster from E. coli J96 (23) shares 91% identity with P1 and 92% identity with P2 of E. coli CFT073 across the whole cluster and 96 and 95% identity, respectively, across the papI-papB regulatory region. All plasmid transformations into E. coli CFT073 (wild type and derivatives) were carried out by electroporation. The following strategy was used to generate plasmids and strains for all single-copy reporter fusions. First, DNA used for fusions was amplified by PCR and cloned into BamHI-KpnI sites in exchange vectors containing either lacZ (pAJR36 and pKC29) or gfp (pAJR28 and pKC47) reporters, flanked by DNA specific for K-12 (pAJR36 and pAJR28) or CFT073 (pKC29 and pKC47) backgrounds. The primers used to generate clones are listed in Table 1. Second, the plasmids were transformed into intermediate allelic-exchange strains (ZAP1164, ZAP957, and ZAP964), and the fusions were integrated onto the chromosome by homologous recombination and counterselection, as described previously (5, 12, 38). The resulting strains are listed in Table 1. A similar strategy was used to generate ZAP594, where pLD1 containing the complete F13 pap cluster was transformed into AAEC090A and integrated into the MG1655 chromosome. Strain ZAP965 was generated through a series of intermediate strains (Table 1) to delete the regulatory region from the 3′ end of papI to the 5′ end of papA for both P1 and P2 operons of E. coli CFT073. The flanking regions incorporating papI were amplified by PCR using primer P1UPfor (CFT073 P1) or P2UPfor (CFT073 P2) together with P12SUPrev (both P1 and P2), and the flanking regions incorporating papA were amplified by PCR using P12DNfor (both P1 and P2) and P1DNrev (CFT073 P1) or P2DNrev (CFT073 P2). The flanking regions were cloned into the SacI/BamHI (papI) and BamHI/XbaI (papA) sites in pUC18 to generate pNJH107 (CFT073 P1) and pNJH117 (CFT073 P2). The flanking regions were subcloned into the SacI and XbaI sites in pIB307 and the sac-kan cassette was cloned in the BamHI site to generate pMT11 (CFT073 P1) and pMT17 (CFT073 P2). Plasmid pMT24 contains a transcriptional fusion of the papI promoter to gfp+ and was generated by cloning the promoter region of papI (primers papI P1for and papI P1rev) into the XbaI site of pKC26.
TABLE 1.
Strains, plasmids, and primers
| Strain, plasmid, or primer | Genotype or sequence | Reference, source, or use |
|---|---|---|
| Strains | ||
| AAEC185A | F− λ−supE44 hsdR17 mcrA endA1 thi-1 Δ(fimBEACDFGH) ΔrecA | 5 |
| MG1655 | K-12 F− λ− | Guyer et al.a |
| J96 | E. coli pyelonephritis isolate, pap prs | 23 |
| CFT073 | E. coli pyelonephritis isolate, P1 pap, P2 pap | 31 |
| AAEC090A | Intermediate allelic-exchange strain with sacB-neo cassette placed in the lac locus of MG1655 | 5 |
| ZAP593 | Allelic exchange of papAJ96F13::lacZ from pLD7 in ZAP1164 | This study |
| ZAP594 | Allelic exchange of papJ96 F13 cluster from pLD1 in AAEC090A | This study |
| ZAP595 | Allelic exchange of papACFT072 P2::lacZ from pMT06 in ZAP964 | This study |
| ZAP957 | Intermediate allelic-exchange strain with sacB-neo cassette placed in the lac locus of CFT073 | This study |
| ZAP714 | Allelic exchange of papACFT073 P1::gfp from pMT01 in ZAP1164 | This study |
| ZAP833 | Allelic exchange of papACFT073 P1::gfp from pMT32 in ZAP957 | This study |
| ZAP834 | Allelic exchange of papACFT073 P2::gfp from pMT22 in ZAP957 | This study |
| ZAP838 | Allelic exchange of papACFT073 P1::lacZ from pMT31 in ZAP957 | This study |
| ZAP843 | Allelic exchange of papACFT073 P1::lacZ from pMT31 in ZAP964 | This study |
| ZAP955 | Allelic exchange of papACFT073 P2::gfp from pKC10 in ZAP1164 | This study |
| ZAP964 | Intermediate allelic-exchange strain with sacB-neo cassette placed in the lac locus of ZAP965 | This study |
| ZAP965 | Intermediate strain with sacB-neo removed from ZAP966 to generate ΔpapI-BCFT073 P2 ΔpapI-BCFT073 P1 | This study |
| ZAP966 | Intermediate strain with sacB-neo cassette placed in papI-BCFT073 P2 of ZAP969 | This study |
| ZAP969 | Intermediate strain with sacB-neo removed from ZAP972 to generate ΔpapI-BCFT073 P1 | This study |
| ZAP972 | Intermediate strain with sacB-neo cassette placed in papI-BCFT073 P1 of CFT073 | This study |
| ZAP992 | Allelic exchange of papACFT073 P1::lacZ from pKC41 in ZAP1164 | This study |
| ZAP996 | Allelic exchange of papAJ96F13::lacZ from pKC37 in ZAP957 | This study |
| ZAP1137 | Allelic exchange of papACFT073 P2::lacZ from pMT06 in ZAP957 | This study |
| ZAP1164 | Intermediate allelic-exchange strain, lacZYA replaced with sacB-neo cassette in MG1655 | 38 |
| Plasmids | ||
| pUC18 | Commercial cloning vector (Ampr) (NEB, United States) | Our stock |
| pBR322 | Commercial cloning vector (Ampr) (NEB, United States) | Our stock |
| pACYC184 | Commercial cloning vector (Cmr) (NEB, United States) | Our stock |
| pPap5 | pBR322 with J96 F13 pap cluster, Apr | 28 |
| pAJR25 | pIB307 with MG1655 lacI and lacA regions | 38 |
| pAJR28 | pAJR25 with promoterless gfp gene placed between MG1655 lacI and lacA | 20 |
| pAJR32 | pAJR25 with sac-kan cassette placed between MG1655 lacI and lacA | 38 |
| pAJR36 | pAJR25 with promoterless lacZ gene placed between MG1655 lacI and lacA | 38 |
| pAJR145 | pACYC184 with rpsM::gfp+ transcriptional fusion, Cmr | 41 |
| pHGM98 | pACYC184 with inducible papI, Tcr | 14 |
| pIB307 | Allelic-exchange temperature-sensitive vector, pSC101 replicon, Cmr | 5 |
| pIB462 | pIB307 with MG1655 lac flanking regions | 5 |
| pKC8 | pIB307 with CFT073 lacI and lacA regions | 20 |
| pCK10 | pAJR28 with papI-ACFT073 P2 placed between MG1655 lacI and lacA | This study |
| pKC11 | pKC8 with sac-kan cassette placed between CFT073 lacI and lacA | 20 |
| pKC26 | pAJR145 with rpsM removed | This study |
| pKC29 | pKC8 with promoterless lacZ placed between CFT073 lacI and lacA | This study |
| pKC37 | pKC29 with papI-AJ96 F13 placed between CFT073 lacI and lacA | This study |
| pKC41 | pAJR36 with papI-ACFT073 P1 placed between MG1655 lacI and lacA | This study |
| pKC47 | pKC8 with promoterless gfp placed between CFT073 lacI and lacA | This study |
| pLD1 | pIB462 with 9.6-kb fragment containing the papJ96 F13 cluster from pPap5 subcloned into EcoRI-BamHI sites | This study |
| pLD7 | pAJR36 with papI-AJ96 F13 placed between MG1655 lacI and lacA | This study |
| pMT01 | pAJR28 with papI-ACFT073 P1 placed between MG1655 lacI and lacA | This study |
| pMT06 | pKC29 with papI-ACFT073 P2 placed between CFT073 lacI and lacA | This study |
| pMT11 | pIB307 with sac-kan cassette placed between papCFT073 P1 flanking regulatory regions | This study |
| pMT17 | pIB307 with sac-kan cassette placed between papCFT073 P2 flanking regulatory regions | This study |
| pMT22 | pKC8 with papI-ACFT073 P2 placed between CFT073 lacI and lacA | This study |
| pMT24 | pKC26 with papICFT073 P1 promoter fused to gfp | This study |
| pMT31 | pKC29 with papI-ACFT073 P1 placed between CFT073 lacI and lacA | This study |
| pMT32 | pKC47 with papI-ACFT073 P1 placed between CFT073 lacI and lacA | This study |
| pNJH107 | pUC18 with upstream and downstream papCFT073 P1 flanking regulatory regions | This study |
| pNJH117 | pUC18 with upstream and downstream papCFT073 P2 flanking regulatory regions | This study |
| Primersb | ||
| J96 P for | 5′-CGCGGATCCGGCCATGCAGTAAAACCGG | pLD7 |
| J96 P rev | 5′-CGGGTACCCCCCTGTGGAATAGTTGGAG | |
| CFT P1 for | 5′-CGCGGATCCGAAGTTTATGGCGTTTGTATTTTG | pMT01 |
| CFT P1 rev | 5′-CGGGTACCCCCCTGAGGAATAGTTGG | |
| CFT P2 for | 5′-CGCGGATCCCTGATTCGTCATTCTATTCTTATTGA | pKC10 |
| CFT P2 rev | 5′-CGGGTACCGCCTTGAGGGATAGATGCA | |
| CFTP1+2 for | 5′-CGCGGATCCGTTTCAGTGAAGCATGCCCAC | pMT32, pMT22 |
| CFTP1+2 rev | 5′-CGGGTACCCATAAATAACAACCTCTTTTTCATTAC | pMT21, pMT06 |
| papIP1for | 5′-CGCTCTAGACATATATTCACTCATCTCACTG | pMT24 |
| papIP1rev | 5′-CGCTCTAGAGTTTCCCCCTTCTGTCGGGC | |
| P1UPfor | 5′-GCTGAGCTCCGGTTCAGTAATATCTGA | pNJH107 |
| P2UPfor | 5′-GCTGAGCTCGTGCCGACGATCCCCTGA | pNJH117 |
| P12SUPrev | 5′-GCTGGATCCTTCACTGAAACAGATAAAWGT | pNJH107, pNJH117 |
| P12DNfor | 5′-CGGGATCCATGGTACCTCGGTTATTGCCGGTGCG | pNJH107, pNJH117 |
| P1DNrev | 5′-CGCTCTAGACCATCTTTTCTGACGGCAGC | pNJH107 |
| P2DNrev | 5′-CGCTCTAGACTATTATCTTTCTTAACAAATGC | pNJH117 |
M. S. Guyer, R. R. Reed, J. A. Steitz, and K. B. Low, presented at the Cold Spring Harbor Symposium on Quantitative Biology, 1981.
Restriction sites are underlined.
Culture conditions.
The medium used for culturing strains and plasmids was Luria-Bertani broth or agar (BDH Merck) supplemented with 25 μg ml−1 tetracycline, 25 μg ml−1 chloramphenicol, or 25 μg ml−1 ampicillin when necessary. Colonization factor antigen (CFA) medium was used for optimal expression of P fimbriae (13). M9 medium contained M9 salts supplemented with thiamine (20 mM), glycerol, or glucose (0.2%). Essential and nonessential amino acids (Sigma-Aldrich, Dorset, United Kingdom) were added to generate rich defined (RD) medium. Human urine was obtained from eight healthy volunteers with no history of UTI or antibiotic usage in the previous 6 months. The urine was pooled, filtered sterilized, stored in aliquots at −20°C, and used within 2 weeks. Urine was added at a final concentration of 0.5× to agar to make plates or was used neat for static growth experiments.
Phase transition determination using lacZ reporter strains.
To determine phase transition frequencies under a number of different conditions, the following strategy was used. Bacteria were grown on CFA agar containing 5-bromo-4-chloro-3-indolyl-β-d-galactosylpyranoside (X-Gal) (40 μg ml−1) at 37°C, from which either blue (mostly phase-on) or white (mostly phase-off) colonies were picked, serially diluted in phosphate-buffered saline (PBS), and plated onto the appropriate medium also containing X-Gal. At least six colonies from the various media were then serially diluted in PBS and plated onto RD M9 medium with glycerol and X-Gal to allow the proportions of phase-on and phase-off colonies to be determined. The frequency of phase transition was calculated using the equation used by Blyn et al. (7). Briefly, to determine the level of off-to-on transition per cell per generation, the proportion of phase-on CFU was divided by the number of generations that arose from the starting CFU. To determine the on-to-off transition per cell per generation, the proportion of phase-off CFU was used. The various conditions used were as follows: for phase transition on rich undefined media, CFA agar containing X-Gal; for different environmental conditions, minimal M9 medium with glycerol, RD M9 medium with glycerol, or RD M9 medium with glucose; and for phase transition on urine, urine plates. To determine the off-to-on phase transition in static urine, single colonies that were phase off were selected from RD M9 medium containing glucose and diluted to a concentration of approximately 5 CFU ml−1 in PBS, and paired samples were grown statically at 37°C in 5 ml urine or 5 ml RD M9 medium with glycerol for 24 h at 37°C. Bacteria were then serially diluted and plated onto RD M9 medium with glycerol to determine the proportions of phase-on and phase-off bacteria. The starting phase of the initial colonies was also determined by plating samples onto RD M9 medium with glycerol. The colonies that were demonstrated to contain >2% phase-on cells from RD M9 medium with glucose were excluded from the analysis.
Indirect immunofluorescence assay for P fimbriae.
For labeling P fimbriae on the bacterial surface, UPEC colonies were collected from CFA agar plates after overnight growth at 37°C using 3 ml of PBS. Bacterial suspensions having an optical density at 600 nm of 0.6 were prepared and washed twice in 1 ml PBS and then mixed with the appropriate primary anti-P rabbit polyclonal serum diluted 1:50 in PBS and incubated for 30 min at room temperature. For detection of F13 P fimbriae an anti-PapA antibody supplied by B. E. Uhlin was used, whereas for detection of the E. coli CFT073 P1 and P2 fimbriae polyclonal serum provided by T. Korhonen was used (OM12) (36). Different primary antibodies were used, reflecting the different Pap types being detected. Excess primary antibody was removed with three washes in 1 ml PBS, and cells were then mixed with the secondary antibody goat Alexa Fluor 488 conjugated with anti-rabbit immunoglobulin G (1:500 in PBS; Molecular Probes) for 30 min at room temperature. Excess secondary antibody was removed with three washes in 1 ml PBS.
Flow cytometry and microscopy.
Fluorescence from either serum-labeled P-fimbriate bacteria or strains possessing a single-copy fluorescent reporter fusion was detected using a FACSCalibur flow cytometer (Becton Dickinson). Acquisition and analysis of flow cytometry data were performed using the CELLQuest software. The selected R1 region was optimized in order to exclude small particles and debris using sized beads (Becton Dickinson) and multiple E. coli strains. The gate for the detection of fluorescence signals was set such that cells under investigation were considered positive when their fluorescence intensity (FL-1 height) exceeded that of all but a very small fraction (0.5%) of the negative control population of the same UPEC strain grown under the same conditions but labeled with only the secondary antibody or the nonfluorescent parent of the fusion strain. Fluorescence microscopy image acquisition and analysis were performed using Improvision OpenLab software. Samples were prepared as described above and fixed onto slides with 4% paraformaldehyde.
Whole-population fluorescence measurements.
pKC26 and pMT24 were transformed into AAEC185A, CFT073, and ZAP965, and single transformants were cultured for approximately 18 h in CFA medium supplemented with 25 μg ml−1 chloramphenicol. Samples were diluted to an optical density at 600 nm of 1.0, and replicate samples (200 μl per well) were assayed with a Flurstar Optima fluorimeter, using absorbance at 485 nm and emission at 520 nm, at a gain of 1,500. The results were corrected for background autofluorescence by subtraction of pKC26 levels at the equivalent optical densities.
RESULTS
Comparison of P fimbriation in UPEC clinical isolates with a cloned pap operon in E. coli K-12.
P fimbriation was measured in two well-characterized clinical E. coli UPEC isolates that both contain two homologous pap operons, isolates J96 (23) and CFT073 (31), and compared with P fimbriation in an E. coli K-12 derivative containing a complete pap operon. Measurements were obtained using single colonies cultured on CFA medium, culture conditions known to promote P fimbria expression (13, 20). Colonies of the pyelonephritis isolate E. coli J96 (Fig. 1A to C) were shown by flow cytometry to contain an average of 18% ± 2% P-fimbriate bacteria labeled with a polyclonal anti-PapA antiserum (32). In E. coli CFT073, the positive proportion determined by flow cytometry was found to be 4.8% ± 0.4% using an anti-P polyclonal serum (Fig. 1F), OM12 (35). This percentage is an underestimate as this polyclonal serum caused a degree of bacterial aggregation. Counting P-fimbria-positive bacteria by immunofluorescence microscopy produced proportions between 10 and 20% depending on the colony measured (Fig. 1D and E). For comparison to assess fimbriation levels in E. coli clinical isolates, the complete F13 pap operon (papI-papG) from E. coli J96 (28, 30) was inserted into the E. coli K-12 chromosome at the lac locus, generating ZAP594 (Table 1). In this strain, only 0.26% ± 0.02% of the bacterial population fell within the gate for fluorescence detection (Fig. 1I). While this value is at the limit of detection by flow cytometry, positively stained bacteria were clearly detectable by microscopy at a low frequency (micrographs are shown in Fig. 1G and H), and the geometric mean for the gated population (Fig. 1I) was higher than that for a negative control (data not shown). E. coli MG1655 transformed with pPap5, a multicopy plasmid that contains the complete F13 pap operon from E. coli J96, was included as a positive control and resulted in an 82.8%-positive subpopulation with the anti-PapA antiserum (Fig. 1L).
FIG. 1.
Immunofluorescence microscopy and flow cytometry of P fimbriation in different E. coli strains. (A, D, G, and J) Phase-contrast images. (B, E, H, and K) Fluorescence images. (C, F, I, and L) Flow cytometry histograms. The gate for fluorescence detection is indicated by M1 and was set as defined in Materials and Methods. The proportion of fluorescent events is indicated in the upper right corner of each histogram. (A, B, and C) E. coli J96; (D, E, and F) E. coli CFT073; (G, H, and I) E. coli ZAP594; (J, K, and L) E. coli MG1655 transformed with pPap5. For panel H, multiple fields had to be scanned to detect a single P-fimbria-positive bacterium, indicating the low F13 Pap off-to-on transition frequency in the E. coli K-12 background.
Reporter fusions to GFP reflect fimbriation levels in clinical isolates and the E. coli K-12 background.
Green fluorescent protein (GFP) reporter fusions to both the E. coli CFT073 pap operons (P1 and P2) were made and inserted into both the E. coli CFT073 and E. coli K-12 chromosomes at lac, generating strains ZAP833 and ZAP834 and strains ZAP714 and ZAP955, respectively (Table 1). The pap regulatory regions used included both papI and papB and the first codon of papA (Fig. 2). The proportions of fluorescent bacteria were then determined by flow cytometry. The proportions of E. coli CFT073 cells expressing P1 and P2 papA::gfp were 24% ± 1.9% and 32% ± 3.6%, respectively. In contrast, the proportions of E. coli K-12 cells expressing P1 and P2 papA::gfp were 0.23% ± 0.04% and 0.37% ± 0.13%, respectively. These data confirmed that there were marked differences in P fimbria expression levels between the E. coli CFT073 and E. coli K-12 genetic backgrounds.
FIG. 2.
Genetic organization of pap gene cluster regulatory region. The open reading frames for papI, papB, and papA are shown, together with the promoter regions for papBA and papI. The black bars represent the distal and proximal GATC Dam methylation sites, and the gray bars represent the Lrp binding sites. The region used for regulatory fusions to LacZ and GFP is also shown.
Measurement of pap phase transition frequencies in E. coli CFT073.
In order to measure phase transition frequencies in a clinical isolate background, fusions of the E. coli CFT073 P1 and P2 and E. coli J96 F13 regulatory regions to lacZ were made and inserted into the E. coli CFT073 chromosome at lac, generating ZAP838, ZAP1137, and ZAP996, respectively. The J96 pap operon fusion phase varied at a high frequency, >1 × 10−2 per cell per generation. The off-to-on phase transition frequency of the CFT073 P1 operon was 4.4 × 10−3 ± 0.3 × 10−3 per cell per generation, and the off-to-on phase transition frequency was 6.1 × 10−3 ± 0.6 × 10−3 per cell per generation for the P2 operon. For comparison, a fusion to P1 was constructed in E. coli K-12, and the off-to-on frequency determined was <1 × 10−5 per cell per generation. Phase transition was also measured in the on-to-off direction and was 3.3 × 10−2 ± 0.08 × 10−2 per cell per generation for CFT073 P1 and 3.5 × 10−2 ± 0.02 × 10−2 per cell per generation for P2 in the CFT073 background. These on-to-off frequencies were found to be consistent when they were measured under a number of different conditions (data not shown).
Correlation of fimbriation levels and phase transition frequencies.
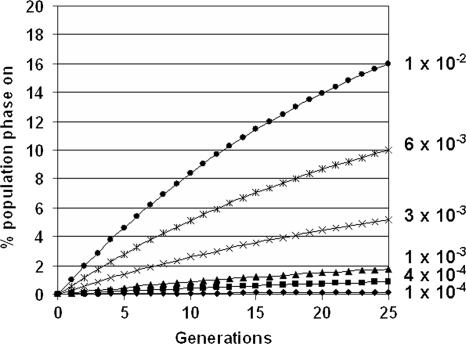
In the current study the frequencies of P phase variation were determined, as were the proportions of fimbriate bacteria in both E. coli K-12 and clinical isolate backgrounds. As these two parameters are linked, the proportion of fimbriate bacteria in the population can be calculated from phase transition frequencies and vice versa. Figure 3 shows the expected proportions of phase-on (fimbriate) bacteria in colonies plotted against the number of generations. These proportions were calculated using a range of off-to-on transition frequencies and a single on-to-off frequency of 3 ×10−2 per cell per generation that was based on the measurements obtained in this study. From this information, it can be estimated that the two pap operons in J96 turn on at a combined frequency of >1 × 10−2 per cell per generation, while the pap operons in E. coli CFT073 vary from off to on at a frequency between 3 × 10−3 and 1 × 10−2 per cell per generation. In comparison, the estimated frequency for the single J96-derived operon in the E. coli K-12 background was 1 × 10−4 per cell per generation based on 0.26% fimbriation. These frequency estimates based on fimbriation levels are in agreement with the actual transition frequencies measured using the papA::lacZ fusions.
FIG. 3.
Correlation between the proportion of phase-on bacteria in a population and phase transition frequencies. Different frequencies of off-to-on phase transition were plotted against the number of generations, using a constant on-to-off transition frequency of 3 × 10−2 per cell per generation. The resultant proportions of the population that are phase-on are expressed as percentages of the total population. The phase-on transition frequencies used for the correlation are indicated as follows (with the expected percentages of phase-on bacteria at equilibrium in parentheses): •, 1 × 10−2 per cell per generation (25%);  , 6 × 10−3 per cell per generation (16.7%); ×, 3 × 10−3 per cell per generation (9.09%); ▴, 1 × 10−3 per cell per generation (3.2%); ▪, 5 × 10−4 per cell per generation (1.64%); and ⧫, 1 × 10−4 per cell per generation (0.33%). All cultures would take more than 200 generations to reach an apparent steady state.
, 6 × 10−3 per cell per generation (16.7%); ×, 3 × 10−3 per cell per generation (9.09%); ▴, 1 × 10−3 per cell per generation (3.2%); ▪, 5 × 10−4 per cell per generation (1.64%); and ⧫, 1 × 10−4 per cell per generation (0.33%). All cultures would take more than 200 generations to reach an apparent steady state.
Environmental signals have differential effects on off-to-on transition frequencies of P1 and P2 pap operons from E. coli CFT073.
Previous research has shown that pap regulation is responsive to a number of environmental signals in an E. coli K-12 background, such as carbon source and temperature (7). In addition, recent studies have suggested that there is differential regulation of these pap clusters in human urine and during murine UTI (43). We therefore wanted to investigate the effect of environmental signals on the transition frequencies of the two pap operons from CFT073 (P1 and P2) in the context of the clinical isolate background. Frequencies were determined using the P1 and P2 lacZ reporter fusions (ZAP838 and ZAP1137, respectively [Table 1]). Table 2 shows the phase transition frequencies for CFT073 P1 and P2 pap operons in the off-to-on orientation when organisms were cultured under different conditions. In contrast to the results obtained with a complex medium (CFA medium), the off-to-on transition frequencies were lower in defined media (Table 2). As expected based on previous results, both operons were catabolite repressed to similar extents (Table 2); the decrease was highly significant for both P1 and P2 papA::lacZ fusions (P < 0.001). The frequencies were also higher in the presence of amino acids (Table 2), although P1 was more responsive (fourfold) than P2 (twofold) (P < 0.001 and P = 0.03, respectively). A temperature decrease from 37 to 28°C only moderately affected the P1 transition frequency and had no effect on P2 (Table 2). However, it was noted that the phase-on colonies were paler, perhaps indicating that there were reduced transcription initiation levels at 28°C, despite the fact that there was no effect on the off-to-on phase transition frequency.
TABLE 2.
Phase transition frequencies of Pap clusters P1 and P2 from E. coli CFT073, measured under different environmental conditions as described in Materials and Methodsa
| Conditions | Off-to-on transition frequency (10−3 per cell per generation) (SEM)
|
On-to-off transition frequency (10−3 per cell per generation) (SEM)
|
||
|---|---|---|---|---|
| P1 cluster | P2 cluster | P1 cluster | P2 cluster | |
| RD M9 glycerol medium, 37°C | 1.92 (0.14) | 1.01 (0.12) | ||
| RD M9 glucose medium, 37°C | 0.94 (0.11) | 0.32 (0.05) | ||
| Minimal M9 glycerol medium, 37°C | 0.79 (0.19) | 0.60 (0.11) | ||
| RD M9 glycerol medium, 28°C | 1.25 (0.20) | 1.02 (0.08) | ||
| Static urine, 37°C | 2.55 (0.31) | 1.25 (0.14) | ||
| Urine plates, 37°C | 4.31 (0.53) | 1.28 (0.22) | 10.16 (2.02) | 14.74 (1.00) |
| CFA medium, 37°C | 4.36 (0.26) | 6.12 (0.64) | 32.60 (0.79) | 35.30 (1.77) |
Frequencies were determined from at least six different colonies derived from 22 to 28 generations, depending on the conditions tested.
The effect of human urine on phase transition frequencies of E. coli CFT073 P1 and P2 was measured on plates at 37°C (Table 2) and in static liquid urine at 37°C (Table 2). On urine plates the off-to-on frequencies for P1 were equivalent to those on CFA medium, whereas the frequency for P2 was approximately fivefold lower. The frequency was significantly higher for P1 (P < 0.001). In liquid urine the frequencies were slightly lower than those on plates, although the difference between P1 and P2 was still evident. On-to-off phase transition frequencies were determined on urine plates (Table 2) and were similar to those measured on CFA medium (approximately 3 × 10−2 per cell per generation [see above]). The phase-off transition frequency on urine plates was marginally higher for P2 (1.5 × 10−2 per cell per generation) than for P1 (1 × 10−2 per cell per generation). Taken together, these results indicate that P1 is expressed at higher levels than P2 in human urine.
PapI in trans can increase the pap off-to-on transition frequency in the E. coli K-12 background.
PapI is an established positive regulator of pap off-to-on phase transition (2, 18, 24, 33). Changes in the amount of PapI may explain the background differences reported in this study, and in a clinical isolate background these differences may arise as a consequence of the presence of multiple pap operons and/or as a consequence of differences in the regulatory network that differentially affect expression from each operon. To determine whether increasing papI expression in E. coli K-12 could increase pap off-to-on phase transition frequencies and fimbriation, plasmid-based papI was transformed into two E. coli K-12 strains, one containing an integrated single-copy fusion of the J96 F13 pap regulatory region to lacZ (ZAP593 [Table 1]) and the other containing the complete J96 F13 pap operon (ZAP594 [Table 1]). The off-to-on transition frequency increased 34-fold in the presence of induced PapI (Fig. 4A). This correlated well with the 53-fold increase in the proportion of P-fimbriate bacteria detected using immunostaining and flow cytometry (Fig. 4B). Taken together, these data suggest that low PapI levels in the E. coli K-12 background could in part explain the lower off-to-on phase transition frequencies in this background.
FIG. 4.
Effect of addition of papI in trans on pap phase transition and fimbriation. (A) Off-to-on phase transition frequency of strain ZAP593. (B) Fimbriation of strain ZAP594 stained with anti-PapA antibody, determined by flow cytometry. In both cases the strains were transformed with pACYC184 (control) or pHMG98 (papI).
Effect of multiple pap operons on pap phase transition frequencies in E. coli CFT073.
To investigate whether the presence of two papI gene copies in the CFT073 chromosome is responsible for the higher pap expression observed in this isolate, a CFT073 mutant strain in which both P1 and P2 regulatory regions and regulators were deleted was constructed. The P1 and P2 lacZ reporter fusions were then assayed in this background (ZAP843 and ZAP595, respectively [Table 1]). The off-to-on transition frequency on CFA medium for P1 was reduced slightly from 4.4 × 10−3 per cell per generation in the CFT073 wild-type background to 3.3 × 10−3 per cell per generation in the CFT073 mutant background, while for P2 there was a 2.4-fold reduction from 6.1 × 10−3 to 2.6 × 10−3 per cell per generation (P < 0.001). When the P1 and P2 lacZ reporter fusions in the CFT073 mutant background were assayed under alternative conditions (28°C, RD M9 medium containing glycerol), the impact of the genetic background was again more evident in the P2 fusion. The off-to-on transition frequency for the P2 fusion was reduced eightfold, while the P1 frequency was reduced twofold. While these data indicate that there was some cross-regulation by the homologous regulators, they cannot account for the marked background differences between the E. coli K-12 and CFT073 strains demonstrated in this study.
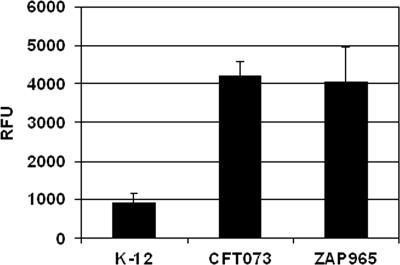
To investigate whether the background affected papI expression per se, a plasmid-based transcriptional fusion of the P1 papI promoter region to gfp was transformed in E. coli K-12, wild-type strain CFT073, and the CFT073 pap regulatory region mutant strain. Expression of papI was higher (4.4-fold) in both wild-type strain CFT073 and the pap regulatory region mutant of CFT073 (Fig. 5) than in E. coli K-12. Increased activation of papI in the clinical isolate background in combination with cross-regulation with homologous clusters accounted for the observed P fimbriation in E. coli CFT073 and J96.
FIG. 5.
Expression of papI::gfp plasmid-based fusion. Expression from the fusion is shown for pMT24 transformed into E. coli K-12, CFT073, and ZAP965. The background fluorescence level determined for pKC26 in each strain was subtracted from the corresponding level for pMT24. The data are expressed in relative fluorescence units (RFU).
DISCUSSION
P fimbriae are an established virulence factor utilized by UPEC to promote colonization of urinary and gastrointestinal tracts (22, 36, 40, 47). Like expression of most fimbrial adhesins produced by E. coli, expression of Pap is phase variable. The mechanism underlying the variation and the genetic and environmental inputs that affect it have been the focus of many years of research. The majority of the studies have been carried out on regulatory regions cloned into the E. coli K-12 chromosome, and the frequencies of phase transition have been determined using lacZ fusions in this nonnative background (i.e., a background that does not normally support expression of a pap cluster) (7). The current study reports for the first time regulation of P-fimbrial phase transition frequencies in a UPEC strain and demonstrates the importance of the genetic background for these frequencies. In particular, high off-to-on transition frequencies may be accounted for by elevated papI expression levels.
Early studies using immunoelectron microscopy and indirect immunofluorescence reported high levels of P fimbria expression in UPEC isolate populations cultured in vitro and demonstrated that phase variation was extremely rapid, although no actual frequency measurements were obtained. The pyelonephritis E. coli isolate KS71 possesses two pap operons and, when it was cultured on CFA agar, 17% of the population had P fimbriae (43). A different UPEC strain, C1212, also carrying two pap operons, produced colonies in which 84% of the bacteria expressed P fimbriae, although in this case one cluster (pilin-21) was dominant (29). Pere et al. showed that populations of E. coli strains found in urine from patients with symptomatic UTI were between 0.01 and 95% P fimbriate (36). Overall, the reported high levels of P fimbria expression predict that the pap off-to-on transition frequencies in UPEC strains are higher than the frequencies determined in the E. coli K-12 background (7). These studies reported pap frequencies of 1 in 10,000 cells transitioning from phase off to phase on in each generation. This frequency would generate a population in which less than 1% of the cells are phase on (Fig. 3), which is not large enough to account for the P-fimbriate subpopulations reported in the studies described above (29, 34).
To address this question in detail, phase transition frequencies were determined for the two Pap clusters present in E. coli CFT073 using lacZ and gfp fusions to the papI-papBA regulatory regions. As anticipated (2, 7, 16, 50), the frequencies were dependent on the culture conditions but ranged from 6 × 10−4 per cell per generation on minimal M9 glycerol medium to 6 × 10−3 per cell per generation on CFA medium. On-to-off transition frequencies were also measured and remained fairly constant at 3 × 10−2 per cell per generation. Based on these frequencies, the expected proportion of fimbriate bacteria cultured on CFA medium would be between 10 and 30% depending on the amount of cross-regulation between the two clusters and the rate at which bacteria that turn off expression lose fimbriae from their surfaces. The predicted level of fimbriation, based on the measured frequencies, was entirely consistent with the level of fimbriation detected for E. coli CFT073 in the present study (10 to 20%) (Fig. 1C and D). It should be noted that construction of the chromosomally integrated reporter fusions does introduce an extra copy of the regulatory region into E. coli CFT073 but that the rates and levels of expression were consistent with fimbriation under the conditions tested.
P1 and P2 are homologous pap clusters in E. coli CFT073, and both encode a PapG class II adhesin. Here we demonstrate that these clusters are differentially regulated by certain environmental conditions. The P1 cluster was more responsive to amino acids and temperature. By contrast, both clusters showed the same level of catabolite repression. Urine has been shown to contain levels of amino acids high enough to support bacterial growth, and the increased off-to-on phase transition frequency of P1 relative to that of P2 may be due to an amino acid response (1). In addition, the P2 on-to-off transition frequency was higher, indicating that the overall fimbriation level was higher for P1 in human urine. This is the first time that phase transition frequencies have been determined for a UPEC isolate cultured in human urine, the physiological environment most similar to human UTI.
The off-to-on phase transition frequencies determined in the current study are similar to those determined for type 1 fimbriae. While type 1 fimbriae are regulated by a totally different phase-variable mechanism, the off-to-on transition frequencies at 37°C are between 8 × 10−4 and 9 × 10−3 per cell per generation, depending on the culture conditions (15). When the data are taken together, it may be that this range of frequencies is optimal for maintaining surface factor heterogeneity in populations and persistence during colonization and infection. Studies of diverse phase-variable systems have also shown similar levels of phase variation frequencies. For example, slip-stranded mispairing is a mechanism used for generating variation and is common in Haemophilus influenzae and Neisseria meningitidis. The frequencies were shown to be in the range from 2 × 10−4 to 5 × 10−3 per cell per generation, depending on the genetic background (46). Only one instance of slip-stranded mispairing has been reported as a mechanism for variation in E. coli, for the AhpC enzyme. The frequency of variation was shown to be in the same range, as demonstrated for phase variation of fimbrial clusters (0.5 × 10−3 per cell per generation) (39).
The present study showed that pap clusters exhibit significantly reduced frequencies of phase variation when they are measured in an E. coli K-12 background. This background difference was confirmed using a complete pap cluster inserted into the E. coli K-12 chromosome at lac. The fimbriation levels of this strain were 100-fold lower than those of the parental strain, J96 (Fig. 1). Based on our current understanding of the pap phase variation mechanism (4), all the required regulators are present in both backgrounds. The higher frequency in the clinical isolate must be explained by altered levels of one or more of the established regulators or novel regulators of the system. While off-to-on transition frequencies were much higher in the isolate background, the transition frequencies in the opposite direction were equivalent. This indicates that the difference is due to a factor or factors that promote only off-to-on phase transition. A key candidate is the positive regulator PapI. Higher total levels of PapI could be achieved by increased expression from individual papI promoters and/or by the presence of multiple homologous pap clusters and therefore an increased papI copy number. To determine the significance of cross-activation in the clinical isolate background, the two papI-papBA regulatory regions from P1 and P2 in E. coli CFT073 were deleted, and expression of pap from a regulatory region fusion engineered into the lac locus was examined. Deletion of the pap regulatory regions made no significant difference to the off-to-on phase transition of P1 but did reduce the P2 transition frequency. These results indicate that cross-activation between P fimbira-related clusters alone cannot account for the frequency differences in the different backgrounds. Subsequently, papI expression was measured in the two backgrounds and was shown to be higher in the clinical isolate than in E. coli K-12. Furthermore, papI expression was the same in the papI-papBA double deletion strain, which suggests that signals over and above native pap regulators influence papI expression in the clinical isolate. While plasmid copy number or GFP stability differences may account for the higher apparent expression level in E. coli CFT073, elevated papI expression would explain the higher off-to-on phase transition rates and fimbriation levels observed in the clinical isolate background. Not surprisingly, the results also indicate that the regulatory network controlling papI expression is different in a clinical isolate, and this is likely to be due to both the evolution of common signal transduction systems and regulatory cross talk with other specifically acquired virulence determinants and their regulators. At present, there is little published research on the regulation of papI, and further work is required in this area.
Phase-variable expression of P fimbriae has two proposed roles in aiding UPEC colonization of the human urinary tract. As P fimbriae are surface antigens, phase variation is thought to limit immune exposure of the specific antigen and assist in evasion of innate and adaptive host responses (25, 44). At the same time, phase variation generates a heterogeneous bacterial population with a subpopulation of P-fimbriate bacteria that are equipped to colonize cells expressing Gal-α(1-4)β-Gal receptors (26-28). Another proposed function of phase variation is to coordinate expression of surface factors that are required in complex microenvironments, such as the colonized epithelium (19). Surface factors can block or hinder each other, and so clusters expressing surface factors, such as fimbrial adhesins, nonfimbrial adhesins, flagella, outer membrane proteins, and capsule, are connected via a regulatory network that is driven in part by phase variation (17). Phase variation frequencies, therefore, not only are important to control expression levels of a specific local factor but also impact the regulation of a wide spectrum of other surface components expressed during infection.
Acknowledgments
This work was supported by Wellcome Trust grant 069091/Z/02/Z to D.L.G. and N.H., by a Wellcome Trust Prize Studentship to M.T., and by an MRC studentship to L.D.
Bernt-Eric Uhlin and Ian Blomfield are thanked for helpful discussions, and T. Korhonen is thanked for supplying polyclonal serum.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Asscher, A. W., M. Sussman, W. E. Waters, R. H. Davis, and S. Chick. 1966. Urine as a medium for bacterial growth. Lancet ii:1037-1041. [DOI] [PubMed] [Google Scholar]
- 2.Baga, M., M. Goransson, S. Normark, and B. E. Uhlin. 1985. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 4:3887-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco, M., J. E. Blanco, M. P. Alonso, A. Mora, C. Balsalobre, F. Munoa, A. Juarez, and J. Blanco. 1997. Detection of pap, sfa and afa adhesin-encoding operons in uropathogenic Escherichia coli strains: relationship with expression of adhesins and production of toxins. Res. Microbiol. 148:745-755. [DOI] [PubMed] [Google Scholar]
- 4.Blomfield, I. C. 2001. The regulation of Pap and type 1 fimbriation in Escherichia coli. Adv. Microb. Physiol. 45:1-49. [DOI] [PubMed] [Google Scholar]
- 5.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 5:1447-1457. [DOI] [PubMed] [Google Scholar]
- 6.Blyn, L. B., B. A. Braaten, and D. A. Low. 1990. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 9:4045-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blyn, L. B., B. A. Braaten, C. A. White-Ziegler, D. H. Rolfson, and D. A. Low. 1989. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 8:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouza, E., R. San Juan, P. Munoz, A. Voss, and J. Kluytmans. 2001. A European perspective on nosocomial urinary tract infections. II. Report on incidence, clinical characteristics and outcome (ESGNI-004 study). European Study Group on Nosocomial Infection. Clin. Microbiol. Infect. 7:532-542. [DOI] [PubMed] [Google Scholar]
- 9.Braaten, B. A., L. B. Blyn, B. S. Skinner, and D. A. Low. 1991. Evidence for a methylation-blocking factor (mbf) locus involved in Pap pilus expression and phase variation in Escherichia coli. J. Bacteriol. 173:1789-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braaten, B. A., J. V. Platko, M. W. van der Woude, B. H. Simons, F. K. de Graaf, J. M. Calvo, and D. A. Low. 1992. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc. Natl. Acad. Sci. USA 89:4250-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell, I., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmerson, J. R., D. L. Gally, and A. J. Roe. 2006. Generation of gene deletions and gene replacements in Escherichia coli O157:H7 using a temperature sensitive allelic exchange system. Biol. Proced. Online 8:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans, D. G., D. J. Evans, Jr., and W. Tjoa. 1977. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect. Immun. 18:330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsman, K., M. Goransson, and B. E. Uhlin. 1989. Autoregulation and multiple DNA interactions by a transcriptional regulatory protein in E. coli pili biogenesis. EMBO J. 8:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gally, D. L., J. A. Bogan, B. I. Eisenstein, and I. C. Blomfield. 1993. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J. Bacteriol. 175:6186-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goransson, M., K. Forsman, and B. E. Uhlin. 1989. Regulatory genes in the thermoregulation of Escherichia coli pili gene transcription. Genes Dev. 3:123-130. [DOI] [PubMed] [Google Scholar]
- 17.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernday, A. D., B. A. Braaten, and D. A. Low. 2003. The mechanism by which DNA adenine methylase and Papl activate the pap epigenetic switch. Mol. Cell 12:947-957. [DOI] [PubMed] [Google Scholar]
- 19.Holden, N. J., and D. L. Gally. 2004. Switches, cross-talk and memory in Escherichia coli adherence. J. Med. Microbiol. 53:585-593. [DOI] [PubMed] [Google Scholar]
- 20.Holden, N. J., M. Totsika, E. Mahler, A. J. Roe, K. Catherwood, K. Lindner, U. Dobrindt, and D. L. Gally. 2006. Demonstration of regulatory cross-talk between P fimbriae and type 1 fimbriae in uropathogenic Escherichia coli. Microbiology 152:1143-1153. [DOI] [PubMed] [Google Scholar]
- 21.Holden, N. J., B. E. Uhlin, and D. L. Gally. 2001. PapB paralogues and their effect on the phase variation of type 1 fimbriae in Escherichia coli. Mol. Microbiol. 42:319-330. [DOI] [PubMed] [Google Scholar]
- 22.Hull, R. A., W. H. Donovan, M. Del Terzo, C. Stewart, M. Rogers, and R. O. Darouiche. 2002. Role of type 1 fimbria- and P fimbria-specific adherence in colonization of the neurogenic human bladder by Escherichia coli. Infect. Immun. 70:6481-6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hull, R. A., R. E. Gill, P. Hsu, B. H. Minshew, and S. Falkow. 1981. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect. Immun. 33:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaltenbach, L. S., B. A. Braaten, and D. A. Low. 1995. Specific binding of PapI to Lrp-pap DNA complexes. J. Bacteriol. 177:6449-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantele, A., T. Mottonen, K. Ala-Kaila, and H. S. Arvilommi. 2003. P fimbria-specific B cell responses in patients with urinary tract infection. J. Infect. Dis. 188:1885-1891. [DOI] [PubMed] [Google Scholar]
- 26.Korhonen, T. K., R. Virkola, and H. Holthofer. 1986. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect. Immun. 54:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leffler, H., and C. Svanborg-Eden. 1981. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect. Immun. 34:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindberg, F. P., B. Lund, and S. Normark. 1984. Genes of pyelonephritogenic Escherichia coli required for digalactoside-specific agglutination of human cells. EMBO J. 3:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low, D., E. N. Robinson, Jr., Z. A. McGee, and S. Falkow. 1987. The frequency of expression of pyelonephritis-associated pili is under regulatory control. Mol. Microbiol. 1:335-346. [DOI] [PubMed] [Google Scholar]
- 30.Marklund, B. I., J. M. Tennent, E. Garcia, A. Hamers, M. Baga, F. Lindberg, W. Gaastra, and S. Normark. 1992. Horizontal gene transfer of the Escherichia coli Pap and Prs pili operons as a mechanism for the development of tissue- specific adhesive properties. Mol. Microbiol. 6:2225-2242. [DOI] [PubMed] [Google Scholar]
- 31.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson, P., S. Naureckiene, and B. E. Uhlin. 1996. Mutations affecting mRNA processing and fimbrial biogenesis in the Escherichia coli pap operon. J. Bacteriol. 178:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nou, X. W., B. Braaten, L. Kaltenbach, and D. A. Low. 1995. Differential binding of Lrp to 2 sets of pap DNA-binding sites mediated by papI regulates pap phase variation in Escherichia coli. EMBO J. 14:5785-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowicki, B., M. Rhen, V. Vaisanenrhen, A. Pere, and T. K. Korhonen. 1984. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J. Bacteriol. 160:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pere, A. 1986. P fimbriae on uropathogenic Escherichia coli O16:K1 and O18 strains. FEMS Microbiol. Lett. 37:19-26. [Google Scholar]
- 36.Pere, A., B. Nowicki, H. Saxen, A. Siitonen, and T. K. Korhonen. 1987. Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis. 156:567-574. [DOI] [PubMed] [Google Scholar]
- 37.Plos, K., T. Carter, S. Hull, R. Hull, and C. Svanborg Eden. 1990. Frequency and organization of pap homologous DNA in relation to clinical origin of uropathogenic Escherichia coli. J. Infect. Dis. 161:518-524. [DOI] [PubMed] [Google Scholar]
- 38.Porter, M. E., P. Mitchell, A. J. Roe, A. Free, D. G. Smith, and D. L. Gally. 2004. Direct and indirect transcriptional activation of virulence genes by an AraC-like protein, PerA from enteropathogenic Escherichia coli. Mol. Microbiol. 54:1117-1133. [DOI] [PubMed] [Google Scholar]
- 39.Ritz, D., J. Lim, C. M. Reynolds, L. B. Poole, and J. Beckwith. 2001. Conversion of a peroxiredoxin into a disulfide reductase by a triplet repeat expansion. Science 294:158-160. [DOI] [PubMed] [Google Scholar]
- 40.Roberts, J. A., M. B. Kaack, G. Baskin, M. R. Chapman, D. A. Hunstad, J. S. Pinkner, and S. J. Hultgren. 2004. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J. Urol. 171:1682-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roe, A. J., S. W. Naylor, K. J. Spears, H. M. Yull, T. A. Dransfield, M. Oxford, I. J. McKendrick, M. Porter, M. J. Woodward, D. G. Smith, and D. L. Gally. 2004. Co-ordinate single-cell expression of LEE4- and LEE5-encoded proteins of Escherichia coli O157:H7. Mol. Microbiol. 54:337-352. [DOI] [PubMed] [Google Scholar]
- 42.Samuelsson, P., L. Hang, B. Wullt, H. Irjala, and C. Svanborg. 2004. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect. Immun. 72:3179-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder, J. A., A. L. Lloyd, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2006. Role of phase variation of type 1 fimbriae in a uropathogenic Escherichia coli cystitis isolate during urinary tract infection. Infect. Immun. 74:1387-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soto, G. E., and S. J. Hultgren. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres-Cruz, J., and M. W. van der Woude. 2003. Slipped-strand mispairing can function as a phase variation mechanism in Escherichia coli. J. Bacteriol. 185:6990-6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tullus, K., I. Kuhn, I. Orskov, F. Orskov, and R. Mollby. 1992. The importance of P and type 1 fimbriae for the persistence of Escherichia coli in the human gut. Epidemiol. Infect. 108:415-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Woude, M. W., L. S. Kaltenbach, and D. A. Low. 1995. Leucine-responsive regulatory protein plays dual roles as both an activator and a repressor of the Escherichia coli pap fimbrial operon. Mol. Microbiol. 17:303-312. [DOI] [PubMed] [Google Scholar]
- 49.Weyand, N. J., and D. A. Low. 2000. Regulation of pap phase variation. Lrp is sufficient for the establishment of the phase off pap DNA methylation pattern and repression of pap transcription in vitro. J. Biol. Chem. 275:3192-3200. [DOI] [PubMed] [Google Scholar]
- 50.White-Ziegler, C. A., L. B. Blyn, B. A. Braaten, and D. A. Low. 1990. Identification of an Escherichia coli genetic locus involved in thermoregulation of the pap operon. J. Bacteriol. 172:1775-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wullt, B., G. Bergsten, H. Connell, P. Rollano, N. Gebratsedik, L. Hang, and C. Svanborg. 2001. P-fimbriae trigger mucosal responses to Escherichia coli in the human urinary tract. Cell. Microbiol. 3:255-264. [DOI] [PubMed] [Google Scholar]
- 52.Xia, Y., D. Gally, K. Forsman-Semb, and B. E. Uhlin. 2000. Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB protein. EMBO J. 19:1450-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zorc, J. J., D. A. Kiddoo, and K. N. Shaw. 2005. Diagnosis and management of pediatric urinary tract infections. Clin. Microbiol. Rev. 18:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]