Abstract
A combinatorial immunoglobulin gene library was constructed from peripheral blood lymphocytes of eight patients infected with Plasmodium falciparum and was screened for the production of human monoclonal antibody Fab fragments to the C-terminal 19-kDa fragment of P. falciparum merozoite surface protein 1 (MSP-119). Three Fab clones recognized recombinant MSP-119 under nonreducing conditions. Indirect immunofluorescence microscopy demonstrated that three Fab clones stained the surfaces of late trophozoites/schizonts and merozoites of the FCR3 and 3D7 strains, suggesting the Fabs' reactivities to a conserved epitope. Sequence analysis of the heavy-chain genes revealed that the closest germ line V segments were VH1-8 and VH7-81, with 91% to 98% homology. The closest germ line D segment was D3-10, and the closest germ line J segment was JH4 or JH5, with 90% to 97% homology. In the light-chain genes, the closest germ line V segment was A27 for the Jκ2, Jκ4, and Jκ5 segments. The dissociation constants of these Fab fragments for recombinant MSP-119 ranged from 1.09 × 10−9 to 2.66 × 10−9 M. The binding of the three Fab fragments to MSP-119 was competitively inhibited by the anti-MSP-119 mouse monoclonal antibody 12.8, which inhibits erythrocyte invasion by merozoites. However, the human Fab fragment with the highest affinity did not inhibit in vitro growth of P. falciparum. This is the first report of gene analysis and bacterial expression of human monoclonal antibodies to P. falciparum MSP-119. The combinatorial immunoglobulin gene library derived from malaria patients provides a potential tool for producing high-affinity human antibodies specific for P. falciparum.
Malaria caused by Plasmodium falciparum is a major public health problem in tropical countries, where it is responsible for 300 to 500 million cases and more than 1 million deaths annually (36). The development of malaria vaccines is urgently needed for improved malaria control. Proteins expressed on the surface of the merozoite, an invasive form of the parasite, seem to be important targets of host immunity and therefore could be potential candidates for the development of malaria vaccines. The P. falciparum major merozoite surface protein 1 (MSP-1) is a leading vaccine candidate antigen (21). Antibodies against MSP-1 are protective against human, monkey, and rodent malaria parasites, and immunization with MSP-1 affords antiparasite protection in experimental animals (7, 8, 30, 35). MSP-1 is synthesized as a 195-kDa precursor on the surfaces of late trophozoites/schizonts, and it is proteolytically processed to form four fragments, of 83 kDa, 30 kDa, 38 kDa, and 42 kDa, during merozoite maturation (14). The C-terminal 42-kDa fragment is further cleaved into N-terminal 33-kDa and C-terminal 19-kDa fragments (MSP-119) (3). All of the fragments, except for MSP-119, are shed from the merozoite surface upon erythrocyte invasion. MSP-119, which contains two epidermal growth factor-like modules, is anchored to the surface via a glycosylphosphatidylinositol moiety (13, 14). Although the P. falciparum MSP-1 gene (msp1) is highly polymorphic, the msp1 region coding for MSP-119 is well conserved among parasite isolates. There is accumulating evidence suggesting that sera from individuals living in areas where malaria is highly endemic contain antibodies against the 19-kDa fragment that inhibit merozoite invasion into red blood cells (9, 24, 25, 31).
P. falciparum occasionally causes severe malaria in children and individuals who have less immunity to the parasite. The efficacy of antimalarial drugs is becoming limited due to the high prevalence of multidrug resistance of the parasite. Therefore, new therapeutic measures are needed to treat severe malaria cases. In this context, passive immunotherapy using human antibodies specific to MSP-119 may provide a valuable therapeutic alternative. Indeed, mouse monoclonal antibodies to MSP-119 inhibit in vitro growth of P. falciparum (3, 5). Mouse monoclonal antibodies are unsuitable for use in humans; therefore, an immunotherapy method that can be used in humans must be developed. However, little is known about the molecular basis of acquired humoral immunity to MSP-119 in malaria-immune individuals.
Several methods have been developed to produce human monoclonal antibodies (1, 2, 11, 44). We have reported that the bacterial expression system is useful for the preparation of human Fab fragments specific to pathogens (6, 17, 39-42). In the present study, we use a combinatorial immunoglobulin gene library derived from lymphocytes of patients with falciparum malaria to produce human monoclonal antibody Fab fragments that specifically react to P. falciparum MSP-119. Additionally, we analyze immunoglobulin gene usage in these Fab fragments.
MATERIALS AND METHODS
Cultivation of P. falciparum.
Asexual blood-stage parasites of P. falciparum (strains FCR3 and 3D7) were maintained at 37°C in RPMI 1640 medium supplemented with 10% human type O serum (45). Cultures were gassed with 5% CO2, 5% O2, and 90% N2 and maintained by routine passage in fresh human type O erythrocytes. Parasites were synchronized by Percoll and sorbitol treatment (46).
Preparation of recombinant MSP-119.
Genomic DNA of P. falciparum (strain FCR3) was isolated from schizonts by using a DNeasy tissue kit (QIAGEN, Hilden, Germany). The DNA was used as a template for amplification of the msp1 region coding for MSP-119 (nucleotide positions 4819 to 53 downstream of the 3′ noncoding region; positions are given according to the 3D7 msp1 sequence [GenBank accession no. Z35327]) with the following primers: forward, 5′-CCCATATGAACATTTCACAACACCAATGCGT-3′; and reverse, 5′-CCCTCGAGTTAGTTAGAGGAACTGCAGAAAATA-3′. To obtain high-fidelity amplification, Pyrobest DNA polymerase (Takara, Otsu, Japan) was used. Twenty cycles of PCR were performed as follows: denaturation at 94°C for 15 s (135 s in cycle 1), annealing at 55°C for 30 s, and polymerization at 72°C for 60 s (360 s in cycle 20). The PCR product was digested with NdeI and XhoI, purified, and then ligated with the pET19b vector (Novagen, Madison, WI). The plasmid was introduced into competent Escherichia coli JM109 cells, and then a clone containing the insert with the right sequence was selected. E. coli BL21 Star (DE3)pLysS cells (Invitrogen, Carlsbad, CA) were transformed with the cloned plasmid. The bacterial clone was cultured in 800 ml of Luria broth containing ampicillin and chloramphenicol until an optical density at 600 nm of 0.6 was achieved. The expression of recombinant MSP-119 tagged with histidine residues was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 3 h. The protein was purified by affinity chromatography, using His-Bind resin (Novagen) according to the manufacturer's recommendations.
Construction of immunoglobulin gene library.
Approximately 10 ml of peripheral blood was obtained from each of eight hospitalized patients with falciparum malaria (six Japanese and two Africans) at Tokai University Hospital, Tokyo Metropolitan Komagome Hospital, and Tokyo Metropolitan Bokutoh General Hospital (Japan). Lymphocytes were separated from the blood by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. Construction of an immunoglobulin gene library from the lymphocytes was performed as previously described (39). Briefly, total RNA was purified from lymphocytes and subjected to reverse transcription-PCR. Genes encoding the light (κ and λ) chain and the Fd region of the heavy (γ and μ) chain were amplified by 30 cycles of PCR. The light-chain genes were first ligated with an expression vector, pFab-His2, and introduced into Escherichia coli JM109 cells. The vector with inserts was then ligated with the Fd heavy-chain genes and introduced into E. coli cells.
Screening of clones producing anti-P. falciparum antibodies.
The first screening of positive clones producing anti-P. falciparum MSP-119 antibodies was performed as described previously (6). Approximately 5 × 103 E. coli colonies per 90-mm plate were grown on Luria broth agar containing 50 μg/ml of ampicillin. Bacterial colonies were transferred to nitrocellulose filters. The filters were replaced on the surfaces of fresh plates containing 1 mM IPTG and then incubated at 30°C for 6 h. The filters were treated with chloroform vapor and lysis buffer containing 100 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1.5% bovine serum albumin, 1 μg of DNase per ml, and 40 μg of lysozyme per ml overnight. After being washed with phosphate-buffered saline containing 0.05% Tween 20 (PBST), the filter was blocked with PBST containing 5% skim milk. Each filter was incubated with 125 μg of recombinant MSP-119 and then with plasma from a patient. Positive signals on the filters were detected with a horseradish peroxidase (HRP)-conjugated goat antibody to human whole immunoglobulin G (IgG; ICN Pharmaceuticals, Aurora, OH) and a Konica HRP-1000 immunostaining kit. Positive clones were identified in the original plates and then cultured in 10 ml of super broth (30 g tryptone, 20 g yeast extract, and 10 g 4-morpholinepropanesulfonic acid per liter, pH 7.0) containing ampicillin to an optical density at 600 nm of 0.8. IPTG was added to the bacterial culture at a final concentration of 100 μM, and the culture was then incubated overnight at 30°C for 12 h. Bacteria were pelleted by centrifugation, resuspended in 0.5 ml of PBS containing 1 mM phenylmethylsulfonyl fluoride, and then sonicated. The lysates were centrifuged at 10,000 × g for 10 min, and supernatants were subjected to a second screening by an enzyme-linked immunosorbent assay (ELISA). CP2.9 (26), which is a chimera of MSP-119 and domain III of apical membrane antigen 1, was also used for screening. This antigen was kindly provided by W.-Q. Pan, Second Military Medical University, Shanghai, China.
ELISA.
Each ELISA well was treated with recombinant MSP-119 or CP2.9 (50 ng/well) diluted in 50 mM sodium bicarbonate buffer. The plates were washed with PBST and then treated with PBS containing 1% skim milk for 1 h. One hundred microliters of the supernatant was added to the wells and incubated for 1 h at room temperature. After being washed, the wells were incubated with 100 μl of HRP-conjugated goat antibody to human IgG Fab (ICN Pharmaceuticals) for 1 h at room temperature and then treated with 200 μl of substrate (0.04% o-phenylenediamine in citric acid-phosphate buffer [pH 5.0] including 0.001% hydrogen peroxide). The reaction was stopped by the addition of 50 μl 2.5 N H2SO4 after 30 min, and the optical density at 490 nm was determined.
Immunofluorescence microscopy.
Indirect immunofluorescence staining was performed with paraformaldehyde-fixed parasites (29) by using fluorescein isothiocyanate-conjugated goat IgG to human IgG Fab (ICN Pharmaceuticals) as the secondary antibody. Propidium iodine was used for counterstaining.
Purification of Fab fragments.
Positive clones were cultured in 1 liter of super broth medium, and 20 ml of the resultant supernatant was prepared as described above. Fab fragments were purified with Talon metal-affinity resin (BD Biosciences, Palo Alto, CA) according to the manufacturer's instructions.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting.
Purified Fab fragments and P. falciparum schizonts (strain FCR3) were solubilized and electrophoresed in 10% acrylamide gels containing sodium dodecyl sulfate (SDS) under reducing and nonreducing conditions, respectively. Protein bands were then transferred to polyvinylidene difluoride membranes. The Fab fragments were detected by an HRP-conjugated goat antibody to the human kappa chain and with HRP-conjugated Ni-nitrilotriacetic acid as previously described (6). Proteins from P. falciparum were incubated with 10 μg of purified human Fab fragments and a 1:200 dilution of patient plasma for 1 h and then detected by HRP-conjugated goat antibodies to human IgG Fab and human whole IgG for 1 h. Development was performed with a Konica immunostaining kit. Normal human Fab (OEM Concepts, Toms River, NJ) and normal human sera were used as negative controls.
Measurement of affinity of Fab fragments.
The affinity constants of the Fab fragments were assessed by surface plasmon resonance, using a BIAcore 3000 instrument (Biacore AB, Uppsala, Sweden). Recombinant MSP-119 was immobilized onto a CM5 chip (Biacore). Association and dissociation constants were determined by using BIAevaluation 3.1.
DNA sequencing.
Plasmid DNAs were isolated from immunofluorescence assay-positive clones. Light-chain genes in the expression vector were subcloned into the sequencing vector. Sequencing reactions in both directions were performed with a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA), using M13 primers. The sequences were obtained using an ABI PRISM 3100 genetic analyzer (Applied Biosystems).
Competitive inhibition assay.
Each ELISA well was coated with recombinant MSP-119 as described above. For competition between human Fab and a mouse monoclonal antibody, various concentrations of purified Fab fragments (0.01, 0.1, 1, and 10 μg per 50 μl PBS) were mixed with 50 μl of anti-MSP-119 mouse monoclonal antibody 12.8 or 2.2 (3, 12, 22) and then added to the wells. The mouse monoclonal antibodies were kindly provided by J. S. McBride, University of Edinburgh. The plates were incubated for 1 h at room temperature and washed with PBST. Reactions were detected as described above. As a control, normal human Fab fragments (OEM Concepts) were used. Competition ELISA between human Fab and sera or plasmas from patients with malaria was also performed. Human sera immune to P. falciparum were obtained from individuals living in the Solomon Islands, where malaria is highly endemic (34). Ten nanograms of Fab labeled with sulfosuccinimidobiotin (Pierce, Rockford, IL) per 50 μl PBS and equal volumes of immune sera (n = 10) diluted 1:10 were premixed and added to the wells of ELISA plates, which were coated as described above. Plasmas from lymphocyte donors (n = 8), diluted 1:10, were also tested by competition ELISA. As controls, serum samples from healthy Japanese individuals (n = 10) were used. The plates were incubated for 1 h and then detected by incubation with streptavidin-biotinylated HRP (GE Healthcare, Buckinghamshire, England) for 1 h. Reactions were also developed as described above and expressed as values relative to those of the control.
Growth inhibition assay.
The effect of human Fab fragments on the growth of P. falciparum (strain FCR3) was examined in vitro (27, 28). Erythrocytes infected with late trophozoites/schizonts were diluted with complete RPMI 1640 and uninfected human erythrocytes to a final hematocrit of 4% and final parasitemia of 0.5%. A total of 160 μl of this suspension was transferred to wells of a 96-well flat-bottomed microplate, and then 40 μl of PBS containing 40 μg of purified recombinant human Fab was mixed with the suspension. As controls, normal human Fab fragments (OEM Concepts) and PBS only were used. Each treatment was tested in two wells. After 24 h of incubation, parasitemia was determined by counting the number of infected erythrocytes in 10,000 total erythrocytes by Giemsa staining. Experiments were repeated three times.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper have been deposited in the DDBJ, EMBL, and GenBank databases under accession numbers AB289325 to AB289330.
RESULTS
Reactivity of human Fab clones.
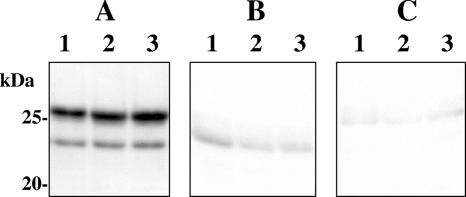
A combinatorial immunoglobulin gene library constructed from peripheral lymphocytes of eight patients with falciparum malaria contained approximately 5 × 107 clones. Colony blotting of 8 × 105 clones yielded 62 positive clones (0.008%). Secondary screening of the positive clones with ELISA, using MSP-119 and CP2.9, followed by screening with immunofluorescence microscopy, identified three positive clones. The positive Fab clones, designated Pf25, Pf143, and Pf227, were reactive to both MSP-119 and CP2.9. Affinity chromatography-purified Fab fragments showed two bands, with molecular masses of 24 and 25 kDa, under reducing conditions (Fig. 1) by SDS-PAGE. These bands were identified as light and heavy chains by Western immunoblot analysis.
FIG. 1.
SDS-PAGE (A) and Western immunoblot analysis (B and C) of purified recombinant Fab fragments from clones Pf25 (lanes 1), Pf143 (lanes 2), and Pf227 (lanes 3). The Fab fragments were subjected to SDS-PAGE in a 10% polyacrylamide gel under reducing conditions and then transferred to polyvinylidene difluoride membranes. The protein bands in panel A were stained with Coomassie brilliant blue. The membranes were treated with an HRP-conjugated goat antibody to the human kappa chain (B) or with HRP-conjugated Ni-nitrilotriacetic acid (C). The numbers on the left indicate the molecular masses of size markers.
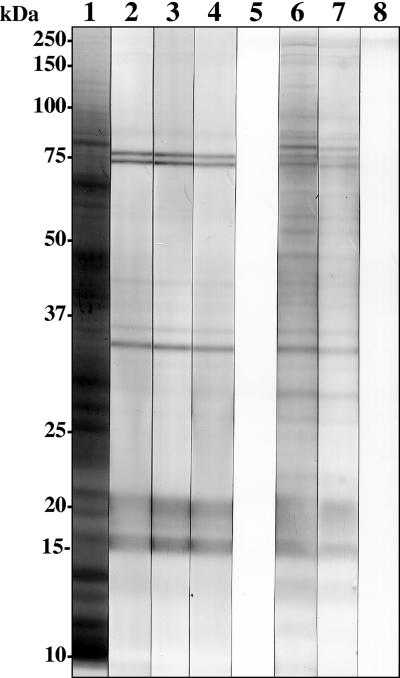
Indirect immunofluorescence microscopy revealed the localization of antigens recognized by these purified Fab fragments on the surfaces of late trophozoites/schizonts and merozoites (Fig. 2). This surface staining was demonstrated on strains FCR3 and 3D7, which are representatives with dimorphic allelic variants in MSP-119, suggesting the Fab fragments' reactivity to a conserved region. Western immunoblot analysis under nonreducing conditions showed that these Fab fragments were reactive to proteins with apparent molecular masses of 16 and 21 kDa (Fig. 3). In addition, 35-, 74-, and 76-kDa bands were also detected. All of these bands were also detected by plasmas from the eight malaria patients used to construct the library in this study.
FIG. 2.
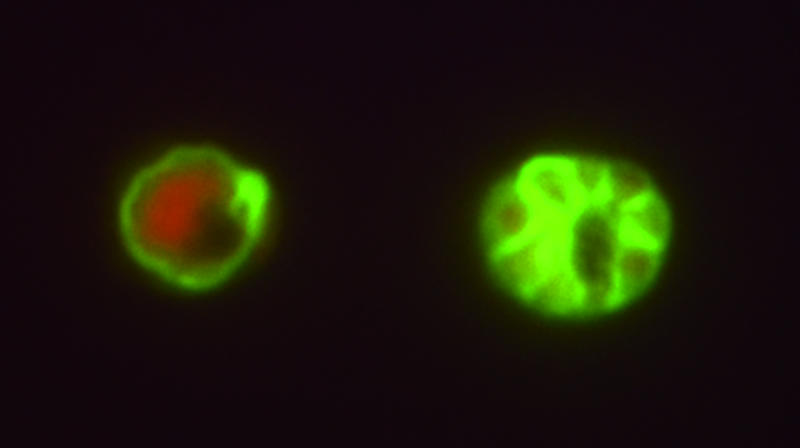
Indirect immunofluorescence staining of P. falciparum (strain FCR3)-infected human erythrocytes with recombinant Fab Pf25. The surfaces of a late trophozoite (left) and of merozoites in the schizont stage (right) were stained. Propidium iodide was used for counterstaining.
FIG. 3.
Western immunoblot analysis of reactivities of human monoclonal antibody Fab fragments to a crude antigen of P. falciparum (strain FCR3). Cell lysates were subjected to SDS-PAGE in a 10% polyacrylamide gel under nonreducing conditions and then transferred to polyvinylidene difluoride membranes. The protein bands in lane 1 were stained with Coomassie brilliant blue. Lanes 2 to 8 were treated as follows: lane 2, Pf25; lane 3, Pf143; lane 4, Pf227; lane 5, control human Fab; lanes 6 and 7, plasmas from patients with falciparum malaria; and lane 8, serum from a healthy patient (control). The preparations in lanes 2 to 5 and lanes 6 to 8 were treated with an HRP-conjugated goat antibody to human Fab and an HRP-conjugated goat antibody to human whole IgG, respectively. The numbers on the left indicate the molecular masses of size markers.
Primary structure and gene usage of human Fab clones.
Deduced amino acid sequences of the heavy- and light-chain immunoglobulin genes of clones Pf25, Pf143, and Pf227 are shown in Fig. 4. The three complementarity-determining regions (CDRs) in the heavy chains of Pf25 and Pf227 were identical. The light-chain CDR1 and CDR2 sequences were identical in the three Fab clones. Only one amino acid was different in CDR3 sequences among these clones. The sequence homology of these clones with germ line sequences was analyzed by IgBLAST at the NCBI website (http://www.ncbi.nlm.nih.gov/igblast/) and by V-QUEST at the international Immunogenetics database (http://imgt.cines.fr:8104/textes/vquest/). For the heavy-chain genes, the closest germ line sequence of the V segments in Pf25 and Pf227 was VH1-8, and that of the V segment in Pf143 was VH7-81 (Table 1). The closest germ line sequence of the D segments was D3-10 for these clones. For the J segment, JH4 was used in Pf25 and Pf227, and JH5 was used in Pf143. All of the light chains belonged to the Vκ1 family. The closest germ line sequence of the V segment was A27 for all three clones, but the closest germ line sequence of the J segment was different for each of the three clones (Table 2).
FIG. 4.
Deduced amino acid sequences of genes coding for heavy- and light-chain variable regions of human anti-P. falciparum MSP-119 Fab fragments. FR, framework regions. The dots indicate identical residues.
TABLE 1.
Comparison of gene usage for heavy-chain variable regions of anti-P. falciparum MSP-119 human Fab fragments
| Clone | V segment
|
D segment
|
J segment
|
|||
|---|---|---|---|---|---|---|
| Closest germ line | % Identity | Closest germ line | % Identity | Closest germ line | % Identity | |
| Pf25-H | VH1-8 | 97 | D3-10 | 100 | JH4 | 94 |
| Pf143-H | VH7-81 | 91 | D3-10 | 100 | JH5 | 90 |
| Pf227-H | VH1-8 | 98 | D3-10 | 100 | JH4 | 97 |
TABLE 2.
Comparison of gene usage for light-chain variable regions of anti-P. falciparum MSP-119 human Fab fragments
| Clone | V segment
|
J segment
|
||
|---|---|---|---|---|
| Closest germ line | % Identity | Closest germ line | % Identity | |
| Pf25-L | A27 | 100 | Jκ5 | 100 |
| Pf143-L | A27 | 99 | Jκ2 | 100 |
| Pf227-L | A27 | 99 | Jκ4 | 100 |
Affinities of human Fab clones.
The affinities of Pf25, Pf143, and Pf227 for recombinant MSP-119 were measured by surface plasmon resonance. The dissociation constants of the three Fab clones ranged from 1.09 × 10−9 to 2.66 × 10−9 M (Table 3). The affinity of Pf25 was approximately two to three times higher than those of Pf143 and Pf227.
TABLE 3.
Association and dissociation constants for binding of recombinant human Fabs to P. falciparum MSP-119, measured by surface plasmon resonancea
| Fab | KA (1/M) | KD (M) |
|---|---|---|
| Pf25 | 9.17 × 108 | 1.09 × 10−9 |
| Pf143 | 5.86 × 108 | 1.71 × 10−9 |
| Pf227 | 3.76 × 108 | 2.66 × 10−9 |
KA, association constant; KD, dissociation constant.
Analysis of an epitope recognized by human Fab fragments.
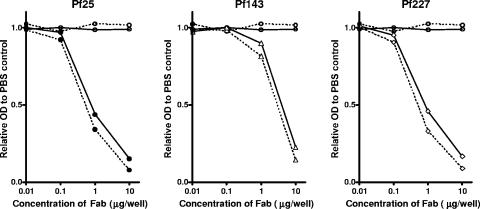
To examine whether the three Fab fragments recognized inhibitory epitopes on MSP-119, a competition assay was performed using an anti-MSP-119 mouse monoclonal antibody, 12.8, which is known to inhibit parasite growth (3, 22). Although competition between the three human Fab fragments and the monoclonal antibody 12.8 was observed, comparable competition was also detected between the Fab fragments and the blocking monoclonal antibody 2.2, which is known to block the binding of the inhibitory monoclonal antibody 12.8 to MSP-119 (12) (Fig. 5).
FIG. 5.
Competitive binding between recombinant human Fab fragments and mouse monoclonal antibodies to MSP-119 in ELISA. Various concentrations of recombinant Fab fragments (Pf25, •; Pf143, ▵; Pf227, ◊; and control Fab, ○) in PBS or PBS only was premixed with 200 ng per well of the inhibitory antibody 12.8 (solid lines) or 20 ng per well of the blocking antibody 2.2 (broken lines) and then added to wells. Reactions were detected with an HRP-conjugated goat antibody to mouse IgG and with a substrate. Optical densities (OD) were measured at 490 nm and expressed as relative values to the PBS control values.
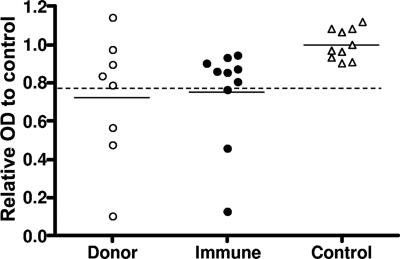
To examine whether the epitope for these Fabs was recognized by immune sera, competition ELISA was also performed using sera from 10 malaria-immune individuals from the Solomon Islands or plasmas from eight donors of lymphocytes. Only three of the immune sera and three of the donor plasmas showed significant inhibition compared with control sera. No significant difference in mean inhibition levels was demonstrated between the donor and the immune groups (Fig. 6).
FIG. 6.
Competitive binding of MSP-119 in ELISA between recombinant human Fab Pf25 and plasmas from donors of lymphocytes, sera from immune inhabitants of the Solomon Islands, or sera from healthy control individuals. Donor, plasmas from donors of lymphocytes (○); immune, immune sera from individuals with P. falciparum infections (•); control, sera from healthy controls (▵). Horizontal bars indicate mean values. The dashed line indicates the cutoff value (mean of controls minus 3 standard deviations).
Inhibitory activity of Fab fragments on parasite growth.
To evaluate whether Pf25, which had the highest affinity among the three Fab clones, inhibits parasite growth, it was added to the culture of P. falciparum at a concentration of 200 μg/ml. Although Pf25 suppressed parasite growth compared with the PBS control (Table 4), no difference was seen between Pf25 and a control human Fab fragment.
TABLE 4.
In vitro effect of Fab fragments on growth of P. falciparum
| Antibody | Concn (mg/ml) | % Parasitemiaa | % Inhibition |
|---|---|---|---|
| PBS only | 0 | 2.48 ± 0.34 | 0 |
| Control Fab | 0.2 | 1.69 ± 0.18 | 32 |
| Pf25 | 0.2 | 1.74 ± 0.34 | 30 |
Mean ± standard deviation of data from three experiments.
DISCUSSION
To the best of our knowledge, this is the first report of the successful production of human monoclonal antibodies reactive to P. falciparum MSP-119, a malaria vaccine candidate. Previous attempts to produce human monoclonal antibodies to P. falciparum with Epstein-Barr virus-transformed lymphocytes did not induce stable secretion of antibodies (16, 18, 47). Recombinant technology has been used to produce human Fab and scFv fragments to P. falciparum proteins (19, 32, 37, 49), and a human scFv fragment to the N-terminal block 2 region has been reported (37). However, the scFv fragment was reactive to only a limited number of parasite isolates, probably because of extensive sequence polymorphism in N-terminal block 2 of MSP-1 (43). In contrast, the three human Fab clones, Pf25, Pf143, and Pf227, obtained in the present study are reactive to a conserved region of MSP-119 (14, 15, 33) because the clones were reactive with strains FCR3 and 3D7. These two strains are representatives of dimorphic allelic variants, showing five amino acid substitutions in MSP-119 (K. Tanabe, unpublished data). Therefore, the epitope recognized by these Fabs seems to be conserved in P. falciparum isolates. The binding of the three Fab fragments was competitively inhibited by two mouse monoclonal antibodies, 12.8 and 2.2, both of which react to conserved epitopes in MSP-119 (3, 22), also suggesting that the three Fabs recognize a conserved epitope in MSP-119.
The three Fab fragments share a similar CDR structure in both the light and heavy chains. Therefore, the epitopes recognized by these Fabs are considered identical. Since the three human Fab fragments did not react with MSP-119 under reducing conditions, the epitope is likely formed by the conformation of the two epidermal growth factor-like domains in MSP-119. The equilibrium dissociation constants (KDs) of the three human Fab fragments ranged from 1.1 to 2.7 nM. These values are considerably higher than those reported for other human Fab fragments. For example, the KD of a human Fab fragment to the recombinant spike protein of severe acute respiratory syndrome-associated coronavirus, which we produced using the same expression system, is 19.8 nM (17), and the KDs of neutralizing human Fab fragments to the recombinant LecA domain of Entamoeba histolytica lectin are 7.7 to 13.9 nM (41). The KDs of anti-P. falciparum MSP-3 human Fab fragments reported by Lundquist et al. (19) are 20 to 46 nM.
The three Fab clones reacted not only to 16- and 21-kDa proteins but also to 74-, 76-, and 35-kDa proteins. We believe that the 16-kDa protein is the C-terminal 19-kDa molecule under nonreducing conditions. The 16-kDa protein may be a fragment processed from the 21-kDa protein after excision of the C-terminal sequence for glycosylphosphatidylinositol anchoring. The 35-kDa band observed in Western immunoblots seems to be the 42-kDa molecule under nonreducing conditions, as reported previously (3). The 74- and 76-kDa proteins may share the same epitope as the N-terminal 83-kDa molecule of MSP-1, which is further processed into slightly smaller fragments of 67 to 75 kDa (20, 38). MSP-119 contains the following three distinct types of epitopes: (i) epitopes recognized by inhibitory antibodies that inhibit processing of the C-terminal 42-kDa molecule and erythrocyte invasion by the merozoite, (ii) epitopes recognized by blocking antibodies that block the binding of the inhibitory monoclonal antibodies to MSP-119, and (iii) epitopes recognized by antibodies which are neither inhibitory nor blocking (4, 48). In the present study, the binding of three human Fabs to MSP-119 was competitively inhibited by both inhibitory and blocking mouse monoclonal antibodies to MSP-119, suggesting that the epitope recognized by these human Fab fragments overlaps epitopes recognized by these mouse monoclonal antibodies. Importantly, the binding of inhibitory monoclonal antibodies is blocked by naturally acquired human antibodies specific to the 83-kDa fragment of MSP-1 (12). In Western immunoblot analysis using our human Fab fragments as well as reactivity with patient plasmas, approximately 74- and 76-kDa bands were detected under nonreducing conditions. Therefore, we cannot exclude the possibility that the three Fab fragments may cross-react with the 83-kDa molecule.
In the present study, a human Fab clone did not inhibit the parasite's growth at a concentration of 200 μg/ml. It has been reported that the mouse monoclonal antibody 12.8 inhibits processing of MSP-142 by 96% at a concentration of 300 μg/ml (12). This concentration of whole IgG molecule is equivalent to 200 μg/ml of Fab fragments. The reason for the failure in obtaining inhibitory antibodies could be the source of our immunoglobulin gene library. We constructed the library from lymphocytes of eight malaria patients having clinical symptoms. It is generally known that individuals with asymptomatic malaria—but not those with clinical malaria—are immune to P. falciparum, and such protective immunity is acquired after repeated infections in areas where malaria is endemic, such as Tanzania (10). In the present study, the epitope for three human Fabs was strongly recognized by only 3 of 10 immune sera. Therefore, construction of a new library from peripheral blood lymphocytes of asymptomatic individuals highly immune to malaria may be required to obtain an inhibitory Fab clone, in addition to further screening of the library prepared for the present study. Indeed, we previously observed that a library constructed with cells from asymptomatic cyst passers of E. histolytica demonstrates a higher positive ratio of antibodies recognizing the adherence-inhibiting epitope of a surface lectin than that for a library prepared from a symptomatic patient with amebiasis (41). Recently, the preparation of whole IgG molecules from Fab fragments was reported (19). Whole IgG containing the cloned Fab fragments may induce antibody-mediated killing of merozoites by complement, antibody-dependent cellular cytotoxicity, and growth inhibition by cross-linking of antigen molecules (28, 29). Since the human Fabs reported in the present study possessed high affinities and specificities for a conserved epitope in MSP-1, we believe that there remains the possibility that these human Fabs are still candidates for malaria immunotherapy.
Meanwhile, the human Fab clones are useful for epitope mapping of antigens by analysis of the antigen-Fab fragment complex (23). An important observation in this context was the identification of the primary structure of anti-MSP-119 human antibodies. The usage of germ line sequences in antibodies to MSP-1 would be useful for understanding the molecular basis of acquired humoral immunity to malaria and for analyzing the epitopes of MSP-119. We believe that the present study will set the stage for further production and development of human anti-MSP-119 monoclonal antibodies for the possible development of passive immunotherapy for falciparum malaria.
Acknowledgments
We thank G. Masuda and K. Ohnishi for their help in preparation of immunoglobulin libraries and Y. Matsumoto for valuable discussions. We also thank J. S. McBride for providing mouse monoclonal antibodies 12.8 and 2.2, W.-Q. Pan for providing CP2.9, and S. Ihara for providing the expression vector.
This work was supported by a grant-in-aid for scientific research from the Japanese Society for the Promotion of Science and by grants from the Ministry of Health, Labor, and Welfare of Japan. X.-J.C. is a recipient of a Japanese Society for the Promotion of Science postdoctoral fellowship for foreign researchers.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Barbas, C. F., III, E. Bjorling, F. Chiodi, N. Dunlop, D. Cababa, T. M. Jones, S. L. Zebedee, M. A. Persson, P. L. Nara, E. Norrby, et al. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 89:9339-9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird, R. E., K. D. Hardman, J. W. Jacobson, S. Johnson, B. M. Kaufman, S. M. Lee, T. Lee, S. H. Pope, G. S. Riordan, and M. Whitlow. 1988. Single-chain antigen-binding proteins. Science 242:423-426. [DOI] [PubMed] [Google Scholar]
- 3.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappel, J. A., and A. A. Holder. 1993. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognise the first growth factor-like domain of merozoite surface protein-1. Mol. Biochem. Parasitol. 60:303-311. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, X. J., S. Ihara, M. Takekoshi, and H. Tachibana. 2000. Entamoeba histolytica: bacterial expression of a human monoclonal antibody which inhibits in vitro adherence of trophozoites. Exp. Parasitol. 96:52-56. [DOI] [PubMed] [Google Scholar]
- 7.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 8.Egan, A. F., P. Burghaus, P. Druilhe, A. A. Holder, and E. M. Riley. 1999. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21:133-139. [DOI] [PubMed] [Google Scholar]
- 9.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173:765-769. [DOI] [PubMed] [Google Scholar]
- 10.Färnert, A., I. Rooth, Å. Svensson, G. Snounou, and A. Björkman. 1999. Complexity of Plasmodium falciparum infections is consistent over time and protects against clinical disease in Tanzanian children. J. Infect. Dis. 179:989-995. [DOI] [PubMed] [Google Scholar]
- 11.Green, L. L., M. C. Hardy, C. E. Maynard-Currie, H. Tsuda, D. M. Louie, M. J. Mendez, H. Abderrahim, M. Noguchi, D. H. Smith, Y. Zeng, N. E. David, H. Sasai, D. Garza, D. G. Brenner, J. F. Hales, R. P. McGuinness, D. J. Capon, S. Klapholz, and A. Jakobovitsand. 1994. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat. Genet. 7:13-21. [DOI] [PubMed] [Google Scholar]
- 12.Guevara Patiño, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holder, A. A., M. J. Blackman, P. A. Burghaus, J. A. Chappel, I. T. Ling, N. McCallum-Deighton, and S. Shai. 1992. A malaria merozoite surface protein (MSP1)—structure, processing and function. Mem. Inst. Oswaldo Cruz 87(Suppl. III):37-42. [DOI] [PubMed] [Google Scholar]
- 14.Holder, A. A., J. S. Sandhu, Y. Hillman, L. S. Davey, S. C. Nicholls, H. Cooper, and M. J. Lockyer. 1987. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology 94:199-208. [DOI] [PubMed] [Google Scholar]
- 15.Jongwutiwes, S., K. Tanabe, and H. Kanbara. 1993. Sequence conservation in the C-terminal part of the precursor to the major merozoite surface proteins (MSP1) of Plasmodium falciparum from field isolates. Mol. Biochem. Parasitol. 59:95-100. [DOI] [PubMed] [Google Scholar]
- 16.Khusmith, S., S. Tharavanij, M. Chongsa-Nguan, P. Tapchaisri, A. Sabchareon, and D. Bunnag. 1987. Human monoclonal anti-Plasmodium falciparum antibodies produced by stable EBV-transformed lymphocytes from patients with falciparum malaria. Southeast Asian J. Trop. Med. Public Health 18:24-32. [PubMed] [Google Scholar]
- 17.Liu, J., H. Shao, Y. Tao, B. Yang, L. Qian, X. Yang, B. Cao, G. Hu, H. Tachibana, and X. Cheng. 2006. Production of an anti-severe acute respiratory syndrome (SARS) coronavirus human monoclonal antibody Fab fragment by using a combinatorial immunoglobulin gene library derived from patients who recovered from SARS. Clin. Vaccine Immunol. 13:594-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren, K., M. Wahlgren, M. Troye-Blomberg, K. Berzins, H. Perlmann, and P. Perlmann. 1983. Monoclonal anti-parasite and anti-RBC antibodies produced by stable EBV-transformed B cell lines from malaria patients. J. Immunol. 131:2000-2003. [PubMed] [Google Scholar]
- 19.Lundquist, R., L. K. Nielsen, A. Jafarshad, D. Soesoe, L. H. Christensen, P. Druilhe, and M. H. Dziegiel. 2006. Human recombinant antibodies against Plasmodium falciparum merozoite surface protein 3 cloned from peripheral blood leukocytes of individuals with immunity to malaria demonstrate antiparasitic properties. Infect. Immun. 74:3222-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyon, J. A., J. D. Haynes, C. L. Diggs, J. D. Chulay, C. G. Haidaris, and J. Pratt-Rossiter. 1987. Monoclonal antibody characterization of the 195-kilodalton major surface glycoprotein of Plasmodium falciparum malaria schizonts and merozoites: identification of additional processed products and a serotype-restricted repetitive epitope. J. Immunol. 138:895-901. [PubMed] [Google Scholar]
- 21.Mahanty, S., A. Saul, and L. H. Miller. 2003. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J. Exp. Biol. 206:3781-3788. [DOI] [PubMed] [Google Scholar]
- 22.McBride, J. S., and H. G. Heidrich. 1987. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71-84. [DOI] [PubMed] [Google Scholar]
- 23.Morgan, W. D., M. J. Lock, T. A. Frenkiel, M. Grainger, and A. A. Holder. 2004. Malaria parasite-inhibitory antibody epitopes on Plasmodium falciparum merozoite surface protein-119 mapped by TROSY NMR. Mol. Biochem. Parasitol. 138:29-36. [DOI] [PubMed] [Google Scholar]
- 24.Nwuba, R. I., O. Sodeinde, C. I. Anumudu, Y. O. Omosun, A. B. Odaibo, A. A. Holder, and M. Nwagwu. 2002. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect. Immun. 70:5328-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-119 are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan, W., D. Huang, Q. Zhang, L. Qu, D. Zhang, X. Zhang, X. Xue, and F. Qian. 2004. Fusion of two malaria vaccine candidate antigens enhances product yield, immunogenicity, and antibody-mediated inhibition of parasite growth in vitro. J. Immunol. 172:6167-6174. [DOI] [PubMed] [Google Scholar]
- 27.Pandey, K. C., S. Singh, P. Pattnaik, C. R. Pillai, U. Pillai, A. Lynn, S. K. Jain, and C. E. Chitnis. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol. Biochem. Parasitol. 123:23-33. [DOI] [PubMed] [Google Scholar]
- 28.Pang, X. L., and T. Horii. 1998. Complement-mediated killing of Plasmodium falciparum erythrocytic schizont with antibodies to the recombinant serine repeat antigen (SERA). Vaccine 16:1299-1305. [DOI] [PubMed] [Google Scholar]
- 29.Pang, X. L., T. Mitamura, and T. Horii. 1999. Antibodies reactive with the N-terminal domain of Plasmodium falciparum serine repeat antigen inhibit cell proliferation by agglutinating merozoites and schizonts. Infect. Immun. 67:1821-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera, K. L., S. M. Handunnetti, I. Holm, S. Longacre, and K. Mendis. 1998. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect. Immun. 66:1500-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perraut, R., L. Marrama, B. Diouf, C. Sokhna, A. Tall, P. Nabeth, J. F. Trape, S. Longacre, and O. Mercereau-Puijalon. 2005. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J. Infect. Dis. 191:264-271. [DOI] [PubMed] [Google Scholar]
- 32.Roeffen, W. F. G., J. M. H. Raats, K. Teelen, R. M. A. Hoet, W. M. Eling, W. J. van Venrooij, and R. W. Sauerwein. 2001. Recombinant human antibodies specific for the Pfs48/45 protein of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 276:19807-19811. [DOI] [PubMed] [Google Scholar]
- 33.Sakihama, N., M. Kimura, K. Hirayama, T. Kanda, K. Na-Bangchang, S. Jongwutiwes, D. Conway, and K. Tanabe. 1999. Allelic recombination and linkage disequilibrium within Msp-1 of Plasmodium falciparum, the malignant human malaria parasite. Gene 230:47-54. [DOI] [PubMed] [Google Scholar]
- 34.Sakihama, N., H. Ohmae, B. Bakote'e, M. Kawabata, K. Hirayama, and K. Tanabe. 2006. Limited allelic diversity of Plasmodium falciparum merozoite surface protein 1 gene from populations in the Solomon Islands. Am. J. Trop. Med. Hyg. 74:31-40. [PubMed] [Google Scholar]
- 35.Siddiqui, W. A., L. Q. Tam, K. J. Kramer, G. S. Hui, S. E. Case, K. M. Yamaga, S. P. Chang, E. B. Chan, and S. C. Kan. 1987. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 84:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sowa, K. M., D. R. Cavanagh, A. M. Creasey, J. Raats, J. McBride, R. Sauerwein, W. F. Roeffen, and D. E. Arnot. 2001. Isolation of a monoclonal antibody from a malaria patient-derived phage display library recognising the block 2 region of Plasmodium falciparum merozoite surface protein-1. Mol. Biochem. Parasitol. 112:143-147. [DOI] [PubMed] [Google Scholar]
- 38.Stafford, W. H., M. J. Blackman, A. Harris, S. Shai, M. Grainger, and A. A. Holder. 1994. N-terminal amino acid sequence of the Plasmodium falciparum merozoite surface protein-1 polypeptides. Mol. Biochem. Parasitol. 66:157-160. [DOI] [PubMed] [Google Scholar]
- 39.Tachibana, H., X. J. Cheng, K. Watanabe, M. Takekoshi, F. Maeda, S. Aotsuka, Y. Kaneda, T. Takeuchi, and S. Ihara. 1999. Preparation of recombinant human monoclonal antibody Fab fragments specific for Entamoeba histolytica. Clin. Diagn. Lab. Immunol. 6:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tachibana, H., N. Matsumoto, X. J. Cheng, H. Tsukamoto, and E. Yoshihara. 2004. Improved affinity of a human anti-Entamoeba histolytica Gal/GalNAc lectin Fab fragment by a single amino acid modification of the light chain. Clin. Diagn. Lab. Immunol. 11:1085-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tachibana, H., K. Watanabe, X. J. Cheng, H. Tsukamoto, Y. Kaneda, T. Takeuchi, S. Ihara, and W. A. Petri, Jr. 2003. VH3 gene usage in neutralizing human antibodies specific for the Entamoeba histolytica Gal/GalNAc lectin heavy subunit. Infect. Immun. 71:4313-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takekoshi, M., F. Maeda, H. Tachibana, H. Inoko, S. Kato, I. Takakura, T. Kenjyo, S. Hiraga, Y. Ogawa, T. Horiki, and S. Ihara. 1998. Human monoclonal anti-HCMV neutralizing antibody from phage display libraries. J. Virol. Methods 74:89-98. [DOI] [PubMed] [Google Scholar]
- 43.Tanabe, K., M. Mackay, M. Goman, and J. G. Scaife. 1987. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 195:273-287. [DOI] [PubMed] [Google Scholar]
- 44.Tomizuka, K., H. Yoshida, H. Uejima, H. Kugoh, K. Sato, A. Ohguma, M. Hayasaka, K. Hanaoka, M. Oshimura, and I. Ishida. 1997. Functional expression and germline transmission of a human chromosome fragment in chimaeric mice. Nat. Genet. 16:133-143. [DOI] [PubMed] [Google Scholar]
- 45.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 46.Trager, W., and H. N. Lanners. 1984. Initial extracellular development in vitro of merozoites of Plasmodium falciparum. J. Protozool. 31:562-567. [DOI] [PubMed] [Google Scholar]
- 47.Udomsangpetch, R., K. Lundgren, K. Berzins, B. Wahlin, H. Perlmann, M. Troye-Blomberg, J. Carlsson, M. Wahlgren, P. Perlmann, and A. Bjorkman. 1986. Human monoclonal antibodies to Pf 155, a major antigen of malaria parasite Plasmodium falciparum. Science 231:57-59. [DOI] [PubMed] [Google Scholar]
- 48.Uthaipibull, C., B. Aufiero, S. E. Syed, B. Hansen, J. A. Guevara Patino, E. Angov, I. T. Ling, K. Fegeding, W. D. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. A. Lyon, and A. A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381-1394. [DOI] [PubMed] [Google Scholar]
- 49.Wajanarogana, S., T. Prasomrothanakul, R. Udomsangpetch, and S. Tungpradabkul. 2006. Construction of a human functional single-chain variable fragment (scFv) antibody recognizing the malaria parasite Plasmodium falciparum. Biotechnol. Appl. Biochem. 44:55-61. [DOI] [PubMed] [Google Scholar]