Abstract
Neutrophils play a prominent role in host defense. Phagocytosis of bacteria leads to the formation of an active NADPH oxidase complex that generates reactive oxygen species for bactericidal purposes. A critical step in the resolution of inflammation is the uptake of neutrophils by macrophages; however, there are conflicting reports on the mechanisms leading to the apoptosis of phagocytic neutrophils. The aim of this study was to clarify the role of effector caspases in these processes. Caspase activity was measured by DEVDase activity assays or immunofluorescence detection of active caspase-3. With normal human and wild-type murine neutrophils there was no caspase activation following phagocytosis of Staphylococcus aureus. However, caspase activity was observed in phagocytic neutrophils with a defective NADPH oxidase, including neutrophils isolated from X-linked gp91phox knockout chronic granulomatous disease mice. These results indicate that a functional NADPH oxidase and the generation of oxidants in the neutrophil phagosome prevent the activation of the cytoplasmic caspase cascade.
Neutrophils play an integral role in the eradication of pathogens from the body. These cells contain a range of toxic compounds, and it is essential that they remain intact without releasing intracellular contents that might damage host tissue. Following maturation and release from the bone marrow, the circulating neutrophil has a life span of 1 to 2 days before it undergoes apoptosis (26). This process has been well characterized and includes caspase activation, phosphatidylserine (PS) exposure, and phagocytosis of the dying cell (8, 9, 27). The rate of this spontaneous apoptosis is susceptible to modulation. If neutrophils are exposed to proinflammatory cytokines, such as interleukins, granulocyte-macrophage colony-stimulating factor, or bacterial lipopolysaccharide, the apoptotic process is delayed (3, 5), thereby prolonging the neutrophil life span and presumably the ability of neutrophils to contribute to pathogen removal.
At an inflammatory site, neutrophils ingest pathogens into intracellular compartments called phagosomes, where killing occurs (11). Phagocytic neutrophils are themselves ingested by macrophages before they disintegrate. However, the mechanism of apoptosis in phagocytic neutrophils appears to be more complicated and controversial than the spontaneous apoptosis of unstimulated cells. Both acceleration (18, 21, 23, 34-36) and retardation (1, 23, 31) of neutrophil apoptosis in actively phagocytosing neutrophils have been reported.
Stimulated neutrophils generate vast amounts of oxidants upon activation of the NADPH oxidase membrane complex within the phagosome (11), and several studies have demonstrated that these oxidants promote the onset of neutrophil apoptosis (7, 14, 23, 24, 28, 34). However, the role of the caspases in this process is unclear. These enzymes play a key role in the cascade of proteolytic cleavage that occurs during apoptosis. Their activation is redox sensitive (12, 13), and we have previously shown that triggering the oxidative burst with phorbol myristate acetate (PMA) blocks caspase activation (8). The situation during phagocytosis, however, differs from that with the artificial PMA stimulus. In particular, oxidant production is generally considered to be restricted to the internal phagosome during phagocytosis, compared with NADPH oxidase activation over the entire plasma membrane with PMA (32). The duration and extent of the oxidative burst also vary in the two systems. There have been two reports suggesting that in contrast to the PMA model, caspases are activated during the apoptosis of phagocytic neutrophils (24, 35).
In this study we investigated the effect of NADPH oxidase on caspase activation in phagocytic neutrophils. The model used involved phagocytosis of Staphylococcus aureus by neutrophils isolated from X-linked gp91phox knockout mice with a nonfunctional NADPH oxidase. We found that while neutrophil oxidant generation is required for PS exposure and uptake by macrophages, the oxidants clearly prevented caspase activation. Indeed, inhibition of the oxidative burst led to enhanced caspase activation but decreased clearance by macrophages. This indicates that the clearance of actively phagocytosing neutrophils is a caspase-independent process.
MATERIALS AND METHODS
Materials.
S. aureus strain 502a (ATCC 27217) was obtained from the New Zealand Communicable Disease Centre (Porirua, New Zealand), and Trypticase soy broth was obtained from Becton Dickinson (Cockeysville, MD). Cell culture medium was supplied by Gibco-BRL (Grand Island, NY), and normal mouse serum was supplied by Biomeda (Foster City, CA). The caspase-3 substrate DEVD-AMC was obtained from Peptide Institute Inc. (Osaka, Japan), and the caspase inhibitor z-VAD-fmk was obtained from Enzyme Systems Products (Livermore, CA). An annexin V-fluorescein isothiocyanate (FITC) Apoptest kit was obtained from Dakocytomation (Glostrup, Denmark [for NeXins Research, Kattendijke, The Netherlands]). Monoclonal rabbit anti-human cleaved caspase-3 antibody was obtained from Cell Signaling Technology Inc. (Beverly, MA), and Diff-Quik was obtained from Dade Behring AG (Dudingen, Switzerland). Diphenyleneiodonium (DPI), paraformaldehyde, o-dianisidine, and thioglycolate were all obtained from Sigma Chemical Co. (St. Louis, MO).
Preparation of S. aureus.
S. aureus was cultured overnight in Trypticase soy broth, harvested by centrifugation, washed, and resuspended in Hanks balanced salt solution (HBSS) (10 mM phosphate buffer [pH 7.4] containing 140 mM NaCl, 0.5 mM MgCl2, 1 mM CaCl2, and 1 mg/ml glucose). Bacterial cell density was measured spectrophotometrically at 550 nm, and the cell number was calculated using a standard curve based on CFU counts. Heat-killed propidium iodide (PI)-labeled S. aureus was prepared by heating 109 S. aureus cells/ml at 90°C for 30 min before adding 2 μg/ml PI. Bacteria were then pelleted, the supernatant was discarded to remove excess PI, and the cells were resuspended in HBSS. All bacteria were opsonized with 10% autologous human serum or commercially obtained mouse serum and rotated end over end for 20 min at 37°C immediately before addition to neutrophils at defined ratios.
Isolation of human and mouse neutrophils.
Neutrophils were isolated from heparinized peripheral blood of healthy adult donors under sterile conditions by Ficoll-Hypaque centrifugation, dextran sedimentation, and hypotonic lysis. Mouse neutrophils were obtained from the peritoneal cavities of X-linked gp91phox knockout and C57BL/6 wild-type mice 18 h after injection of 1 ml of 4% thioglycolate, as approved by the University of Otago Animal Ethics Committee. Cells collected in HBSS supplemented with 0.1% bovine serum albumin were spun through 1.5 ml of Ficoll-Hypaque at 1,000 × g for 20 min to concentrate the neutrophils.
Neutrophil stimulation.
Human or murine neutrophils (107 cells/ml) were incubated at 37°C in RPMI 1640 medium with 10% autologous or mouse serum for 10 min in the presence or absence of 10 μM DPI. DPI-pretreated or untreated neutrophils were then incubated at 37°C in 5% CO2 for up to 5 h with occasional mixing with opsonized S. aureus to obtain a ratio of 2, 10, 20, or 50 bacteria per neutrophil. At various intervals cells were harvested, and cytospins were prepared by first removing any nonphagocytosed bacteria by differential centrifugation at 100 × g (15), followed by cytocentrifugation for 5 min at 250 rpm onto high-binding microscope glass slides. Each spot was fixed with 4% (wt/vol) paraformaldehyde (pH 7.4) and stained with Diff-Quik. The number of cells that had phagocytosed S. aureus was determined, and the morphology was observed.
Fluorometric analysis of effector caspase activity.
Neutrophils (106 cells) that were untreated or pretreated with DPI were incubated without S. aureus or with S. aureus at ratios of 1:2, 1:10, 1:20, and 1:50 and centrifuged at selected times to remove the medium. The pellets were washed with phosphate-buffered saline, and caspase-3-like activity was determined by assessment of DEVD-AMC cleavage. Briefly, pellets were transferred to a microtiter plate and resuspended in 100 μl (final volume) of a caspase buffer solution containing 100 mM HEPES, 10% sucrose, 0.1% NP-40, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), and 5 mM dithiothreitol (pH 7.2) supplemented with 50 μM of the fluorogenic peptide substrate Ac-DEVD-AMC. The cleavage of the caspase substrate was monitored over a 30-min period at 37°C in a Fluoroscan II plate reader using an excitation wavelength of 390 nm and an emission wavelength of 460 nm.
Immunofluorescence analysis and quantification of caspase-3.
Neutrophils (107 cells/ml) pretreated with or without DPI were incubated with or without PI-labeled S. aureus (1:20) for 3 h at 37°C. Nonphagocytic neutrophils and phagocytic neutrophils at a cell/bacterium ratio of 1:20 were cytospun onto the same microscope slide to obtain a mixed population of cells. The active form of caspase-3 was detected by immunofluorescence using a previously described method (4), a monoclonal rabbit anti-human cleaved caspase-3 antibody, and FITC-conjugated goat anti-rabbit immunoglobulin G. A Leitz Aristoplan fluorescence microscope was used to capture images of cleaved caspase-3 immunofluorescence (green fluorescence) and red fluorescent PI-labeled neutrophils. Color composite images were then processed using a high-throughput image analysis program (Metamorph V.6.2.6; Molecular Devices) to determine the average fluorescence intensity of cleaved caspase-3 staining in phagocytic (red fluorescence) and nonphagocytic (no red fluorescence) neutrophils. A “journal” was written to automatically color separate the red and green channels, convert each image into a 16-bit image format for analysis, and then insert each pair of 16-bit images into the Cell Scoring application of Metamorph. The Cell Scoring algorithm determined the average fluorescence intensity of cleaved caspase-3 staining in phagocytic (red fluorescence) and nonphagocytic (no red fluorescence) neutrophils and automatically logged the data into Excel spreadsheets. The advantages of this method for quantifying imaging data are that it is fully automated, objective, and standardized. Further information about this high-throughput image analysis platform can be found at http://www.health.auckland.ac.nz/pharmacology/discovery-1/.
Exposure of PS.
The externalization of PS was assessed using annexin V-FITC according to the protocol outlined in the Apoptest-FITC kit. Untreated or DPI-pretreated wild-type or chronic granulomatous disease (CGD) murine neutrophils were incubated with S. aureus at a 1:20 ratio in the presence or absence of the caspase inhibitor z-VAD-fmk (10 μM) for 4 h at 37°C. Binding was assessed using calcium-free HEPES buffer with EGTA (10 mM HEPES-NaOH [pH 7.4], 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM EGTA) to confirm that fluorescence increases were due to annexin V binding, which is calcium dependent, and was also assessed without any addition of annexin V-FITC. Flow cytometry was performed with a FACS Vantage from Becton Dickinson (San Jose, CA), and the data were analyzed using CellQuest software. Ten thousand events were analyzed, and both the geometric mean fluorescence of viable cells and the percentage of cells displaying more than 10 fluorescent units were recorded. PI-positive necrotic neutrophils (10 to 17%) were gated out of the final analysis.
Uptake of phagocytic neutrophils by macrophages.
Human monocyte-derived macrophages were prepared by adhering the peripheral blood monocyte layer after Ficoll-Hypaque centrifugation for 2 h on a 24-well tissue culture plate (5 × 106 cells/well) (14). Following vigorous washing with HBSS, adherent monocytes were cultured in Iscove's modified Dulbecco's medium with 10% serum for 7 days. Mouse neutrophils (106 cells) that were not treated or were pretreated with DPI were incubated with or without S. aureus (1:20) for 4 h at 37°C, harvested, and resuspended in 500 μl HBSS. Iscove's modified Dulbecco's medium was removed from the 7-day macrophage wells and replaced with the neutrophils in HBSS, and the preparations were incubated for 1 h at 37°C. Wells were then fixed with 4% paraformaldehyde for 10 min, and neutrophils were visualized with a myeloperoxidase stain (1 mM o-dianisidine, 5 mM H2O2, 50 mM sodium phosphate buffer; pH 6). The number of neutrophils phagocytosed per 100 macrophages was determined.
Statistics.
Statistical analysis was performed with the SigmaStat software package from Jandel Scientific (SPSS Science, Chicago, IL) using repeated-measures analysis of variance, followed by the Bonferroni test for multiple comparisons.
RESULTS
Caspase activation in human phagocytic neutrophils.
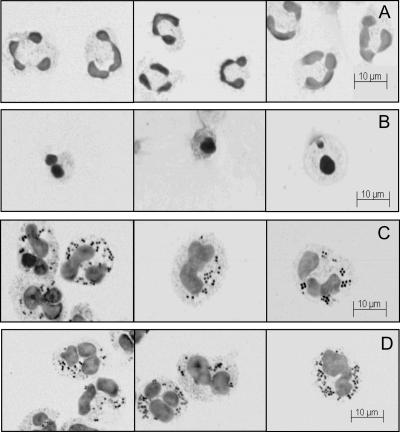
We have previously established that artificial stimulation of the oxidative burst in neutrophils by a phorbol ester can prevent activation of the redox-sensitive caspases (8). To determine whether this phenomenon is physiological, we investigated the effect of NADPH oxidase-derived oxidants on caspase activity in neutrophils phagocytosing bacteria. In our system neutrophils were incubated with opsonized S. aureus at ratios ranging from 2 to 50 bacteria per neutrophil and harvested at various intervals. We have previously demonstrated that with continual mixing S. aureus is rapidly internalized (half-life, 9 min [15]). In this system we used occasional mixing to prevent excessive neutrophil damage, and all neutrophils had bacteria in their cytoplasm within 30 min. None of the inhibitors used in this study had any effect on the kinetics of uptake. Phagocytic neutrophils lost their characteristic multilobed nuclei at 3 h (Fig. 1C) and instead displayed nuclei that were swollen and irregular in shape. These nuclei were very different from the shrunken and condensed nuclei in neutrophils that had undergone spontaneous apoptosis at 48 h (Fig. 1B). At 5 h swollen nuclei were still apparent (Fig. 1D), and the results at later time points were impossible to assess because the phagocytic neutrophils were too fragile to survive the cytospin process.
FIG. 1.
Phagocytosis of S. aureus by human neutrophils and subsequent changes in morphology. Photographs from a representative experiment for cytospins of human unstimulated neutrophils at 3 h (A), of untreated neutrophils after 48 h of incubation (B), of neutrophils with S. aureus at 3 h (C), and of neutrophils with S. aureus at 5 h (D).
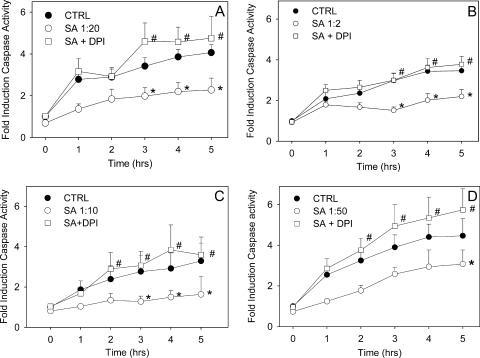
There was a three- to fourfold increase in the activity of effector caspases measured with the fluorescent substrate DEVD-AMC in unstimulated human neutrophils over 5 h (Fig. 2A). This reflected initiation of spontaneous apoptosis. In contrast, caspase activation was delayed following phagocytosis of S. aureus (Fig. 2A). DPI treatment of phagocytic neutrophils resulted in enhanced caspase activation (Fig. 2A). DPI alone had no effect on the rate of spontaneous caspase activity. S. aureus itself had no detectable caspase activity, and it was also not able to inhibit caspases, as demonstrated by the significant levels of caspase activity seen in DPI-treated phagocytic neutrophils. Addition of the supernatant of cultured S. aureus had no influence on the levels of caspase activation observed in nonphagocytic neutrophils (not shown), demonstrating that the inhibition of caspase activity observed in neutrophils coincubated with S. aureus was indeed due to phagocytosis and not due to a soluble factor secreted by the bacteria.
FIG. 2.
Caspase-3 activation is blocked by NADPH oxidase activity in phagocytic human neutrophils. Caspase activity was assessed hourly in neutrophils incubated alone (CTRL), in neutrophils incubated with S. aureus (SA) at ratios of 1:20 (A), 1:2 (B), 1:10 (C), and 1:50 (D), and in neutrophils treated with DPI prior to coincubation with S. aureus (SA + DPI). The active caspase-3 activity was measured by monitoring the arbitrary units of fluorescence liberated following cleavage of the fluorogenic peptide substrate DEVD-AMC. The means and standard errors of the means of four to nine experiments are shown. An asterisk indicates that the P value is <0.05 for a comparison with the control, and a number sign indicates that the P value is <0.05 for a comparison with phagocytic neutrophils with a functional NADPH oxidase.
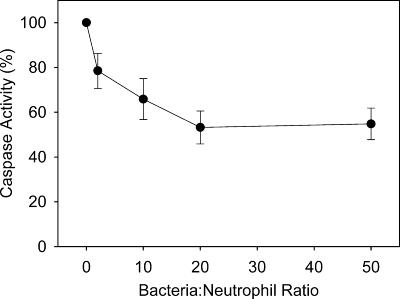
We explored the effects of different neutrophil/S. aureus ratios on caspase activation. After 1 h of incubation ratio-dependent inhibition of caspase activity was observed (Fig. 3). However, over the 5-h time course there was little difference between ratios (Fig. 2B to D). Cytospins at 3 h showed that although the cultures began at a ratio of 1:2, bacterial growth during this period meant that the ratio was higher at later time points (not shown). Phagocytic neutrophils pretreated with DPI at all ratios showed levels of caspase activity either equivalent to or slightly greater than the levels of cells undergoing spontaneous apoptosis (Fig. 2A to D).
FIG. 3.
Dose-dependent inhibition of caspase activity. Caspase activity in neutrophils incubated with S. aureus at ratios of 1:2, 1:10, 1:20, and 1:50 after 1 h of incubation is expressed as a percentage of the activity expressed at 1 h in unstimulated neutrophils.
Immunofluorescence analysis of active caspase-3.
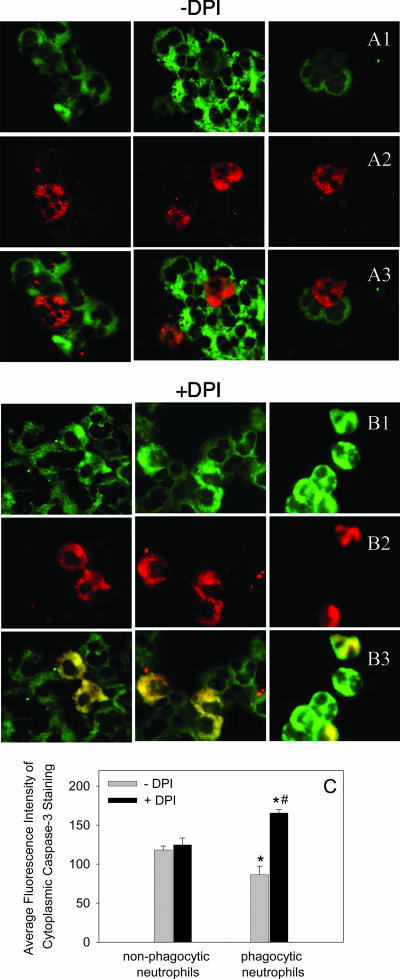
To confirm the caspase activity assays, we used an immunofluorescence technique that detects the active form of caspase-3 in individual cells. Neutrophils were incubated with or without PI-labeled S. aureus for 3 h, and the two populations of cells were mixed immediately prior to cytospin to enable direct comparison between phagocytic and unstimulated neutrophils. Nonphagocytic neutrophils undergoing spontaneous apoptosis showed strong active caspase-3 staining within the cytoplasm (Fig. 4A). In contrast, neutrophils that had phagocytosed PI-labeled S. aureus displayed reduced cytosolic active caspase-3 staining (Fig. 4A). Inhibition of the NADPH oxidase by DPI, however, resulted in the phagocytic neutrophils demonstrating caspase-3 fluorescence staining equal to or greater than that of nonphagocytic cells (Fig. 4B).
FIG. 4.
Immunofluorescence staining for active caspase-3. Neutrophils were incubated either alone or with PI-labeled S. aureus (1:20) for 3 h, and then both phagocytic and nonphagocytic neutrophils were mixed and cytospun onto the same microscope slide. Cells were treated with a cleaved caspase-3 antibody, followed by a secondary antibody conjugated to FITC, and were visualized with a fluorescent microscope. Images of the green fluorescence (A1 and B1) showing positive caspase-3 staining localized in the cytosol and the red fluorescence (A2 and B2) identifying phagocytic neutrophils were captured from the same field, and the pairs of images were superimposed (A3 and B3). Untreated phagocytic neutrophils (A2) surrounded by nonphagocytic neutrophils (A3) show negligible caspase-3 activity compared to the activity of the neighboring nonphagocytic cells (A1). When phagocytic neutrophils were pretreated with DPI (B1 to B3) to inhibit the NADPH oxidase, the phagocytic cells adjacent to nonphagocytic neutrophils (B3) displayed enhanced caspase activation (B1). The photographs are from representative experiments. (C) Quantification of caspase-3 fluorescence within untreated and phagocytic neutrophils with or without DPI treatment, analyzed using the Discovery-1 high-throughput and high-content screening machine. Fluorescence intensity was assessed in 300 cells from 27 photographs taken in three different experiments. An asterisk indicates that the P value is <0.05 for a comparison with the control, and a number sign indicates that the P value is <0.05 for a comparison with phagocytic neutrophils with a functional NADPH oxidase.
To quantify these observations, we used a high-throughput image analysis program (Metamorph V.6.2.6; Molecular Devices) to determine the average fluorescence intensities of cleaved caspase-3 staining in PI-labeled phagocytic (red fluorescence) and nonphagocytic (no red fluorescence) neutrophils (Fig. 4C). The results of this objective analysis confirmed our initial observations and showed that there was a statistically significant reduction in cleaved caspase-3 in phagocytic neutrophils compared with nonphagocytic neutrophils, as well as a reversal of the suppression by DPI (Fig. 4C).
Caspase activation in murine phagocytic neutrophils.
DPI is not a specific inhibitor of the NADPH oxidase and is known to have a variety effects on cells (20, 25). To validate our finding that caspase-3 activation is blocked by NADPH oxidase-derived oxidants upon phagocytosis, we assessed caspase activation in phagocytic murine CGD neutrophils with a nonfunctional NADPH oxidase. Neutrophils from the mouse peritoneal cavity were incubated with opsonized S. aureus at a ratio of 1:20 and harvested at selected times. The extents of phagocytosis of S. aureus were comparable for wild-type and CGD murine neutrophils, and no apparent difference in nuclear morphology was observed (not shown).
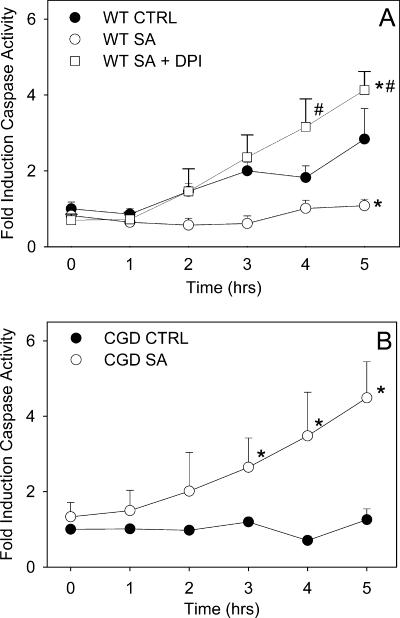
There was a threefold increase in caspase-3 activation in unstimulated wild-type murine neutrophils, which was blocked in the phagocytic neutrophils (Fig. 5A). When the oxidative burst was inhibited by DPI, increased caspase-3 activity was induced in phagocytic cells (Fig. 5A). Unstimulated CGD mouse neutrophils showed no spontaneous caspase-3 activation during the 5-h incubation, but following phagocytosis of S. aureus the levels of caspase activity were comparable to those of DPI-treated phagocytic wild-type neutrophils and increased more than fourfold (Fig. 5B). In combination, these results suggest that the process of phagocytosis can result in caspase activation but that an active NADPH oxidase prevents this from occurring.
FIG. 5.
Caspase-3 activation in phagocytic murine neutrophils. Neutrophils were incubated either alone (CTRL) or with S. aureus (SA) (1:20) or were treated with DPI prior to coincubation with S. aureus (SA + DPI). Caspase-3 activity was assessed hourly by monitoring the increase in fluorescence with excitation at 390 nm and emission at 460 nm following cleavage of the fluorogenic peptide substrate DEVD-AMC. The means and standard errors of the means of 3 to 10 experiments are shown. An asterisk indicates that the P value is <0.05 for a comparison with the control, and a number sign indicates that the P value is <0.05 for a comparison with phagocytic neutrophils with a functional NADPH oxidase. WT, wild type.
PS exposure and uptake of murine phagocytic neutrophils by macrophages.
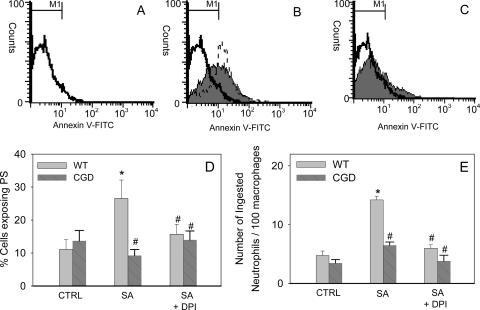
We have previously shown by using pharmacological inhibition that a functional NADPH oxidase is important for PS exposure in human phagocytic neutrophils (14). Therefore, we sought to investigate PS externalization and neutrophil uptake by macrophages in our CGD mouse model, which we have now demonstrated to have elevated caspase activity. PS exposure was assessed by annexin V-FITC labeling in wild-type and CGD mouse neutrophils. Phagocytosis of S. aureus triggered a threefold increase in annexin V-FITC-positive wild-type murine neutrophils (Fig. 6B and D). This increase in fluorescence was not observed in the absence of annexin V-FITC or when a calcium-free buffer was used to prevent the binding of annexin V-FITC to PS (not shown). The increased PS exposure was blocked upon treatment with DPI (Fig. 6C and D). Similarly, neutrophils isolated from CGD mice did not express PS after phagocytosis of S. aureus (Fig. 6D), confirming the importance of a functional NADPH oxidase for PS externalization. The presence of the general caspase inhibitor z-VAD-fmk had no significant effect on the NADPH oxidase-dependent externalization of PS observed in phagocytic neutrophils (Fig. 6B).
FIG. 6.
Phagocytosis triggers PS exposure and uptake by macrophages in murine neutrophils and requires a functional NADPH oxidase. After 4 h of incubation with S. aureus (1:20) cells were stained with annexin V-FITC and PI (A to C). The flow cytometry histograms show the results of a single representative experiment with mouse wild-type neutrophils. (A) Unstimulated neutrophils; (B) neutrophils with S. aureus (shaded area); (C) DPI-treated neutrophils with S. aureus (shaded area). The results of treatment of neutrophils with S. aureus in the presence of the caspase inhibitor z-VAD-fmk are indicated by the dashed line in panel B. (D) Percentage of murine wild-type (WT) and CGD neutrophils exposing PS following incubation alone (CTRL), following incubation with S. aureus (SA), or following pretreatment with DPI (SA + DPI). (E) For macrophage uptake studies, murine neutrophils were incubated with S. aureus, harvested, and layered onto monocyte-derived macrophages, the medium was removed, and the wells were fixed and stained for myeloperoxidase with o-dianisidine, enabling visualization of neutrophils. The number of neutrophils phagocytosed per 100 macrophages was determined. The means and standard errors of the means of three to eight experiments are shown. An asterisk indicates that the P value is <0.05 for a comparison with the control, and a number sign indicates that the P value is <0.05 for a comparison with phagocytic neutrophils with a functional NADPH oxidase.
The key apoptotic process leading to clearance of cells from an inflammatory site is uptake by macrophages. To determine whether macrophages were able to recognize and engulf the phagocytic cells, neutrophils from wild-type and CGD mice were coincubated with S. aureus and then layered onto human monocyte-derived macrophages. We found that macrophages actively engulfed wild-type neutrophils that had phagocytosed S. aureus and that this was also blocked by DPI treatment (Fig. 6E). Furthermore, we observed impaired uptake of phagocytic CGD mouse neutrophils by macrophages (Fig. 6E).
DISCUSSION
Neutrophils undergo apoptosis following the phagocytosis and killing of pathogens, but it is not clear whether apoptosis proceeds via a conventional caspase-dependent pathway or an alternate pathway. Using enzymatic and immunofluorescence assays of effector caspases in phagocytic murine and human neutrophils, we demonstrated that caspase activation does not occur following phagocytosis. Caspases were detected only when the NADPH oxidase was inhibited or absent. However, it is known that the oxidative burst is critical for PS exposure and macrophage uptake. Therefore, caspase activation and the oxidative burst appear to be mutually exclusive events in the phagocytic neutrophil.
We have previously shown that sustained oxidant production can interfere with caspase activation (12, 13), and the same phenomenon was observed in neutrophils stimulated with the artificial stimulus PMA (8). It is possible that phagosomal production and consumption of oxidants could spare cytoplasmic caspases, but our data clearly show that phagocytic cells have reduced caspase activity, as assessed by activity assays and immunohistochemistry. Our results differ from the reports of Zhang et al. (35) and Perskvist et al. (24), which demonstrated that there was caspase activation in phagocytic neutrophils and suggested that this activity is a requirement for apoptosis (24, 35). The basis of the apparent discrepancy between the studies is unclear, although it might be related to the nature of the microorganism used to activate the neutrophils. We explored the effects of different neutrophil/bacterium ratios and saw no difference, and significant inhibition of caspase activation occurred even at low ratios. There was also no difference in the effect of phagocytosis on caspase activation due to live or dead bacteria, as demonstrated in our immunofluorescence study, where neutrophils phagocytosed heat-killed S. aureus. This rules out any contribution of killing defects in provoking the different responses. Others have reported that inhibition of the NADPH oxidase had no effect on the Entamoeba-induced cleavage of caspase-3 in human neutrophils (28) and that phagocytosis results in a reduction of caspase activity (31) and caspase-3 gene expression (2), thus supporting our results.
It is possible that phagocytic neutrophils secrete factors that dampen caspase activity in surrounding neutrophils. However, the immunofluorescence assay clearly illustrated that the NADPH oxidase-dependent block in caspase activation occurs in the individual phagocytic cells. DPI-treated or CGD neutrophils were the only phagocytic neutrophils to show significant caspase activity. However, there was no PS exposure or uptake of these cells. Furthermore, PS exposure in phagocytic neutrophils occurred in the presence of a caspase inhibitor. This implies that caspases are not involved in the clearance of phagocytic neutrophils but that an oxidant-dependent event is crucial. This is consistent with the observation of Kagan and colleagues that oxidation of PS is necessary for its externalization (16, 22, 30); more recently, Tyurina et al. showed that in HL-60 cells exposed to nitrosative stress PS exposure was dissociated from the common apoptotic pathway (29). We have also recently reported that 24-h ascorbate-deficient neutrophils fail to undergo PS exposure and uptake by macrophages despite activation of caspases (33).
It has been proposed that the accumulation of neutrophils associated with the pathology of CGD is in part due to impaired clearance of these cells (8, 14, 19). Our observations now indicate that these neutrophils also have active caspases. One possibility is that caspase activation in these neutrophils could enhance the structural dismantling of the cell, increasing the potential for release of neutrophil proteins and exacerbation of local tissue damage. Such a phenomenon is consistent with the increased gastric atrophy observed in CGD mice colonized with Helicobacter pylori (17).
Many studies have used morphological changes to the neutrophil nucleus as a key marker of apoptosis (2, 18, 34, 35). However, we found that nuclei of phagocytic neutrophils underwent morphological changes clearly different from those of nuclei of neutrophils undergoing spontaneous apoptosis. Zhang and colleagues thought that morphological features of apoptosis, such as nuclear condensation, could become distorted by an overabundance of yeast and therefore used only two yeast particles per human neutrophil (35). Although the loss of the characteristic multilobed nuclei could result from space constraints within the neutrophil, the same morphology was observed in neutrophils incubated with S. aureus at lower ratios containing only one or two bacteria within the cytoplasm. Coxon et al. have also reported NAPDH oxidase-dependent changes in nuclear morphology in neutrophils phagocytosing serum-opsonized target particles (6), and Fuchs and colleagues recently described morphology distinct from apoptosis and necrosis in stimulated neutrophils during neutrophil extracellular trap formation that also showed irregular swollen nuclei and required oxidants derived from the NADPH oxidase (10). Therefore, we do not believe that nuclear morphological changes can be used to assess apoptosis in phagocytic neutrophils.
In summary, our studies indicate that the roles of oxidants generated by the neutrophil NADPH oxidase upon phagocytosis extend beyond the exclusive task of bacterial killing. We show that these neutrophil oxidants also serve as important signaling molecules in the phagocytic neutrophil. NADPH oxidase-derived oxidants trigger cell surface changes that result in macrophage recognition and engulfment of phagocytic cells. This phagocytic neutrophil clearance is a caspase-independent process; indeed, the neutrophil-derived oxidants prevent caspase involvement.
Acknowledgments
This work was made possible by the award of a Sir Charles Hercus Fellowship from the Health Research Council of New Zealand to M.H. and of a Ph.D. scholarship from the University of Otago to R.W.
Editor: F. C. Fang
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Baran, J., K. Guzik, W. Hryniewicz, M. Ernst, H. D. Flad, and J. Pryjma. 1996. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect. Immun. 64:4242-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borjesson, D. L., S. D. Kobayashi, A. R. Whitney, J. M. Voyich, C. M. Argue, and F. R. Deleo. 2005. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J. Immunol. 174:6364-6372. [DOI] [PubMed] [Google Scholar]
- 3.Brach, M. A., S. deVos, H. J. Gruss, and F. Herrmann. 1992. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80:2920-2924. [PubMed] [Google Scholar]
- 4.Cheah, F. C., M. B. Hampton, B. A. Darlow, C. C. Winterbourn, and M. C. Vissers. 2005. Detection of apoptosis by caspase-3 activation in tracheal aspirate neutrophils from premature infants: relationship with NF-kappaB activation. J. Leukoc. Biol. 77:432-437. [DOI] [PubMed] [Google Scholar]
- 5.Colotta, F., F. Re, N. Polentarutti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 6.Coxon, A., P. Rieu, F. J. Barkalow, S. Askari, A. H. Sharpe, U. H. von Andrian, M. A. Arnaout, and T. N. Mayadas. 1996. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5:653-666. [DOI] [PubMed] [Google Scholar]
- 7.Engelich, G., M. White, and K. L. Hartshorn. 2001. Neutrophil survival is markedly reduced by incubation with influenza virus and Streptococcus pneumoniae: role of respiratory burst. J. Leukoc. Biol. 69:50-56. [PubMed] [Google Scholar]
- 8.Fadeel, B., A. Ahlin, J. I. Henter, S. Orrenius, and M. B. Hampton. 1998. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood 92:4808-4818. [PubMed] [Google Scholar]
- 9.Fadok, V. A., J. S. Savill, C. Haslett, D. L. Bratton, D. E. Doherty, P. A. Campbell, and P. M. Henson. 1992. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149:4029-4035. [PubMed] [Google Scholar]
- 10.Fuchs, T. A., U. Abed, C. Goosmann, R. Hurwitz, I. Schulze, V. Wahn, Y. Weinrauch, V. Brinkmann, and A. Zychlinsky. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 12.Hampton, M. B., and S. Orrenius. 1997. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 414:552-556. [DOI] [PubMed] [Google Scholar]
- 13.Hampton, M. B., I. Stamenkovic, and C. C. Winterbourn. 2002. Interaction with substrate sensitises caspase-3 to inactivation by hydrogen peroxide. FEBS Lett. 517:229-232. [DOI] [PubMed] [Google Scholar]
- 14.Hampton, M. B., M. C. Vissers, J. I. Keenan, and C. C. Winterbourn. 2002. Oxidant-mediated phosphatidylserine exposure and macrophage uptake of activated neutrophils: possible impairment in chronic granulomatous disease. J. Leukoc. Biol. 71:775-781. [PubMed] [Google Scholar]
- 15.Hampton, M. B., M. C. Vissers, and C. C. Winterbourn. 1994. A single assay for measuring the rates of phagocytosis and bacterial killing by neutrophils. J. Leukoc. Biol. 55:147-152. [DOI] [PubMed] [Google Scholar]
- 16.Kagan, V. E., B. Gleiss, Y. Y. Tyurina, V. A. Tyurin, C. Elenstrom-Magnusson, S. X. Liu, F. B. Serinkan, A. Arroyo, J. Chandra, S. Orrenius, and B. Fadeel. 2002. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J. Immunol. 169:487-499. [DOI] [PubMed] [Google Scholar]
- 17.Keenan, J. I., R. A. Peterson, and M. B. Hampton. 2005. NADPH oxidase involvement in the pathology of Helicobacter pylori infection. Free Radic. Biol. Med. 38:1188-1196. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, S. D., K. R. Braughton, A. R. Whitney, J. M. Voyich, T. G. Schwan, J. M. Musser, and F. R. DeLeo. 2003. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. USA 100:10948-10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi, S. D., J. M. Voyich, K. R. Braughton, A. R. Whitney, W. M. Nauseef, H. L. Malech, and F. R. DeLeo. 2004. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J. Immunol. 172:636-643. [DOI] [PubMed] [Google Scholar]
- 20.Li, Y., and M. A. Trush. 1998. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 253:295-299. [DOI] [PubMed] [Google Scholar]
- 21.Lundqvist-Gustafsson, H., S. Norrman, J. Nilsson, and A. Wilsson. 2001. Involvement of p38-mitogen-activated protein kinase in Staphylococcus aureus-induced neutrophil apoptosis. J. Leukoc. Biol. 70:642-648. [PubMed] [Google Scholar]
- 22.Matsura, T., B. F. Serinkan, J. Jiang, and V. E. Kagan. 2002. Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett. 524:25-30. [DOI] [PubMed] [Google Scholar]
- 23.Nilsdotter-Augustinsson, A., A. Wilsson, J. Larsson, O. Stendahl, L. Ohman, and H. Lundqvist-Gustafsson. 2004. Staphylococcus aureus, but not Staphylococcus epidermidis, modulates the oxidative response and induces apoptosis in human neutrophils. APMIS 112:109-118. [DOI] [PubMed] [Google Scholar]
- 24.Perskvist, N., M. Long, O. Stendahl, and L. Zheng. 2002. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J. Immunol. 168:6358-6365. [DOI] [PubMed] [Google Scholar]
- 25.Pullar, J. M., and M. B. Hampton. 2002. Diphenyleneiodonium triggers the efflux of glutathione from cultured cells. J. Biol. Chem. 277:19402-19407. [DOI] [PubMed] [Google Scholar]
- 26.Savill, J., and C. Haslett. 1994. Fate of neutrophils, p. 295-314. In P. J. Hellewell and T. J. Williams (ed.), Immunopharmocology of neutrophils. Academic Press, London, United Kingdom.
- 27.Savill, J. S., A. H. Wyllie, J. E. Henson, M. J. Walport, P. M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Investig. 83:865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sim, S., T. S. Yong, S. J. Park, K. I. Im, Y. Kong, J. S. Ryu, D. Y. Min, and M. H. Shin. 2005. NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J. Immunol. 174:4279-4288. [DOI] [PubMed] [Google Scholar]
- 29.Tyurina, Y. Y., L. V. Basova, N. V. Konduru, V. A. Tyurin, A. I. Potapovich, P. Cai, H. Bayir, D. Stoyanovsky, B. R. Pitt, A. A. Shvedova, B. Fadeel, and V. E. Kagan. 2007. Nitrosative stress inhibits the aminophospholipid translocase resulting in phosphatidylserine externalization and macrophage engulfment: implications for the resolution of inflammation. J. Biol. Chem. 282:8498-8509. [DOI] [PubMed] [Google Scholar]
- 30.Tyurina, Y. Y., F. B. Serinkan, V. A. Tyurin, V. Kini, J. C. Yalowich, A. J. Schroit, B. Fadeel, and V. E. Kagan. 2004. Lipid antioxidant, etoposide, inhibits phosphatidylserine externalization and macrophage clearance of apoptotic cells by preventing phosphatidylserine oxidation. J. Biol. Chem. 279:6056-6064. [DOI] [PubMed] [Google Scholar]
- 31.van Zandbergen, G., J. Gieffers, H. Kothe, J. Rupp, A. Bollinger, E. Aga, M. Klinger, H. Brade, K. Dalhoff, M. Maass, W. Solbach, and T. Laskay. 2004. Chlamydia pneumoniae multiply in neutrophil granulocytes and delay their spontaneous apoptosis. J. Immunol. 172:1768-1776. [DOI] [PubMed] [Google Scholar]
- 32.Vissers, M. C., W. A. Day, and C. C. Winterbourn. 1985. Neutrophils adherent to a nonphagocytosable surface (glomerular basement membrane) produce oxidants only at the site of attachment. Blood 66:161-166. [PubMed] [Google Scholar]
- 33.Vissers, M. C., and R. P. Wilkie. 2007. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1α. J. Leukoc. Biol. 81:1236-1244. [DOI] [PubMed] [Google Scholar]
- 34.Watson, R. W., H. P. Redmond, J. H. Wang, C. Condron, and D. Bouchier-Hayes. 1996. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J. Immunol. 156:3986-3992. [PubMed] [Google Scholar]
- 35.Zhang, B., J. Hirahashi, X. Cullere, and T. N. Mayadas. 2003. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J. Biol. Chem. 278:28443-28454. [DOI] [PubMed] [Google Scholar]
- 36.Zheng, L., M. He, M. Long, R. Blomgran, and O. Stendahl. 2004. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J. Immunol. 173:6319-6326. [DOI] [PubMed] [Google Scholar]