Abstract
Chagas’ heart disease (CHD), caused by the parasite Trypanosoma cruzi, is the most common form of myocarditis in Central America and South America. Some humans and experimental animals develop both humoral and cell-mediated cardiac-specific autoimmunity during infection. Benznidazole, a trypanocidal drug, is effective at reducing parasite load and decreasing the severity of myocarditis in acutely infected patients. We hypothesized that the magnitude of autoimmunity that develops following T. cruzi infection is directly proportional to the amount of damage caused by the parasite. To test this hypothesis, we used benznidazole to reduce the number of parasites in an experimental model of CHD and determined whether this treatment altered the autoimmune response. Infection of A/J mice with the Brazil strain of T. cruzi leads to the development of severe inflammation, fibrosis, necrosis, and parasitosis in the heart accompanied by vigorous cardiac myosin-specific delayed-type hypersensitivity (DTH) and antibody production at 21 days postinfection. Mice succumbed to infection within a month if left untreated. Treatment of infected mice with benznidazole eliminated mortality and decreased disease severity. Treatment also reduced cardiac myosin-specific DTH and antibody production. Reinfection of treated mice with a heart-derived, virulent strain of T. cruzi or immunization with myosin led to the redevelopment of myosin-specific autoimmune responses and inflammation. These results provide a direct link between the levels of T. cruzi and the presence of autoimmunity and suggest that elimination of the parasite may result in the reduction or elimination of autoimmunity in the chronic phase of infection.
Chagas’ disease is one of several diseases, along with leishmaniasis and African sleeping sickness, caused by parasites of the family Trypanosomatidae. Endemic to Central America and South America, the parasite that causes Chagas’ disease, Trypanosoma cruzi, is the world's leading cause of myocarditis (28). The World Health Organization estimates that 16 to 18 million people are infected with T. cruzi, with about 100 million people at risk in 21 countries (41, 42). In spite of recent advances in the control of the vectorial and transfusional transmission of T. cruzi (66), Chagas’ disease remains a serious infectious disease in Latin America due to its prevalence, morbidity, and mortality (49). Despite its obvious clinical importance and the efforts of many investigators, the pathogenesis of Chagas’ heart disease (CHD) is still incompletely understood.
A variety of explanations have been proposed for the damage observed in CHD. Potential mechanisms include (i) toxin secretion by the parasite (4), (ii) damage to cardiac microvasculature (53), (iii) destruction of heart neuronal tissue (13), (iv) parasite-specific immune responses to T. cruzi antigens persistent in the heart tissue (8, 19, 38, 55, 60, 61, 69, 72), (v) antibody-mediated cytotoxicity and nonspecific neutrophil- and eosinophil-induced damage (26, 27), and (vi) autoimmunity (18, 30-34). Although autoimmunity is defined simply as an immune reaction against an organism's own proteins, the progression from benign to pathogenic autoimmunity, resulting in disease, is a distinction often overlooked. It is this distinction which has spurred controversy among investigators (5, 9, 17, 18, 21, 24, 26, 31-33, 59, 63) about the significance of autoimmunity in disease pathogenesis. This mechanism suggests that T. cruzi-induced cardiac damage and/or molecular mimicry between parasite and host leads to a breakdown in self-tolerance, resulting in eventual autoimmune tissue damage. Our overall hypothesis is that the combination of viable parasite-induced myocardial damage, parasite antigen-specific immunity, and autoimmunity contributes to the inflammation and heart failure of CHD.
Although T. cruzi-induced autoimmunity has been a matter of considerable investigation (10, 22, 23, 25, 31, 34, 36, 37, 51, 57, 58, 65, 67, 68), its role in disease pathogenesis and the mechanism(s) by which it develops remain unclear. Here, we address the role of viable parasites in the induction and persistence of autoimmunity in A/J mice by the administration of benznidazole, a nitroheterocyclic drug employed in the chemotherapy of human Chagas’ disease (50). Although the mechanism of drug action is not entirely understood, it is thought that when T. cruzi metabolizes benznidazole, its lack of catalase and peroxidase enzymes hinders its ability to dispose of newly generated free radicals (15). The presence of a full repertoire of antioxidant enzymes provides mammals the ability to cope with the drug more effectively, though not without detrimental side effects, including abdominal pain, diarrhea, nausea, and vomiting. Benznidazole exerts a number of effects on the host immune response to T. cruzi infection, including the enhancement of macrophage-associated phagocytosis and proinflammatory cytokine production (45), the selective expansion of effector and memory CD8+ T lymphocytes (47), and the decrease of both P-selectin and vascular cell adhesion molecule 1 levels (29). In addition, host immune factors, including interleukin 12 (39) and gamma interferon (52), are important for maximum efficacy of benznidazole therapy during infection. The proven trypanocidal activity of benznidazole, along with its ease of administration in drinking water, enabled us to efficiently reduce the number of parasites at various times during infection.
Benznidazole treatment administered within the first week of infection reduced the magnitude of myosin-specific cellular and humoral immunity compared to untreated controls at 21 days postinfection (d.p.i.) with T. cruzi. Since mice succumbed to disease at 30 d.p.i. in our experimental CHD model, we were unable to include infected untreated controls for long-term experiments. However, by comparing immune responses to baseline uninfected treated animals, we could make conclusions pertaining to the change in myosin-specific autoreactivity at later time points. Using this basis of comparison, we observed that the initiation of treatment within the first or second week of infection eliminated myosin-specific cellular autoimmunity at 60 or 90 d.p.i., respectively. All stages of disease displayed an overall decrease in inflammation and a complete absence of parasites in the heart tissue. Finally, after drug treatment was terminated, reinfection with T. cruzi or immunization with cardiac myosin led to the restoration of strong myosin-specific immunity and inflammation in mice, indicating that cardiac autoimmunity can be regulated indirectly by modulating the levels of the parasite.
MATERIALS AND METHODS
Experimental animals and T. cruzi infections.
Four- to 6-week-old male A/J mice (Jackson Laboratories, Bar Harbor, ME) were housed under specific-pathogen-free conditions. Mice were infected by intraperitoneal injection of 1 × 104 T. cruzi Brazil strain trypomastigotes derived from infection of tissue culture H9C2 rat myoblasts (American Type Culture Collection, Manassas, VA). A cardiotropic substrain of the Brazil strain of T. cruzi was isolated from the heart of an infected mouse and propagated in H9C2 rat myoblasts to generate trypomastigotes for use in reinfection experiments. This strain has since been maintained and termed the “Brazil heart” strain. Parasitemias from tail bleeds were measured by hemacytometry. Uninfected controls received intraperitoneal injections of Dulbecco's phosphate-buffered saline (GibcoBRL, Grand Island, NY) of equal volume. Mice were anesthetized by a single intraperitoneal injection of sodium pentobarbital (60 mg/kg of body weight) for each experimental manipulation. The use and care of mice were conducted in accordance with the guidelines of the Center for Comparative Medicine at Northwestern University.
Preparation of myosin and T. cruzi antigen.
Cardiac myosin heavy chains and T. cruzi antigen were prepared as described previously (34). Briefly, hearts were rinsed in ice-cold saline, minced, homogenized, and stirred for 90 min at 4°C. Muscle residue was removed by centrifugation for 10 min at 12,000 × g. The supernatant was then centrifuged at 140,000 × g for 4 h. This supernatant was added to cold water, and the precipitate was allowed to settle overnight. The cloudy precipitate was centrifuged for 15 min at 12,000 × g, after which the myosin pellet was homogenized and redissolved. Actin contamination was removed by centrifugation for 30 min at 43,000 × g, and myosin was reprecipitated overnight in cold water. This procedure was repeated to remove actomyosin, and myosin was finally redissolved in a glycerol buffer. The final myosin concentration was determined by Bradford assay and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis.
Induction of autoimmune myocarditis.
Mice were immunized with purified cardiac myosin (300 μg) in an emulsion of complete Freund's adjuvant (CFA; Difco, Detroit, MI) in a total volume of 0.1 ml. Three subcutaneous sites in the dorsal flank were injected with equal amounts. Seven days later, mice were boosted in an identical manner.
Drug treatment.
Benznidazole (Roche Chemical and Pharmaceutical Products, Sao Paulo, Brazil) was administered in the drinking water of T. cruzi-infected, myosin-immunized, or saline-injected mice at a concentration of 100 mg/kg/day as described previously (14, 47). Treatment was initiated and terminated at various time points postinfection.
Histopathology.
Hearts were removed, rinsed with saline, and fixed for 24 h in 10% buffered formalin. Fixed hearts were embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy. Two sections were taken from each heart, one including both atria and the other both ventricles. Each section was examined for evidence of mononuclear and polymorphonuclear cell infiltration, necrosis and mineralization, T. cruzi pseudocysts (parasitosis), and fibrosis and was assigned a histologic score of 0 (no involvement noted) to 4 (100% involvement), with 1, 2, and 3 representing 25, 50, and 75% involvement of the histologic section, respectively (35).
Serologic analysis.
Levels of cardiac myosin-specific and T. cruzi-specific immunoglobulin G (IgG) were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (34). Endpoint dilution titers for total IgG were defined as the highest serum dilution that resulted in an absorbance value (optical density at 450 nm) of 2 standard deviations above the mean for a negative control sample (pooled sera from uninfected mice) included on every plate.
DTH.
Myosin-specific and T. cruzi-specific delayed-type hypersensitivity (DTH) was quantified using a standard ear swelling assay (34). Antigen-induced ear swelling was the result of mononuclear cell infiltration and exhibited typical DTH kinetics (i.e., minimal swelling at 4 h and maximal swelling at 24 to 48 h postinjection).
Statistical analyses.
DTH values were log10 transformed prior to statistical analyses if they were not normally distributed. For comparison of two groups, the significance of DTH values was analyzed by Student's t test. For comparison of multiple groups and a control, the significance of DTH values was analyzed by a one-way analysis of variance, followed by adjustment for multiple comparisons by the Dunnett test (post hoc analysis). The control group for comparison is specified in each figure legend. Antibody values were not normally distributed and so were analyzed for significance by the Mann-Whitney U test. P values of <0.05 were considered significant unless otherwise specified.
RESULTS
Early parasiticidal drug treatment reduces cardiac myosin-specific autoimmunity and cardiac inflammation and reduces tissue parasitosis in acute T. cruzi infection.
Autoimmunity is only one mechanism of Chagas’ disease pathogenesis which may develop as a result of antigenic molecular mimicry or bystander activation after parasite-induced tissue destruction. In either case, levels of the parasite might correlate with the presence of autoimmunity. The goal of the present study was to explore this relationship. To test the association of autoimmunity and levels of T. cruzi, we employed our experimental model of CHD in which A/J mice are infected with the Brazil strain of T. cruzi, leading to the development of cardiac inflammation, fibrosis, necrosis, and parasitosis accompanied by vigorous cardiac myosin-specific DTH and antibody production at 21 d.p.i. and mortality within 30 d.p.i (34). We administered the trypanocidal drug benznidazole (100 mg/kg/day) to infected mice at different times postinfection to ascertain the association between levels of parasite, myocarditis, and autoimmunity. All of the experiments included in this paper are diagrammed in Fig. 1. Groups of infected/untreated and saline-injected/benznidazole-treated mice were included as controls. The results that follow are representative of three separate experiments.
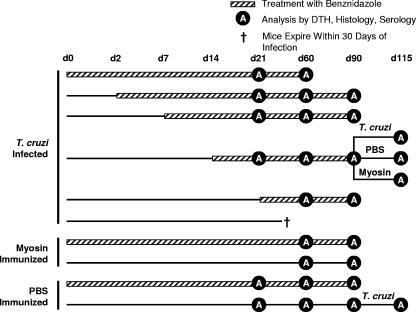
FIG. 1.
Experimental regimens used in this paper. T. cruzi-infected, myosin-immunized, or phosphate-buffered saline (PBS)-immunized mice were treated with a curative dose of 100 mg/kg/day of benznidazole in drinking water for the course of disease indicated (days [d] 21, 60, and 90). The times during which benznidazole was administered are indicated with a crosshatched line, and those for which no treatment was given are indicated with a simple line. Untreated, control mice (infected or immunized) received only water. The d.p.i. at which benznidazole treatment was initiated or mice were sacrificed for analysis (black circle containing white A) are indicated. Analyses included DTH, antibody assays, and cardiac histology. A large group of infected mice treated with benznidazole beginning at 14 d.p.i. was divided into three subgroups at 90 d.p.i. One subgroup was reinfected with T. cruzi, another was immunized with PBS, and a third was immunized with myosin. Additional control groups were included as indicated. The single dagger associated with the infected, untreated group indicates that the mice were sacrificed because they did not survive past 30 d.p.i.
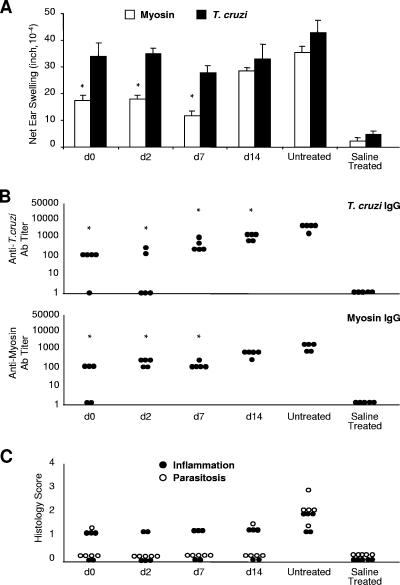
The treatment initiated during the first week of infection (i.e., at 0, 2, or 7 d.p.i.) significantly reduced myosin-specific DTH and antibody titers (Fig. 2A and B). In general, levels of myosin-specific DTH were lower when benznidazole administration was begun earlier. Histopathologic examination of heart sections from these animals revealed a reduction of inflammation and an absence of parasitosis compared to that of heart sections from untreated control animals (Fig. 2C). The cardiac inflammation in all benznidazole-treated animals, however, was consistent despite clear differences in cardiac autoimmunity. As expected, the number of parasites found in the blood was also significantly lower in animals in which treatment was begun within the first week of infection (data not shown). Interestingly, benznidazole treatment caused a significant reduction in parasite-specific antibody production yet had no significant impact on T. cruzi-specific cellular immunity (Fig. 2A and B).
FIG. 2.
Early benznidazole treatment reduces cardiac myosin-specific DTH, antimyosin, and T. cruzi-specific antibody production and inflammation and eliminates parasitosis in acute T. cruzi infection. T. cruzi-infected and saline-injected A/J mice were treated with a curative dose of benznidazole (at the d.p.i. indicated) for the course of disease. Untreated, infected mice were also included (Untreated). At 21 d.p.i., (A) myosin-specific and T. cruzi-specific DTH responses were measured by a 24-hour ear swelling assay (40) and (B) IgG antibody (Ab) titer values were determined by ELISA. Error bars indicate standard errors of the means for a minimum of three animals. Each dot corresponds to one animal. *, P < 0.05 relative to the untreated control group. (C) Inflammation and parasitosis were assessed by analyzing hematoxylin- and eosin-stained heart sections. Each dot corresponds to one animal. See Fig. 1 for a schematic of the experimental design. d, day.
Drug treatment administered during the chronic phase of T. cruzi infection (60 or 90 d.p.i.) results in the elimination of myosin-specific cellular autoimmunity.
For the purpose of this study, we use the term “acute” to refer to disease present at 21 d.p.i. in A/J mice with the Brazil strain of T. cruzi and “chronic” to refer to disease present after animals reach 60 d.p.i. For such long-term experiments, infected, untreated controls cannot be used, since A/J mice do not survive past 30 d.p.i. with the Brazil strain of T. cruzi. To control for the effects of benznidazole on myosin-specific DTH and antibody production, myosin-immunized mice treated with benznidazole were examined to determine the possible effects of the drug on myosin autoimmunity (Fig. 3 and 4). There was no effect, indicating that any effect of the drug on autoimmunity in infected mice would be related to its antiparasitic activity and not to an immunomodulatory action.
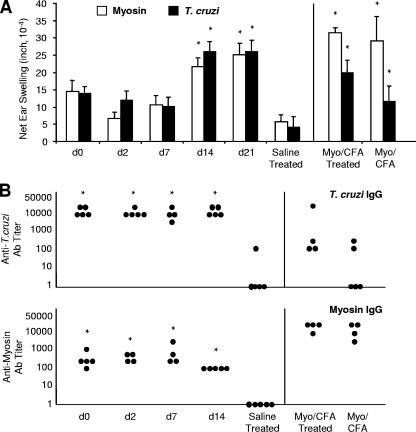
FIG. 3.
Initiating benznidazole treatment within the first week of T. cruzi infection results in an absence of myosin-specific and T. cruzi-specific DTH at 60 d.p.i. but has no significant effect on IgG production. T. cruzi-infected and saline-injected A/J mice were treated with a curative dose of benznidazole beginning at the indicated d.p.i. Mice immunized with myosin (Myo) and CFA, with or without benznidazole treatment, were also included (right). Infected, untreated control animals were not included since they died within 30 d.p.i. At 60 d.p.i., (A) myosin-specific and T. cruzi-specific DTH responses were measured by a 24-hour ear swelling assay (40) and (B) T. cruzi-specific and myosin-specific IgG titers were determined by ELISA. Error bars indicate standard errors of the means for a minimum of three animals, and each dot corresponds to one animal. *, P < 0.05 relative to saline-injected, treated control animals. See Fig. 1 for a schematic of the experimental design. d, day; Ab, antibody.
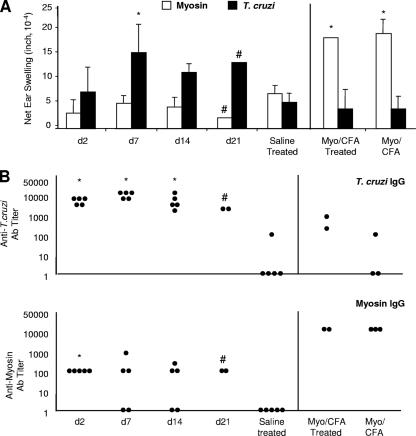
FIG. 4.
Initiating benznidazole treatment within two weeks of T. cruzi infection results in an absence of myosin-specific DTH and a reduction in T. cruzi-specific DTH at 90 d.p.i. but has no significant effect on T. cruzi-specific IgG production. T. cruzi-infected and saline-injected A/J mice were treated with a curative dose of benznidazole beginning at the indicated d.p.i. Mice immunized with myosin (Myo) and CFA, with or without benznidazole treatment, were also included (right). Infected, untreated control animals were not included since they died within 30 d.p.i. At 90 d.p.i., (A) myosin-specific and T. cruzi-specific DTH responses were measured by a 24-hour ear swelling assay and (B) T. cruzi-specific and myosin-specific IgG titers were determined by ELISA. Error bars indicate standard errors of the means for a minimum of three animals, and each dot corresponds to one animal. *, P < 0.05 relative to saline-injected, treated control animals; #, no statistical significance since only two mice were involved. See Fig. 1 for a schematic of the experimental design. d, day; Ab, antibody.
Drug treatment in infected mice was initiated at the time points indicated (Fig. 1) for all infected animals and maintained until 60 or 90 d.p.i., at which time DTH and antibody levels were ascertained and cardiac histopathology was assessed. Since we were unable to maintain viable infected, untreated control animals at 60 and 90 d.p.i., we established a baseline (negative control) by using uninfected, healthy animals expected to yield negligible, if not a complete absence of, autoreactivity. If our experimental group produced autoimmunity not significantly different (using appropriate statistical parameters) from that of negative controls, we referred to the autoimmunity associated with this particular group as “eliminated.” We observed that the myosin-specific DTH in infected mice at 60 d.p.i. was similar to that observed at 21 d.p.i. (Fig. 1 and 3). In both cases, earlier initiation of benznidazole treatment led to lower myosin-specific DTH than did later benznidazole treatment. However, to our surprise, we found that myosin-specific DTH was eliminated in infected/treated mice (Fig. 3). In other words, myosin-specific DTH levels in mice treated within the first week of infection were not significantly different from those in saline controls. At 60 d.p.i., myosin-specific antibodies were present at low levels, which did not differ significantly with the timing of benznidazole initiation (Fig. 3). Interestingly, earlier initiation of benznidazole treatment also led to lower levels of T. cruzi-specific DTH than did later initiation. T. cruzi-specific antibody levels were high and did not differ among the benznidazole-treated groups. At 60 d.p.i., both cardiac inflammation and tissue parasitosis were eliminated in all infected, treated animals (data not shown). We also observed T. cruzi-specific DTH in myosin-immunized mice, in agreement with previous findings (30).
Similar to our findings at 60 d.p.i, we found that myosin-specific DTH was absent at 90 d.p.i. in mice that had been treated within the first 2 weeks of infection (Fig. 4). Myosin-specific antibody levels were also low and were absent in some animals in which treatment was begun early (days 7 and 14) (Fig. 4B). We also observed that T. cruzi-specific DTH levels were lower than those seen at 21 d.p.i., although T. cruzi-specific antibody levels were high and did not differ among treatment groups. At 90 d.p.i., cardiac inflammation and parasitosis were absent from all infected, treated animals (data not shown). In contrast to what was observed at 60 d.p.i., T. cruzi-specific DTH was eliminated in myosin-immunized mice at 90 d.p.i.
Secondary T. cruzi infection or myosin immunization induces cardiac autoimmunity and myocarditis in benznidazole-treated mice.
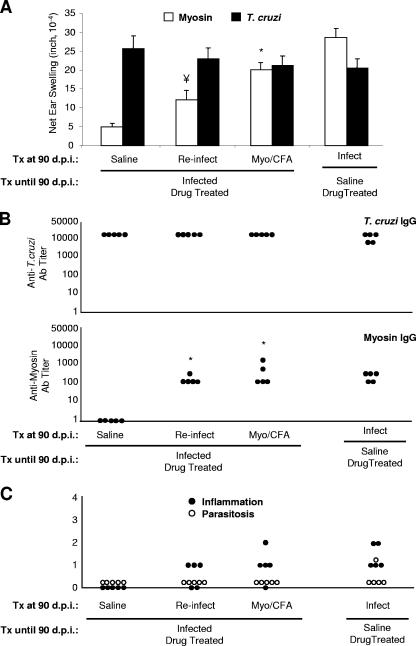
Finally, we investigated the ability of a secondary T. cruzi infection or a noninfectious cardiac insult to initiate a cardiac autoimmune response and/or myocarditis in animals that had been treated with benznidazole and that no longer exhibited myosin-specific DTH. Since we observed the complete disappearance of inflammation, parasitosis, and myosin-specific DTH in T. cruzi-infected mice that had received long-term treatment with benznidazole, we began with these animals for the experiment. After continuous daily treatment of infected mice from 14 d.p.i. to 90 d.p.i., we terminated drug treatment and reinfected mice with a virulent, heart-derived substrain of the T. cruzi Brazil strain, immunized mice with cardiac myosin, or injected mice with saline. As an additional control, we also infected naïve mice that had received benznidazole for 90 days. Twenty-five days later (115 d.p.i.), we found that reinfection or myosin immunization of these mice resulted in the restoration of both cellular and humoral cardiac autoimmunity and mild myocarditis (Fig. 5 and 6). The magnitude of autoimmunity and the severity of myocarditis in reinfected animals were not as great as those seen in animals infected for the first time (Fig. 2). Despite the absence of parasitosis observed after reinfection, parasite-specific DTH and antibody levels were induced to high levels (Fig. 5). Interestingly, in a separate experiment, treated mice reinfected with the original T. cruzi Brazil strain displayed no signs of myocarditis and maintained the absence of myosin-specific DTH (data not shown).
FIG. 5.
T. cruzi reinfection or myosin immunization of infected, benznidazole-treated mice restores myosin-specific autoimmunity and cardiac inflammation. Mice were infected with T. cruzi or injected with saline and treated with benznidazole from 14 d.p.i. through 90 d.p.i. At 90 d.p.i., treatment was terminated and groups of animals were immunized with saline, immunized with myosin, primarily infected with T. cruzi, or reinfected with T. cruzi as indicated. Twenty-five days later (115 days after the initiation of the experiment), (A) myosin-specific and T. cruzi-specific DTH responses were measured by a 24-hour ear swelling assay and (B) IgG titer values were determined by ELISA. Error bars indicate standard errors of the means for a minimum of three animals. (C) Inflammation and parasitosis were assessed by analyzing hematoxylin- and eosin-stained heart sections. Each dot corresponds to one animal (B and C). Statistics are provided relative to the infected, treated group immunized with saline at 90 d.p.i. (*, P < 0.05; ¥, P = 0.057). See Fig. 1 for a schematic of the experimental design. Myo, myosin; Ab, antibody; Tx, treatment.
FIG. 6.
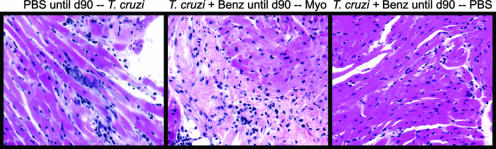
Histologic analysis of mice at 115 d.p.i. Representative cardiac sections from mice in the experiment represented in Fig. 5 are shown. Mice infected primarily with T. cruzi after phosphate-buffered saline (PBS) injection and receiving no drug treatment until day 90 (d90) developed myocardial inflammation and necrosis with the presence of parasite pseudocysts (left). Mice infected with T. cruzi and treated with benznidazole (Benz) until 90 d.p.i. and then immunized with myosin (Myo) developed severe myocarditis and fibrosis (center). Mice infected with T. cruzi and treated with benznidazole until 90 d.p.i. and then immunized with PBS had normal cardiac histology (right).
DISCUSSION
Autoimmunity develops in some humans and experimental animals as a result of T. cruzi infection. However, the role of autoimmunity in disease pathogenesis and the mechanism(s) by which it is induced remain obscure. In this study, we addressed the importance of live T. cruzi-induced damage to the induction and persistence of cardiac autoimmunity by reducing the number of parasites in infected animals with the trypanocidal drug benznidazole. Infection of A/J mice with the Brazil strain of T. cruzi typically leads to the development of severe inflammation and parasitosis in the heart along with strong cardiac myosin-specific cellular and humoral immunity at 21 d.p.i. and mortality by 30 d.p.i. The early administration of benznidazole decreased the severity of myocarditis, eliminated mortality in infected animals, and permitted us to conduct long-term experiments (60 and 90 d.p.i.). Unfortunately, the early mortality associated with our disease model prevented us from analyzing infected, untreated controls for the duration of the experiment (specifically at 60 or 90 d.p.i.). However, because autoimmunity persists throughout the course of long-term infection when other strains of parasite and mouse are employed (16, 20, 48, 51), it is highly unlikely that autoimmunity in A/J mice infected with the Brazil strain would spontaneously resolve within several months of infection. Further, our finding that autoimmunity can be “restored” by infection or immunization of treated and cured mice at 90 d.p.i. shows that these animals retain their autoimmune potential upon treatment. Finally, it should be noted that autoimmunity persists in some humans with chronic T. cruzi infection (1, 9, 56, 64).
Depending on the time of drug treatment initiation, myosin-specific immunity, measured by DTH and antibody production, was significantly reduced or eliminated in both acute (21 d.p.i.) and chronic (60 and 90 d.p.i.) phases of experimental CHD. The elimination of both cardiac parasitosis and parasitemia (data not shown) illustrated the effective reduction of the parasite in all drug-treated, infected animals. Additionally, strong myosin-specific immune responses observed in myosin-immunized animals treated with drug clearly showed that benznidazole has no inherent impact on the development of autoimmunity.
The mechanism of bystander activation posits that viable parasites destroy heart tissue, causing the release of host antigens (54) and subsequent stimulation of autoreactive cells. Bystander activation has also been invoked to explain the presence of myosin-specific T cells in patients after myocardial infarction (43). In other words, any cause of cardiac myocyte damage, including parasite-induced cytolysis, can cause bystander autoimmunity. Molecular mimicry, on the other hand, states that an infectious agent (parasite, bacterium, or virus) possesses epitopes that are immunologically similar to host determinants, and due to minor antigenic differences between the two, the pathogen epitope is able to induce an immune response that breaks tolerance to the host peptide (46). This mechanism has been attributed to a variety of cases of infection-induced autoimmunity, including streptococcus-induced myocarditis (11, 12) and even T. cruzi-induced Chagas’ disease (9, 23).
We found that the reduction of infectious parasites drastically lessens or eliminates myosin-specific immunity, lending strong support to the bystander activation mechanism. However, benznidazole treatment also caused significant reductions in T. cruzi-specific antibody titers (Fig. 2B) as well as parasite-specific DTH (Fig. 3A). Furthermore, the myosin-immunized animals displayed significant parasite-specific immunity (Fig. 3), consistent with previous results (34) showing cross-reactive immune responses. These results suggest the possibility that reduction of parasite load can eliminate cardiac autoimmunity (i) by attenuating the extent of cardiomyocyte damage and (ii) by lowering the number of pathogenic mimic epitopes (T. cruzi antigens) to which cross-reactive T cells can respond. The development of strong myosin-specific immunity and the absence of detectable cardiac damage observed upon immunization with T. cruzi protein extract (30) suggest that molecular mimicry is a likely mechanism of autoimmunity during infection, although bystander activation may be required for the development of myocarditis. Considered together with previous findings, the results of the current study pointing to bystander activation as vital to autoimmunity in CHD led us to hypothesize that viable parasites together with parasite-specific immunity cooperatively contribute to the onset and maintenance of cardiac autoimmunity.
We observed that levels of both T. cruzi and myosin-specific antibody were higher the later benznidazole treatment was initiated (Fig. 2). One hypothesis to explain this result is that the levels of antigen-specific antibodies may be associated with the severity of myocarditis and the level of T. cruzi. These, in turn, are related to the day of benznidazole treatment initiation. This hypothesis may also explain why myosin-specific DTH levels increase the later the day of initial benznidazole treatment. However, it does not explain how T. cruzi-specific DTH is high regardless of when benznidazole was administered (Fig. 2). The maintenance of this parasite-specific immunity along with low, residual levels of autoreactivity could account for the mild inflammation observed in all drug-treated animals in the acute phase of disease (Fig. 2C). The negligible parasitosis observed in the treated animals at this stage also suggests a potential role for minor parasite-mediated damage, resulting in mild inflammation.
Treatment of infected mice with benznidazole resulted in the elimination of myosin-specific DTH and a decrease in T. cruzi-specific DTH by 60 and 90 d.p.i. (Fig. 3 and 4). The decrease in antigen-specific DTH, months after removal of the antigenic stimulus, may suggest that levels of antigen-specific memory T cells decrease over time, especially in the absence of continuous antigen stimulation, but this remains to be investigated. This theory is controversial; some contend that antigen persistence is not required for the maintenance of long-lived memory (44), while others have found that antigen persistence is linked to the persistence of T-cell memory in cases of Plasmodium exposure (2, 71) and coronavirus-induced encephalitis (6). This hypothesis may also explain why T. cruzi-specific DTH levels are eliminated in myosin-immunized mice over time (Fig. 3 and 4). We also observed a decrease in myosin-specific antibodies in treated mice over time, which may support the hypothesis of a decrease in the autoimmune memory response over time. We did not observe a similar decrease in T. cruzi-specific antibody levels over time. The high levels of T. cruzi antibodies, seemingly unaffected by benznidazole initiation, may be explained by (i) the insensitivity of our IgG assay in detecting differences in T. cruzi-specific IgG at high titers, (ii) the persistence of T. cruzi-specific memory B cells (3), or (iii) the long half-life of T. cruzi-specific IgG (62).
The elimination of cardiac autoimmunity observed in the late stage of disease prompted us to investigate whether this reestablishment of self-tolerance could be maintained in the presence of a secondary infection or general cardiac-specific insult. One potential mechanism to explain the elimination of autoimmunity in treated mice is that the reduction of parasites caused a reduction of parasite-associated damage and concomitant immune exposure to myosin. The absence of immune exposure to myosin and the return to a state of normalcy, free from infection-induced inflammatory conditions, cause the dampening of the self-directed immune response, resulting in the restoration of myosin tolerance. If this is the case, secondary infection or cardiac insult resulting in the presentation of cardiac myosin to autoreactive T cells should not elicit an autoimmune response. Reinfection with a virulent, cardiotropic parasite strain caused the reappearance of myosin-specific autoimmunity and mild myocarditis. This suggests that, while a large number of myosin-specific T cells may become unresponsive after the eradication of infection, a strong enough secondary stimulus is sufficient to reactivate this population. Histologic analysis of the cardiac tissue also revealed reduced parasitosis in the hearts of mice reinfected with the hypervirulent strain compared to that seen either in the acute phase of disease or in those mice infected primarily. This suggests that the reactivation of the autoreactive cells, together with elevated parasite-specific immunity, is largely responsible for the mild, chronic inflammation. While not completely protected from T. cruzi, these “immunized” mice may have an enhanced ability to clear the parasite. In fact, reinfection with the original Brazil strain did not induce myosin-specific DTH (data not shown). This may be due to the strong anti-T. cruzi Brazil strain immunity (high specific DTH and antibody levels), which protected these animals (no parasitosis, parasitemia, or myocarditis). The absence of myocarditis after reinfection with the original Brazil strain could mean no damage, no presentation of myosin, no myosin autoimmunity, and therefore no myosin-specific DTH. From the perspective of treating human infections in which individuals can be infected or reinfected with various parasitic strains at any time, these results suggest that elimination of the parasite may be the best option to eliminate autoimmunity and maintain self-tolerance. Of course, these results also suggest that an effective T. cruzi vaccine must prevent T. cruzi-associated damage due to exposure to highly virulent strains, which may involve autoimmunity.
The overall importance of this last suggestion is debatable since it is not at all clear whether autoimmunity associated with T. cruzi infection in humans is pathogenic. The controversy surrounding autoimmunity as a major mechanism of pathogenesis in Chagas’ disease is currently under investigation by a number of laboratories. This study establishes a direct association between the parasite load and the magnitude of cardiac autoimmunity in experimental T. cruzi infection. This line of experimentation encourages the future exploration of T. cruzi infection-induced autoimmunity and the relationship of inflammation and damage to autoimmunity in all autoimmune diseases. Areas of current study include the mechanism by which autoimmunity resolves upon drug treatment and the functional immunology of parasite-specific and myosin-specific lymphocytes in this powerful model of infection-induced myocarditis. Finally, because treatment with the trypanocidal drug trifluralin (70) or TAK-187 also prevents cardiac damage in an experimental model of Chagas’ disease (7), it will be interesting to know whether autoimmunity is reduced in these animals as well.
Acknowledgments
There is no conflict of interest for any of the authors of this report due to either commercial or other affiliations.
This work was supported by NIH grant HL075822 (to D.M.E.), NIH training grant AI007476 (to M.D.D.), predoctoral fellowships from the American Heart Association (to K.V.H. and J.S.L.), and a postdoctoral fellowship from the Irvington-Dana Institute (to J.S.L.).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Abel, L. C., J. Kalil, and E. Cunha Neto. 1997. Molecular mimicry between cardiac myosin and Trypanosoma cruzi antigen B13: identification of a B13-driven human T cell clone that recognizes cardiac myosin. Braz. J. Med. Biol. Res. 30:1305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Achtman, A. H., P. C. Bull, R. Stephens, and J. Langhorne. 2005. Longevity of the immune response and memory to blood-stage malaria infection. Curr. Top. Microbiol. Immunol. 297:71-102. [DOI] [PubMed] [Google Scholar]
- 3.Acosta Rodriguez, E. V., E. Zuniga, C. L. Montes, and A. Gruppi. 2003. Interleukin-4 biases differentiation of B cells from Trypanosoma cruzi-infected mice and restrains their fratricide: role of Fas ligand down-regulation and MHC class II-transactivator up-regulation. J. Leukoc. Biol. 73:127-136. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, N. W., and M. B. Whitlow. 1989. Secretion by Trypanosoma cruzi of a hemolysin active at low pH. Mol. Biochem. Parasitol. 33:249-256. [DOI] [PubMed] [Google Scholar]
- 5.Avila, J. L. 1992. Molecular mimicry between Trypanosoma cruzi and host nervous tissues. Acta Cient. Venez. 43:330-340. [PubMed] [Google Scholar]
- 6.Chen, A. M., N. Khanna, S. A. Stohlman, and C. C. Bergmann. 2005. Virus-specific and bystander CD8 T cells recruited during virus-induced encephalomyelitis. J. Virol. 79:4700-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrales, M., R. Cardozo, M. A. Segura, J. A. Urbina, and M. A. Basombrio. 2005. Comparative efficacies of TAK-187, a long-lasting ergosterol biosynthesis inhibitor, and benznidazole in preventing cardiac damage in a murine model of Chagas’ disease. Antimicrob. Agents Chemother. 49:1556-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings, K. L., and R. L. Tarleton. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 129:53-59. [DOI] [PubMed] [Google Scholar]
- 9.Cunha-Neto, E., V. Coelho, L. Guilherme, A. Fiorelli, N. Stolf, and J. Kalil. 1996. Autoimmunity in Chagas’ disease. Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas’ cardiomyopathy patient. J. Clin. Investig. 98:1709-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunha-Neto, E., M. Duranti, A. Gruber, B. Zingales, I. de Messias, N. Stolf, G. Bellotti, M. E. Patarroyo, F. Pilleggi, and J. Kalil. 1995. Autoimmunity in Chagas’ disease cardiomyopathy: biological relevance of a cardiac myosin-specific epitope crossreactive to an immunodominant Trypanosoma cruzi antigen. Proc. Natl. Acad. Sci. USA 92:3541-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham, M. W. 2003. Autoimmunity and molecular mimicry in the pathogenesis of post-streptococcal heart disease. Front. Biosci. 8:s533-s543. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham, M. W. 2004. T cell mimicry in inflammatory heart disease. Mol. Immunol. 40:1121-1127. [DOI] [PubMed] [Google Scholar]
- 13.Davila, D. F., R. O. Rossell, and J. H. Donis. 1989. Cardiac parasympathetic abnormalities: cause or consequence of Chagas heart disease? Parasitol. Today 5:327-329. [DOI] [PubMed] [Google Scholar]
- 14.de Gaspari, E. N., E. S. Umezawa, B. Zingales, A. M. Stolf, W. Colli, and I. A. Abrahamsohn. 1990. Trypanosoma cruzi: serum antibody reactivity to the parasite antigens in susceptible and resistant mice. Mem. Inst. Oswaldo Cruz 85:261-270. [DOI] [PubMed] [Google Scholar]
- 15.Docampo, R., and S. N. Moreno. 1984. Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev. Infect. Dis. 6:223-238. [DOI] [PubMed] [Google Scholar]
- 16.dos Santos, R. R., M. A. Rossi, J. L. Laus, J. S. Silva, W. Savino, and J. Mengel. 1992. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J. Exp. Med. 175:29-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisen, H., and S. Kahn. 1991. Mimicry in Trypanosoma cruzi: fantasy and reality. Curr. Opin. Immunol. 3:507-510. [DOI] [PubMed] [Google Scholar]
- 18.Engman, D. M., and J. S. Leon. 2002. Pathogenesis of Chagas heart disease: role of autoimmunity. Acta Trop. 81:123-132. [DOI] [PubMed] [Google Scholar]
- 19.Frank, F. M., P. B. Petray, S. I. Cazorla, M. C. Munoz, R. S. Corral, and E. L. Malchiodi. 2003. Use of a purified Trypanosoma cruzi antigen and CpG oligodeoxynucleotides for immunoprotection against a lethal challenge with trypomastigotes. Vaccine 22:77-86. [DOI] [PubMed] [Google Scholar]
- 20.Gattass, C. R., M. T. Lima, A. F. Nobrega, M. A. Barcinski, and G. A. Dos Reis. 1988. Do self-heart-reactive T cells expand in Trypanosoma cruzi-immune hosts? Infect. Immun. 56:1402-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gironès, N., and M. Fresno. 2003. Etiology of Chagas disease myocarditis: autoimmunity, parasite persistence, or both? Trends Parasitol. 19:19-22. [DOI] [PubMed] [Google Scholar]
- 22.Girones, N., C. I. Rodriguez, B. Basso, J. M. Bellon, S. Resino, M. A. Munoz-Fernandez, S. Gea, E. Moretti, and M. Fresno. 2001. Antibodies to an epitope from the Cha human autoantigen are markers of Chagas’ disease. Clin. Diagn. Lab. Immunol. 8:1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwai, L. K., M. A. Juliano, L. Juliano, J. Kalil, and E. Cunha-Neto. 2005. T-cell molecular mimicry in Chagas disease: identification and partial structural analysis of multiple cross-reactive epitopes between Trypanosoma cruzi B13 and cardiac myosin heavy chain. J. Autoimmun. 24:111-117. [DOI] [PubMed] [Google Scholar]
- 24.Kalil, J., and E. Cunha-Neto. 1996. Autoimmunity in Chagas disease cardiomyopathy: fulfilling the criteria at last? Parasitol. Today 12:396-399. [DOI] [PubMed] [Google Scholar]
- 25.Kerner, N., P. Liegeard, M. J. Levin, and M. Hontebeyrie-Joskowicz. 1991. Trypanosoma cruzi: antibodies to a MAP-like protein in chronic Chagas’ disease cross-react with mammalian cytoskeleton. Exp. Parasitol. 73:451-459. [DOI] [PubMed] [Google Scholar]
- 26.Kierszenbaum, F. 1999. Chagas’ disease and the autoimmunity hypothesis. Clin. Microbiol. Rev. 12:210-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kierszenbaum, F. 1996. Chronic chagasic tissue lesions in the absence of Trypanosoma cruzi: a proposed mechanism. Parasitol. Today 12:414-415. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhoff, L. V., L. M. Weiss, M. Wittner, and H. B. Tanowitz. 2004. Parasitic diseases of the heart. Front. Biosci. 9:706-723. [DOI] [PubMed] [Google Scholar]
- 29.Laucella, S. A., E. L. Segura, A. Riarte, and E. S. Sosa. 1999. Soluble platelet selectin (sP-selectin) and soluble vascular cell adhesion molecule-1 (sVCAM-1) decrease during therapy with benznidazole in children with indeterminate form of Chagas’ disease. Clin. Exp. Immunol. 118:423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leon, J. S., M. D. Daniels, K. M. Toriello, K. Wang, and D. M. Engman. 2004. A cardiac myosin-specific autoimmune response is induced by immunization with Trypanosoma cruzi proteins. Infect. Immun. 72:3410-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon, J. S., and D. M. Engman. 2001. Autoimmunity in Chagas heart disease. Int. J. Parasitol. 31:554-560. [DOI] [PubMed] [Google Scholar]
- 32.Leon, J. S., and D. M. Engman. 2003. The contribution of autoimmunity to Chagas disease?, p. 97-106. In K. M. Tyler and M. A. Miles (ed.), World class parasites: American trypanosomiasis, vol. 7. Kluwer Academic Publishers, Boston, MA. [Google Scholar]
- 33.Leon, J. S., and D. M. Engman. 2003. The significance of autoimmunity in the pathogenesis of Chagas heart disease. Front. Biosci. 8:e315-e322. [DOI] [PubMed] [Google Scholar]
- 34.Leon, J. S., L. M. Godsel, K. Wang, and D. M. Engman. 2001. Cardiac myosin autoimmunity in acute Chagas’ heart disease. Infect. Immun. 69:5643-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon, J. S., K. Wang, and D. M. Engman. 2003. Myosin autoimmunity is not essential for cardiac inflammation in acute Chagas’ disease. J. Immunol. 171:4271-4277. [DOI] [PubMed] [Google Scholar]
- 36.Levin, M. J., E. Mesri, R. Benarous, G. Levitus, A. Schijman, P. Levy-Yeyati, P. A. Chiale, A. M. Ruiz, A. Kahn, M. B. Rosenbaum, H. N. Torres, and E. L. Segura. 1989. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas’ heart disease. Am. J. Trop. Med. Hyg. 41:530-538. [DOI] [PubMed] [Google Scholar]
- 37.Levitus, G., M. Hontebeyrie-Joskowicz, M. H. Van Regenmortel, and M. J. Levin. 1991. Humoral autoimmune response to ribosomal P proteins in chronic Chagas heart disease. Clin. Exp. Immunol. 85:413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin, D. L., and R. L. Tarleton. 2005. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J. Immunol. 174:1594-1601. [DOI] [PubMed] [Google Scholar]
- 39.Michailowsky, V., S. M. Murta, L. Carvalho-Oliveira, M. E. Pereira, L. R. Ferreira, Z. Brener, A. J. Romanha, and R. T. Gazzinelli. 1998. Interleukin-12 enhances in vivo parasiticidal effect of benznidazole during acute experimental infection with a naturally drug-resistant strain of Trypanosoma cruzi. Antimicrob. Agents Chemother. 42:2549-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minoprio, P., M. C. el Cheikh, E. Murphy, M. Hontebeyrie-Joskowicz, R. Coffman, A. Coutinho, and A. O'Garra. 1993. Xid-associated resistance to experimental Chagas’ disease is IFN-gamma dependent. J. Immunol. 151:4200-4208. [PubMed] [Google Scholar]
- 41.Moncayo, A. 2003. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem. Inst. Oswaldo Cruz 98:577-591. [DOI] [PubMed] [Google Scholar]
- 42.Moncayo, A. 1999. Progress towards interruption of transmission of Chagas disease. Mem. Inst. Oswaldo Cruz 94(Suppl. 1):401-404. [DOI] [PubMed] [Google Scholar]
- 43.Moraru, M., A. Roth, G. Keren, and J. George. 2006. Cellular autoimmunity to cardiac myosin in patients with a recent myocardial infarction. Int. J. Cardiol. 107:61-66. [DOI] [PubMed] [Google Scholar]
- 44.Müllbacher, A., and K. Flynn. 1996. Aspects of cytotoxic T cell memory. Immunol. Rev. 150:113-127. [DOI] [PubMed] [Google Scholar]
- 45.Murta, S. M., C. Ropert, R. O. Alves, R. T. Gazzinelli, and A. J. Romanha. 1999. In-vivo treatment with benznidazole enhances phagocytosis, parasite destruction and cytokine release by macrophages during infection with a drug-susceptible but not with a derived drug-resistant Trypanosoma cruzi population. Parasite Immunol. 21:535-544. [DOI] [PubMed] [Google Scholar]
- 46.Oldstone, M. B. 1987. Molecular mimicry and autoimmune disease. Cell 50:819-820. [DOI] [PubMed] [Google Scholar]
- 47.Olivieri, B. P., V. Cotta-De-Almeida, and T. Araujo-Jorge. 2002. Benznidazole treatment following acute Trypanosoma cruzi infection triggers CD8+ T-cell expansion and promotes resistance to reinfection. Antimicrob. Agents Chemother. 46:3790-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pontes-de-Carvalho, L., C. C. Santana, M. B. Soares, G. G. Oliveira, E. Cunha-Neto, and R. Ribeiro Dos Santos. 2002. Experimental chronic Chagas’ disease myocarditis is an autoimmune disease preventable by induction of immunological tolerance to myocardial antigens. J. Autoimmun. 18:131-138. [DOI] [PubMed] [Google Scholar]
- 49.Prata, A. 1994. Chagas’ disease. Infect. Dis. Clin. N. Am. 8:61-77. [PubMed] [Google Scholar]
- 50.Revelli, S., C. Le Page, E. Piaggio, J. Wietzerbin, and O. Bottasso. 1999. Benznidazole, a drug employed in the treatment of Chagas’ disease, down-regulates the synthesis of nitrite and cytokines by murine stimulated macrophages. Clin. Exp. Immunol. 118:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzo, L. V., E. Cunha-Neto, and A. R. Teixeira. 1989. Autoimmunity in Chagas’ disease: specific inhibition of reactivity of CD4+ T cells against myosin in mice chronically infected with Trypanosoma cruzi. Infect. Immun. 57:2640-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romanha, A. J., R. O. Alves, S. M. Murta, J. S. Silva, C. Ropert, and R. T. Gazzinelli. 2002. Experimental chemotherapy against Trypanosoma cruzi infection: essential role of endogenous interferon-gamma in mediating parasitologic cure. J. Infect. Dis. 186:823-828. [DOI] [PubMed] [Google Scholar]
- 53.Rossi, M. A. 1990. Microvascular changes as a cause of chronic cardiomyopathy in Chagas’ disease. Am. Heart J. 120:233-236. [DOI] [PubMed] [Google Scholar]
- 54.Santos-Buch, C. A., and A. M. Acosta. 1985. Pathology of Chagas disease, p. 145-184. In I. Tizard (ed.), Immunology and pathogenesis of trypanosomiasis. CRC Press, Boca Raton, FL.
- 55.Schijman, A. G., C. A. Vigliano, R. J. Viotti, J. M. Burgos, S. Brandariz, B. E. Lococo, M. I. Leze, H. A. Armenti, and M. J. Levin. 2004. Trypanosoma cruzi DNA in cardiac lesions of Argentinean patients with end-stage chronic Chagas heart disease. Am. J. Trop. Med. Hyg. 70:210-220. [PubMed] [Google Scholar]
- 56.Soares, M. B., L. Pontes-De-Carvalho, and R. Ribeiro-Dos-Santos. 2001. The pathogenesis of Chagas’ disease: when autoimmune and parasite-specific immune responses meet. An. Acad. Bras. Cienc. 73:547-559. [DOI] [PubMed] [Google Scholar]
- 57.Sterin-Borda, L., and E. Borda. 2000. Role of neurotransmitter autoantibodies in the pathogenesis of chagasic peripheral dysautonomia. Ann. N. Y. Acad. Sci. 917:273-280. [DOI] [PubMed] [Google Scholar]
- 58.Sterin-Borda, L., C. Perez Leiros, M. Wald, G. Cremaschi, and E. Borda. 1988. Antibodies to beta 1 and beta 2 adrenoreceptors in Chagas’ disease. Clin. Exp. Immunol. 74:349-354. [PMC free article] [PubMed] [Google Scholar]
- 59.Tarleton, R. L. 2003. Chagas disease: a role for autoimmunity? Trends Parasitol. 19:447-451. [DOI] [PubMed] [Google Scholar]
- 60.Tarleton, R. L. 2001. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 31:550-554. [DOI] [PubMed] [Google Scholar]
- 61.Tarleton, R. L., M. J. Grusby, and L. Zhang. 2000. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J. Immunol. 165:1520-1525. [DOI] [PubMed] [Google Scholar]
- 62.Tarleton, R. L., and R. E. Kuhn. 1983. Changes in cell populations and immunoglobulin-producing cells in the spleens of mice infected with Trypanosoma cruzi: correlations with parasite-specific antibody response. Cell. Immunol. 80:392-404. [DOI] [PubMed] [Google Scholar]
- 63.Tarleton, R. L., and L. Zhang. 1999. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol. Today 15:94-99. [DOI] [PubMed] [Google Scholar]
- 64.Teixeira, A. R. L., G. Teixeira, V. Macedo, and A. Prata. 1978. Trypanosoma cruzi sensitized T-lymphocyte mediated 51Cr release from human heart cells in Chagas’ disease. Am. J. Trop. Med. Hyg. 27:1097-1107. [DOI] [PubMed] [Google Scholar]
- 65.Tibbetts, R. S., T. S. McCormick, E. C. Rowland, S. D. Miller, and D. M. Engman. 1994. Cardiac antigen-specific autoantibody production is associated with cardiomyopathy in Trypanosoma cruzi-infected mice. J. Immunol. 152:1493-1499. [PubMed] [Google Scholar]
- 66.Urbina, J. A., and R. Docampo. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 19:495-501. [DOI] [PubMed] [Google Scholar]
- 67.Van Voorhis, W. C., and H. Eisen. 1989. Fl-160: a surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J. Exp. Med. 169:641-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Voorhis, W. C., L. Schlekewy, and H. L. Trong. 1991. Molecular mimicry by Trypanosoma cruzi: the F1-160 epitope that mimics mammalian nerve can be mapped to a 12-amino acid peptide. Proc. Natl. Acad. Sci. USA 88:5993-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wizel, B., M. Palmieri, C. Mendoza, B. Arana, J. Sidney, A. Sette, and R. L. Tarleton. 1998. Human infection with Trypanosoma cruzi induces parasite antigen-specific cytotoxic T lymphocyte responses. J. Clin. Investig. 102:1062-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaidenberg, A., T. Luong, D. Lirussi, J. Bleiz, M. B. Del Buono, G. Quijano, R. Drut, L. Kozubsky, A. Marron, and H. Buschiazzo. 2006. Treatment of experimental chronic Chagas disease with trifluralin. Basic Clin. Pharmacol. Toxicol. 98:351-356. [DOI] [PubMed] [Google Scholar]
- 71.Zevering, Y., C. Khamboonruang, K. Rungruengthanakit, L. Tungviboonchai, J. Ruengpipattanapan, I. Bathurst, P. Barr, and M. Good. 1994. Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax. Proc. Natl. Acad. Sci. USA 91:6118-6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, L., and R. L. Tarleton. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J. Infect. Dis. 180:480-486. [DOI] [PubMed] [Google Scholar]