Abstract
Marginal zone B (MZB) cells are a B-cell subset that produces T-cell-independent antibodies to blood-borne antigens. In this study, we examined the effects of MZB cell depletion on the immune response to the Lyme disease spirochete Borrelia burgdorferi, an extracellular pathogen for which T-cell-independent antibody is an important host defense. MZB cell depletion of C3H/HeJ mice using monoclonal antibody to LFA-1 and α4β1 integrins reduced B. burgdorferi-specific immunoglobulin M (IgM) titers, enhanced pathogen burden, and led to more severe arthritis assessed within the first 2 weeks of infection. In addition, MZB cell-depleted mice had reduced levels of B. burgdorferi-specific IgG, which correlated with diminished splenic CD4+ T-cell-activation, proliferation, and cytokine production. Passive transfer of immune mouse serum from infected control mice into infected MZB cell-depleted mice reduced pathogen burden but did not alter the expression of T-cell activation markers on splenic CD4+ T cells. These findings demonstrate that MZB cells not only are a source of pathogen-specific IgM important for limiting spirochete burden and pathology but also play a prominent role in the priming of splenic T-cell responses to a blood-borne pathogen.
Spirochetes of the genus Borrelia are arthropod-borne agents of both human and domestic animal diseases and are transmitted to vertebrate hosts during vector feeding (4). Borrelia burgdorferi is the etiologic agent of Lyme disease, the most common vector-borne disease in the United States (25). After the initial deposition in the skin during tick feeding, B. burgdorferi replicates locally and then disseminates through the blood and tissues to distant sites where disease can be seen. The transient blood-borne phase of B. burgdorferi infection within the vertebrate host promotes wider colonization of the skin, thereby facilitating the acquisition of the pathogen by feeding ticks (21). Blood-borne infection, however, can lead to systemic manifestations of disease, including arthritis, myocarditis, and neurologic disease (25). Control of the pathogen during the early hematogenous stage of infection is an important host defense against the development of more widespread pathological sequelae (27).
Laboratory mice experimentally infected with B. burgdorferi by intradermal inoculation develop systemic infection, arthritis, and myocarditis and serve as a model for acute disseminated Lyme disease in humans (5). Within 4 days of intradermal inoculation, spirochetes become blood borne and induce myocarditis and inflammatory arthritis that peak in severity by 14 to 30 days of infection (5). Spirochetes are most abundant in tissues during periods of disease and can be detected in the bloodstream by culture throughout this period. Both myocarditis and arthritis undergo immune-mediated regression by 45 to 60 days of infection even though spirochetes persist at low levels in multiple tissues, including hearts and joints. Humoral immunity is an essential mammalian defense against Borrelia spirochetes (1, 6) and is required for the resolution of B. burgdorferi-induced arthritis (6). Antibodies produced in the absence of T-cell help are sufficient to prevent infection and to induce arthritis resolution, as has been shown by passive immunization of SCID mice and in experimental infection of mice deficient in T cells, major histocompatibility complex class II molecules, or CD40, a molecule required for T-cell-dependent B-cell responses (13, 20). The sources of protective and disease-resolving antibodies have not been fully delineated but likely involve innate and follicular B-cell subsets to various degrees throughout the course of infection (6, 20).
Marginal zone B (MZB) cells comprise an innate B-cell subset localized in mice to the marginal sinuses of the spleen, where they provide early immune responses to blood-borne particulate antigen (Ag) (19, 22). These cells serve to bridge the innate and adaptive immune systems because they have the capacity to respond to specific foreign Ag more rapidly than follicular B cells (26). In addition to being an early source of pathogen-specific T-cell-independent immunoglobulin M (IgM), MZB cells are potent Ag-presenting cells (APCs) that can present particulate Ag to activate naïve CD4+ T cells after immunization (3, 17, 24). MZB cells can be distinguished from follicular B cells by their high levels of CD1d and complement receptor CD21 expression (2) and lower levels of CD23, a negative regulator of B cells. Reduced levels of CD23 on MZB cells may allow them to produce a rapid antibody (Ab) response in comparison to follicular B cells, as has been reported previously with inert particles and heat-killed bacteria as Ag (22).
Using the relapsing fever spirochete Borrelia hermsii, we have previously shown that MZB cells directly associate with blood-borne Borrelia spirochetes and are activated in vivo to produce pathogen-specific IgM within 72 h of infection (7). In the absence of CD1d, however, MZB production of B. hermsii-specific IgM was impaired, resulting in an accentuated bacteremic phase (7). Paradoxically, MZB cells from CD1d-deficient mice had prolonged upregulation of activation markers, including CD25 and B7.1, in comparison to wild-type (WT) MZB cells, yet their production of pathogen-specific IgM in vitro and in vivo was reduced (7). We previously observed diminished resistance to B. burgdorferi infection and arthritis in CD1d−/− mice, with spirochete DNA found at earlier time points in urinary bladders after intradermal infection in comparison to similarly infected WT mice (16). This altered containment of the pathogen during the first week of infection in CD1d−/− mice may be the result of impaired production of B. burgdorferi-specific IgM by innate B-cell subsets, including CD1dhigh MZB cells.
In this study, we depleted MZB cells from mice to determine their contribution to host defense against B. burgdorferi and to investigate their role in the induction of adaptive immune responses during the first 2 weeks of B. burgdorferi infection. In addition to reduced B. burgdorferi-specific IgM, elevated pathogen burden, and an earlier appearance of arthritis, we found reduced levels of B. burgdorferi-specific IgG that correlated with diminished splenic CD4+ T-cell responses. These results demonstrate a role for MZB cells not only in Borrelia-specific IgM production but also in promoting the early adaptive T- and B-cell responses to B. burgdorferi infection.
MATERIALS AND METHODS
Mice.
C3H/HeJ mice, 6 to 8 weeks of age, were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in filter frame cages and administered food and water ad libitum according to Yale University animal care and use guidelines. Mice were euthanized by carbon dioxide asphyxiation. The Yale University Institutional Animal Care and Use Committee approved all procedures.
Infection of mice.
Frozen aliquots of low-passage B. burgdorferi N40 were grown to logarithmic phase in BSK-H medium (Sigma-Aldrich, St. Louis, MO) and enumerated by dark-field microscopy using a Petroff-Hausser chamber. Each mouse was inoculated intradermally with 104 spirochetes in 100 μl of BSK-H medium. At the time of mouse sacrifice, infection was confirmed by culturing the blood or a portion of the urinary bladder in BSK-H medium for 14 days, after which the presence of spirochetes was determined by dark-field microscopy.
Flow cytometry analysis of MZB cells and T cells.
Splenocytes were isolated from mice at the time of sacrifice. After red blood cell lysis and Fc receptor blockade with anti-CD16/32 monoclonal Ab (mAb) (BD Pharmingen, San Diego, CA), MZB cells were identified by flow cytometry using four-color immunofluorescence staining. Splenocytes were labeled with allophycocyanin- conjugated anti-B220, phycoerythrin-conjugated anti-CD23, and fluorescein isothiocyanate-labeled anti-CD21/CD35 mAbs, which allow MZB cells to be distinguished from follicular B cells (BD Pharmingen). The MZB cell population was identified by its B220+ CD23low/negative CD21high phenotype, and the follicular B-cell population was identified by its B220+ CD23high CD21intermediate phenotype using a FACSCalibur flow cytometer (BD Pharmingen). T cells were labeled with allophycocyanin or cychrome-conjugated anti-Thy1.2 and phycoerythrin-conjugated or cychrome-conjugated anti-CD4 mAb. The T-cell activation marker CD44 was identified with either cychrome-conjugated anti-CD44 or biotinylated anti-CD44 mAb detected with cychrome-conjugated streptavidin (BD Pharmingen). The CD45Rb activation marker was detected with fluorescein isothiocyanate-CD45Rb, and the CD62L activation marker was detected with allophycocyanin-conjugated anti-CD62L mAb. All conjugated mAbs and streptavidin were used at a 1:50 dilution.
MZB cell depletion.
MZB cells were depleted from mice with a single treatment of 100 μg each of anti-LFA (αLβ2) and anti-CD49d (anti-α4) mAb (BD Pharmingen) as described previously (7, 18). Controls were treated with isotype-matched mAb (Pharmingen). In each experiment, the efficacy of depletion was assessed by flow cytometry of splenocytes from one mouse sacrificed 7 days after Ab treatment (at the start of infection), as described above.
ELISA.
B. burgdorferi-specific enzyme-linked immunosorbent assays (ELISAs) were performed using sera from infected animals. Ninety-six-well microtiter plates were coated with B. burgdorferi lysate (3 μg in 50 μl 100% ethanol per well) by overnight incubation at 4°C. After blocking in phosphate-buffered saline (PBS) containing 5% bovine serum albumin, serial twofold dilutions of sera in PBS-0.5% bovine serum albumin-0.5% Tween 20 were added to the wells and incubated for 1 h at room temperature. Secondary biotinylated anti-mouse IgM and IgG (Vector Labs, Burlingame, CA) were used at a 1:1,000 dilution, and bound Abs were detected with the ABC Elite peroxidase detection and ABTS [2,2′-azino-bis(ethylbenzthiazolinesulfonic acid)] substrate kits (Vector Labs). The READY-SET-GO! Mouse Interferon Gamma ELISA kit (eBioscience, San Diego, CA), was used according to the manufacturer's instructions to measure gamma interferon (IFN-γ) in culture supernatants. The concentration of IFN-γ was determined in serial twofold dilutions using a standard curve, and the average values were determined for each sample.
Quantitative PCR of B. burgdorferi DNA.
DNA was isolated from 100 μl of whole blood using the Isoquick DNA isolation kit (ORCA Research Inc., Bothell, WA) and from urinary bladder using the DNeasy kit (QIAGEN Inc, Valencia, CA) according to the manufacturer's instructions. The copy number of B. burgdorferi in each sample was determined by quantitative PCR of the B. burgdorferi recA gene using an iCycler (Bio-Rad, Hercules, CA), the Brilliant SYBR green kit (Stratagene, Cedar Creek, TX), and the following primers: 5′ primer 5′-GTGGATCTATTGTATTAGATGAGGCTCTCG-3′ and 3′ primer 5′-GCCAAAGTTCTGCAACATTAACACCTAAAG-3′. The recA copy number was normalized to the mouse actin gene amplified using the following primers and probe: 5′ primer 5′-ATCAGGTAGTCGGTCAGG-3′, 3′ primer 5′-GGTATCTATCTCGACTC-3′, and probe 6-carboxyfluorescein-TCCAGCAGATCTGGATCAGCAAGCA-carboxytetramethylrhodamine (Applied Biosystems, Foster City, CA). A 60°C annealing temperature and 45 or 50 cycles were used for the recA and actin gene reactions, respectively. Standard curves were generated for both PCRs using known quantities of DNA. Reactions were performed in duplicate, and the quantities of PCR products generated were determined from the standard curves.
Histopathology.
Bilateral hindlimb joints (knee and tibiotarsal joints) were processed and stained with hematoxylin and eosin by routine histologic techniques performed by HSRL Laboratories (Mount Jackson, VA) or the Section of Comparative Medicine, Yale School of Medicine. The knee and tibiotarsal joints were scored for arthritis severity on a scale of 0 (negative) to 3 (severe) in a blinded fashion as described previously (6).
Passive immunization.
Groups of four or five control and MZB cell-depleted mice were inoculated intradermally with 104 B. burgdorferi N40 spirochetes. At 10 days of infection, groups of WT and MZB cell-depleted mice were inoculated intraperitoneally with either 200 μl of serum from the 10-day-infected control mice or normal mouse serum (NMS) diluted with PBS to a final volume of 300 μl. A third group received PBS alone. Four days after passive immunization, mice were sacrificed, and T-cell activation, spirochete burdens, and Ab titers were measured as described above.
Splenocyte culture and ex vivo T-cell activation.
Spleens were fragmented between the frosted ends of two glass slides to retrieve splenocytes. After red blood cell lysis, the splenocytes from mice within the isotype control group were combined, and the splenocytes from each MZB cell-depleted mouse were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) using the Cell Trace CFSE proliferation kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. The labeled cells from the combined control group and each MZB cell-depleted mouse were aliquoted into 24-well tissue culture plates at a density of 5 × 106 cells in 1 ml of Click's medium (Irvine Scientific, Santa Ana, CA) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 pg/ml). Cells were stimulated for 72 h with 10 μg/ml of B. burgdorferi sonicate or PBS, after which they were stained with immunofluorescent Ab for analysis by flow cytometry as described above. The cell supernatants were collected and stored at −80°C for later cytokine analysis by ELISA, as described above.
RESULTS
Depletion of MZB cells decreases B. burgdorferi-specific Ab titers and enhances arthritis early in infection.
To examine the role of MZB cells in host resistance to B. burgdorferi in its blood-borne phase, we treated mice with mAb to LFA-1 and α4β1 integrins, two integrins that tether MZB cells to the marginal sinuses in the spleen. This treatment results in the selective depletion of MZB cells without affecting other lymphocyte populations in the spleen (7, 18). The treatment regimen used results in MZB cell depletion for 2 to 3 weeks, after which time newly generated MZB cells from the bone marrow begin to repopulate the spleen. Mice were infected with B. burgdorferi 1 week after mAb treatment, a time previously determined to correlate with at least an 80% reduction in the number of MZB cells (18). Mice depleted of MZB cells had lower B. burgdorferi-specific IgM levels in the serum 7 days after infection (Fig. 1A) and had increased numbers of spirochetes in the blood (Fig. 1B), similar to our previous findings with mice infected with B. hermsii spirochetes (7). B. burgdorferi-specific IgG titers were also reduced in MZB cell-depleted mice in comparison to infected control mice treated with isotype-matched mAb (Fig. 1C). Enhanced pathogen burden in MZB cell-depleted mice was associated with more severe arthritis assessed at 10 and 14 days of infection (Table 1).
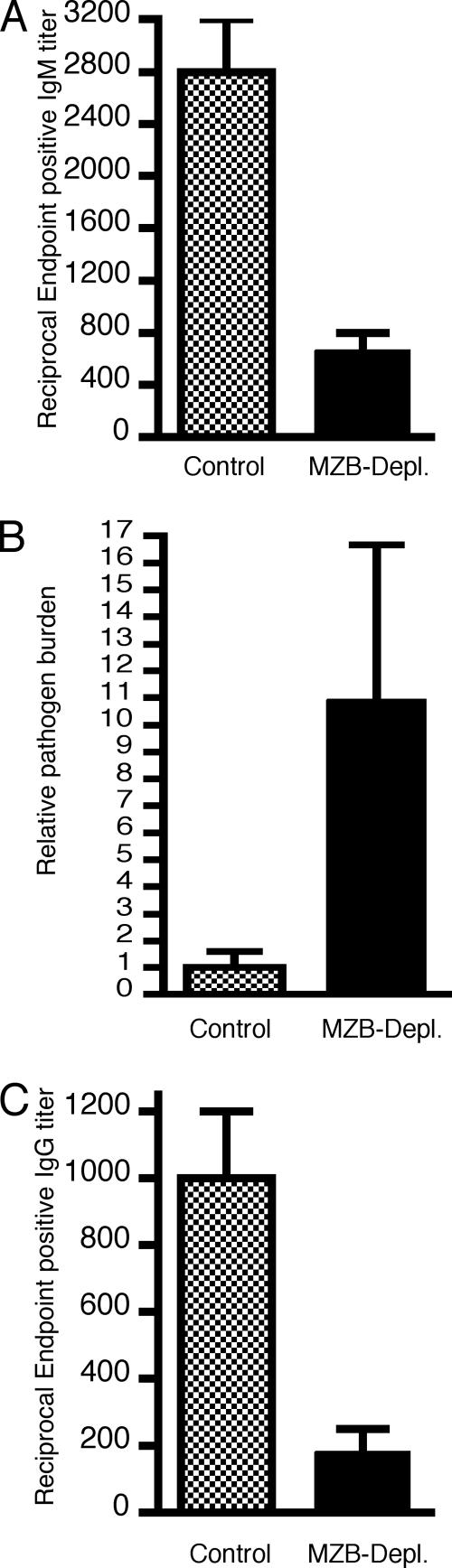
FIG. 1.
MZB cell depletion reduces B. burgdorferi-specific IgM and IgG titers and increases pathogen burden at 7 days of infection. Ab titers are the reciprocal endpoint positive titers of B. burgdorferi-specific IgM (A) or IgG (C) in serum obtained from 7-day-infected control and MZB cell-depleted mice (MZB-Depl.). (B) The pathogen burden in the blood of each mouse was determined by quantitative PCR as described in Materials and Methods; the copy number of the B. burgdorferi recA gene was normalized to 1 ng of the mouse actin gene product. Results were derived from two separate experiments using four to five mice per group. For comparison purposes, the relative pathogen burden was set to 1 for the control mouse group in each experiment (the raw values for the control groups were 41,000 and 1,250 copies of B. burgdorferi recA/ng mouse actin gene, respectively). The pathogen burden calculated for each mouse is the average of duplicates from two separate assays. The differences between the control and the MZB cell-depleted serum titers for IgM (P = 0.03) and IgG (P = 0.03) as well as the pathogen burdens (P = 0.01) were significant (Mann-Whitney test).
TABLE 1.
Depletion of MZB cells exacerbates arthritis in B. burgdorferi-infected mice
| Day of infection | Mouse group | Arthritis prevalence (no. of inflamed joints/ total no. of joints)a | Mean arthritis severity score ± SDb |
|---|---|---|---|
| 10 | Isotype control | 3/10 | 0.4 ± 0.7 |
| MZB cell depleted | 11/11c | 1.6 ± 0.7d | |
| 14 | Isotype control | 12/28 | 0.5 ± 0.7 |
| MZB cell depleted | 17/23e | 1.1 ± 0.9f |
Arthritis prevalence was reported as the number of joints with inflammation over the total number of joints examined in each group.
Arthritis was scored on a scale of 0 (negative) to 3 (severe) and is reported as the average score of the inflamed joints in each mouse group. Three to seven mice were examined in each group. The data for the arthritis at 14 days is combined from two separate experiments.
MZB cell-depleted mice versus control mice, P = 0.001, Fisher's exact test.
P = 0.001, two-tailed Student's t test.
P = 0.046, Fisher's exact test.
P = 0.016, two-tailed Student's t test.
Depletion of MZB cells impairs T-cell activation and pathogen-specific IgG production.
MZB cells produce pathogen-specific Ab within a few days of blood-borne infection, after which time follicular B cells become activated to produce Ab. After day 7 of infection, IgM titers began to increase, but the IgG titers remained low in MZB cell-depleted mice. At day 10 of infection, the serum B. burgdorferi-specific IgM titers were comparable to those of infected control mice (reciprocal endpoint IgM titers of 1,067 ± 267 for MZB-depleted mice and 800 ± 400 for isotype control mice) and trended higher at 14 days of infection (1,360 ± 538 versus 720 ± 180, respectively). The B. burgdorferi-specific IgG levels, however, were still reduced at day 14 (2,720 ± 999 for MZB cell-depleted mice versus 8,320 ± 1,920 for isotype control mice; P = 0.032). All IgG subtypes were represented (data not shown). Because the serum titers of B. burgdorferi-specific T-cell-dependent Abs were reduced in MZB cell-depleted mice, we examined the expression levels of the T-cell activation markers CD44, CD45Rb, and CD62L (11, 15). When examined directly ex vivo, splenic CD4+ T cells from 7-day-infected control mice had increased expression of CD44 in comparison to T cells from MZB cell-depleted mice (Fig. 2A). The differences in CD44 expression were still apparent at 14 days of infection (Fig. 2B), at which time CD45Rb expression was diminished to a greater extent on T cells from infected control mice than on those from infected MZB cell-depleted mice (Fig. 2C). No differences were noted in the expression of CD62L, which was diminished on T cells from both infected control and MZB cell-depleted mice (Fig. 2D). T cells from 7-day-infected MZB cell-depleted mice stimulated in vitro with B. burgdorferi lysate did not alter the expression of CD44 (Fig. 3A) or CD45Rb (Fig. 3B) in comparison to unstimulated cells from either infected control or infected MZB cell-depleted mice. In contrast, stimulated T cells from infected control mice exhibited an increase in CD44 and a decrease in CD45Rb expression. In vitro stimulation also reduced CD62L expression by T cells from infected MZB cell-depleted mice to a lesser degree than T cells from infected control mice (Fig. 3C). T cells from 14-day-infected MZB cell-depleted mice stimulated in vitro with B. burgdorferi lysate still expressed low levels of the activation marker CD44, whereas this marker could be detected in a significant proportion of stimulated T cells from infected control mice (Fig. 3D). The levels of CD62L and CD45Rb, however, were similarly diminished on T cells from both groups of mice after in vitro stimulation (data not shown).
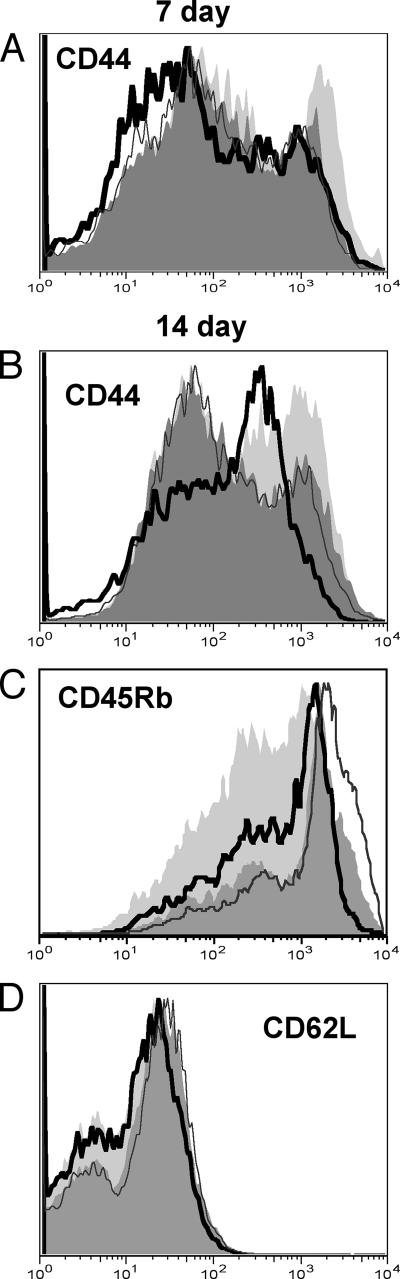
FIG. 2.
T cells from infected MZB cell-depleted mice have reduced activation markers in comparison to controls. Splenocytes from 7-day-infected (A) and 14-day-infected (B and C) mice were labeled and analyzed by flow cytometry as described in Materials and Methods. Gates were set on Thy1.2+ CD4+ cells. The activation marker expression on the gated cells from one representative mouse per group is shown in the histograms: light gray solid histogram, cells from an infected control mouse; black line open histogram, cells from an infected MZB cell-depleted mouse; dark gray solid histogram, cells from an uninfected control mouse; gray line, open histogram, cells from an uninfected MZB cell-depleted mouse. The averages of the mean fluorescent intensities (MFIs) from the five infected MZB cell-depleted mice were significantly different from those of the five infected control mice at days 7 and 14 of infection (P = 0.02 [A], P = 0.01 [B], and P = 0.004 [C], as determined by the two-tailed Student t test). There were no statistical differences in the MFIs between the uninfected MZB cell-depleted and control mouse groups.
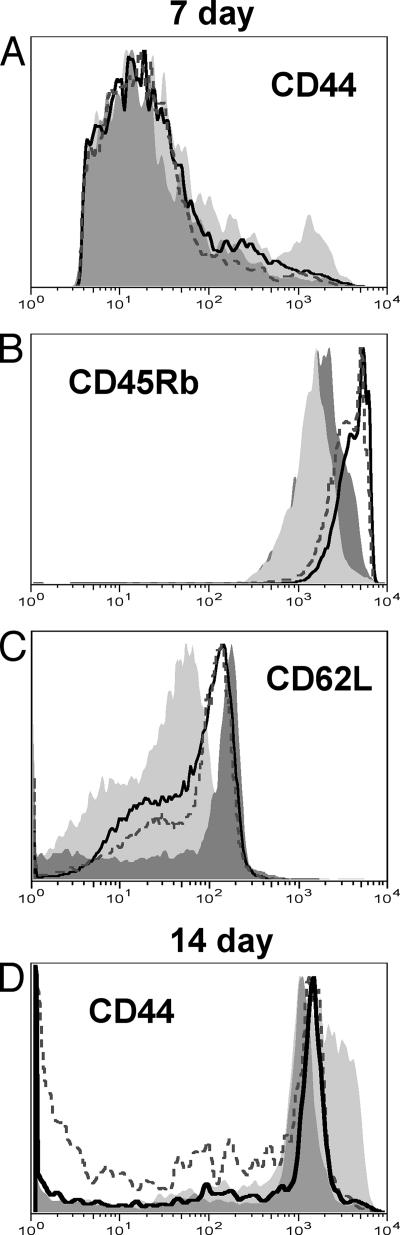
FIG. 3.
CD4+ T cells from MZB cell-depleted mice have reduced responses to B. burgdorferi after in vitro stimulation. Splenocytes from 7-day-infected (A to C) and 14-day-infected (D) mice were stimulated with B. burgdorferi lysate for 72 h prior to immunofluorescent staining, as described in Materials and Methods. Gates were set on Thy1.2+ CD4+ cells. The activation marker expression on the gated cells from one representative mouse per group is shown in the histograms: light gray solid histogram, stimulated cells from an infected control mouse; black line, open histogram, stimulated cells from an infected MZB cell-depleted mouse; dark gray solid histogram, unstimulated cells from an infected control mouse; gray line, open histogram, unstimulated cells from an infected MZB cell-depleted mouse. The averages of the MFIs from the stimulated cells from five MZB cell-depleted mice were significantly different from those of five control mice (P = 0.02 [A], P = 0.01 [B], P = 0.003 [C], and P = 0.005 [D], as determined by a two-tailed Student's t test).
T cells from B. burgdorferi-infected MZB cell-depleted mice proliferate less and produce lower amounts of IFN-γ than T cells from infected control mice.
To assess the effects of MZB cell depletion on T-cell proliferation, splenocytes from 7-day-infected mice were labeled with CFSE and stimulated in vitro with B. burgdorferi lysate for 72 h. A higher percentage of splenic CD4+ T cells from control mice proliferated in response to B. burgdorferi lysate stimulation, and those cells underwent more divisions than cells from MZB cell-depleted mice (Table 2). Stimulated splenic CD4+ T cells from MZB cell-depleted mice proliferated to a similar extent as unstimulated cells from infected mice, with 74.7% and 75.3% of unstimulated cells remaining quiescent from control and MZB cell-depleted mice, respectively (data not shown). CD4+ T cells from 7-, 10-, and 14-day-infected MZB cell-depleted mice stimulated with B. burgdorferi lysate produced lower levels of IFN-γ than those from infected control mice (Table 3). IFN-γ could not be detected in culture supernatants of unstimulated splenocytes from either infected control or MZB cell-depleted mice (data not shown). The percentages of CD4+ T cells in the cultured splenocytes were not statistically different between MZB cell-depleted and control mice (Table 3).
TABLE 2.
Proliferation of CD4+ T cells from 7-day-infected mice after in vitro restimulationa
| Mouse group | % of CD4+ T cells for no. of cell divisions:
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| MZB cell depleted | 76.2b | 21.2c | 2.03d | 0.42e |
| Isotype control | 21.0 | 50.7 | 25.3 | 2.08 |
A total of 5 × 106 CFSE-labeled splenocytes were cultured with 10 μg of B. burgdorferi lysate for 72 h. The percentage of CD4+ T cells in each division, based on the intensity of CFSE staining, was calculated using Flowjo software (Tree Star Inc., Ashland, OR). The numbers represent the percentages of CD4+ T cells in each generation. P values were determined by a two-tailed Student's t test comparing values for MZB cell-depleted mice with those of isotype control mice.
P = 0.002.
P = 0.016.
P < 0.0001.
P < 0.0001.
TABLE 3.
Splenocytes from MZB cell-depleted mice produce less IFN-γa
| Mouse group | Mean IFN-γ (ng/ml) ± SD at day of infection:
|
||
|---|---|---|---|
| 7 | 10 | 14 | |
| MZB cell depleted | 13.1 ± 2.5b | 55.4 ± 5.4c | 21.6 ± 7.0d |
| Isotype control | 74.2 ± 9.8 | 250 ± 15.0 | 106.7 ± 31.7 |
Values are the mean levels of IFN-γ (ng/ml) in culture supernatants ± standard deviations. A total of 5 × 106 splenocytes were cultured with 10 μg of B. burgdorferi lysate for 72 h (days 7 and 10 of infection) or for 48 h (day 14 of infection). IFN-γ levels in the cell supernatants were determined by ELISA. P values were determined by a two-tailed Student's t test. The percentages of CD4+ T cells in both groups at each time point were similar (57.3 and 50.7, 71.3 and 52.9, and 60.8 and 59.4% for MZB cell-depleted and isotype control groups at days 7, 10, and 14, respectively).
P = <0.0001.
P = <0.0001.
P = 0.002.
B. burgdorferi-infected MZB cell-depleted mice have elevated pathogen burdens that can be reduced by immune serum from infected control mice.
To assess the effects of reduced B. burgdorferi-specific Ab titers on pathogen burden, we measured B. burgdorferi DNA in urinary bladders of mice 14 days after infection. Urinary bladders were chosen because they are a representative site of disseminated infection and are reproducibly positive by culture at this stage of infection. At 14 days, even though the B. burgdorferi-specific IgM titers in MZB cell-depleted and control mice were comparable, the pathogen burdens remained elevated in the urinary bladders of MZB-depleted mice (Fig. 4A). To determine whether the elevated pathogen burden was a consequence of the reduced anti-B. burgdorferi-specific Ab titers, we treated 10-day-infected MZB-depleted mice with immune serum from similarly infected WT mice. Four days after treatment, the immune sera did in fact lower the pathogen burden in the MZB-depleted mice to levels found in the control mice, whereas NMS and PBS had no effect (Fig. 4B).
FIG. 4.
Passive transfer of immune serum from 10-day-infected control mice into MZB cell-depleted mice reduces pathogen burden. The relative pathogen burden in urinary bladders was determined as described in the legend of Fig. 1 and performed on specimens from two separate experiments in which the control mice had an average of 29,000 and 112,800 copies of B. burgdorferi recA gene/ng mouse actin gene, respectively. At day 14 (A), MZB cell-depleted mice (MZB-Depl.) had significantly more B. burgdorferi DNA than controls (P = 0.025, Mann-Whitney test). (B) Ten-day-infected MZB cell-depleted mice (Depl) were treated with either immune mouse serum (IMS) from 10-day-infected control mice (Cntr), NMS, or PBS and analyzed for pathogen burden at infection day 14. The results from the NMS and PBS treatment groups were combined because no differences were observed. Comparison of groups was performed using the Mann-Whitney test with the following results: a P value of 0.55 for IMS-treated control versus IMS-treated MZB cell-depleted groups, a P value of 0.003 for the combined PBS/NMS-treated control versus PBS/NMS-treated MZB cell-depleted groups, and a P value of 0.019 for the IMS-treated MZB cell-depleted group versus the PBS/NMS-treated MZB cell-depleted groups.
DISCUSSION
MZB cells are a first-line defense against blood-borne pathogens entering the spleen freely through the blood or transported by circulating APCs. These cells are poised to secrete IgM and IgG3 to foreign Ags before the development of adaptive immune responses. In addition, recent immunization studies have demonstrated that MZB cells can transport immune complexes to follicular dendritic cells and deposit them on their surface (12) or process Ag for direct presentation on major histocompatibility complex class II molecules to naïve CD4+ T cells (3). Few studies, however, have investigated the response of MZB cells during the course of an active infection. Borrelia spirochetes are a particularly suitable pathogen with which to examine MZB cell involvement in host defense because they have a distinct blood-borne phase in the mouse, and T-cell-independent Ab is sufficient to control infection. Using B. hermsii infection of mice, which manifests as periodic episodes of bacteremia in which spirochetes are visible in the blood, we previously showed that MZB cells produce pathogen-specific IgM and upregulate T-cell costimulatory molecules within 72 h of infection (7). In the current study, we sought to determine the contribution of MZB cells to the evolving host response to B. burgdorferi, a subacute pathogen in mice that becomes blood borne within 4 days of intradermal inoculation but rapidly exits the vasculature to infect other tissues. We used mAb to selectively deplete MZB cells from normal mice prior to infection so as to avoid confounding issues of immunologic defects associated with mutant mouse strains that lack MZB cells (14). The mAbs employed to deplete MZB cells are not expected to impact B. burgdorferi interactions with host tissue since this organism does not bind LFA-1 or α4β1 integrins (10). MZB cell depletion is only transient, as newly formed MZB cells repopulate the spleen by day 14 of infection (data not shown) (18). Our results provide evidence not only that MZB cells are an important source of pathogen-specific IgM but also that they play a critical role in stimulating T-cell-dependent Ab responses during the initial stages of infection.
Deficiency in MZB cells led to reduced pathogen-specific IgM in the serum at 7 days. The low level of IgM detected at this stage of infection may arise from other B-cell subsets, such as bone marrow-derived perisinusoidal B cells or B1 B cells, that participate in T-cell-independent immune responses to blood-borne Ags (9). At later time points, the B. burgdorferi-specific IgM titers approached those of infected control mice and were about twofold higher by day 14. The IgM detected at day 14 may arise from newly developed MZB cells that have repopulated the spleen (18), other innate B-cell populations, and/or follicular B cells. In contrast, the IgG titers in MZB cell-depleted mice were low at day 7 and remained low at day 14 of infection, a time point at which T-cell-dependent pathogen-specific Ab responses are normally readily detectable in B. burgdorferi-infected mice. These findings suggested that MZB cell depletion had affected not only the T-cell-independent B-cell response to B. burgdorferi but also the activation of CD4+ T cells that facilitate B-cell isotype switching.
MZB cell-depleted mice had impaired splenic T-cell responses when examined directly ex vivo and after in vitro restimulation with B. burgdorferi lysate. Although immunization studies have revealed that MZB cells can be potent APCs and can directly activate T-helper cells (3), MZB cells have not previously been shown to have a direct impact on T-cell-dependent Ab production during infection. MZB cells are capable of transporting and depositing IgM-containing immune complexes onto follicular dendritic cells (12). Both the decrease in the number of MZB cells after depletion and the early decrease in IgM titers would negatively impact this method of Ag presentation and subsequent T-cell activation. The phenotype of MZB cell-depleted mice contrasts with that of CD1d−/− mice, even though in both types of mice, Borrelia-specific IgM responses are impaired (7). We have previously shown that MZB cells from B. hermsii-infected CD1d−/− mice increase the expression of T-cell costimulatory molecules, suggesting that CD1d−/− MZB cells are still capable of priming and activating helper T cells. When we directly compared B. burgdorferi-specific IgM and IgG titers from 7-day-infected WT, MZB cell-depleted, and CD1d−/− mice, we found that IgG titers were preserved in CD1d−/− mice but not in MZB cell-depleted mice (reciprocal endpoint positive IgM titers of 1,000 ± 200 for WT mice, 450 ± 126 for MZB cell-depleted mice, and 425 ± 287 for CD1d−/− mice and IgG titers of 1,920 ± 739 for WT mice, 720 ± 403 for MZB cell-depleted mice, and 2,133 ± 739 for CD1d−/− mice). Thus, in the case of Borrelia infection, the APC contributions of MZB cells to T-helper-cell activation appear to be CD1d independent, but pathogen-specific IgM secretion by MZB cells is CD1d dependent.
Reduction in Borrelia-specific Ab likely contributes to the 5- to 10-fold increase in pathogen burden noted in the MZB-depleted mice, as Ab is a key defense mechanism against infection with B. burgdorferi. In support of this interpretation, we found that the passive transfer of immune serum to infected MZB cell-depleted mice reduced their pathogen load, consistent with the known effects of pathogen-specific Ab on B. burgdorferi infection in mice (6, 20). Passive immunization, however, had no impact on the reduced levels of activation marker expression by the T cells in MZB cell-depleted mice (data not shown).
Our results also show that B. burgdorferi infection of MZB cell-depleted mice results in the earlier development of arthritis. In C3H mice, arthritis peaks in severity at 4 weeks of infection, but evidence of arthritis can be seen earlier. In our study, all of the joints examined from the 10-day-infected MZB cell-depleted mice exhibited arthritis, whereas only a few joints from the control animals had inflammation at that time point, suggesting that MZB cell depletion altered the kinetics of disease development. Even at day 14, a time point when C3H mice normally have developed arthritis, the MZB cell-depleted mice had more pronounced disease. B. burgdorferi-induced arthritis severity is known to be equivalent in T-cell-deficient and immunocompetent mice but is more severe in B-cell-deficient mice (8, 20, 23). Our results suggest that a deficiency in the MZB cell subset and its production of pathogen-specific IgM can exacerbate arthritis early in infection.
In summary, we found reduced pathogen-specific IgG and significant impairment in the activation, proliferation, and cytokine production by splenic T cells from MZB cell-depleted mice infected with B. burgdorferi. These findings reveal an important role for this B-cell subset in priming splenic T cells and the subsequent development of T-cell-dependent IgG responses during infection, extending similar observations after immunization (3). Our study also supports our previous finding that MZB cells are a source of pathogen-specific IgM during the early stages of Borrelia infection and shows that a deficiency in this response can exacerbate arthritis. These findings may have relevance to other blood-borne pathogens for which the timely production of Ab and/or T-cell-mediated immunity is important for limiting pathogen burden and pathological sequelae in the host.
Acknowledgments
We thank Deborah Beck and Jialing Mao for excellent technical assistance.
This work was supported by grants from the NIH (AI50604 and AR47058) and the Arthritis Foundation.
Editor: D. L. Burns
Footnotes
Published ahead of print on 30 April 2007.
REFERENCES
- 1.Alugupalli, K. R., S. Akira, E. Lien, and J. M. Leong. 2007. MyD88- and Bruton's tyrosine kinase-mediated signals are essential for T cell-independent pathogen-specific IgM responses. J. Immunol. 178:3740-3749. [DOI] [PubMed] [Google Scholar]
- 2.Amano, M., N. Baumgarth, M. D. Dick, L. Brossay, M. Kronenberg, L. A. Herzenberg, and S. Strober. 1998. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J. Immunol. 161:1710-1717. [PubMed] [Google Scholar]
- 3.Attanavanich, K., and J. F. Kearney. 2004. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J. Immunol. 172:803-811. [DOI] [PubMed] [Google Scholar]
- 4.Barbour, A. G., and S. F. Hayes. 1986. Biology of Borrelia species. Microbiol. Rev. 50:381-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 6.Barthold, S. W., S. Feng, L. K. Bockenstedt, E. Fikrig, and K. Feen. 1997. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin. Infect. Dis. 25(Suppl. 1):S9-S17. [DOI] [PubMed] [Google Scholar]
- 7.Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. [DOI] [PubMed] [Google Scholar]
- 8.Bockenstedt, L. K., I. Kang, C. Chang, D. Persing, A. Hayday, and S. W. Barthold. 2001. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect. Immun. 69:5264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cariappa, A., I. B. Mazo, C. Chase, H. N. Shi, H. Liu, Q. Li, H. Rose, H. Leung, B. J. Cherayil, P. Russell, U. von Andrian, and S. Pillai. 2005. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity 23:397-407. [DOI] [PubMed] [Google Scholar]
- 10.Coburn, J., J. M. Leong, and J. K. Erban. 1993. Integrin alpha IIb beta 3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc. Natl. Acad. Sci. USA 90:7059-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeGrendele, H. C., M. Kosfiszer, P. Estess, and M. H. Siegelman. 1997. CD44 activation and associated primary adhesion is inducible via T cell receptor stimulation. J. Immunol. 159:2549-2553. [PubMed] [Google Scholar]
- 12.Ferguson, A. R., M. E. Youd, and R. B. Corley. 2004. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int. Immunol. 16:1411-1422. [DOI] [PubMed] [Google Scholar]
- 13.Fikrig, E., S. W. Barthold, M. Chen, C. H. Chang, and R. A. Flavell. 1997. Protective antibodies develop, and murine Lyme arthritis regresses, in the absence of MHC class II and CD4+ T cells. J. Immunol. 159:5682-5686. [PubMed] [Google Scholar]
- 14.Guinamard, R., M. Okigaki, J. Schlessinger, and J. V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31-36. [DOI] [PubMed] [Google Scholar]
- 15.Huet, S., H. Groux, B. Caillou, H. Valentin, A. M. Prieur, and A. Bernard. 1989. CD44 contributes to T cell activation. J. Immunol. 143:798-801. [PubMed] [Google Scholar]
- 16.Kumar, H., A. Belperron, S. W. Barthold, and L. K. Bockenstedt. 2000. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165:4797-4801. [DOI] [PubMed] [Google Scholar]
- 17.Lopes-Carvalho, T., J. Foote, and J. F. Kearney. 2005. Marginal zone B cells in lymphocyte activation and regulation. Curr. Opin. Immunol. 17:244-250. [DOI] [PubMed] [Google Scholar]
- 18.Lu, T. T., and J. G. Cyster. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297:409-412. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan, I. C., and Y. J. Liu. 1991. Marginal zone B cells respond both to polysaccharide antigens and protein antigens. Res. Immunol. 142:346-351. [DOI] [PubMed] [Google Scholar]
- 20.McKisic, M. D., and S. W. Barthold. 2000. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 68:5190-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuttall, P. A. 1998. Displaced tick-parasite interactions at the host interface. Parasitology 116:S65-S72. [DOI] [PubMed] [Google Scholar]
- 22.Oliver, A. M., F. Martin, G. L. Gartland, R. H. Carter, and J. F. Kearney. 1997. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 27:2366-2374. [DOI] [PubMed] [Google Scholar]
- 23.Schaible, U. E., S. Gay, C. Museteanu, M. D. Kramer, G. Zimmer, K. Eichmann, U. Museteanu, and M. M. Simon. 1990. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137:811-820. [PMC free article] [PubMed] [Google Scholar]
- 24.Song, H., and J. Cerny. 2003. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 198:1923-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vos, Q., A. Lees, Z. Q. Wu, C. M. Snapper, and J. J. Mond. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176:154-170. [DOI] [PubMed] [Google Scholar]
- 27.Wormser, G. P., D. McKenna, J. Carlin, R. B. Nadelman, L. F. Cavaliere, D. Holmgren, D. W. Byrne, and J. Nowakowski. 2005. Brief communication: hematogenous dissemination in early Lyme disease. Ann. Intern. Med. 142:751-755. [DOI] [PubMed] [Google Scholar]