Abstract
Campylobacter jejuni CG8486, which belongs to the HS4 complex, was isolated from a patient with inflammatory diarrhea in Thailand. This strain caused a diarrheal disease in ferrets comparable to that caused by C. jejuni strain 81-176, but it was much less invasive for epithelial cells in vitro than 81-176. Complete genome sequencing of CG8486 revealed a 1.65-Mb genome that was very similar to the other two published genomes of clinical isolates of C. jejuni, the genomes of 81-176 and NCTC 11168, with a limited number of CG8486-specific genes mapping outside the hypervariable carbohydrate biosynthesis loci. These data suggest that the genes required for induction of inflammatory diarrhea are among the genes shared by CG8486 and 81-176 but that either major changes in the carbohydrate loci and/or more subtle changes in other genes may modulate virulence.
Campylobacter jejuni is a leading bacterial cause of food-borne diarrhea worldwide (8). The human disease is associated principally with consumption of poultry, although other sources, such as water and milk, have been identified (14). Infection with strains of C. jejuni with sialyated lipooligosaccharide (LOS) cores that mimic human gangliosides in structure can result in development of Guillain-Barré syndrome as a sequela to diarrheal disease (6). Symptoms of campylobacteriosis can range from mild watery diarrhea to severe dysentery (41). This range of symptoms is likely due in part to the immune status of the host, but genome diversity may also play a role. Despite extensive studies little is known about the molecular pathogenesis of C. jejuni diarrheal disease. The only well-characterized virulence factor is the cytolethal distending toxin, but the role of this toxin, which is shared with numerous, diverse pathogens, in diarrheal disease remains uncertain. The publication of the genome of a clinical isolate of C. jejuni from the United Kingdom, NCTC 11168 (31), elucidated much about the biology of this bacterium but revealed no novel virulence factors that it has in common with other bacteria other than a previously unrecognized capsular polysaccharide (31). Whole-genome microarrays have been used extensively to explore the genomic diversity of C. jejuni (9, 21, 22, 30, 32-34, 38), and recently the complete genomes of a second C. jejuni strain, RM1221, which was isolated from chicken skin but whose virulence properties are unknown, and strains of Campylobacter coli, Campylobacter lari, and Campylobacter upsaliensis have been published (10). Ten additional strains of Campylobacter spp. are being sequenced by The Institute for Genomic Research (D. Fouts, personal communication), and sequences of nine of these strains have been deposited in the GenBank database. These organisms include three strains from Guillain-Barré syndrome cases and one isolate, strain 81-176, from a diarrhea outbreak. The latter strain has also been sequenced independently using a new pyrosequencing technology based on a microfabricated high-density picoliter reactor (23). C. jejuni 81-176 is a well-characterized strain that originated from an outbreak of diarrheal disease in school children following consumption of raw milk at a dairy farm (19). This strain has subsequently been fed to adult human volunteers twice, and the clinical features associated with this strain are well documented (4, 7), in contrast to those of the other sequenced strains. Moreover, 81-176 is more invasive for intestinal epithelial cells than other C. jejuni strains (18, 27), and it has been shown to cause diarrheal disease in multiple animal models (1, 3, 35).
C. jejuni and C. coli are major causes of diarrheal disease in Southeast Asia. Several studies of deployed United States military personnel in Thailand have shown that these Campylobacter species are identified in 30 to 60% of patients presenting with acute diarrhea (37). A clinic-based study of United States military personnel presenting with acute diarrhea was undertaken during a 14-day period in May 1999. Several C. jejuni isolates from patients with distinct diarrheal disease symptoms were identified and subsequently were characterized. We used the newly available pyrosequencing technology (23) to sequence the genome of an isolate of C. jejuni that came from one of these patients with well-defined clinical symptoms. Here we compare the genome sequence of this strain to those of two other diarrheal isolates, NCTC 11168 and 81-176.
MATERIALS AND METHODS
Strain isolation and characterization.
Strain CG8486 was isolated from a United States military service member with acute dysentery in Khorat, Thailand, in May 1999. For primary isolation in the field laboratory a membrane filter method with nonselective brucella agar containing 5% sheep blood agar (18) was used. Suspect colonies were subcultured onto blood agar following transport to the Armed Forces Research Institute of Medical Sciences laboratory in Bangkok for species identification. Antibiotic susceptibility testing of the isolate, designated CG8486, by the disk diffusion method (6) revealed azithromycin sensitivity and resistance to ofloxacin and tetracycline. CG8486 was identified as type Lior 5 at the Armed Forces Research Institute of Medical Sciences and as a member of the HS4 complex by Eva Nielsen of the Danish Veterinary Laboratory.
Other bacterial strains and growth conditions.
Strains 81-176 and OH4384 have been described previously (7, 11). C. jejuni was grown in Mueller-Hinton broth or on Mueller-Hinton agar at 37°C under microaerophilic conditions.
Determination of inflammatory markers in blood and stools.
Plasma and fecal extracts (2 ml extraction buffer/g of stool) were used to determine inflammatory cytokines by a capture enzyme-linked immunosorbent assay (Endogen, Woburn, MA). The C-reactive protein (CRP) (Dade Behring, Marburg, Germany) content in plasma and the lactoferrin (Leukotest; TechLab, Blacksburg, VA) content in stools were determined by using commercially available kits. The extraction buffer was phosphate-buffered saline containing 0.05% Tween 20, 1 mg/ml EDTA, 1 mg/ml bovine serum albumin, 1 mg/ml phenylmethylsulfonyl fluoride, and 200 mg/ml trypsin soybean inhibitor.
DNA extraction.
Genomic DNA was prepared using the method of Sambrook et al. (36).
DNA sequence analysis.
The C. jejuni CG8486 genome was sequenced using a Genome Sequencer 20 sequencing instrument (454 Life Sciences, Branford, CT). Two sequencing rounds yielded approximately 596,000 sequences with an average read length of 109 bases for a total of approximately 65 Mb of data.
Contig assembly and annotation.
Assembly was performed by using the newbler Assembler software provided by 454 Life Sciences. The analysis yielded 86 large contigs ranging from 557 to 155,249 bp long representing a total of ∼1.65 Mb. Each of the contigs was independently screened for open reading frames (ORFs) at least 80 bp long using Artemis software (http://www.sanger.ac.uk/Software/Artemis/). Overlapping ORFs were manually eliminated. A total of 1,628 chromosomal ORFs have been found. ORFs were locally aligned using BLASTN software (http://www.ncbi.nlm.nih.gov/BLAST/download.shtml) against the whole C. jejuni NCTC 11168 genome. ORFs with no significant match were selected, and the corresponding proteins were compared to a nonredundant database using a local BLASTP program (http://www.ncbi.nlm.nih.gov/BLAST/download.shtml) for putative functional attribution.
Gap closure.
After ordering of the CG8486 contigs on the sequence of C. jejuni NCTC 11168, many of the contigs could be tentatively aligned based on a shared gene at either end. Primers were designed for the end of each putative contiguous contig to close the gaps by PCR. After reassembly 30 of the gaps (40%) appeared to be separated by only a few base pairs. Following this analysis 58 gaps were successfully closed, leaving a total of 18 contigs. Some of the remaining gaps that were not closed are found in regions with repetitive sequences in other C. jejuni genomes.
Comparative genomics.
All nucleotide and predicted protein comparisons were performed locally using the local BLAST package against the respective submitted C. jejuni genome updated nt and nr database. An e value of 10−2 was selected as a threshold for ORF function attribution.
Oligonucleotide synthesis.
DNA primers were synthesized with an Applied Biosystems model 392 DNA synthesizer.
Conventional DNA sequencing.
Some regions of the DNA sequence obtained from pyrosequencing were confirmed using dideoxy sequence analysis with an Applied Biosystems model 3100 DNA sequencer.
Invasion assays.
Invasion assays using INT407 or Caco2 cells were done as previously described (27). Basically, approximately ∼2 × 106 bacterial cells were added to a monolayer of ∼7 × 104 epithelial cells. Following centrifugation at 200 × g for 5 min, the assay mixtures were incubated for 2 h at 37°C in 5% CO2-95% air. Each monolayer was washed twice with Hanks' balanced salt solution, and fresh, prewarmed medium containing 100 μg/ml gentamicin was added to kill extracellular bacteria. After two additional hours of incubation, the monolayer was washed twice with Hanks' balanced salt solution, and the bacteria were lysed in 0.01% Triton X-100 for 30 min. The released intracellular bacteria were enumerated by plate counting on Mueller-Hinton agar. The invasion ability was expressed as the percentage of the input inoculum surviving the gentamicin treatment.
Ferret model.
Experiments with the ferret model were performed as previously described (2, 15). Campylobacter-free, 6-week-old female ferrets were purchased from Triple F Farms (Sayre, PA). After 1 week of acclimation, the animals were infected with 1.8 × 1010 to 2.0 × 1010 bacteria by oral gavage and observed for signs of diarrheal disease for 4 days. Seven animals per strain were infected. The experiments were conducted in compliance with the Animal Welfare Act and according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (18a).
Project accession number.
This whole-genome shotgun project has been deposited in the EMBL/GenBank under project accession number ASY00000000. The version described in this paper is the first version, AASY01000000.
RESULTS AND DISCUSSION
Clinical history.
A 28-year-old male active duty soldier presented with a 3-day history of diarrhea (three episodes in the 24 h prior to presentation), abdominal cramps, gross blood in stools, and headaches without fever, nausea, or vomiting. He consented to study enrollment and provided a stool specimen prior to antibiotic therapy, from which C. jejuni strain CG8486 was the only pathogen isolated. The clinical history was notable for a previous episode of mild watery Salmonella-associated diarrhea that had responded promptly (<12 h) to a 3-day course of ofloxacin therapy. The patient had been without symptoms for 4 days prior to new-onset dysentery. He denied use of doxycycline for malaria prophylaxis. Physical examination was notable for an oral temperature of 99.2°F, no orthostasis, and mild diffuse abdominal tenderness. The patient was positive for a number of inflammatory markers in both blood and stool (20, 25), as shown in Table 1. The patient was treated with azithromycin, and the diarrhea significantly improved by 72 h after the first antibiotic dose. A total of 13 diarrhea stools were documented during the episode lasting approximately 6 days.
TABLE 1.
Concentrations of inflammatory markers in stools and plasma of patients infected with CG8486a
| Specimens | Inflammatory response
|
||||||
|---|---|---|---|---|---|---|---|
| CRP concn (mg/ml) | Gamma interferon concn (pg/ml) | Interleukin-1β concn (pg/ml) | Interleukin-6 concn (pg/ml) | Interleukin-8 concn (pg/ml) | Tumor necrosis factor alpha concn (pg/ml) | Lactoferrin | |
| Plasma | 192 | <2.5 | <2.5 | <2.5 | 208 | 7 | NA |
| Stool | NA | <2.5 | 1,744 | <2.5 | <2.5 | <2.5 | + |
The detection limit for CRP was 6 mg/ml, and for cytokines the detection limit was 2.5 pg/ml; the concentration of interleukin-12 was below the level of detection. The lactoferrin assay showed agglutination at a stool dilution of 1:200. NA, not applicable.
In vitro and in vivo characterization of CG8486 virulence.
Since C. jejuni strains are considered invasive for intestinal epithelial cells, we compared the ability of CG8486 to invade INT407 cells to that of the well-studied, invasive strain 81-176. In triplicate experiments, 81-176 invaded INT407 cells at 1.49% ± 0.51% of the input inoculum. In contrast, CG8486, which was fully motile, invaded cells at 0.001% ± 0.001% of the input inoculum, which was >1000-fold lower than the value for 81-176 (P < 0.001). Strain 81-176 also invaded Caco2 cells at a higher level (0.42% ± 0.31%) than CG8486 (0.016% ± 0.001%), although the difference did not reach statistical significance (P = 0.08).
When strains 81-176 and CG8486 were compared using the ferret model of diarrhea, they produced comparable disease patterns (five of seven animals infected with each strain developed diarrhea). Thus, CG8486 appeared to be as virulent as 81-176 in the ferret model, despite being less invasive than 81-176 in vitro. All previous studies with 81-176 indicated that invasiveness in vitro correlated with disease in the ferret model (2, 12, 42).
General comparative genomics with other C. jejuni strains.
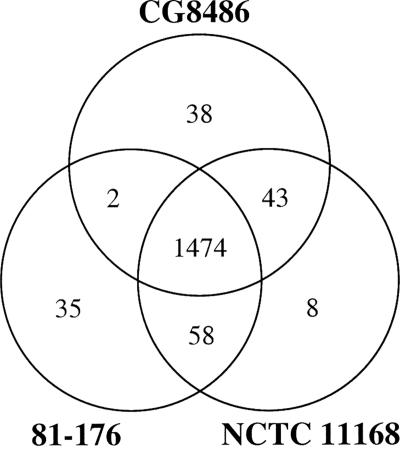
The G+C content of the CG8486 genome is 30.43%, which is very similar to the G+C contents of the NCTC 11168 (30.55%), RM1221 (30.31%), and 81-176 (30.61%) genomes. The chromosome size of CG8486, 1.61 Mb, is closer to those of NCTC 11168 (1.64 Mb) and 81-176 (1.59 Mb) than to that of RM1221 (1.78 Mb), which contains insertions of phage or plasmids in four genomic islands, none of which were observed in CG8486. The genomes of CG8486, 81-176, and NCTC 11168, the three clinical isolates, are summarized in Fig. 1. The three strains share 1,474 genes. There are 35 genes that are unique to 81-176, 38 genes that are unique to CG8486, and 8 genes that are unique to NCTC 11168. Strains 81-176 and CG8486 share only two genes not found in NCTC 11168, which encode a type I restriction-modification (R-M) system, as discussed below. Excluding the carbohydrate loci, CG8486 and NCTC 11168 share 43 genes not found in 81-176 (see Table S1 in the supplemental material), and 81-176 and NCTC 11168 share 58 genes not found in CG8486.
FIG. 1.
Venn diagram comparing the gene contents of C. jejuni strains CG8486, NCTC 11168, and 81-176. The gene content of 81-176 is based on the results of Hofreuter et al. (17). These estimations exclude the capsule, LOS, and flagellin posttranslational modification loci. The two genes present in both strain CG8486 and strain 81-176 are Cj8486_0771 (cju16) and Cj8486_0774 (cju20) coding for a type I R-M system (HsdM and HsdR, respectively). The eight genes unique to strain NCTC 11168 are listed in Table S2 in the supplemental material.
In general, as has been seen with other C. jejuni genomes, the vast majority of genes shared by CG8486 and reference strain NCTC 11168 are very similar. The CG8486 chromosome also appeared to have a high degree of synteny with the NCTC 11168 chromosome, with the exception of insertions or deletions of various sizes and a large chromosomal inversion between the intergenic region between the homologs of Cj0936 and Cj0937 (Cj8486_0953c and Cj1152c) and Cj1116c (Cj8486_954 and Cj8486_1154c). This inversion resulted in truncation, and most likely inactivation, of Cj1116c, which encodes a probable membrane-bound zinc metallopeptidase. The position of this inversion between Cj0936 and Cj0937 is also the position of an insertion of plasmid DNA in strain C. jejuni 81-176 (17), suggesting that this region may define a hot spot for recombinational activity.
NCTC 11168 genes missing in CG8486.
A total of 87 ORFs found in NCTC 11168 (5.5% of the genome) are absent in CG8486 (see Table S2 in the supplemental material). Fifty-six (64%) of these ORFs coded for proteins <200 amino acid residues long, and 66 (76%) were smaller than the average ORF size for the NCTC 11168 genome (948 bp) (31). The bias for the absence of the small NCTC 11168 ORFs in CG8486 is due in part to the absence of 41 (47.0%) ORFs classified as hypothetical. Forty-seven (54%) of the missing genes were found in hypervariable regions (see Table S2 in the supplemental material) as defined by Taboada et al. (38), including the carbohydrate loci discussed below. Microarray comparative genomic assays revealed up to 20% variability between C. jejuni strains (9), but the values represent both missing and highly divergent genes. In comparison, 119 NCTC 11168 ORFs were missing in the RM1221 genome (10). The C. jejuni 81-176 genome appears to be missing a total of 51 ORFs compared to the NCTC 11168 genome, excluding the hypervariable carbohydrate loci. Of these 51 ORFs, 8 are also missing in CG8486 (Fig. 1). These genes are Cj0008, Cj0568, Cj1520, and Cj1722c coding for hypothetical proteins, Cj1376 and Cj1723c coding for putative periplasmic proteins, and Cj0422c and Cj1051c coding for a putative helix-turn-helix and an R-M protein, respectively.
CG8486 ORFs absent from NCTC 11168 and 81-176 genomes.
There are 40 genes unique to strain CG8486 compared to NCTC 11168, and these genes are located in 15 zones (Table 2). The majority of these unique genes represent gene replacements, and the minority appear to be simple insertions. This observation corroborates the hypothesis of Taboada et al. that C. jejuni strains have a low level of genome plasticity, with only specific regions showing diversity (38). The variability in CG8486 is similar to that seen in 81-176 (17), in which 37 unique genes were located in 11 regions on the chromosome. As mentioned above, the only genes shared by 81-176 and CG8486 that are not found in NCTC 11168 are those corresponding to a type I R-M system in zone 6 (Table 2; see below). Based on these observations, it appears that the pools of unique genes of 81-176 and CG8486 are different and that these genes are physically located at distinct locations on the chromosome (see Table S3 in the supplemental material).
TABLE 2.
C. jejuni GC8486 ORFs absent from the NCTC 11168 genome
| Insertion location | Locus tag | Protein size (residues) | Closest relationship (accession no.)a | No. of identical residues/total no. of residues (% identity) |
|---|---|---|---|---|
| Zone 1 (Cj0032/Cj0034) | Cj8486_0031 | 397 | Hypothetical protein CCO0074 (ZP_00368158) | 367/398 (92) |
| Zone 2 (Cj0054c/Cj0057) | Cj8486_0050c | 382 | Hypothetical protein CJE0051 (AAW34647) | 259/260 (99) |
| Cj8486_0051c | 164 | Hypothetical protein CJE0052 (AAW34648) | 89/90 (98) | |
| Zone 3 (methyltransferase) (Cj0259/Cj0261) | Cj8486_0245 | 1,037 | N6 adenine-specific DNA methyltransferase CJE0310 (AAW34900) | 1,062/1,085 (97) |
| Zone 4 (R-M type III) (Cj0288c/Cj0289c) | Cj8486_0274 | 384 | Serine/threonine protein kinase (YP_529411) | 76/279 (27) |
| Cj8486_0275 | 637 | Adenine-specific DNA methyltransferase (YP_665145) | 299/628 (47) | |
| Cj8486_0276 | 892 | Type III restriction enzyme R protein (ZP_00370006) | 550/859 (64) | |
| Zone 5 (Cj0493/Cj0495) | Cj8486_0486c | 82 | Unknown | |
| Zone 6 (R-M type I) (Cj0759/Cj0760) | Cj8486_0771 | 636 | Putative type I restriction enzyme MjaXP M protein (HsdM) | 619/636 (97) |
| Cj8486_0772 | 375 | Type I restriction enzyme specificity protein (HsdS) | 231/252 (91) | |
| Cj8486_0773 | 324 | Hypothetical protein Tgh129 | 104/105 (99) | |
| Cj8486_0774 | 971 | Type I site-specific DNase chain R (HsdR) | 962/971 (99) | |
| Zone 7 (methyltransferase) (Cj0761/Cj0762) | Cj8486_0777cb | 96 | Methyltransferase FkbM (YP_534624) | 45/94 (47) |
| Cj8486_0778c | 177 | Methyltransferase FkbM (YP_534624) | 82/173 (47) | |
| Zone 8 (divergence zone) (Cj0967/Cj0975) | Cj8486_1110 | 283 | Conserved domain protein CJE1054 (ZP_00369667) | 193/286 (67) |
| Cj8486_1111 | 293 | Hypothetical protein CJE1052 (AAW35382) | 19/62 (30) | |
| Cj8486_1112 | 170 | Filamentous hemagglutinin domain protein CLA0151 (EAL54392) | 62/118 (52) | |
| Cj8486_1113c | 283 | Filamentous hemagglutinin domain protein CLA0151 (EAL54392) | 157/164 (95) | |
| Zone 9 (Cj1050c/Cj1052) | Cj8486_1024b | 309 | Polysaccharide deacetylase family protein (AAW35941) | 165/306 (53) |
| Cj8486_1123 | 345 | Hypothetical protein CJE1501 (AAW35942) | 157/332 (47) | |
| Zone 10 (LOS) (Cj1136/Cj1139c) | Cj8486_1176 | 352 | β-1,4-N-Acetylgalactosaminyltransferase (AAX45337) | 163/313 (52) |
| Zone 11 (VacA) (Cj1359/Cj1361c) | Cj8486_1401 | 157 | Toxin-like outer membrane protein, putative (ZP_00367410) | 131/132 (99) |
| Cj8486_1402 | 184 | Vacuolating cytotoxin precursor, putative (ZP_00367411) | 77/78 (98) | |
| Cj8486_1403 | 239 | Vacuolating cytotoxin precursor, putative (ZP_00367411) | 208/232 (89) | |
| Cj8486_1404 | 160 | Hypothetical protein CJJ26094_1412 (ZP_01069043) | 136/149 (91) | |
| Zone 12 (capsular polysaccharide) (Cj1427c/Cj1428c) | Cj8486_1469cb | 92 | Putative GDP-mannoheptose-4,6-dehydratase (AAR01886) | 340/344 (98) |
| Zone 13 (R-M type I) (Cj1548/Cj1555c) | Cj8486_1586 | 796 | HsdR (AAM00880) | 726/769 (94) |
| Cj8486_1587 | 420 | RloA (AAM00881) | 420/420 (100) | |
| Cj8486_1588 | 226 | RloB (AAM00882) | 180/210 (85) | |
| Cj8486_1589 | 403 | Putative type I specificity subunit HsdS (AAN33149) | 403/403 (100) | |
| Cj8486_1590 | 251 | HsdM (AAM00858) | 475/494 (96) | |
| Cj8486_1591c | 149 | 4-Carboxymuconolactone decarboxylase, putative (ZP_01071025) | 121/124 (97) | |
| Cj8486_1592c | 80 | Hypothetical protein CJE1727 (YP_179702) | 135/139 (97) | |
| Cj8486_1593c | 122 | Transporter, putative CJE1728 (AAW36155) | 79/80 (98) | |
| Cj8486_1594c | 299 | Transporter, putative CJE1728 (AAW36155) | 286/293 (97) | |
| Zone 14 (arsenite resistance) (Cj1560/Cj1563) | Cj8486_1598 | 105 | Arsenical resistance operon repressor CJE1731 (AAW36157) | 105/105 (100) |
| Cj8486_1599 | 140 | Arsenate reductase CJE1732 (AAW36158) | 140/140 (100) | |
| Cj8486_1600 | 347 | Arsenical resistance protein, putative CJE1733 (AAW36159) | 347/347 (100) | |
| Zone 15 (Cj1726c/Cj1727c) | Cj8486_1774 | 73 | Conserved hypothetical protein CCO0025 (EAL56179) | 73/73 (100) |
| Cj8486_1775 | 113 | Conserved hypothetical protein CCO0026 (EAL56180) | 96/96 (100) |
The closest relationship is based on a BLASTP analysis of the gene product of CG8486.
Genes containing homopolymeric G·C tracts.
Homopolymeric G·C tracts.
Variable poly(G·C) tracts in C. jejuni were first reported in the genome sequence of NCTC 11168 (31), and subsequent studies have confirmed high levels of phase variation of surface antigens by slipped-strand mispairing (2, 11, 16). C. jejuni CG8486 had a total of 23 poly(G·C) tracts (Table 3), including three tracts in ORFs that are specific to this strain and five that map to intergenic regions. The first of the G·C tracts that are unique to CG8486 is found at the 3′ end of Cj8486_777c, encoding a putative methyltransferase. The other two genes unique to CG8486 with homopolymeric tracts are Cj8486_1024c, encoding a putative polysaccharide deacetylase, and Cj8486_1469c, encoding a sequence which is in the capsule locus. Seven of the CG8486 homopolymeric tracts are found in genes shared with NCTC 11168, but the corresponding NCTC 11168 allele lacks the repeats. Conversely, NCTC 11168 contains 14 G·C tracts that are not present in the corresponding genes in CG8486. Although slipped-strand mispairing has not been observed directly for any of these CG8486 genes, these observations suggest that the repertoire of potential phase-variable genes in C. jejuni is more extensive than previously thought.
TABLE 3.
Localization of homopolymeric G·C tracts of C. jejuni CG8486
| Homopolymeric repeat | Contig | Position | Locus tag affected | Putative function | G·C tract present in NCTC 11168a | G·C tract present in RM1221a |
|---|---|---|---|---|---|---|
| C (11) | Scontig01 | 79195-79205 | Intergenic | No | Yes (intergenic) | |
| C (10) | Scontig02 | 20153-20162 | Cj8486_0044c | Putative iron binding | Yes (Cj0045c) | Yes (CJE0044) |
| G (7) | Scontig03b | 3585-3591 | Cj8486_0258 | ATP-binding subunit | Yes (Cj0275) | Yes (CJE0324) |
| G (11) | Scontig05 | 127553-127563 | Intergenic | Yes (intergenic) | Yes (intergenic) | |
| G (9) | Scontig05 | 177163-177771 | Cj8486_0628 | Unknown | Yes (Cj0617) | No |
| G (10) | Scontig07 | 40362-40371 | Cj8486_0688 | Potassium-transporting ATPase B chain | No | NA |
| G (8) | Scontig07 | 49392-49399 | Cj8486_0699c | Possible sugar transferase | Yes (Cj0685c) | Yes (CJE0783) |
| G (10) | Scontig07 | 100000-100009 | Cj8486_0758 | Putative periplasmic protein | No | Yes (CJE0835) |
| G (9) | Scontig07 | 104411-104419 | Cj8486_0764 | Unknown | No | NA |
| C (10) | Scontig08 | 17763-17772 | Cj8486_0777cb | Methyltransferase FkbM | NA | NA |
| C (10) | Scontig10b | 63188-63197 | Cj8486_1024cb | Polysaccharide deacetylase | NA | Yes (CJE1500) |
| G (9) | Scontig15 | 17057-17065 | Cj8486_1181 | β-1,4-N-Acetylgalactosaminyltransferase | No | No |
| C (10) | Scontig15 | 124805-124814 | Intergenic | No | Yes (intergenic) | |
| G (11) | Scontig15 | 165473-165483 | Cj8486_1333c | Unknown | No | Yes (CJE1487) |
| G (11) | Scontig15 | 166944-166954 | Cj8486_1345 | Unknown | Yes (Cj1297) | NA |
| G (9) | Scontig16 | 10109-10117 | Cj8486_1364 | Unknown | Yes (Cj1318) | NA |
| G (9) | Scontig16 | 14904-14912 | Cj8486_1368 | Formyltransferase | No | No |
| C (9) | Scontig20 | 690-698 | Cj8486_1379c | Unknown | Yes (Cj1342c) | Yes (CJE1531) |
| C (9) | Scontig20 | 21002-21009 | Intergenic | No | Yes (intergenic) | |
| C (9) | Scontig20 | 80827-80835 | Cj8486_1463c | Unknown | Yes (Cj1420c) | NA |
| C (7) | Scontig22 | 48-54 | Intergenic | No | NA | |
| C (8) | Scontig22 | 3653-3660 | Cj8486_1469cb | GDP-mannose 4,6-dehydratase | NA | NA |
| G (11) | Scontig22 | 10033-10043 | Cj8486_1475 | Sugar transferase | Yes (Cj1422c) | NA |
The localization of CG8486 G·C homopolymeric tracts was compared with the localization in the NCTC 11168 and RM1221 genomes. NA, not applicable (genes in the NCTC 11168 and RM1221 genomes that are absent in CG8486).
C. jejuni CG8486 genes absent from the NCTC 11168 reference genome (see Table 2).
Surface-exposed carbohydrate loci.
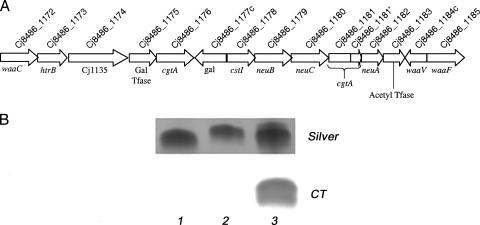
The genes encoding the three surface-exposed carbohydrate structures of C. jejuni, the LOS, capsule, and flagellin glycosylation structures, are the most variable regions of the chromosome (9, 32, 38), and CG8486 also shows divergence in these regions. The LOS gene cluster of CG8486 is shown in Fig. 2A, and the genes are listed in Table 4. Based on the recently described genotyping scheme of Parker et al. (29), CG8486, like 81-176, belongs to class B, a class of organisms containing sialic acid biosynthetic genes. Full-length pairwise alignment yielded 85.1% identity between the loci of C. jejuni CG8486 and ATCC 43449, the class B prototype strain. The C. jejuni CG8486 LOS locus is composed of 15 ORFs from Cj8486_1172 (waaC) to Cj8486_1185 (waaF) spanning 13.9 kb. Compared to the class B prototype strain, CG8486 has several interesting changes. There are two copies of GalNAc transferase genes, Cj8486_1176 and Cj8486_1181/Cj8486_1181′, the latter of which appears to be out of frame due to slipped-strand mispairing at a run of G residues. The full-length ORF (Cj8486_1176) encodes a protein with 34% sequence identity at the N-terminal end with the product of Cj8486_1181; the C-terminal end of the product of Cj8486_1176, however, shows only 11% identity with the Cj8486_1181′ product. There are no other G·C tracts in any of the other LOS genes. Figure 2B shows that the LOS core of CG8486 migrates at a higher electrophoretic Mr than that of 81-176 and that the core of CG8486 does not bind cholera toxin, indicating that it lacks GM1 ganglioside mimicry.
FIG. 2.
LOS region of CG8486. (A) Diagram of the LOS genes. Each gene name is indicated below the arrow, and the CG8486 locus tag is indicated above the arrow. The cgtA gene between neuC and neuA apparently undergoes slipped-strand mismatch repair at the site indicated by the vertical line. (B) Characterization of the LOS core of CG8486 (lane 2) by silver staining and binding to cholera toxin. The cores of 81-176 (Parker class B) (lane 1), which is a mixture of GM2 and GM3 ganglioside mimics (29), and OH4384 (lane 3), which expresses a GM1 (11) mimic, are compared. CT, cholera toxin.
TABLE 4.
Gene content of C. jejuni CG8486 LOS locus
| Gene locus | Gene name | Protein size (residues) | Function |
|---|---|---|---|
| Cj8486_1172 | waaC | 342 | Putative lipopolysaccharide heptosyltransferase |
| Cj8486_1173 | htrB | 274 | Putative lipid A biosynthesis lauroyl acyltransferase |
| Cj8486_1174 | Cj1135 | 515 | Putative two-domain glycosyltransferase |
| Cj8486_1175 | Cj1136 | 293 | Putative galactosyltransferase |
| Cj8486_1176 | cgtA | 352 | β-1,4-N-Acetylgalactosaminyltransferase |
| Cj8486_1177c | Cj1139c | 297 | Putative galactosyltransferase |
| Cj8486_1178 | cstI | 247 | α-2,3-Sialyltransferase |
| Cj8486_1179 | neuB | 346 | N-Acetylneuraminic acid synthetase |
| Cj8486_1180 | neuC | 374 | Putative N-acetylglucosamine-6-phosphate 2-epimerase |
| Cj8486_1181 | cgtA | 205 | β-1,4-N-Acetylgalactosaminyltransferase |
| Cj8486_1181′ | cgtA | 110 | β-1,4-N-Acetylgalactosaminyltransferase |
| Cj8486_1182 | neuA | 226 | CMP-Neu5Ac synthetase |
| Cj8486_1183 | 210 | Sialate-O-acetyltranferase | |
| Cj8486_1184c | waaV | 298 | Putative glucosyltransferase |
| Cj8486_1185 | waaF | 345 | ADP-heptose-lipopolysaccharide heptosyltransferase |
The strain-specific capsule locus, located between kpsC and kpsF, is 26 kb long, a size similar to the size of the 81-176 locus but smaller than the NCTC 11168 locus. Most of the genes encode proteins with similarity to other proteins involved in sugar synthesis or transferases. The capsule structure of CG8486, which was identified as a member of the HS4 complex, is being determined (M. A. Monteiro et al., unpublished data), and a full discussion of the capsule locus will be included in that study.
The flagellin glycosylation locus of CG8486 appears to be very similar to that of NCTC 11168, but the region was located on five different contigs, as sequenced. This likely reflects the high degree of repetitive DNA known to be present in this locus and highlights a limitation of this sequencing technique. There is, however, evidence that strain CG8486 contains genes for synthesis of pseudaminic acid (the pse pathway) (13, 15, 40) and genes for synthesis of legionaminic acid (24) (the ptm pathway). Strain NCTC 11168 also has both pathways (31), but 81-176 lacks the legionaminic acid pathway (15).
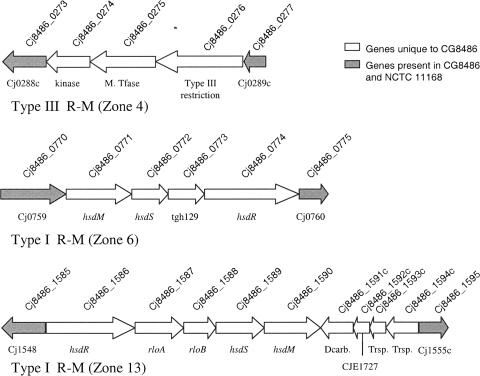
DNA R-M genes.
C. jejuni NCTC 11168 contains one complete type I system and one putative type II system for R-M. An additional type I R-M system has been described for the newly sequenced 81-176 genome (17) In contrast, two complete type I R-M systems and one type III R-M system are present in CG8486, as summarized in Fig. 3. The first type I system occurs in zone 6 between Cj0759 and Cj0760, the same physical location as the additional type I R-M system of 81-176. The only two genes found in both CG8486 and 81-176 that are absent from the NCTC 11168 genome are located in this zone. These genes code for the subunit HsdM and HsdR (Cj8486_0771 and Cj8486_0774, respectively, for CG8486 and cju16 and cju20, respectively, for 81-176). Since the subunit HsdS is different, it is likely that this R-M system has different DNA recognition patterns in strains CG8486 and 81-176.
FIG. 3.
Schematic representation of C. jejuni CG8486 R-M loci. Zone 4 corresponds to a type III R-M system. In the CG8486 genome this locus appears to be localized between the genes corresponding to Cj0288c and Cj0289c of NCTC 11168. Zone 6 corresponds to a type I R-M system localized between the genes corresponding to Cj0759 and Cj0760 of NCTC 11168. Zone 13 corresponds to a type I R-M system and replaces the NCTC 11168 type I R-M system located between Cj1548 and Cj1555. M. Tfase, DNA methyltransferase; Dcarb., putative 4-carboxymuconolactone decarboxylase; Trsp., putative transporter.
The second type I R-M system is located between Cj1548 and Cj1555 (zone 13 in Fig. 3) and replaces the corresponding NCTC 11168 ORFs that are missing in the CG8486 genome. This is part of the commonly reported hypervariable Cj1543c-Cj1563 region (38).
In addition, C. jejuni CG8486 also has a potential functional type III R-M system between Cj0288c and Cj0289c (zone 4, including Cj8486_274 to Cj8486_276 [Fig. 3]). This locus contains three ORFs with high similarity to ORFs found in C. upsaliensis strain RM3195 and is at the same chromosomal location (adjacent to lpxB). Cj8486_0275 and Cj8486_0276 are similar to an adenine-specific DNA methyltransferase of Helicobacter pylori 26695 (47% identity) and CPU0800, a type III restriction enzyme R protein (64% identity). In addition to these R-M loci the C. jejuni CG8486 genome has alleles encoding two putative DNA methylases found in NCTC 11168, Cj0208 (Cj8486_0205) and Cj1461 (Cj8486_1499), and an additional putative methylase that replaces Cj0260 (Cj8486_0245; zone 3). C. jejuni RM1221 also contains the latter insertion (CJE0310), which is 97% identical to Cj8486_0245. The protein encoded by this gene has a high level of identity with a putative N6 adenine-specific DNA methyltransferase and also has a high degree of conservation with the homolog in H. pylori.
Natural transformation assays have shown that CG8486 can be readily transformed with DNA from 81-176 and can be readily mutated by in vitro transposition of its own DNA (C. P. Ewing and P. Guerry, unpublished data). However, electroporation and natural transformation of CG8486 DNA cloned in Escherichia coli are greatly reduced compared to electroporation and natural transformation of DNA of other C. jejuni strains (data not shown), consistent with the extensive repertoire of R-M genes present in this strain.
Degenerate virulence genes.
CG8486 contains remnants of two potential virulence genes that have apparently degenerated. Seven small ORFs with unknown functions (Cj0968 to Cj0974) in NCTC 11168 are replaced by four ORFs in CG8486 (zone 8 containing Cj8486_1110 to Cj8486_1113c), an organization that was confirmed by conventional sequencing of this region. These CG8486 ORFs showed significant identity with ORFs encoding different domains of a protein annotated as a filamentous hemagglutinin in C. lari RM2100 (CLA0151; 1,085 amino acids; 119 kDa). The bordering genes, Cj0967 to Cj0975, also appear to be pseudogenes in CG8486 (Cj8486_1106c and Cj8486_1114c, respectively).
Four ORFS located between Cj1359 and Cj1361 (zone 11, including Cj8486_1401 to Cj8486_1404) code for proteins that have identity with different domains of VacA of C. coli RM2228 and H. pylori. Similar genes were also found in C. jejuni strains 260.94 and RM1221 (CJE1550-CJE1551 and CJE1552). VacA is a toxin that is secreted by a type V secretion mechanism from H. pylori (39) and has three functional domains: an N-terminal signal sequence, a passenger domain, and an autotransporter domain in the C terminus. ORFs Cj8486_1401 to Cj8486_1404 of C. jejuni CG8486 encode putative proteins that share similarities with putative VacA proteins of C. coli RM2228 and C. jejuni 260.94 and the autotransporter domain of VacA from H. pylori.
The presence of the hemagglutinin and a VacA-like gene provides additional evidence that C. jejuni has exchanged genes with other pathogens, including H. pylori (28), but the horizontally acquired genes appear to have lost function.
Antibiotic and heavy metal resistance genes.
Like C. jejuni RM1221 and C. coli RM2228, C. jejuni CG8486 contains a putative arsenic resistance operon that appears to be in the same physical location between Cj1560 and Cj1563 on the NCTC 11168 chromosome (zone 14, including Cj8486_1598 through Cj8486_1600; CJE1730 to CJE1733 in the RM1221 genome). This operon contains homologs of arsR, encoding a putative repressor, arsB, encoding a putative efflux pump protein, and arsC, encoding an arsenate reductase (genes Cj8486_1598, Cj8486_1599, and Cj8486_1600, respectively). It seems that NCTC 11168 has a degenerate relic of this operon. It is interesting that the two C. jejuni strains that contain putative arsenate resistance genes, RM1221 and CG8486, were isolated in the western United States and Thailand, respectively, areas reported by the World Health Organization to have water contaminated with arsenic at levels of >0.01 mg/liter (http://www.who.int/mediacenter/factsheets/fs210/en/index.html).
Plasmid sequences.
A total of 12 contigs showed a high degree of similarity with the pTet conjugative R plasmid of C. jejuni 81-176 (5). These contigs cover 37 kb of the 45.2 kb of pTet, and experimental analysis confirmed the presence of an approximately 45-kb plasmid in CG8486 (data not shown). The presence of a tetO gene on the CG8486 plasmid is consistent with the observed resistance of this strain to tetracycline and with the reported high incidence of tetracycline resistance among C. jejuni strains from Asia (26). The cpp2, cpp3, cpp4, cpp6, cpp7, cpp24, cpp25, and cmgB7 genes, which code for proteins with unknown functions, and repA, encoding a putative replication initiator protein, appear to be missing from the CG8486 plasmid, as sequenced. In addition, the CG8486 plasmid has an additional 627-bp ORF between cpp23 and cpp26 (c351.07). This ORF codes for a protein that is similar to a conserved, hypothetical protein of 81-176 (CJJ81176_0778). The CG8486 plasmid contains all of the genes involved in a putative type IV conjugation system of pTet.
Conclusions.
High-throughput pyrosequencing is a powerful technology for rapidly sequencing bacterial genomes (23). When a high level of redundancy of sequence data is obtained (in this study, chromosomal DNA was sequenced to an average of about 30 sequence reads at each nucleotide position), accurate de novo assembly of the nonrepetitive parts of the genome allows identification of novel genes. The major aim of this study was to use this technology to sequence the genome of the CG8486 strain, which caused an inflammatory diarrhea similar to that seen with 81-176 but which was markedly less invasive than 81-176. Hofreuter et al. (17) identified a number of 81-176-specific genes. Moreover, they demonstrated that mutation of a number of these genes (dmsA, which encodes a putative dimethyl sulfoxide reductase; cytC, which encodes a component of a cytochrome c oxidase; and ggt, encoding γ-glutamyltranspeptidase) failed to reduce invasion but suggested that some of these 81-176 unique genes may make the strain more robust in vivo by counteracting oxidative stress. However, none of these genes are found in CG8486. This suggests that genes necessary for induction of inflammatory diarrhea are among the genes shared by 81-176 and GC8486 and that the reasons for the higher level of in vitro invasion by 81-176 remain obscure (17). Collectively, multiple genome data indicate that the major genetic differences among C. jejuni strains are in the three carbohydrate loci (10, 17, 31, 33, 34), and it may be that differences in these surface antigens affect virulence both in vivo and in vitro. However, the possibility that more subtle changes in the nucleotide sequences of genes common to Campylobacter strains may also modulate pathogenesis cannot be ruled out.
Supplementary Material
Acknowledgments
We thank Eva Nielsen for serotyping, Gary Majam, Dawn Pattarini, and Cheryl P. Ewing for technical assistance, and Lorrin W. Pang and Chittima Pitarangsi of the Armed Forces Research Institute of Medical Sciences in Bangkok for their microbiology expertise and assistance during the Cobra Gold exercise. We also thank the medical staff during the exercise. Nichole Nolan and Shannon Lentz prepared the genomic library and performed Genome Sequencer 20 sequencing runs.
This work was funded by Military Infectious Disease Research Program work units 6000.RAD1.DA3.A0308 and 643807A.849.D.A0002.
The clinical data and specimen isolate analyzed in this study were derived from a research protocol (DOD 30596) approved by the institutional review board at the Naval Medical Research Center in compliance with all applicable federal regulations governing the protection of human subjects. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Navy, the Department of Defense, or the United States Government. This work was prepared as part of official duties of the government employees. Title 17 U.S.C. 101 defines United States Government work as work prepared by a military service member or employee of the United States Government as part of that person's official duties.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 16 April 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bacon, D. J., R. A. Alm, D. H. Burr, L. Hu, D. J. Kopecko, C. P. Ewing, T. J. Trust, and P. Guerry. 2000. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 68:4384-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. [DOI] [PubMed] [Google Scholar]
- 3.Baqar, S., A. L. Bourgeois, L. A. Applebee, A. S. Mourad, M. T. Kleinosky, Z. Mohran, and J. R. Murphy. 1996. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect. Immun. 64:4933-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baqar, S., B. Rice, L. Lee, A. L. Bourgeois, A. N. El Din, D. R. Tribble, G. P. Heresi, A. S. Mourad, and J. R. Murphy. 2001. Campylobacter jejuni enteritis. Clin. Infect. Dis. 33:901-905. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor, R. A., B. M. Pearson, L. M. Friis, P. Guerry, and J. M. Wells. 2004. Nucleotide sequences and comparison of two large conjugative plasmids from different Campylobacter species. Microbiology 150:3507-3517. [DOI] [PubMed] [Google Scholar]
- 6.Bereswill, S., and M. Kist. 2003. Recent developments in Campylobacter pathogenesis. Curr. Opin. Infect. Dis. 16:487-491. [DOI] [PubMed] [Google Scholar]
- 7.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 8.Blaser, M. J. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176(Suppl. 2):S103-S105. [DOI] [PubMed] [Google Scholar]
- 9.Dorrell, N., J. A. Mangan, K. G. Laing, J. Hinds, D. Linton, H. Al-Ghusein, B. G. Barrell, J. Parkhill, N. G. Stoker, A. V. Karlyshev, P. D. Butcher, and B. W. Wren. 2001. Whole genome comparison of Campylobacter jejuni human isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 11:1706-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouts, D. E., E. F. Mongodin, R. E. Mandrell, W. G. Miller, D. A. Rasko, J. Ravel, L. M. Brinkac, R. T. DeBoy, C. T. Parker, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, S. A. Sullivan, J. U. Shetty, M. A. Ayodeji, A. Shvartsbeyn, M. C. Schatz, J. H. Badger, C. M. Fraser, and K. E. Nelson. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple campylobacter species. PLoS Biol. 3:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert, M., M. F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A. M. Cunningham, and W. W. Wakarchuk. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277:327-337. [DOI] [PubMed] [Google Scholar]
- 12.Goon, S., C. P. Ewing, M. Lorenzo, D. Pattarini, G. Majam, and P. Guerry. 2006. A σ28-regulated nonflagella gene contributes to virulence of Campylobacter jejuni 81-176. Infect. Immun. 74:769-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goon, S., J. F. Kelly, S. M. Logan, C. P. Ewing, and P. Guerry. 2003. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol. Microbiol. 50:659-671. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths, P. L., and R. W. Park. 1990. Campylobacters associated with human diarrhoeal disease. J. Appl. Bacteriol. 69:281-301. [DOI] [PubMed] [Google Scholar]
- 15.Guerry, P., C. P. Ewing, M. Schirm, M. Lorenzo, J. Kelly, D. Pattarini, G. Majam, P. Thibault, and S. Logan. 2006. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 60:299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerry, P., C. M. Szymanski, M. M. Prendergast, T. E. Hickey, C. P. Ewing, D. L. Pattarini, and A. P. Moran. 2002. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofreuter, D., J. Tsai, R. O. Watson, V. Novik, B. Altman, M. Benitez, C. Clark, C. Perbost, T. Jarvie, L. Du, and J. E. Galan. 2006. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect. Immun. 74:4694-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu, L., and D. J. Kopecko. 1999. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect. Immun. 67:4171-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Institute of Laboratory Animals Resources. 1996. Guide for the care and use of laboratory animals. Institute of Laboratory Animals Resources, National Research Council, National Academy Press, Washington, DC.
- 19.Korlath, J. A., M. T. Osterholm, L. A. Judy, J. C. Forfang, and R. A. Robinson. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 152:592-596. [DOI] [PubMed] [Google Scholar]
- 20.Legrand, D., E. Elass, M. Carpentier, and J. Mazurier. 2005. Lactoferrin: a modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 62:2549-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard, E. E., T. Takata, M. J. Blaser, S. Falkow, L. S. Tompkins, and E. C. Gaynor. 2003. Use of an open-reading frame-specific Campylobacter jejuni DNA microarray as a new genotyping tool for studying epidemiologically related isolates. J. Infect. Dis. 187:691-694. [DOI] [PubMed] [Google Scholar]
- 22.Leonard, E. E., L. S. Tompkins, S. Falkow, and I. Nachamkin. 2004. Comparison of Campylobacter jejuni isolates implicated in Guillain-Barre syndrome and strains that cause enteritis by a DNA microarray. Infect. Immun. 72:1199-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margulies, M., M. Egholm, W. E. Altman, S. Attiya, J. S. Bader, L. A. Bemben, J. Berka, M. S. Braverman, Y. J. Chen, Z. Chen, S. B. Dewell, L. Du, J. M. Fierro, X. V. Gomes, B. C. Godwin, W. He, S. Helgesen, C. H. Ho, G. P. Irzyk, S. C. Jando, M. L. Alenquer, T. P. Jarvie, K. B. Jirage, J. B. Kim, J. R. Knight, J. R. Lanza, J. H. Leamon, S. M. Lefkowitz, M. Lei, J. Li, K. L. Lohman, H. Lu, V. B. Makhijani, K. E. McDade, M. P. McKenna, E. W. Myers, E. Nickerson, J. R. Nobile, R. Plant, B. P. Puc, M. T. Ronan, G. T. Roth, G. J. Sarkis, J. F. Simons, J. W. Simpson, M. Srinivasan, K. R. Tartaro, A. Tomasz, K. A. Vogt, G. A. Volkmer, S. H. Wang, Y. Wang, M. P. Weiner, P. Yu, R. F. Begley, and J. M. Rothberg. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNally, D. J., A. J. Aubrey, J. P. Hui, N. H. Khieu, D. Whitfield, C. P. Ewing, P. Guerry, J. R. Brisson, S. M. Logan, and E. C. Soo. 19 March 2007. Targeted metabolomics analysis of Campylobacter coli VC167 reveals legionaminic acid derivatives as novel flagellar glycans. J. Biol. Chem. doi: 10.1074/jbc.M611027200. [DOI] [PubMed]
- 25.Munoz, C., S. Baqar, L. van de Verg, J. Thupari, S. Goldblum, J. G. Olson, D. N. Taylor, G. P. Heresi, and J. R. Murphy. 1995. Characteristics of Shigella sonnei infection of volunteers: signs, symptoms, immune responses, changes in selected cytokines and acute-phase substances. Am. J. Trop. Med. Hyg. 53:47-54. [PubMed] [Google Scholar]
- 26.Nirdnoy, W., C. J. Mason, and P. Guerry. 2005. Mosaic structure of a multiple-drug-resistant, conjugative plasmid from Campylobacter jejuni. Antimicrob. Agents Chemother. 49:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 90:6884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyarzabal, O. A., R. Rad, and S. Backert. 2007. Conjugative transfer of chromosomally encoded antibiotic resistance from Helicobacter pylori to Campylobacter jejuni. J. Clin. Microbiol. 45:402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker, C. T., S. T. Horn, M. Gilbert, W. G. Miller, D. L. Woodward, and R. E. Mandrell. 2005. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J. Clin. Microbiol. 43:2771-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker, C. T., B. Quinones, W. G. Miller, S. T. Horn, and R. E. Mandrell. 2006. Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J. Clin. Microbiol. 44:4125-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 32.Pearson, B. M., C. Pin, J. Wright, K. I'Anson, T. Humphrey, and J. M. Wells. 2003. Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett. 554:224-230. [DOI] [PubMed] [Google Scholar]
- 33.Poly, F., D. Threadgill, and A. Stintzi. 2004. Identification of Campylobacter jejuni ATCC 43431-specific genes by whole microbial genome comparisons. J. Bacteriol. 186:4781-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poly, F., D. Threadgill, and A. Stintzi. 2005. Genomic diversity in Campylobacter jejuni: identification of C. jejuni 81-176-specific genes. J. Clin. Microbiol. 43:2330-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell, R. G., M. J. Blaser, J. I. Sarmiento, and J. Fox. 1989. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect. Immun. 57:1438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Sanders, J. W., D. W. Isenbarger, S. E. Walz, L. W. Pang, D. A. Scott, C. Tamminga, B. A. Oyofo, W. C. Hewitson, J. L. Sanchez, C. Pitarangsi, P. Echeverria, and D. R. Tribble. 2002. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: presentation and outcome of Campylobacter infection. Am. J. Trop. Med. Hyg. 67:533-538. [DOI] [PubMed] [Google Scholar]
- 38.Taboada, E. N., R. R. Acedillo, C. D. Carrillo, W. A. Findlay, D. T. Medeiros, O. L. Mykytczuk, M. J. Roberts, C. A. Valencia, J. M. Farber, and J. H. Nash. 2004. Large-scale comparative genomics meta-analysis of Campylobacter jejuni isolates reveals low level of genome plasticity. J. Clin. Microbiol. 42:4566-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Telford, J. L., A. Covacci, P. Ghiara, C. Montecucco, and R. Rappuoli. 1994. Unravelling the pathogenic role of Helicobacter pylori in peptic ulcer: potential new therapies and vaccines. Trends Biotechnol. 12:420-426. [DOI] [PubMed] [Google Scholar]
- 40.Thibault, P., S. M. Logan, J. F. Kelly, J. R. Brisson, C. P. Ewing, T. J. Trust, and P. Guerry. 2001. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276:34862-34870. [DOI] [PubMed] [Google Scholar]
- 41.Wassenaar, T. M., and M. J. Blaser. 1999. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1:1023-1033. [DOI] [PubMed] [Google Scholar]
- 42.Yao, R., D. H. Burr, and P. Guerry. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 23:1021-1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.