Abstract
Coxiella burnetii, the etiological agent of Q fever, has two phase variants. Phase I has a complete lipopolysaccharide (LPS), is highly virulent, and causes Q fever in humans and pathology in experimental animals. Phase II lacks an LPS O side chain, is avirulent, and does not grow well in immunocompetent animals. To understand the pathogenicity of Q fever, we investigated the roles of immune components in animals infected with Nine Mile phase I (NM I) or Nine Mile phase II (NM II) bacteria. Immunodeficient mice, including SCID mice (deficient in T and B cells), SCIDbg mice (deficient in T, B, and NK cells), nude mice (deficient in T cells), muMT mice (deficient in B cells), bg mice (deficient in NK cells), mice deficient in tumor necrosis factor alpha (TNF-α−/− mice), and mice deficient in gamma interferon (IFN-γ−/− mice), were compared for their responses to infection. SCID, SCIDbg, nude, and IFN-γ−/− mice showed high susceptibility to NM I, and TNF-α−/− mice showed modest susceptibility. Disease caused by NM I in SCID, SCIDbg, and nude mice progressed slowly, while disease in IFN-γ−/− and TNF-α−/− mice advanced rapidly. B- and NK-cell deficiencies did not enhance clinical disease development or alter bacterial clearance but did increase the severity of histopathological changes, particularly in the absence of B cells. Mice infected with NM II showed no apparent clinical disease, but T-cell-deficient mice had histopathological changes. These results suggest that T cells are critical for clearance of C. burnetii, either NM I or NM II, that IFN-γ and TNF-α are essential for the early control of infection, and that B cells are important for the prevention of tissue damage.
Coxiella burnetii is an obligate intracellular bacterium and the etiological agent of Q fever. Q fever is a worldwide zoonosis with a broad host range including humans, wild and domestic animals, birds, and arthropods (32). Naturally infected animals rarely demonstrate disease except for reproductive disorders, including late-term abortion (4). The disease in humans can present in either an acute or chronic form (27). Acute Q fever is mainly a flu-like syndrome that is primarily self-limiting and can be readily treated with antibiotics when the appropriate diagnosis is made (30). The chronic form frequently manifests as endocarditis, a serious and difficult-to-treat outcome of infection (15).
Immunocompetent laboratory animals, such as guinea pigs and mice, are routinely used as animal models for Q fever (33, 34). Guinea pigs are sensitive to the infection, show a fever response and pathology, and have a higher mortality rate than mice (24, 28, 33). Mice do not generally manifest significant clinical disease, but some strains are more sensitive than others, as shown by differences in their mortality rates. Among this range of differences, the A/J mouse is considered a sensitive strain (high mortality) and the C57BL/6J mouse is considered a resistant strain (low mortality) (33, 34). Nude and severe combined immunodeficient mice (SCID; T and B cell deficient) are highly sensitive to the infection (2, 26). In addition, it has been shown that immunosuppressive treatments can alter the sensitivity of mice to infection (3, 25). The observation that C. burnetii phase I infection causes lethal disease in SCID mice supports the notion that acquired immunity is essential for the host defense against the infection (2). However, the immune components that are required for the successful control of infection have not been defined.
Macrophages are the major target cells during C. burnetii infection. Cytokines that regulate macrophage bactericidal activity, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), have been shown to be important factors in infection control. Indeed, failure to produce IFN-γ has been reported in chronic Q fever patients (30), and there is one case report of effective IFN-γ treatment of a Q fever patient (29). In vitro studies showed effective killing of intracellular C. burnetii with a supplement of IFN-γ and TNF-α (6, 20).
The phase variation phenotype parallels the smooth-to-rough lipopolysaccharide (LPS) transition of many gram-negative enterobacteria (1). Upon serial in vitro passage of virulent C. burnetii, avirulent LPS phase variants outgrow virulent organisms, allowing a population shift in culture (4). Phase I is highly virulent, replicates in immunocompetent animals, and causes Q fever in humans and pathology in experimental animals. Phase II is an avirulent variant that does not replicate in immunocompetent animals (28). Uptake of phase I bacteria by human monocytes is mediated by αvβ3 integrin, resulting in interference with CR3 activation and a reduction in phagocytic activity, whereas uptake of phase II bacteria is mediated by both αvβ3 integrin and CR3 and does not affect CR3 activation (8). In contrast to what occurs in in vivo conditions, phase II bacteria can replicate in an in vitro culture lacking host immunity (28). Nine Mile phase I (NM I) (strain RSA 493) organisms encode a complete LPS with the O side chain, while Nine Mile phase II (NM II) (strain RSA 439) organisms do not express the O side chain or several terminal sugar residues because of a major deletion of genes involved in LPS biosynthesis (19). Based on the difference in virulence of these phase variants, phase I bacteria require biosafety level 3 (BL3) containment, while phase II bacteria can be cultured under BL2 conditions. However, the factors that are responsible for the difference in virulence between phase I and phase II have not been clearly demonstrated.
In this study, we investigated the in vivo role of immune system components (T, B, and NK cells, IFN-γ, and TNF-α) in NM I and NM II infections in mice. Clinical signs, bacteremia, splenomegaly, quantification of bacteria in the spleen, histopathology, and immunohistochemistry were used as indicators to evaluate the pathogenicity of NM I and NM II. This study provides novel information for understanding the mechanisms of host defense against C. burnetii infection.
MATERIALS AND METHODS
Bacteria.
Two cloned isolates, C. burnetii RSA 493 (NM I) and C. burnetii RSA 439 (NM II) were used. NM I was cultured in embryonated chicken eggs, and NM II was cultured in L929 cells; both were purified by gradient centrifugation and stored at −80°C until use. Enumeration of bacteria was performed by quantitative PCR as described below.
Animals.
Five- to 6-week-old female mice were purchased from Taconic (Germantown, NY) or Jackson Laboratory (Bar Harbor, ME). The various mouse strains and their relevant immunological characteristics are shown in Table 1. Two wild-type (wt) mouse strains (CB17 and B6) were used as controls appropriately for each experiment. Mice were housed under sterile conditions in BL3 facilities. All animal experiments were performed under an animal protocol approved by the University Laboratory Animal Care Committee of Texas A&M University.
TABLE 1.
Immunological characteristics of mouse strains used
| Designation | Defect | Strain | Source |
|---|---|---|---|
| SCID | T and B cells | C.B-Igh-1b/IcrTac-Prkdcscid | Taconic |
| SCIDbg | T, B, and NK cells | C.B-Igh-1bGbmsTac-Prkdcscid-LystbgN7 | Taconic |
| Nude | T cells | B6.Cg-Foxn1nu/J | Jackson |
| muMT | B cells | B6.129S2-Igh-6tm1Cgn/J | Jackson |
| bg | NK cells | C57BL/6J-Lystbg-J/J | Jackson |
| IFN-γ−/− | IFN-γ | B6.129S7-Ifngtm1Ts/J | Jackson |
| TNF-α−/− | TNF-α | B6.129S6-Tnftm1Gkl/J | Jackson |
| CB17 | None (wt) | C.B-Igh-1b/IcrTac | Taconic |
| B6 | None (wt) | C57BL/6J | Jackson |
Experimental infection in mice.
Four mice per group were infected intraperitoneally with a low dose (102 genome copies/mouse) or high dose (105 genome copies/mouse) of C. burnetii. Phosphate-buffered saline (PBS) was used as a sham infection control. Apparent clinical signs were observed daily, and body weights were recorded at 0, 7, 14, 21, and 28 days postinfection (DPI). Body weight changes were expressed as the body weight index. The body weight index is the relative body weight on a day divided by the relative body weight of sham-infected mice on the same day; the relative body weight is the body weight on each day divided by the body weight at zero time. Blood was collected from the lateral saphenous vein at 7, 14, and 21 DPI and by cardiac puncture at 28 DPI after euthanasia. Bacteremia was determined by detecting C. burnetii DNA in 10 μl of whole blood. At necropsy, spleens were weighed, small pieces were collected for DNA extraction, and the DNA was used for C. burnetii gene detection by quantitative PCR to confirm growth of the bacteria. Hearts, lungs, livers, and spleens were fixed in 10% formalin-PBS for histopathology.
Quantitative PCR.
Purified bacteria or blood was directly mixed with lysis buffer. Small pieces of spleen were weighed and homogenized with lysis buffer, and parts of the homogenates were used for DNA extraction. DNA extraction and real-time PCR were performed as described previously (7). Briefly, samples were digested with proteinase K, followed by DNA extraction using a High Pure PCR template preparation kit (Roche Molecular Biochemicals, Indianapolis, IN). DNA was used as a template for quantifying the number of C. burnetii com1 gene copies. The bacterial genome copy number per whole spleen was extrapolated from the copy number of each PCR and the weights of spleen pieces and whole spleens.
Serology.
Serum samples were diluted from 1:16 to 1:2,048 by twofold dilution. C. burnetii phase I and II specific antibody titers were measured by an indirect immunofluorescent assay using whole-cell antigen fixed in 24-spot slides and goat Alexafluor 488-labeled anti-mouse immunoglobulin G(H+L) [IgG(H+L)] antibody (Molecular Probes, Carlsbad, CA).
Histopathology.
Formalin-fixed tissues were mounted in paraffin, sliced, and stained with hematoxylin and eosin (HE). The degree of inflammation was scored as follows: 0, no appreciable inflammatory changes; 1, mild inflammation, consisting of a slight increase in the numbers of lymphocytes and macrophages; 2, moderate inflammation, characterized by scattered accumulations of lymphocytes and macrophages, occasionally accompanied by mild necrosis; 3, marked inflammation, consisting of locally extensive macrophage and lymphocyte accumulations with hyperemia and hemorrhage and scattered mild to moderate necrosis; and 4, severe inflammation, characterized by dense accumulations of macrophages and lymphocytes with hyperemia and hemorrhage and moderate to severe necrosis. Extramedullary hematopoiesis (EMH) was defined as random in distribution, containing erythrocytic series cells and including megakaryocytes, which can be distinguished from inflammation that is accompanied by hyperemia, hemorrhage, and necrosis and does not include megakaryocytes. Immunohistochemistry (IHC) analysis was performed with rabbit anti-C. burnetii phase I serum and a VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA) used according to the manufacturer's protocol. The degree of C. burnetii antigen distribution was scored as follows: 0, none; 1, mild, rare immunopositive cells (less than 10 positive cells per tissue); 2, moderate, a few immunopositive cells (more than 10 positive cells); 3, marked, several, and/or multifocal distribution of immunopositive cells in each field; and 4, severe, diffuse distribution of immunopositive cells in each field.
Statistics.
Results expressed as means ± standard deviations were compared with Student's t test. Differences were considered significant at a P value of <0.05.
RESULTS
Low-dose infection with phase I and II C. burnetii in multiple-immune-component-deficient mice.
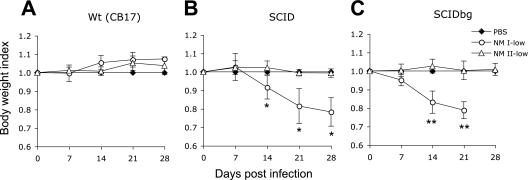
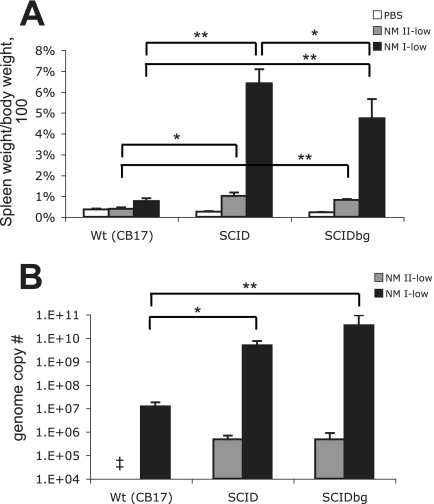
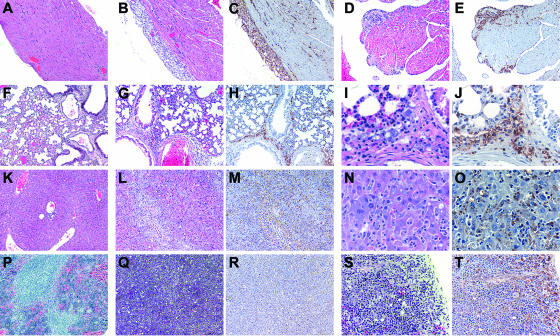
To evaluate the in vivo growth and virulence of NM I and NM II bacteria in mice lacking acquired cell-mediated immunity, we first compared low-dose infections in mice with deficiencies in multiple immune system components. SCID and SCIDbg mice infected with the low dose of NM I showed severe persistent illness. Animals exhibited ruffled fur, a hunched posture, and severe weight loss (Fig. 1). SCIDbg mice died at 27.8 ± 0.5 DPI. Bacteremia was detected in both SCID and SCIDbg mice at 14, 21, and 28 DPI (data not shown). wt mice infected with the low dose of NM I exhibited only transient ruffled fur, and no bacteremia was detected. Splenomegaly was extremely severe in SCIDbg and SCID mice (the splenomegaly in SCID mice was more severe than that in SCIDbg mice [P < 0.05]) but was mild in wt mice (Fig. 2A). White necrotic foci were found in the livers of SCID and SCIDbg mice. The bacterial loads in the spleens were similar in SCID and SCIDbg mice, and they were higher than those in wt mice (Fig. 2B). Coxiella DNA was undetectable in PBS control groups. Histopathologically, SCIDbg and SCID mice had moderate to severe cellular inflammation (Table 2). Distribution of C. burnetii antigen, detected as brown granules by IHC staining, was associated with cellular infiltration (Fig. 3). The heart had accumulations of macrophages in subepicardial, valvular, and subendocardial regions, and there were scattered macrophages within the myocardium, but not all sites were necessarily infiltrated in an individual specimen (Fig. 3B to E). Subepicardial mineralization was occasionally noted. Lungs had multifocal perivascular accumulations of macrophages, which were milder in SCID mice than in SCIDbg mice, and scattered to diffuse areas of interstitial neutrophil accumulation (Fig. 3G to J). Livers had marked multifocal to extensive accumulations of macrophages and multifocal random to marked diffuse neutrophil accumulation (Fig. 3L to O). Additionally, SCIDbg mice had multifocal hepatocellular coagulative necrosis. Spleens had marked multifocal diffuse accumulations of macrophages and moderate to marked diffuse neutrophil accumulations (Fig. 3Q to T). Extensive infiltration of inflammatory cells in livers and spleens obscured or distorted the normal tissue architecture. wt mice infected with the low dose of NM I had mild mineralization in the heart and mild multifocal cellular coagulation in the liver.
FIG. 1.
Body weight changes of mice infected with the low dose of NM I or NM II. (A) wt (CB17) mice; (B) SCID mice; (C) SCIDbg mice. Data for SCIDbg mice infected with the low dose of NM I at 28 DPI are not available because of death of the mice. The results (means ± standard deviations) are expressed as the body weight index. One asterisk indicates that the P value is <0.05 and two asterisks indicate that the P value is <0.001 for comparisons of infected mice with PBS-inoculated control mice.
FIG. 2.
Spleens of mice infected with the low dose of NM I or NM II: splenomegaly (A) and bacterial genome numbers (B) in the spleen at 28 DPI. The results (means ± standard deviations) are expressed as the percentage of spleen weight compared with the body weight (A) and the number of C. burnetii genomes in the whole spleen (B). One asterisk indicates that the P value is <0.05, and two asterisks indicate that the P value is <0.001. Double dagger, not detected.
TABLE 2.
Degree of inflammation and C. burnetii antigen distribution in mice infected with the low dose of NM I or NM II at 28 DPI
| Mice | Mean inflammation score (mean antigen distribution score) witha:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NM I infection
|
NM II infection
|
PBS (heart, lung, liver, and spleen) | |||||||
| Heart | Lung | Liver | Spleen | Heart | Lung | Liver | Spleen | ||
| SCIDbg | 2.6b (3.3b) | 2.3b (3.0b) | 3.8b (3.7b) | 4.0b (4.0b) | 0.3 (0.0) | 0.0 (0.0) | 1.8b (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| SCID | 2.6b (2.3b) | 3.8b (2.7b) | 3.0b (3.7b) | 4.0b (4.0b) | 0.0 (0.0) | 0.0 (0.0) | 1.3b (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| wt (CB17) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
0, none; 1, mild; 2, moderate; 3, marked; 4, severe.
P < 0.05 for a comparison with the wt group with the same infection.
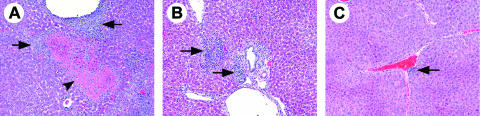
FIG. 3.
Histopathology of SCIDbg mice infected with the low dose of NM I at 28 DPI. (A to E) Heart; (F to J) lung; (K to O) liver; (P to T) spleen. Panels A, F, K, and P show the results for control mice from the PBS group. HE staining or IHC staining (C, E, H, J, M, O, R, and T) was used. (A to H, K to M, and P to R) Original magnification, ×100; (S and T) original magnification, ×200; (I, J, N, and O) original magnification, ×400. Severe cellular infiltrations associated with C. burnetii antigen (brown granules with IHC staining) were observed in all tissues from infected mice. Subepicardial lesions (B and C), subendocardial lesions (D and E), and peribronchial and some perialveolar lesions (G to J) were also observed, and extensive cellular infiltrations obscured the tissue architecture in the liver and spleen associated with diffuse antigen distribution (L to O and Q to T).
In contrast to NM I infection, no mice infected with NM II showed clinical illness or bacteremia. However, SCID and SCIDbg mice infected with NM II had splenomegaly (Fig. 2A). Coxiella DNA in the spleen was detected in both SCID and SCIDbg mice but not in wt mice (Fig. 2B). SCID and SCIDbg mice infected with NM II had mild histopathological changes in the heart, liver, and spleen but not in the lungs (Table 2). In the heart, mild multifocal subendocardial mineralization with mild subjacent accumulation of mainly macrophages was observed in one SCIDbg mouse, and focal or moderate locally extensive subepicardial mineralization was observed in three of four SCID mice and in one wt mouse. In the liver, SCIDbg mice had multifocal, mild to moderate macrophage and neutrophil accumulations associated with necrotic foci and multifocal hepatocellular coagulative necrosis; SCID mice had random, multifocal scattered macrophage accumulations. Spleens of both SCIDbg and SCID mice exhibited increased or moderate to marked EMH. No specific histopathological change or C. burnetii antigen was found in wt mice infected with NM II and in PBS groups.
These results confirmed the high susceptibility of multiple-immune-component-deficient mice to phase I C. burnetii, as previously reported (2), and that NK cells have an accessory role in control of the infection through innate immunity. These results also showed that phase II C. burnetii replicates in vivo in the absence of acquired cell-mediated immunity, although not as efficiently as phase I.
High-dose infection with phase II C. burnetii in multiple-immune-component-deficient mice.
Low-dose challenge with phase II bacteria resulted in no apparent clinical illness, even though animals were permissive for replication of NM II. To confirm the reduced virulence of phase II bacteria, we used high-dose infections (105 genome copies) with NM II in multiple-immune-component-deficient mice.
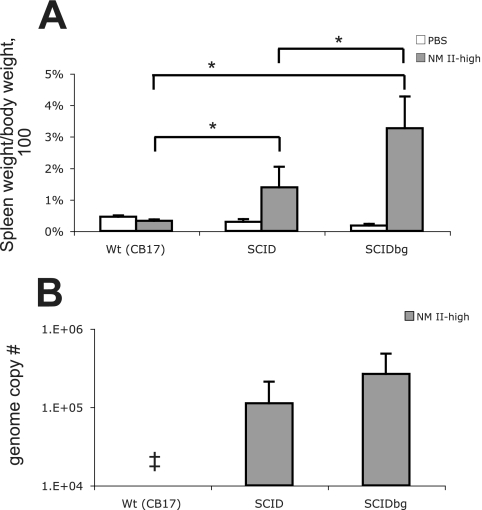
No animals infected with the high dose of NM II showed clinical signs of illness, and no bacteremia was detected. Splenomegaly was found in SCID and SCIDbg mice, and it was more severe in SCIDbg mice (P < 0.05) (Fig. 4A). SCIDbg mice had numerous white necrotic foci in the liver. Similar amounts of bacteria were detected in spleens of SCID and SCIDbg mice (Fig. 4B). wt mice infected with the high dose of NM II did not have splenomegaly or detectable bacterial DNA. Histopathologic changes were noted in the liver (Table 3). Examination of livers of SCID and SCIDbg mice revealed multifocal random macrophage accumulations, and there was occasional coagulative necrosis of hepatocytes in SCIDbg mice (Fig. 5A and B). Livers of wt mice had focal and perivascular inflammation consisting of macrophages and lymphocytes (Fig. 5C). Spleens had moderate EMH in all mouse strains; additionally, small discrete accumulations of macrophages were noted in SCID mice. Hearts of SCID and wt mice had occasional mild to marked subepicardial mineralization. In lungs, SCIDbg mice had no specific changes; SCID mice had occasional focal vascular thrombosis, and wt mice had focal dense accumulations of macrophages. No specific histopathological change, except occasional mild to moderate subepicardial mineralization in the heart, or C. burnetii antigen was found in PBS groups.
FIG. 4.
Spleens of mice infected with the high dose of NM II: splenomegaly (A) and bacterial genome numbers (B) in the spleen at 28 DPI. The results (means ± standard deviations) are expressed as the percentage of spleen weight based on the body weight (A) and the number of C. burnetii genomes in the whole spleen (B). An asterisk indicates that the P value is <0.05. SCID and SCIDbg mice were significantly different from PBS-inoculated groups (P < 0.05) for splenomegaly (A). Double dagger, not detected.
TABLE 3.
Degree of inflammation and C. burnetii antigen distribution in mice infected with the high dose of NM I or NM II at 28 DPI
| Mice | Mean inflammation score (mean antigen distribution score) witha:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NM I infection
|
NM II infection
|
PBS (heart, lung, liver, and spleen) | |||||||
| Heart | Lung | Liver | Spleen | Heart | Lung | Liver | Spleen | ||
| SCIDbg | 0.0 (0.0) | 0.0 (0.0) | 2.8b (1.0b) | 0.0 (2.0b) | 0.0 (0.0) | ||||
| SCID | 0.0 (0.0) | 0.0 (0.0) | 2.0b (0.8b) | 1.0b (2.0b) | 0.0 (0.0) | ||||
| wt (CB17) | 0.0 (0.0) | 0.3 (0.0) | 0.8 (0.0) | 0.0 (0.0) | 0.0 (0.0) | ||||
| Nude | 0.0 (1.5b) | 0.0 (1.9b) | 1.5 (3.5b) | 0.0b (4.0)b | 0.0 (0.0) | 1.0b (0.0) | 1.0 (0.3) | 1.0b (0.7) | 0.0 (0.0) |
| muMT | 1.3 (0.0) | 1.3b (0.0) | 3.0b (0.0) | 3.3b (0.0) | 0.1 (0.0) | 0.3 (0.0) | 2.3b (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| bg | 0.9 (0.0) | 0.1 (0.0) | 2.8b (0.0) | 3.0 (1.7) | 0.0 (0.0) | 0.1 (0.0) | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| TNF-α−/− | 3.2b (1.3b) | 0.0 (0.0) | 2.7 (3.0b) | 1.7 (2.0b) | 0.0 (0.0) | 0.1 (0.0) | 1.0 (0.0) | 0.0 (0.5) | 0.0 (0.0) |
| IFN-γ−/− | 1.5b (1.3b) | 1.1b (1.8b) | 3.3b (3.5b) | 2.3 (2.0) | 0.0 (0.0) | 0.1 (0.0) | 0.0b (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| wt (B6) | 0.4 (0.0) | 0.1 (0.0) | 2.0 (1.0) | 2.3 (1.0) | 0.3 (0.0) | 0.0 (0.0) | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
0, none; 1, mild; 2, moderate; 3, marked; 4, severe. High-dose NM I infection of SCIDbg, SCID, and wt (CB17) mice was not performed.
P < 0.05 for a comparison with the wt group with the same infection.
FIG. 5.
Liver histopathology of mice infected with the high dose of NM II at 28 DPI. (A) SCIDbg mice had hepatocellular coagulative necrosis (arrowhead) and severe cellular accumulation (arrows). (B) SCID mice had granuloma-like cellular accumulation (arrows). (C) wt (CB17) mice had mild cellular coagulation (arrow). HE staining was used. Original magnification, ×100.
These results revealed that there was low-level virulence of phase II C. burnetii in immunocompromised mice compared to the avirulence in immunocompetent mice. The splenomegaly, bacterial load in spleen, and histopathology scores for the NM II high-dose infection were lower than those for the NM I low-dose infection, indicating the relatively low but detectable virulence of NM II.
High-dose infection with phase I and II C. burnetii in mice deficient in a single immune component.
To investigate the roles of selected immune components in C. burnetii infection, we performed high-dose infections using NM I and NM II in several single-immune-component-deficient mice. Based on the critical role that IFN-γ and TNF-α play in macrophage bactericidal activity, IFN-γ- and TNF-α-deficient mice were used. Additionally, based on the phenotypic differences observed in wt, SCID, and SCIDbg mice, we compared infections in T-cell-, B-cell-, and NK-cell-deficient mice.
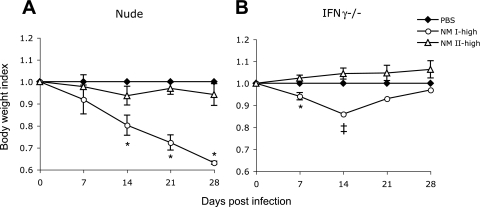
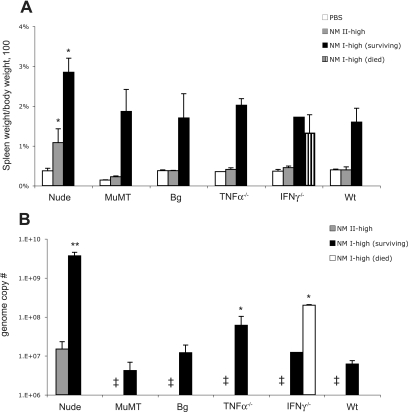
After high-dose NM I infection, only nude and IFN-γ−/− mice showed body weight loss (Fig. 6). Mortality occurred only in IFN-γ−/− mice. Three of four IFN-γ−/− mice died at 12.3 ± 0.6 days; the surviving IFN-γ−/− mouse lost body weight until 14 DPI but recovered by 28 DPI. Bacteremia was detected in nude, TNF-α−/−, and IFN-γ−/− mice infected with the high dose of NM I; in nude mice, bacteremia appeared after 14 DPI, while in TNF-α−/− and IFN-γ−/− mice it developed early in infection (Table 4). Splenomegaly was found in all mouse strains, but only nude mice had significantly more severe splenomegaly than wt mice (Fig. 7A). Coxiella DNA was detected in the spleen in all mouse strains, and the bacterial load was significantly higher in nude, TNF-α−/−, and IFN-γ−/− (mortality group) mice than in wt mice (Fig. 7B). After high-dose NM II infection, body weight loss and bacteremia were not found in any mice, and splenomegaly and Coxiella DNA detection occurred only in nude mice (Fig. 6 and 7).
FIG. 6.
Body weight changes of mice infected with the high dose of NM I or NM II. (A) Nude mice; (B) IFN-γ−/− mice. The double dagger indicates that the data for IFN-γ−/− mice infected with the high dose of NM I at 14, 21, and 28 DPI are from a single mouse that survived (three of four mice died before 14 DPI). The results (means ± standard deviations) are expressed as the body weight index. An asterisk indicates that the P value is <0.05 for comparisons of infected mice with PBS-inoculated control mice.
TABLE 4.
Bacteremia and mortality in single-immune-component-deficient mice infected with the high dose of NM I
| Mice | No. with bacteremia/total no.
|
No. that died/total no. | |||
|---|---|---|---|---|---|
| 7 DPI | 14 DPI | 21 DPI | 28 DPI | ||
| Nude | 0/4 | 2/4 | 4/4 | 4/4 | 0/4 |
| muMT | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| bg | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| TNF-α−/− | 3/3 | 0/3 | 0/3 | 1/3 | 0/3 |
| IFN-γ−/− | 4/4 | 1/1 | 1/1 | 1/1 | 3/4 |
| wt (B6) | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
FIG. 7.
Spleens of mice infected with the high dose of NM I or NM II: splenomegaly (A) and bacterial genome numbers (B) in the spleen at 28 DPI. The results (means ± standard deviations) are expressed as the percentage of spleen weight based on the body weight (A) and the number of C. burnetii genomes in the whole spleen (B). One asterisk indicates that the P value is <0.05 and two asterisks indicate that the P value is <0.001 for the comparison of infected mice with wt mice infected with the same inoculum. (A) Every mouse group infected with NM I had significant differences from the control group of the same mouse strain treated with PBS (P < 0.05 for wt [B6], muMT, and bg mice; P < 0.001 for nude and TNF-α−/− mice). Double dagger, not detected.
Antibody detection showed high titers of anti-phase II antibody and moderate titers of anti-phase I antibody in wt, bg, TNF-α−/−, and IFN-γ−/− mice infected with NM I (Table 5). bg, TNF-α−/−, IFN-γ−/−, and wt mice infected with NM I had higher IgG titers than mice infected with NM II. Nude mice, however, had no detectable IgG titer after NM I infection, but IgG was detected following NM II infection.
TABLE 5.
C. burnetii phase I and II specific IgG titers of mice at 28 DPI detected by theindirect immunofluorescence assay
| Mice | NM I infection
|
NM II infection
|
||||||
|---|---|---|---|---|---|---|---|---|
| Anti-phase I titer
|
Anti-phase II titer
|
Anti-phase I titer
|
Anti-phase II titer
|
|||||
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| Nude | NTa | NT | NT | 40 | 32-64 | |||
| muMT | NT | NT | NT | NT | ||||
| bg | 512 | 256-1,024 | 2,048 | 2,048 | 38 | 32-64 | 1,024 | 1,024 |
| TNF-α−/− | 406 | 256-512 | 2,048 | 2,048 | 27 | 16-32 | 609 | 256-2,048 |
| IFN-γ−/− | 128b | 2,048b | 64 | 64 | 1,024 | 1,024 | ||
| wt (B6) | 724 | 512-1,024 | 2,048 | 2,048 | 45 | 32-64 | 724 | 512-1,024 |
NT, not detectable.
Value for a single serum from a surviving mouse.
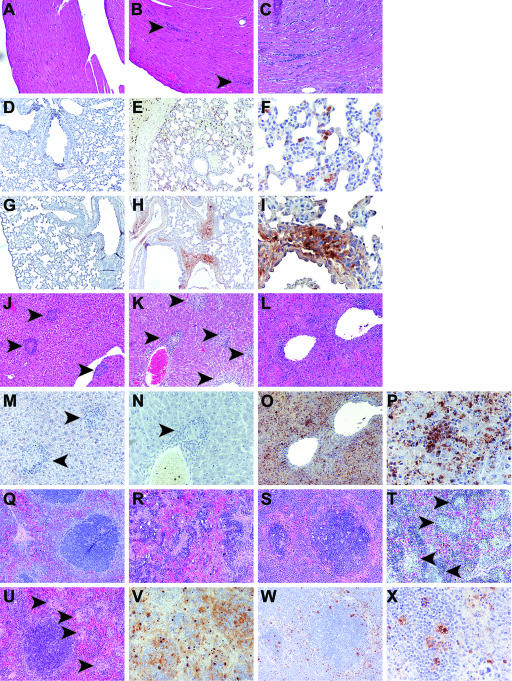
Histopathological changes, mainly macrophage accumulations, were found in all mice infected with NM I (Table 3). IFN-γ−/− mice had the most substantial systemic inflammatory response. The antigen distribution in IFN-γ−/− mice was diffuse, and antigen did not accumulate in perivascular or periportal regions or in cellular accumulations in the lungs, livers, and spleens (Fig. 8). Nude mice had mild to moderate, random to perivascular inflammation in the liver and scattered necrotic hepatocytes (Fig. 8H, I, R, S, and V). muMT and bg mice had cellular inflammation in all organs (Fig. 8K and T). However, C. burnetii antigen was not detected in muMT mice (Fig. 8N). TNF-α−/− mice had the most severe inflammation score for the heart, in which cellular infiltration was found in the endocardium and epicardium and was diffuse in the myocardium (Fig. 8C). The inflammation scores of wt mice were mild for the heart and lung (Fig. 8A and 8B) and marked for the liver and spleen (Fig. 8J and U). Immunodeficient mice infected with NM II showed histopathologic changes in the lungs and livers (Table 3). The spleens had slight to mild EMH, and lung inflammation was seen principally in alveolar walls containing macrophages. In nude mice, the lungs had thickened alveolar walls containing macrophages; the livers contained mild random and perivascular accumulations of macrophages and scattered small foci of hepatocellular necrosis; and the spleens had mild excess macrophages. In muMT mice, the livers had multifocal accumulations of inflammatory cells with scattered hepatocellular necrosis. bg mice had scattered minor foci of inflammatory cell accumulations, which were sometimes associated with vasculature, and occasional single hepatocytic necrosis in the liver. TNF-α−/− mice had mild foci of inflammatory cell accumulations in the liver. wt mice had scattered minor foci of inflammatory cell accumulations with occasional necrosis of single hepatocytes. C. burnetii antigen in NM II-infected animals was barely detected (Table 3). No specific histopathological change or C. burnetii antigen was found in PBS groups (Fig. 8D and Q), except for occasional EMH in the liver or spleen.
FIG. 8.
Histopathology of mice infected with the high dose of NM I at 28 DPI. (A to C) Heart; (D to I) lung; (J to P) liver; and (Q to X) spleen. (D and Q) Tissue section of PBS group. (A to C, J to L, and Q to U) HE staining; (D to I, M to P, and V to X) IHC staining. (A to E, G, H, J to L, O, Q to W) Original magnification, ×100; (M and N) original magnification, ×200; (F, I, P, and X) original magnification, ×400. (A and B) Hearts of wt mice had few small areas of cellular coagulation in the myocardium (arrowhead). (C) Hearts of TNF-α−/− mice had extensive cellular infiltration in the myocardium. (D) C. burnetii antigen was not detectable in PBS groups, and there was no nonspecific IHC staining. (E and F) Lungs of IFN-γ−/− mice had C. burnetii antigen distributed in the parenchyma and not accumulated in perivascular areas. (G and M) C. burnetii antigen in wt mice was barely detectable. (H and I) Lungs of nude mice had abundant antigen distributed primarily in perivascular areas. (J and M) Livers of wt mice had perivascular, periportal, and random formation of small cellular accumulations (arrowheads) but no detectable C. burnetii antigen. (K and N) Livers of muMT mice had perivascular, periportal, and random cellular accumulation but no detectable C. burnetii antigen. (L, O, and P) Livers of IFN-γ−/− mice had diffuse cellular infiltration and antigen distribution. (Q) Spleens of nude mice had smaller follicles than wt mice but no characteristic pathological changes. (R and V) Spleens of nude mice had diffuse cellular infiltration and antigen distribution that obscured tissue architecture. (S, W, and X) Spleens of IFN-γ−/− mice had random cellular accumulations and scattered C. burnetii antigen distribution. (T) Spleens of muMT mice had marked random cellular accumulations (arrowheads).
DISCUSSION
In both NM I and NM II infections, T cells have a critical role in the clearance of the bacterium, and B cells have an important role in protecting against tissue damage. IFN-γ and TNF-α have a key role in the early stage of the infection, and IFN-γ expression is essential for effective clearance and survival in NM I-challenged animals. Several studies have demonstrated that immunosuppressed or immunodeficient mice, such as cyclophosphamide-treated mice, nude mice, SCID mice, and SCIDbg mice, have dramatically heightened sensitivity to C. burnetii infection (2, 3, 9, 17, 25, 26). Our results provide the first evidence demonstrating that T cells and IFN-γ are essential immune components for controlling the infection. Three mouse strains with T-cell deficiency showed persistent clinical disease with large bacterial loads in the spleen, suggesting a major role for T cells in C. burnetii clearance. This hypothesis is supported by an in vitro study showing that T lymphocytes are the major contributor to the immune response to C. burnetii, as measured by their lymphoproliferative response (22).
Splenomegaly has been routinely used as an indicator of C. burnetii infection in experimental animals. In wt mice, the degree of splenomegaly is correlated with the bacterial load in the spleen (41). Splenomegaly is only a correlate of bacterial colonization and is not highly sensitive for discriminating differences between mouse strains. Detection of bacterial antigen using immunohistochemistry is useful to localize bacteria, but it is not suitable to evaluate numbers of bacteria because of low sensitivity. Quantification of viable bacteria by tissue culture infection is time-consuming and has a low sensitivity. Therefore, we believe that detection of bacterial genome copies is the optimal tool for the evaluation of infection.
The high mortality rate and short survival time of IFN-γ−/− mice infected with NM I suggests that the mice failed to control replication of NM I early in the infection. In vitro studies have shown that IFN-γ restored impairment of phagosome maturation of macrophages infected with C. burnetii (16) and promoted apoptosis of the infected cells (12), resulting in C. burnetii killing. IFN-γ-mediated control of NM I has been reported in mouse cells (J774 and peritoneal macrophages) (6) and also in human cells (THP-1 and peripheral blood mononuclear cells) (16). These reports support the conclusions in this study that IFN-γ is crucial for the innate immune response to NM I infection. IFN-γ has also been shown to be important for the control of NM II infection in vitro (40); however, we did not detect this requirement in our study, suggesting that NM II replication could be controlled in vivo without IFN-γ. The distribution of antigen in the tissues of IFN-γ−/− mice infected with NM I was diffuse and different from that in other mice, where antigens were more likely to be distributed in perivascular areas. IFN-γ increases expression of cell adhesion molecules on immune cells and functions to increase their extravasation from vascular sites to inflammatory areas (21). Deficiencies in inflammatory cell recruitment and a lack of a defined circumscript quality of granulomas in IFN-γ−/− mice have been reported in Mycobacterium avium infection (18). The diffuse antigen distribution seen in IFN-γ−/− mice infected with NM I could be due to a lack of proper cellular recruitment. Therefore, the early death of IFN-γ−/− mice can be explained by the lack of macrophage bactericidal activation and cell migration to the site of inflammation.
TNF-α deficiency increased the bacterial load in NM I-infected mice. TNF-α partially mediates IFN-γ-dependent killing of C. burnetii and cell death (11). Bacteremia was seen in all TNF-α−/− mice at the earliest time point but only in a single mouse at later time points. We speculate that this was due to an early failure of macrophage activation that can be rescued when significant acquired cell-mediated immunity is developed. TNF-α−/− mice infected with NM I had the most severe heart lesions. TNF-α is known to upregulate expression of adhesion receptors, including intercellular adhesion molecule 1 in cardiomyocytes and V- and P-selectin and vascular cell adhesion molecule 1 in endothelial cells that mediate efficient binding of circulating leukocytes (10, 14). We speculate that the severe lesions found in TNF-α−/− mice were due to the combination of bacteremia and a lack of efficient leukocyte recruitment. The influence of tissue-specific macrophages was apparent in the heart, an organ that normally lacks resident macrophages. The results for IFN-γ−/− and TNF-α−/− mice suggest the importance of macrophage activation at early times postinfection and immune cell recruitment to the site of infection.
B-cell deficiency did not affect bacterial clearance but did alter histopathology. This provides evidence that supports the hypothesis that B cells play an important role in regulation of inflammation against C. burnetii infection. A direct comparison of SCID and nude mice with NM I infections showed similar clinical signs in both mouse strains, such as loss of activity, cachexia, and body weight loss, but longer survival times in nude mice (M. Andoh, G. Zhang, and K. Hirai, unpublished data), which supports a role for B cells in resistance against the infection. B cells have been reported to produce interleukin-10, which is an important negative regulator of cell-mediated immunity that inhibits IFN-γ secretion from activated Th1 and NK cells (13, 37). For many bacterial pathogens, Th1 cells are important in the activation of macrophages, and activated macrophages are critical for the clearance of pathogens. However, without careful regulation of this inflammatory response, self-damage of host tissues can occur, as seen in chronic infection with the intracellular bacterium Chlamydia trachomatis (5). Our recent findings demonstrate that heat-killed NM I induces a strong Th1 response, while heat-killed NM II induces a weak Th1 response (G. Zhang, K. E. Russell-Lodrigue, M. Andoh, Y. Zhang, L. R. Hendrix, and J. E. Samuel, submitted for publication). We speculate that the severe histopathologic changes with successful bacterial clearance in muMT mice are due to the inability to downregulate a Th1 response associated with B-cell deficiency. Therefore, our results support a model whereby T-cell activity is critical for the clearance of bacteria and B-cell activity supports the successful control of pathology.
The role of NK cells in C. burnetii infection was not clearly identified in this study. Although histopathology was more severe in SCIDbg mice than SCID mice, the numbers of bacteria in these mice were similar. There was no notable difference between bg and wt mouse infections. Although the bg mutant mouse has abnormalities in all granule-containing cells, including lymphocytes, the most notable defect is in NK cell function (31). We believe that NK cells have an accessory role in the inflammatory response but are not crucial for prevention of bacterial replication. They may provide an immediate supply of IFN-γ for the activation of macrophages in the innate immune response (36).
The three T-cell-deficient mouse strains allowed NM II to establish an infection; the histopathologic changes in several organs demonstrated this ability to cause systemic infections in immunodeficient animals. Although the bacterial load and antigen distribution in NM II-infected mice were limited and clinical signs of disease were never observed, this is the first report of NM II colonization in vivo. In SCID and SCIDbg mice infected with NM II, pathological changes were dose dependent, but the bacterial loads were similar. In a guinea pig model, high infectious doses of NM II caused a short-term fever response, but organisms were not detected in the infected animals (28). These observations suggest that NM II infection can be partially controlled by innate immunity; however, acquired cell-mediated immunity is required for bacterial clearance. Capo et al. reported in vitro data showing that NM II is ingested more effectively than NM I and fails to survive within macrophages (8). However, in vivo without T-cell-mediated immunity, NM II could remain in the organs for 4 weeks. We speculate that the difference between NM I and NM II in the interaction with receptors and trafficking to different intracellular compartments (8) is not the primary cause of the difference in bacterial killing and elimination in vivo.
Nude mice infected with NM II allowed the bacteria to colonize and developed detectable antibody, but the titers were lower than those in other NM II-infected mice. The low antibody titer in spite of bacterial colonization could be explained by the fact that nude mice have an impairment in antibody production due to a lack of helper T cells and a partial defect in B-cell maturation (23). Alternatively, nude mice infected with NM I did not have detectable antibody even though their bacterial load was greatest and clinical signs were the most severe of all the single-immune-component-deficient mice, while other mice infected with NM I had high antibody titers with a smaller bacterial load. In addition to the impairment in nude mice noted above, we believe that there could be two possible explanations for the total lack of antibody to NM I; one explanation is immunosuppresion caused by NM I, reported as a lymphocyte hyporesponsiveness (38). Recently, we observed a hyporesponsiveness to specific recall antigen and mitogens by splenocytes during acute infection in both mice and guinea pigs (G. Zhang, unpublished data; K. E. Russell-Lodrigue, unpublished data). The other explanation may be the difference in the responses of dendritic cells (DCs) between NM I and NM II infections. Recent in vitro data have shown that NM I did not activate DC, while NM II infection led to upregulation of major histocompatibility complex class II and CD40 expression as measures of DC maturation (35). DCs use major histocompatibility complex class II and CD40 to contact and activate B cells (39). In nude mice infected with NM I, B cells likely do not get activated by DCs and lack helper T cells, resulting in no detectable antibody production.
Our study demonstrated the in vivo roles of various immune system components in the control of C. burnetii infection in mice. Each immune component has previously been studied independently, but the components have never been compared directly in an animal model. Comparison of NM I and NM II infections showed that acquired immunity is essential to overcome both infections, and NM I may be able to escape innate immune mechanisms that are designed to control bacterial replication. These observations provide a better understanding of the virulence of C. burnetii and Q fever pathology.
Acknowledgments
This study was supported by NIH grant RO1 AI057768 (to J.E.S.) and by a subaward to J.E.S. from grant U54 AI057156.
We are grateful to Laura R. Hendrix for a critical review of the manuscript and for helpful suggestions. We also thank Maria Labandeira-Rey for critical reading and editing of the manuscript.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Amano, K., and J. C. Williams. 1984. Chemical and immunological characterization of lipopolysaccharides from phase I and phase II Coxiella burnetii. J. Bacteriol. 160:994-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andoh, M., T. Naganawa, A. Hotta, T. Yamaguchi, H. Fukushi, T. Masegi, and K. Hirai. 2003. SCID mouse model for lethal Q fever. Infect. Immun. 71:4717-4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atzpodien, E., W. Baumgartner, A. Artelt, and D. Thiele. 1994. Valvular endocarditis occurs as a part of a disseminated Coxiella burnetii infection in immunocompromised BALB/cJ (H-2d) mice infected with the nine mile isolate of C. burnetii. J. Infect. Dis. 170:223-226. [DOI] [PubMed] [Google Scholar]
- 4.Baca, O. G., and D. Paretsky. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol. Rev. 47:127-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1994. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 2:94-98. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, R. E., K. Russell, G. Zhang, and J. E. Samuel. 2004. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect. Immun. 72:6666-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan, R. E., and J. E. Samuel. 2003. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J. Clin. Microbiol. 41:1869-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capo, C., A. Moynault, Y. Collette, D. Olive, E. J. Brown, D. Raoult, and J. L. Mege. 2003. Coxiella burnetii avoids macrophage phagocytosis by interfering with spatial distribution of complement receptor 3. J. Immunol. 170:4217-4225. [DOI] [PubMed] [Google Scholar]
- 9.Criley, J. M., A. J. Carty, C. L. Besch-Williford, and C. L. Franklin. 2001. Coxiella burnetii infection in C.B-17 scid-bg mice xenotransplanted with fetal bovine tissue. Comp. Med. 51:357-360. [PubMed] [Google Scholar]
- 10.Davani, E. Y., D. R. Dorscheid, C. H. Lee, C. van Breemen, and K. R. Walley. 2004. Novel regulatory mechanism of cardiomyocyte contractility involving ICAM-1 and the cytoskeleton. Am. J. Physiol. Heart Circ. Physiol. 287:H1013-H1022. [DOI] [PubMed] [Google Scholar]
- 11.Dellacasagrande, J., C. Capo, D. Raoult, and J. L. Mege. 1999. IFN-gamma-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J. Immunol. 162:2259-2265. [PubMed] [Google Scholar]
- 12.Dellacasagrande, J., E. Ghigo, D. Raoult, C. Capo, and J. L. Mege. 2002. IFN-gamma-induced apoptosis and microbicidal activity in monocytes harboring the intracellular bacterium Coxiella burnetii require membrane TNF and homotypic cell adherence. J. Immunol. 169:6309-6315. [DOI] [PubMed] [Google Scholar]
- 13.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunon, D., L. Piali, and B. A. Imhof. 1996. To stick or not to stick: the new leukocyte homing paradigm. Curr. Opin. Cell Biol. 8:714-723. [DOI] [PubMed] [Google Scholar]
- 15.Fenollar, F., P. E. Fournier, M. P. Carrieri, G. Habib, T. Messana, and D. Raoult. 2001. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis. 33:312-316. [DOI] [PubMed] [Google Scholar]
- 16.Ghigo, E., C. Capo, C. H. Tung, D. Raoult, J. P. Gorvel, and J. L. Mege. 2002. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J. Immunol. 169:4488-4495. [DOI] [PubMed] [Google Scholar]
- 17.Hall, W. C., J. D. White, R. A. Kishimoto, and R. E. Whitmire. 1981. Aerosol Q fever infection of the nude mouse. Vet. Pathol. 18:672-683. [DOI] [PubMed] [Google Scholar]
- 18.Hansch, H. C., D. A. Smith, M. E. Mielke, H. Hahn, G. J. Bancroft, and S. Ehlers. 1996. Mechanisms of granuloma formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Int. Immunol. 8:1299-1310. [DOI] [PubMed] [Google Scholar]
- 19.Hoover, T. A., D. W. Culp, M. H. Vodkin, J. C. Williams, and H. A. Thompson. 2002. Chromosomal DNA deletions explain phenotypic characteristics of two antigenic variants, phase II and RSA 514 (crazy), of the Coxiella burnetii nine mile strain. Infect. Immun. 70:6726-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howe, D., L. F. Barrows, N. M. Lindstrom, and R. A. Heinzen. 2002. Nitric oxide inhibits Coxiella burnetii replication and parasitophorous vacuole maturation. Infect. Immun. 70:5140-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issekutz, A. C., and T. B. Issekutz. 1993. Quantitation and kinetics of blood monocyte migration to acute inflammatory reactions, and IL-1 alpha, tumor necrosis factor-alpha, and IFN-gamma. J. Immunol. 151:2105-2115. [PubMed] [Google Scholar]
- 22.Izzo, A. A., B. P. Marmion, and T. Hackstadt. 1991. Analysis of the cells involved in the lymphoproliferative response to Coxiella burnetii antigens. Clin. Exp. Immunol. 85:98-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushik, A., G. Kelsoe, and J. C. Jaton. 1995. The nude mutation results in impaired primary antibody repertoire. Eur. J. Immunol. 25:631-634. [DOI] [PubMed] [Google Scholar]
- 24.Kazar, J., M. Lesy, P. Propper, D. Valkova, and R. Brezina. 1993. Comparison of virulence for guinea pigs and mice of different Coxiella burnetii phase I strains. Acta Virol. 37:437-448. [PubMed] [Google Scholar]
- 25.Kazar, J., J. Rajcani, and S. Schramek. 1982. Differential effects of cyclophosphamide on Coxiella burnetii infection in mice. Acta Virol. 26:174-182. [PubMed] [Google Scholar]
- 26.Kishimoto, R. A., H. Rozmiarek, and E. W. Larson. 1978. Experimental Q fever infection in congenitally athymic nude mice. Infect. Immun. 22:69-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moos, A., and T. Hackstadt. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 55:1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morisawa, Y., H. Wakiguchi, T. Takechi, T. Kurashige, and H. Nagaoka. 2001. Intractable Q fever treated with recombinant gamma interferon. Pediatr. Infect. Dis. J. 20:546-547. [DOI] [PubMed] [Google Scholar]
- 30.Raoult, D., and T. Marrie. 1995. Q fever. Clin. Infect. Dis. 20:489-496. [DOI] [PubMed] [Google Scholar]
- 31.Roder, J., and A. Duwe. 1979. The beige mutation in the mouse selectively impairs natural killer cell function. Nature 278:451-453. [DOI] [PubMed] [Google Scholar]
- 32.Sawyer, L. A., D. B. Fishbein, and J. E. McDade. 1987. Q fever: current concepts. Rev. Infect. Dis. 9:935-946. [DOI] [PubMed] [Google Scholar]
- 33.Scott, G. H., G. T. Burger, and R. A. Kishimoto. 1978. Experimental Coxiella burnetii infection of guinea pigs and mice. Lab. Anim. Sci. 28:673-675. [PubMed] [Google Scholar]
- 34.Scott, G. H., J. C. Williams, and E. H. Stephenson. 1987. Animal models in Q fever: pathological responses of inbred mice to phase I Coxiella burnetii. J. Gen. Microbiol. 133:691-700. [DOI] [PubMed] [Google Scholar]
- 35.Shannon, J. G., D. Howe, and R. A. Heinzen. 2005. Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc. Natl. Acad. Sci. USA 102:8722-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thale, C., and A. F. Kiderlen. 2005. Sources of interferon-gamma (IFN-gamma) in early immune response to Listeria monocytogenes. Immunobiology 210:673-683. [DOI] [PubMed] [Google Scholar]
- 37.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waag, D. M., and J. C. Williams. 1988. Immune modulation by Coxiella burnetii: characterization of a phase I immunosuppressive complex differentially expressed among strains. Immunopharmacol. Immunotoxicol. 10:231-260. [DOI] [PubMed] [Google Scholar]
- 39.Wykes, M., and G. MacPherson. 2000. Dendritic cell-B-cell interaction: dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology 100:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamboni, D. S., and M. Rabinovitch. 2003. Nitric oxide partially controls Coxiella burnetii phase II infection in mouse primary macrophages. Infect. Immun. 71:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, G., K. Kiss, R. Seshadri, L. R. Hendrix, and J. E. Samuel. 2004. Identification and cloning of immunodominant antigens of Coxiella burnetii. Infect. Immun. 72:844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]