Abstract
While most vaccines consisting of killed bacteria induce high serum antibody titers, they do not always confer protection as effective as that induced by infection, particularly against mucosal pathogens. Bordetella bronchiseptica is a gram-negative respiratory pathogen that is endemic in many nonhuman mammalian populations and causes substantial disease in a variety of animals. At least 14 different live attenuated vaccines against this pathogen are available for use in a variety of livestock and companion animals. However, there are few published data on the makeup or efficacy of these vaccines. Here we report the use of a genetically engineered double mutant of B. bronchiseptica, which lacks adenylate cyclase and type III secretion, as a vaccine candidate. This strain is safe at high doses, even for highly immunocompromised animals, and induces immune responses that are protective against highly divergent B. bronchiseptica strains, preventing colonization in the lower respiratory tract and decreasing the bacterial burden in the upper respiratory tract. This novel B. bronchiseptica vaccine candidate induces strong local immunity while eliminating damage caused by the two predominant cytotoxic mechanisms.
The lower respiratory tract has a well-developed immunological surveillance system which, during health, maintains this area as a sterile environment despite frequent exposure to microorganisms. However, some microorganisms specialize in infecting the mammalian respiratory tract, suggesting that they have evolved ways to modulate or avoid host defense mechanisms. Since respiratory infections are a major source of morbidity and mortality, the development of vaccines that can protect against these infectious organisms is a priority. Historically, vaccination strategies have focused on the development of strong serum antibody titers as an indicator of efficacy, but serum antibody titers do not always correlate with protection, particularly against mucosal pathogens. While parenteral vaccination against respiratory pathogens often protects against disease, it does not always prevent infection. Additionally, immunity induced by a bolus injection often wanes, leaving individuals susceptible to disease. In many cases, protective immunity generated in response to local mucosal infection is more effective and longer lasting than that generated in response to parenteral immunization (reviewed in references 18 and 19).
Bordetella spp. efficiently and rapidly colonize ciliated respiratory epithelium and are able to persist within the host respiratory tract for several weeks (15). The mouse model provides an ideal system with which to study the potential use of live vaccines in a vigorous infection model in which both the pathogen and host immunity can be manipulated experimentally (7). Attenuated strains of Bordetella bronchiseptica have been used as live vaccines in a variety of domesticated mammals, with limited data on safety and efficacy (reviewed in reference 28). However, the molecular basis for attenuation is either unknown or unpublished. Since the genetic mutations in these strains have not been elucidated, the possibility of reversion to a more virulent form cannot be ruled out, particularly with the wide variety of hosts, environments, exacerbating conditions, and coinfections that may be encountered with their use. As a result, more recent studies have focused on the use of attenuated strains of B. bronchiseptica with stable and genetically defined mutations as live vaccines and as vectors for heterologous antigens (1, 16, 24, 26, 27). Studies using dogs and current live attenuated B. bronchiseptica vaccines have shown mixed results. One study done by Jacobs et al. showed partial protection (11), while other studies indicated that several live attenuated B. bronchiseptica vaccines are ineffectual and cause adverse effects (4).
Since protection is often associated with immune responses to virulence factors, efforts have been focused on generating mutants with metabolic defects which alter the ability to survive in vivo but allow for expression of virulence factors (reviewed in references 22 and 28). The best-studied B. bronchiseptica vaccine strain with defined mutations has a disruption in aroA, which encodes a synthase crucial to the production of aromatic amino acids (27). The aroA mutant is considerably less efficient at colonizing the respiratory tracts of mice, as it is cleared by day 8 postinoculation, than its wild-type parent, which persists until at least day 28 postinoculation (27). Although this mutant seems to generate protective immunity against the parental strain, the anti-B. bronchiseptica titers in mice infected with the mutant strain were 1/100 those of mice infected with the wild-type strain, suggesting that optimal antibody production requires efficient colonization (16). Stevenson and Roberts recently demonstrated the potential of using the aroA mutant to deliver a fragment of the tetanus toxoid (FrgC), as both humoral and mucosal antibody responses to tetanus toxoid were measurable (26). However, this vaccine protected only a minority of mice from challenge with lethal doses of toxin. A vector that is more efficient in colonization and thus induces a more robust immune response might prove to be more efficacious. Here we describe a genetically modified strain of B. bronchiseptica which is nonpathogenic even in highly susceptible mouse strains and provides antibody titers and protection against infection that are similar to those induced by infection with the wild-type bacterium.
MATERIALS AND METHODS
Bacteria.
Bacteria were maintained on Bordet-Gengou agar (Difco) with 10% defibrillated sheep's blood, inoculated into Stainer-Scholte broth at optical densities of 0.1 or lower, and grown to mid-log phase at 37°C on a roller drum. Wild-type strains of B. bronchiseptica (RB50), B. parapertussis (12822), and B. pertussis (BP536) have been described previously (2, 9, 23). B. bronchiseptica strains 1127 and 253 were kind gifts from Robert Livingston at the University of Missouri Research Animal Diagnostic Laboratory. The sequence types of strains 1127 and 253 were determined by sequencing of seven housekeeping genes as previously described (3). The construction of B. bronchiseptica strain AVS, an RB50-derived mutant which lacks adenylate cyclase and the ATPase necessary for type III secretion, has been described previously (7). In brief, an isogenic mutant of RB50 containing an in-frame deletion of the adenylate cyclase toxin gene (cyaA) (7), was constructed with an in-frame deletion of the ATPase (bscN) gene required for type III secretion (32).
Animal experiments.
Wild-type (WT) BALB/c, C3H/HeJ (Toll-like receptor 4 deficient [TLR4def]), and BL/6.129 tumor necrosis factor alpha-knockout (TNF-α−/−) mice were obtained from The Jackson Laboratory. Mice were maintained and treated at the Pennsylvania State University in accordance with IACUC and approved institutional guidelines. Mice were lightly sedated with isoflurane (Abbott Laboratories) and inoculated by pipetting 50 μl of phosphate-buffered saline (PBS) containing the indicated dose of bacteria onto the tip of the external nares. This method reliably distributes the bacteria throughout the respiratory tract, as determined by dissection at 10 min postinoculation (6, 10, 12, 13, 20, 25, 32). For survival curves, groups of 10 mice were infected with the indicated bacteria and euthanized when they displayed signs of deteriorating lethal bordetellosis, which include ruffled fur, hunched backs, and labored breathing, in order to alleviate unnecessary suffering. For time course experiments, groups of four animals were sacrificed at the indicated time points after inoculation. Colonization of respiratory organs was quantified by homogenization of each tissue in PBS, plating onto Bordet-Gengou blood agar containing 20 μg of streptomycin per ml, and colony counting. Low-dose intranasal vaccinations were performed by pipetting approximately 5 μl of PBS containing 100 CFU of either RB50 or the double mutant AVS onto the external nares. Reinfections with the indicated bacteria occurred at 49 days post-primary infection or postvaccination. For passive transfer experiments, WT mice were inoculated with 5 × 105 CFU of B. bronchiseptica strain RB50 or AVS as described above, and sera were collected on day 49 postinoculation. Two hundred microliters of pooled convalescent-phase serum was injected intraperitoneally into mice immediately before inoculation.
Lung histology.
For lung histology, the trachea and lungs were excised and inflated with 10% formaldehyde. The lungs were then sectioned and stained with hematoxylin and eosin at the Animal Diagnostic Laboratory at the Pennsylvania State University. The sections were scored for pathology by a veterinarian with training and experience in rodent pathology who was blinded to experimental treatment. A score of 0 indicates no noticeable inflammation or lesions; a score of 1 indicates few or scattered foci affecting less than 10% of the tissue, typically with a few mild perivascular and/or peribronchial lymphoid aggregates; a score of 2 indicates frequent mild perivascular and/or peribronchial lymphoid aggregates, with or without an occasional small focus of pneumonia, with overall inflammation affecting no more than 10 to 20% of the tissue; a score of 3 indicates moderate lesions, typically with abundant perivascular and peribronchial lymphoid infiltrates and multiple mild to moderate foci of pneumonia, with inflammation affecting approximately 20 to 30% of the tissue; and a score of 4 indicates extensive pneumonia and marked inflammation affecting more than 30% of the tissue.
ELISAs.
Titers of anti-Bordetella antibody in convalescent-phase sera or lung homogenates were determined by enzyme-linked immunosorbent assay (ELISA). In brief, 96-well plates with adhered heat-killed RB50 were probed with the indicated convalescent-phase serum or lung homogenate. The serum or homogenate was serially diluted in 1:2 ratios across the plate. The end-point titer was determined by comparison to similarly treated naïve serum or naïve lung homogenate. Specific classes and isotypes of antibodies were determined by using appropriate horseradish peroxidase-conjugated goat anti-mouse antibodies (Southern Biotechnology Associates and Pharmingen).
Splenocyte restimulation.
Spleens were harvested from groups of three or four naïve, RB50-infected, or AVS-infected WT mice at 28 days postinoculation. Spleens were homogenized through a screen in RPMI medium, and the red blood cells were lysed by treatment with 0.83% NH4Cl. Splenocytes were left unstimulated or stimulated in triplicate with heat-killed RB50 (multiplicity of infection [MOI] of 5), and the supernatants were harvested after 3 days (21). The concentrations of gamma interferon (IFN-γ), interleukin-10 (IL-10), and IL-4 were quantified by sandwich ELISAs using appropriate paired antibodies specific for each cytokine per the manufacturer's directions (R&D Systems).
Statistical analysis.
For all experiments, statistical significance was determined using Student's t test. P values of ≤0.05 are indicated with asterisks.
RESULTS
AVS is avirulent in susceptible mouse strains.
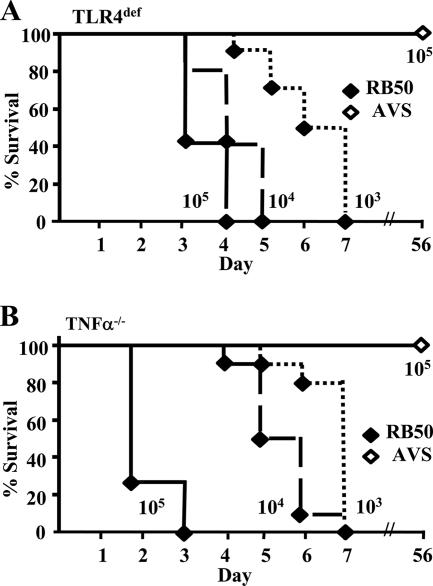
The deletion of adenylate cyclase and the ATPase necessary for type III secretion results in the ablation of in vitro cytotoxicity by AVS compared to that of the parental WT strain, RB50 (7, 29), suggesting that this mutant may have attenuated virulence during infection. However, this mutant bacterium has not previously been examined in vivo. To determine if AVS is less virulent during infection, we examined its ability to cause lethal disease in immunocompromised mice lacking TLR4 or TNF-α. WT B. bronchiseptica infection in immunocompromised mice has been described previously, and the roles of TLR4 and TNF-α in Bordetella infection have been well characterized (13, 14). As such, these mice were used to determine the safety of AVS in a known susceptible model. We intranasally inoculated TLR4def and TNF-α−/− mice with 103, 104, or 105 CFU of RB50 or 105 CFU of AVS in a 50-μl inoculum and observed them for signs of severe disease. WT mice given similar doses of RB50 are able to control the disease and to clear bacteria from the lower respiratory tract (12). As previously observed, TLR4def mice and TNF-α−/− mice rapidly developed signs of bordetellosis, including ruffled fur and hunched backs, and succumbed within 7 days following inoculation with as little as 103 CFU of RB50 (Fig. 1). However, TLR4def mice and TNF-α−/− mice failed to develop signs of bordetellosis following inoculation with 105 CFU of AVS (Fig. 1) and eliminated bacteria from the lower respiratory tract by day 49 postinoculation (data not shown). Therefore, all reinfection experiments were done on day 49 postinoculation.
FIG. 1.
Lethality of B. bronchiseptica strains in susceptible mice. Groups of 5 to 10 (A) TLR4def or (B) TNF-α−/− mice were inoculated intranasally with approximately 5 × 103, 5 × 104, or 5 × 105 CFU of RB50 or 5 × 105 CFU of AVS in a 50-μl volume, as indicated.
AVS induces less pathology in susceptible mouse strains.
To determine if reduced mortality correlated with decreased pathology, we excised the lungs of mice inoculated with 5 × 105 CFU of RB50 or AVS on day 3 postinoculation and examined hematoxylin- and eosin-stained sections. The lungs of WT mice infected with RB50 showed a mean pathology score of 2.6, while those infected with AVS received a score of 1.8 (Fig. 2A). The lungs of TLR4def mice and TNF-α−/− mice infected with RB50 received lung pathology scores of 3.3 and 3.6, respectively, while their counterparts infected with AVS were scored at 1.5 and 1.9 (Fig. 2A). Compared to the lungs of AVS-infected animals, the lungs of WT, TLR4def, and TNF-α−/− mice infected with RB50 showed substantially more inflamed lesions, which were predominantly neutrophilic in nature (Fig. 2B). These results suggest that diminished inflammation and lung pathology may contribute to the decreased virulence of AVS in susceptible mouse strains.
FIG. 2.
Lung pathology in susceptible mouse strains. Groups of four to six WT, TLR4def, or TNF-α−/− mice were inoculated intranasally with approximately 5 × 105 CFU of either RB50 or AVS in a 50-μl volume, as indicated. On day 3 postinoculation, the trachea and lungs were excised, inflated with 10% formaldehyde, sectioned, stained, and examined by a veterinary pathologist blinded to experimental treatment. (A) Pathology scores. (B) Lung histology pictures. *, P value of <0.05.
AVS protects susceptible mouse strains against reinfection.
The ability of TLR4def and TNF-α−/− mice to eliminate AVS from the lower respiratory tract suggests that these mice are able to generate adaptive immunity to this organism. To test whether this provided protection against subsequent infection with WT B. bronchiseptica, we challenged convalescent and naïve TLR4def and TNF-α−/− mice with 5 × 105 CFU of RB50 and determined bacterial numbers in the respiratory organs on day 3 postinoculation. The nasal cavities of convalescent TLR4def and TNF-α−/− mice contained approximately 104 CFU, whereas the same organ in naïve mice contained 108 CFU on day 3 postchallenge (Fig. 3). Similarly, the bacterial numbers present in convalescent TLR4def and TNF-α−/− mice were near or below the lower limit of detection (∼10 CFU) in the trachea and lungs, while naïve mice harbored approximately 107 CFU in the trachea and 109 CFU in the lungs. Unlike naïve TLR4def and TNF-α−/− mice, which eventually succumb to infection with RB50, mice previously infected with AVS did not develop lethal disease following challenge with RB50 (data not shown). These results indicate that previous infection with AVS generates adaptive immunity which is capable of limiting bacterial colonization of susceptible mice.
FIG. 3.
AVS protection in susceptible mouse strains. Groups of four (A) TLR4def or (B) TNF-α−/− mice were inoculated intranasally with approximately 5 × 105 CFU of AVS in a 50-μl volume. On day 49 postinoculation, the mice were then challenged with approximately 5 × 105 CFU of RB50 in a 50-μl volume. On day 52, the mice were sacrificed, and the numbers of RB50 CFU in the nasal cavity, trachea, and lungs were measured. The dashed line indicates the limit of detection. *, P value of <0.05. Error bars indicate standard errors.
AVS is defective in colonizing the lower but not the upper respiratory tract.
In order to examine the usefulness of AVS as a vaccine strain, we sought to better characterize infection of WT mice with this strain. To determine the ability of B. bronchiseptica strain AVS to colonize the respiratory tracts of mice compared to that of the WT parental strain RB50, we intranasally inoculated WT mice with 5 × 105 CFU of either RB50 or AVS in a 50-μl inoculum. Bacterial burdens in the respiratory organs were measured on days 0, 3, 7, 14, and 28 postinoculation. On day 3 postinoculation, the bacterial numbers of both RB50 and AVS were approximately 106 CFU in the nasal cavity, 104 CFU in the trachea, and 105 CFU in the lungs (Fig. 4). Thereafter, RB50 was recovered in numbers 10 to 1,000 times larger than those of AVS in the trachea and lungs while remaining at similar CFU in the nasal cavity. Although AVS was eliminated from the lower respiratory tract faster than RB50, it persisted in the lungs for several weeks prior to clearance by day 28 postinoculation.
FIG. 4.
AVS colonization in WT mice. Groups of four to six WT mice were inoculated intranasally with approximately 5 × 105 CFU of either RB50 or AVS in a 50-μl volume, as indicated. Bacterial numbers were measured in the nasal cavity, trachea, and lungs on days 0, 3, 7, 10, 28, and 56 postinoculation. The dashed line indicates the limit of detection. *, P value of <0.05. Error bars indicate standard errors.
Antibodies raised against AVS are protective.
We previously demonstrated that protection against B. bronchiseptica infection requires Bordetella-specific antibodies, so we investigated the ability of AVS to elicit a serum and mucosal antibody response (12). We collected sera and lung homogenates from WT mice at 49 days postinoculation with 5 × 105 CFU of RB50 or AVS and measured anti-B. bronchiseptica (RB50) titers by ELISA. The overall antibody titers generated in response to infection with AVS were similar to those generated by infection with RB50 (Fig. 5A and B). No substantial differences between RB50- and AVS-generated sera in the titers of antibody isotypes immunoglobulin G (IgG), IgG1, IgG2a, IgG2b, and IgG3 were observed (Fig. 5A and B and data not shown). These results suggest that AVS induces a humoral immune response that is similar in scope to that induced by RB50. In addition, sera and lung homogenates from AVS-infected WT mice that were reinfected with AVS at 49 days postinoculation were examined. Interestingly, the total antibody titers in the sera of the reinfected mice were similar to those for RB50-infected mice but significantly higher than those for AVS-infected mice, indicating that reinfection with AVS has a booster effect on total serum antibodies. In correlation with the booster effect, reinfected mice had significantly less Bordetella-specific serum IgM than either RB50- or AVS-infected mice. However, AVS-reinfected mice had statistically significantly higher titers of IgA in both the serum and lung homogenate (Fig. 5A and B).
FIG. 5.
AVS-induced antibody response and its effect on RB50 colonization. Groups of three or four WT mice were intranasally inoculated with approximately 5 × 105 CFU of either WT RB50 or AVS in a 50-μl volume, as indicated. A group of four WT mice were rechallenged with AVS on day 49 postinoculation. Sera (IS) (A) and lung homogenates (LH) (B) were collected on day 49 postinoculation or day 3 post-secondary challenge. B. bronchiseptica-specific antibody titers were measured by ELISA. (C) Groups of four WT mice were injected intraperitoneally with 200 μl of either naïve serum or immune serum (IS) raised against RB50 or AVS, as indicated, and then intranasally inoculated with approximately 5 × 105 CFU of RB50 in a 50-μl volume. Bacterial numbers in the lungs, trachea, and nasal cavity were measured on day 3 postinoculation and -transfer. The dashed line indicates the limit of detection. *, P value of <0.05. Error bars indicate standard errors.
We previously demonstrated that adoptive transfer of convalescent-phase sera from mice infected with RB50 is sufficient to limit infection of this organism in the lower respiratory tract (12). To measure the protective ability of serum antibodies generated by infection with AVS to protect the lower respiratory tract from colonization with WT B. bronchiseptica, we transferred 200 μl of immune serum obtained from naïve, RB50-infected, or AVS-infected animals into naïve mice and intranasally challenged the mice with 5 × 105 CFU of RB50 in a 50-μl inoculum. Mice were euthanized at 3 days postchallenge, and bacterial burdens were determined as previously described. Passive transfer of immune serum obtained from either RB50- or AVS-infected mice was able to reduce the bacterial numbers in naïve mice approximately 1,000-fold in the lungs (Fig. 5C). These results indicate that the antibodies generated in response to AVS are as protective as those generated in response to RB50.
AVS induces increased IFN-γ production but decreased IL-10 production in splenocytes.
In order to examine the T-cell response to AVS, spleens of WT mice that were naïve or inoculated with AVS or RB50 28 days previously were excised and stimulated with heat-killed B. bronchiseptica (MOI of 5). Representative Th1 cytokine (IFN-γ), anti-inflammatory cytokine (IL-10), and Th2 cytokine (IL-4) production was quantified (Fig. 6). Spleens from AVS-inoculated mice produced similar amounts of IFN-γ and IL-10. In contrast, spleens from RB50-inoculated mice produced higher levels of IL-10, associated with delayed clearance, and lower levels of IFN-γ, which is known to contribute to clearance (21). No difference was detected between any groups for IL-4 production (Fig. 6).
FIG. 6.
AVS-induced splenocyte production of IFN-γ, IL-10, and IL-4. Spleens from groups of three or four naïve, AVS-inoculated, or RB50-inoculated WT mice were restimulated in triplicate with heat-killed RB50 (MOI of 5) for 3 days. IFN-γ, IL-10, and IL-4 production was determined by sandwich ELISA. *, P value of <0.05. Error bars indicate standard errors.
Low-dose intranasal vaccination with AVS protects against subsequent heterologous B. bronchiseptica infection.
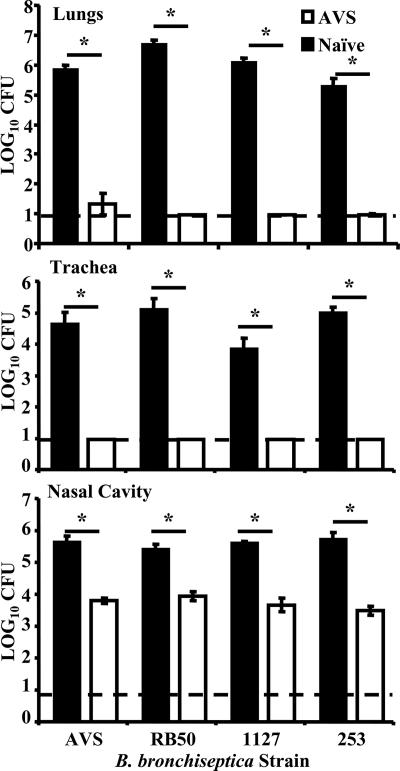
To investigate the efficacy of AVS as a possible live vaccine candidate, we vaccinated groups of WT mice with a single dose of approximately 100 CFU of AVS in a 5-μl volume of PBS. On day 49 postvaccination, vaccinated or naive mice were challenged intranasally with 5 × 105 CFU of AVS, the parental strain RB50 (sequence type 12) (3), or one of two heterologous B. bronchiseptica strains, 1127 (sequence type 6) and 253 (sequence type 27), in a 50-μl inoculum. Mice were euthanized at 3 days postchallenge, and bacterial burdens were determined as previously described. The bacterial numbers for vaccinated groups were at or near the detectable threshold for the trachea and lungs (Fig. 7), suggesting that vaccination with AVS generates a protective immune response that prevents subsequent infection of the lower respiratory tract. In addition, intranasal vaccination with AVS prevented colonization by the challenge strain in the nasal cavity (Fig. 7). Bacteria isolated from the nasal cavities of RB50-challenged, AVS-vaccinated mice were determined to be AVS via a lack of hemolysis seen on Bordet-Gengou plates. Bacteria isolated from the nasal cavities of naïve mice infected with the same inoculum showed hemolysis, indicating that the lack of hemolysis seen in vaccinated animals was not due to mutation prior to infection.
FIG. 7.
Intranasal vaccination with AVS protects WT mice. Groups of four WT mice were vaccinated intranasally with AVS. On day 49 postvaccination, the mice were inoculated intranasally with approximately 5 × 105 CFU of AVS, RB50, 1127, or 253, as indicated, in a 50-μl volume, and bacterial numbers were measured at 3 days postinoculation. The dashed line indicates the limit of detection. *, P value of <0.05. Error bars show standard errors.
To determine if low-dose intranasal vaccination with AVS protects susceptible mouse strains, groups of TLR4def and TNF-α−/− mice were vaccinated and then challenged with RB50 as described above. The mice were sacrificed on day 3 post-secondary challenge, and bacterial burdens were measured (data not shown). Results similar to those for high-dose infection-induced immunity (Fig. 3) were found. The nasal cavities of vaccinated TLR4def mice and TNF-α−/− mice contained 10,000-fold fewer CFU than those of control mice. The tracheae and lungs of vaccinated TLR4def mice and TNF-α−/− mice contained <20 CFU, while the same organs of control mice contained approximately 106 and 108 CFU, respectively. These results indicate that low-dose intranasal vaccination with AVS protects susceptible mice from severe infection by a variety of B. bronchiseptica strains.
Low-dose intranasal vaccination with AVS induces protective immunity against Bordetella pertussis and Bordetella parapertussis in the lower respiratory tract.
To determine if vaccination with AVS generated cross protection against B. pertussis and B. parapertussis, groups of WT mice were vaccinated with a single dose of approximately 100 CFU of AVS as described above. On day 49 postvaccination, the mice were challenged intranasally with 5 × 105 CFU of B. pertussis or B. parapertussis. The mice were euthanized on day 3 postchallenge, and bacterial burdens were measured. The nasal cavities, tracheae, and lungs of control mice infected with B. pertussis contained approximately 104, 103, and 105 CFU, respectively, while the same organs of vaccinated mice contained approximately 103, 101, and 102.5 CFU (Fig. 8A). Similarly, the nasal cavities, tracheae, and lungs of control mice infected with B. parapertussis had approximately 106, 105, and 106 CFU, respectively, while the same organs of vaccinated mice contained approximately 104, 101, and 101 CFU (Fig. 8B). These results suggest that in addition to protecting against B. bronchiseptica infection, low-dose intranasal vaccination with AVS also provides substantial cross immunity to B. pertussis and B. parapertussis.
FIG. 8.
Intranasal vaccination with AVS induces protective immunity against Bordetella pertussis and Bordetella parapertussis in the lower respiratory tract. WT mice were vaccinated intranasally with approximately 100 CFU of AVS in a 5-μl volume. On day 49 postvaccination, the mice were inoculated intranasally with approximately 5 × 105 CFU of either (A) B. pertussis or (B) B. parapertussis in a 50-μl volume. Bacterial numbers were measured at 3 days postinoculation. The dashed line indicates the limit of detection. *, P value of <0.05. Error bars indicate standard errors.
DISCUSSION
An effective vaccination program is critical to limiting the spread and impact of highly transmissible respiratory pathogens. Ideal candidates for widely used vaccines should be safe, effective, and easily administered. B. bronchiseptica is endemic in many mammalian populations, and a particularly high incidence of infections is seen in kennels as well as pig farms, where extensive vaccination is used to prevent disease (5). Therefore, there is a need for an efficacious B. bronchiseptica vaccine that provides effective and long-lasting protection with a single administration.
Current vaccines used against B. bronchiseptica are composed of either killed WT bacterial strains that are administered parenterally or live attenuated vaccine strains that are administered intranasally. Although the general differences in immunity between infection and vaccination with noninfectious components are still being elucidated, the specific mechanisms of clearance that differ between these two types of immunity during B. bronchiseptica infection have been studied. The results indicate that while vaccination with heat-killed B. bronchiseptica administered intraperitoneally induces greater serum antibody responses than those induced by intranasal infection, vaccination-induced antibodies are less protective in an adoptive transfer model (5a). Vaccination with heat-killed bacteria also provides less protection in the upper respiratory tract than does infection-induced immunity (5a). This suggests that the lack of a strong local memory response leads to the need for repeated vaccinations when parenterally delivered killed vaccines are used. Since protective immunity induced by infection is superior to that induced by vaccination with killed or acellular components, the ideal vaccination regimen should consist of infection with a strain that is lacking defined factors that contribute to pathology but are not required for the generation of protective immunity.
Here we describe the use of a mutant of B. bronchiseptica, strain AVS, which lacks adenylate cyclase and type III secretion, as a live attenuated vaccine. The AVS strain has many characteristics that make it an ideal candidate for use as a live vaccine. AVS is safe at high doses, even in immunocompromised hosts, as it induces less pathology (Fig. 2) and mortality (Fig. 1) than the WT strain and also protects animals against infection and disease caused by the virulent parental strain, heterologous B. bronchiseptica strains (Fig. 7), and the human pathogens B. pertussis and B. parapertussis (Fig. 8). Although there is some inflammation associated with high doses of AVS, typically with mild perivascular and peribronchiolar lymphoid aggregates, there are no clinical signs of illness, even in immunocompromised mice. WT and immunocompromised mice infected with AVS show much less pathology than mice infected with RB50 (Fig. 2). The differences are especially striking considering that immunocompromised mice infected with RB50 exhibit extensive pathology and high morbidity and mortality, while immunocompromised mice infected with AVS show no clinical signs and a mild inflammatory response. This suggests that AVS may be safe and suitable for use in immunocompromised individuals, although further testing is warranted.
The protection induced by AVS appears to be mediated by antibodies, as serum induced by either RB50 or AVS is sufficient to protect WT mice against disease and bacterial burden in the lungs following RB50 infection (Fig. 5). Apparently, neither adenylate cyclase nor type III secretion is required for the generation of a protective immune response, and the lack of antibodies directed against adenylate cyclase did not decrease the efficacy of immune serum. Since AVS expresses the type III secretion apparatus, it may contribute to protective antigens without producing type III secretion system-associated pathology. Together, these data suggest that adenylate cyclase and type III secretion are not required for the generation of protective immune responses. While AVS does not survive as long as RB50 in the lower respiratory tract of WT mice (Fig. 4), it does persist in a comparable fashion to RB50 in the upper respiratory tract (Fig. 4). This suggests that the ability of AVS to persist in the upper respiratory tract, a feature that attenuated strains with metabolic mutations may lack, could contribute to its ability to protect animals against WT B. bronchiseptica either by direct competition or by stimulating local immune responses.
Clinical application of this strain is likely to introduce small numbers of bacteria, primarily into the nasal cavity. Importantly, even a single low-dose, low-volume inoculation of AVS administered intranasally was able to protect WT mice against high-dose infections with heterologous WT B. bronchiseptica strains as well as WT strains of B. pertussis and B. parapertussis (Fig. 7 and 8). This protection against human-associated Bordetella spp. could potentially address the issues of waning immunity observed in vaccinated human populations (8, 17, 30). Current whooping cough vaccination strategies also use bolus injection as opposed to mucosal immunization, which may contribute to the known ability of B. pertussis and B. parapertussis to avoid humoral immunity (12, 31) Live, attenuated whooping cough vaccines were recently investigated for use in humans (17). A vaccine with proven efficacy against the three classical Bordetella spp., such as AVS, could potentially replace the current bolus injection vaccination strategies, which involve many vaccinations during the first year of life and periodic repeated booster vaccinations (15).
The means by which infection with AVS can generate protective immunity may reflect the roles of adenylate cyclase and the type III secretion system during interactions with host immune cells. Both of these virulence factors play several roles that contribute to the ability of B. bronchiseptica to cause disease. Type III secretion, as well as adenylate cyclase, is cytotoxic to phagocytes, which probably prevents phagocytosis of B. bronchiseptica (7, 29). In healthy mice, the damage caused by type III secretion-mediated necrosis is controlled, but in immunocompromised mice this can lead to severe pathology and even mortality (20; P. Mann and E. Harvill, unpublished data). Therefore, the lack of these two toxins results in decreased lung pathology, allowing the safe use of AVS in immunocompromised mice. The type III secretion system also contributes to the long-term persistence of B. bronchiseptica in the lower respiratory tract of the host by actively inhibiting the generation of protective, IFN-γ-mediated Th1 responses (21, 25). This results in the early generation of an anti-inflammatory Th2 response and bacterial persistence in the lungs for the first few weeks of infection. Eventually, a Th1 response is generated and, along with antibodies that bind to B. bronchiseptica, facilitates the clearance of bacteria from the lower respiratory tract. Infection with strains that lack type III secretion results in the earlier generation of a protective Th1 response and in faster bacterial clearance (21, 25). By using a rationally designed vaccine strain that does not express toxins which induce pathology and modulate the immune response, an efficient and effective protective immune response is generated without collateral pathology. The consequence is a highly effective vaccine that is safe to use and generates a strong protective immune response against subsequent infection.
Acknowledgments
We thank Robert Livingston at the University of Missouri Research Animal Diagnostic Laboratory for the kind gift of B. bronchiseptica strains 1127 and 253. We also thank Kari Dundore for her technical assistance.
This work was funded by grants from the Pennsylvania Department of Agriculture (ME440678 [to E.H.]), the United States Department of Agriculture (2002-35204-11684 [to E.H.]), and the National Institutes of Health (5-RO1-A1053075-02 [to E.H.]). P.M. was funded by the U.S. Army Medical Service Corp Long Term Health Education and Training Program.
Editor: D. L. Burns
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Bey, R. F., F. J. Shade, R. A. Goodnow, and R. C. Johnson. 1981. Intranasal vaccination of dogs with liver avirulent Bordetella bronchiseptica: correlation of serum agglutination titer and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am. J. Vet. Res. 42:1130-1132. [PubMed] [Google Scholar]
- 2.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diavatopoulos, D. A., C. A. Cummings, L. M. Schouls, M. M. Brinig, D. A. Relman, and F. R. Mooi. 2005. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 1:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edinboro, C. H., M. P. Ward, and L. T. Glickman. 2004. A placebo-controlled trial of two intranasal vaccines to prevent tracheobronchitis (kennel cough) in dogs entering a humane shelter. Prev. Vet. Med. 62:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Gopinathan, L., G. S. Kirimanjeswara, D. N. Wolfe, M. L. Kelley, and E. T. Harvill. 2007. Different mechanisms of vaccine-induced and infection-induced immunity to Bordetella bronchiseptica. Microb. Infect. 9:442-448. [DOI] [PubMed] [Google Scholar]
- 6.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He, Q., M. K. Viljanen, H. Arvilommi, B. Aittanen, and J. Mertsola. 1998. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA 280:635-637. [DOI] [PubMed] [Google Scholar]
- 9.Heininger, U., P. A. Cotter, H. W. Fescemyer, G. Martinez de Tejada, M. H. Yuk, J. F. Miller, and E. T. Harvill. 2002. Comparative phenotypic analysis of the Bordetella parapertussis isolate chosen for genomic sequencing. Infect. Immun. 70:3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inatsuka, C. S., S. M. Julio, and P. A. Cotter. 2005. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc. Natl. Acad. Sci. USA 102:18578-18583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs, A. A., R. P. Theelen, R. Jaspers, L. J. Horspool, D. Sutton, J. G. Bergman, and G. Paul. 2005. Protection of dogs for 13 months against Bordetella bronchiseptica and canine parainfluenza virus with a modified live vaccine. Vet. Rec. 157:19-23. [DOI] [PubMed] [Google Scholar]
- 12.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 71:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann, P. B., K. D. Elder, M. J. Kennett, and E. T. Harvill. 2004. Toll-like receptor 4-dependent early elicited tumor necrosis factor alpha expression is critical for innate host defense against Bordetella bronchiseptica. Infect. Immun. 72:6650-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann, P. B., M. J. Kennett, and E. T. Harvill. 2004. Toll-like receptor 4 is critical to innate host defense in a murine model of bordetellosis. J. Infect. Dis. 189:833-836. [DOI] [PubMed] [Google Scholar]
- 15.Mattoo, S., and J. D. Cherry. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArthur, J. D., N. P. West, J. N. Cole, H. Jungnitz, C. A. Guzman, J. Chin, P. R. Lehrbach, S. P. Djordjevic, and M. J. Walker. 2003. An aromatic amino acid auxotrophic mutant of Bordetella bronchiseptica is attenuated and immunogenic in a mouse model of infection. FEMS Microbiol. Lett. 221:7-16. [DOI] [PubMed] [Google Scholar]
- 17.Mielcarek, N., A. S. Debrie, D. Raze, J. Bertout, C. Rouanet, A. B. Younes, C. Creusy, J. Engle, W. E. Goldman, and C. Locht. 2006. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moylett, E. H., and I. C. Hanson. 2003. Immunization. J. Allergy Clin. Immunol. 111:S754-S765. [DOI] [PubMed] [Google Scholar]
- 19.Neutra, M. R., and P. A. Kozlowski. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148-158. [DOI] [PubMed] [Google Scholar]
- 20.Pilione, M. R., L. M. Agosto, M. J. Kennett, and E. T. Harvill. 2006. CD11b is required for the resolution of inflammation induced by Bordetella bronchiseptica respiratory infection. Cell. Microbiol. 8:758-768. [DOI] [PubMed] [Google Scholar]
- 21.Pilione, M. R., and E. T. Harvill. 2006. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect. Immun. 74:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raupach, B., and S. H. Kaufmann. 2001. Bacterial virulence, proinflammatory cytokines and host immunity: how to choose the appropriate Salmonella vaccine strain? Microbes Infect. 3:1261-1269. [DOI] [PubMed] [Google Scholar]
- 23.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts, M., D. Maskell, P. Novotny, and G. Dougan. 1990. Construction and characterization in vivo of Bordetella pertussis aroA mutants. Infect. Immun. 58:732-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner, J. A., M. R. Pilione, H. Shen, E. T. Harvill, and M. H. Yuk. 2005. Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence. J. Immunol. 175:4647-4652. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson, A., and M. Roberts. 2004. Intranasal immunisation against tetanus with an attenuated Bordetella bronchiseptica vector expressing FrgC: improved immunogenicity using a Bvg-regulated promoter to express FrgC. Vaccine 22:4300-4305. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson, A., and M. Roberts. 2002. Use of a rationally attenuated Bordetella bronchiseptica as a live mucosal vaccine and vector for heterologous antigens. Vaccine 20:2325-2335. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson, A., and M. Roberts. 2003. Use of Bordetella bronchiseptica and Bordetella pertussis as live vaccines and vectors for heterologous antigens. FEMS Immunol. Med. Microbiol. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 29.Stockbauer, K. E., A. K. Foreman-Wykert, and J. F. Miller. 2003. Bordetella type III secretion induces caspase 1-independent necrosis. Cell. Microbiol. 5:123-132. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe, M., and M. Nagai. 2004. Whooping cough due to Bordetella parapertussis: an unresolved problem. Expert Rev. Anti-Infect. Ther. 2:447-454. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe, D. N., G. S. Kirimanjeswara, and E. T. Harvill. 2005. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect. Immun. 73:6508-6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuk, M. H., E. T. Harvill, and J. F. Miller. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945-959. [DOI] [PubMed] [Google Scholar]