Abstract
Dutch-belted and New Zealand White rabbits were passively immunized with AVP-21D9, a human monoclonal antibody to protective antigen (PA), at the time of Bacillus anthracis spore challenge using either nasal instillation or aerosol challenge techniques. AVP-21D9 (10 mg/kg) completely protected both rabbit strains against lethal infection with Bacillus anthracis Ames spores, regardless of the inoculation method. Further, all but one of the passively immunized animals (23/24) were completely resistant to rechallenge with spores by either respiratory challenge method at 5 weeks after primary challenge. Analysis of the sera at 5 weeks after primary challenge showed that residual human anti-PA levels decreased by 85 to 95%, but low titers of rabbit-specific anti-PA titers were also measured. Both sources of anti-PA could have contributed to protection from rechallenge. In a subsequent study, bacteriological and histopathology analyses revealed that B. anthracis disseminated to the bloodstream in some naïve animals as early as 24 h postchallenge and increased in frequency with time. AVP-21D9 significantly reduced the dissemination of the bacteria to the bloodstream and to various organs following infection. Examination of tissue sections from infected control animals, stained with hematoxylin-eosin and the Gram stain, showed edema and/or hemorrhage in the lungs and the presence of bacteria in mediastinal lymph nodes, with necrosis and inflammation. Tissue sections from infected rabbits dosed with AVP-21D9 appeared comparable to corresponding tissues from uninfected animals despite lethal challenge with B. anthracis Ames spores. Concomitant treatment with AVP-21D9 at the time of challenge conferred complete protection in the rabbit inhalation anthrax model. Early treatment increased the efficacy progressively and in a dose-dependent manner. Thus, AVP-21D9 could offer an adjunct or alternative clinical treatment regimen against inhalation anthrax.
Inhalation anthrax is characterized by edema and hemorrhage of the mediastinal lymph nodes, pulmonary edema, and pleural effusion (12). Clinical trials or experimental human studies are not feasible or ethical, because this form of anthrax is highly fatal and the natural incidence of the disease is very low. Consequently, animal models for inhalation anthrax are crucial in the study of disease pathogenesis, as well as in the evaluation of new therapeutics and vaccines. While nonhuman primates are often deemed the most desirable animal model of inhalation anthrax, the high cost and small number of laboratories capable of performing such trials are very limiting. The New Zealand White rabbit is a reliable small animal model (18, 28) that is often used to evaluate vaccines and antitoxic drugs against anthrax, and the associated pathology resembles human inhalation anthrax (1, 11). The prevailing strategy in vaccine design against inhalation anthrax focuses on the inclusion of recombinant protective antigen (PA) as the primary component (3, 9, 10, 15, 16, 18, 28, 29). This important bacterial protein was appropriately named long before its essential role as the receptor-binding component of the anthrax toxins was known (26). Studies in various animal models have shown that vaccination with PA evokes antibodies that neutralize both of the anthrax toxins, lethal toxin and edema toxin (25, 27). Since both lethal toxin and edema toxin are assembled on the surface of target cells from the deposition of PA, lethal factor (LF), and/or edema factor, the mechanism of anti-PA's protective capacity was thought to be largely due to (i) interference with toxin assembly, (ii) inhibition of receptor binding, (iii) blockade of PA pore formation, or (iv) interference of LF or edema factor translocation through the heptameric PA pore inserted in the pinocytotic vesicle (19, 23). Additional protective mechanisms for anti-PA involving spore-bound PA and interactions with phagocytic cells have been formulated (7), but the evidence for them is not well established.
In an earlier study, we showed that a human anti-PA monoclonal antibody (MAb) (AVP-21D9) was highly effective in protecting Dutch-belted rabbits against inhalation anthrax after inoculation with B. anthracis spores via nasal instillation (21). In the present study, we sought to characterize further the protective role of AVP-21D9 and to compare protection conferred to both Dutch-belted and New Zealand White rabbits challenged with B. anthracis Ames spores by nasal instillation versus aerosol administration.
MATERIALS AND METHODS
Human MAb to PA.
AVP-21D9 (Avanir Pharmaceuticals, San Diego, CA) is a human MAb (immunoglobulin G1 [IgG1] κ isotype) specific for PA and was produced by CHO (Chinese hamster ovary)-K1 cells adapted to growth in serum-free medium or cultured in a in a 5-liter Wave shake flask bioreactor (25, 27). The two batches of protein A-purified AVP-21D9 antibodies used in this study showed a purity of >95% as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, and the 50% effective concentrations in a toxin neutralization assay using RAW 264.7 murine macrophages (American Type Culture Collection, Manassas, VA) were 32 and 40 pM, respectively (25). AVP-21D9 was provided as sterile solution in 20 mM Tris-150 mM NaCl-0.01% Tween 80 at a concentration of 6 or 8 mg/ml, respectively.
Serological analyses.
PA enzyme-linked immunosorbent assays (ELISAs) were performed by coating all wells of a 96-well flat-bottom plate (Nalge Nunc International, Rochester, NY) with 50 μl of PA antigen (1 μg/ml) diluted in 50 mM sodium carbonate buffer (pH 9.6) overnight at 4°C. After removal of the PA solution, 200 μl of blocking buffer was added to each well at 25°C and left for 5 min. All wells were then emptied, 100 μl of blocking buffer (10 g powdered milk/liter phosphate-buffered saline [PBS) was added to the wells, and serial twofold dilutions of the rabbit sera were made. The plates were incubated at 25°C for 1 h before being washed three times with 200 μl of 0.05% Tween 20 in PBS. Subsequently, 100 μl of either goat anti-rabbit IgG-alkaline phosphatase (AP) conjugate (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; SC-2057, mouse/human adsorbed) or goat anti-human IgG-AP conjugate was diluted in blocking solution, added to each well, and incubated for 1 h at room temperature. Control experiments demonstrated that the goat anti-rabbit IgG conjugate reacted intensely with rabbit IgG but did not react (optical density at 405, <0.06) with human IgG1 when tested in the PA ELISA using a range of 1 to 200 μg/ml AVP-21D9 (data not shown). The human anti-PA titers were correlated with a standard dilution series of AVP-21D9, enabling the results to be expressed in μg/ml. After washing of the plates, 100 μl of AP yellow (pNPP) liquid substrate (Sigma Chemical Company, St. Louis, MO) was added to each well. The ELISA plates were developed for 15 to 30 min at room temperature, and the plates were read with a plate reader, using the optical density at 405 nm.
The rabbit sera were also tested using the 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) neutralization assay with a kit purchased from the American Type Culture Collection (Manassas, VA) (22). J774A.1 murine monocyte/macrophage-like cells (ATCC) were plated at 5 × 105 cells/ml and grown to 60 to 80% confluence at 37°C overnight in 5% CO2. Twofold dilutions of sera in duplicate were preincubated with PA (0.05 μg/ml) and LF (0.04 μg/ml) for 30 min at 37°C in 5% CO2. The titrated serum dilutions and lethal toxin were then transferred to the cells and incubated for 4 h. After incubation, 10 μl/well of yellow tetrazolium MTT salt was added to the cells and left for 2 h. The salt was reduced by metabolically active cells. The resulting intracellular purple formazan was solubilized overnight in detergent reagent (ATCC catalog no. 30-1010K) provided in the MTT assay kit. The reaction product was measured at 570 nm and quantified. We then calculated the 50% toxin-neutralizing antibody titers by determining the sample dilution that reached the midpoint between the mean absorbance of the PA-LF positive control wells and the reduction in nontreated cells.
Preparation of B. anthracis spores.
Spores were prepared by inoculating B. anthracis Ames strain in Schaeffer's sporulation medium (pH 7.0), consisting of 16 g Difco Nutrient Broth, 0.5 g MgSO4·7H2O, 2.0 g KCl, and 16.7 g MOPS (morpholinepropanesulfonic acid) per liter (21). Before inoculation, the following supplements were filter sterilized using 0.22-μm syringe filters and then added to the medium to the indicated final concentrations: 0.1% glucose, 1 mM Ca(NO3)2, 0.1 mM MnSO4, and 1 μM FeSO4. Cultures were grown in 50-ml aliquots contained in 500-ml plastic Erlenmeyer flasks fitted with Whatman Bug stoppers (Fisher Scientific, Hampton, NH) at 37°C with gentle shaking (180 rpm) for 48 h, after which 100 ml of sterile distilled water was added to dilute the medium and promote sporulation. After 10 to 11 days of continuous shaking, sporulation was confirmed at >99% via phase-contrast microscopy and a modified Wirtz-Conklin spore stain (13), and the spores were centrifuged at 630 × g in a sealed-carrier centrifuge (Jouan Inc., Winchester, VA) at 4°C for 15 min. The spore pellets were then washed four times in Cellgro sterile water (Mediatech, Herndon, VA) and resuspended in sterile water. Subsequently, the spore suspension was layered onto a cushion of 58% Hypaque-76 (GE Healthcare, Piscataway, NJ) at a ratio of 1:2.5 by volume. Without mixing, the tubes were centrifuged in a JA 25.50 rotor at 8,270 × g for 45 min at 4°C in an Avanti J-20XPI refrigerated centrifuge (Beckman Coulter, Fullerton, CA). The Hypaque supernatant was carefully decanted and the spore pellet washed twice with sterile water and finally resuspended in sterile water. Aliquots of the stock spore suspension were stored at −70°C and freshly diluted in water to the desired number of CFU immediately before each animal challenge experiment. Serial plate counts were performed in triplicate to establish the concentration of viable spores in the spore stock vials, usually 1 × 109 to 1 × 1010 CFU/ml. The spore suspensions were homogeneous when examined by phase-contrast microscopy. B. anthracis cultures and spores were prepared and stored in a restricted-access biosafety level 2 laboratory registered with the CDC and inspected by the Department of Defense and the U.S. Department of Agriculture.
Challenge of rabbits with B. anthracis Ames spores.
Specific-pathogen-free (SPF) Dutch-belted dwarf rabbits (2.0 kg) and New Zealand White rabbits (1.4 kg) were purchased from Myrtle's Rabbitry, Inc., Thompson Station, TN. Five weeks after the initial challenge, the Dutch-belted rabbits had grown to 2.3 kg, while the New Zealand rabbits grew to 2.7 kg. Where indicated, 12 rabbits of each breed were challenged for 15 min by exposure to an aerosol of B. anthracis Ames spores generated by a three-jet Collison nebulizer attached to a (whole-body) Madison aerosol chamber (Madison, WI). Using a seven-stage cascade impactor (InTox Products, Moriarty, NM), we determined that the effective cutoff diameter of 93.2% of the B. anthracis Ames spore particles used in these studies was 1.06 to 1.62 μm, with 65% impacted on stage 5 (1.06 μm). The concentration of the spore suspension pipetted into the nebulizer (Cneb) was adjusted to 1 × 1010 CFU/ml, which was based on our earlier empirical observation that a Cneb of 1 × 108 CFU/ml in the Collison nebulizer yielded one 50% lethal dose (LD50) in Dutch-belted rabbits challenged with B. anthracis Ames spores in the aerosol chamber under the parameters set up for the aerosol equipment (e.g., airflow at 50 liters/min). By sampling the aerosol during animal exposure with an all-glass biosampler (SKC Inc., Eighty Four, PA), the presented dose (Dp) for primary challenge of the Dutch-belted dwarf rabbits was estimated to be 1.13 × 107 CFU (100 LD50), while for the New Zealand White rabbits, the Dp was estimated to be 9.78 × 106 CFU (87 LD50). These Dutch-belted and New Zealand White rabbits were subsequently rechallenged after 5 weeks, and the Dp values were estimated to be 1.19 × 107 CFU (106 LD50) and 1.42 × 107 CFU (126 LD50), respectively. These inhalation challenge doses were based on an empirically determined LD50 dose of B. anthracis Ames spores for rabbits having a Dp value of 1.125 × 105 CFU, which corresponded well with the reported average inhalation LD50 dose of B. anthracis Ames spores being 1 × 105 CFU (31). In our computations, the minute lung volume for rabbits was estimated based on Guyton's formula and body weight, and the algorithms for computing the presented dose (Np) were used as described by Roy and Pitt (24). All aerosol challenge procedures were performed in a custom class III biosafety glove cabinet (The Baker Company, Sanford, ME) to which the Madison chamber had been connected to reduce risk to personnel and the laboratory environment. All the equipment was operated within a registered select agent animal biosafety level 3 facility at the University of Texas Medical Branch.
As part of the same protection study, another 12 rabbits of each breed were similarly challenged with 100 LD50 of B. anthracis Ames spores (1 × 107 CFU) by using nasal instillation as described previously (21). For nasal instillation, the anesthetized animals were suspended vertically, using the upper incisors, as described by Comer et al. (6) and Peterson et al. (21), with the bulk of the body weight of the rabbits resting on the base of the platform. The spore suspension (50 μl/naris) was instilled slowly for 2 to 3 min onto the anterior opening of each naris. Subsequently, PBS (50 μl/naris) was used to wash any nonadherent spores from the nasal cavity into the lungs of each animal species. Animals were housed in ventilated rabbit cages (Allentown Caging, Allentown, NJ).
Thus, a total of 48 rabbits (24 New Zealand White and 24 Dutch-belted rabbits) were challenged with lethal respiratory doses of B. anthracis Ames spores by either aerosol or nasal instillation. One-half of the animals served as unimmunized controls while the remaining animals received at the time of challenge a single subcutaneous (s.c.) injection of AVP-21D9 (10 mg/kg), which is a human MAb to PA (Avanir Pharmaceuticals, San Diego, CA). After a period of 5 weeks, the surviving animals were rechallenged with approximately 100 LD50 of B. anthracis Ames spores by the same aerosol or nasal instillation techniques. The rechallenge after 5 weeks was included in the study design to determine whether the animals would still be protected when the levels of AVP-21D9 had decreased. In addition, we analyzed the sera from these animals to determine the levels of human and rabbit antibodies to PA. For rechallenge of surviving vaccinated rabbits, an additional set of 12 weight-matched naïve rabbits of each breed was purchased and challenged with B. anthracis Ames spores similarly to those dosed with AVP-21D9 to verify that a lethal dose was applied in this challenge.
For the pathology and microbiology time course study, seven additional groups (n = 6) of Dutch-belted dwarf rabbits (0.7 to 1.0 kg) were challenged with 100 LD50 of B. anthracis Ames spores (1 × 107 CFU) by nasal instillation. For this procedure, the rabbits were anesthetized with ketamine-HCl (35 mg/kg) and xylazine-HCl (5 mg/kg) by intramuscular injection, and programmable transponders (Bio Medic Data Systems, Seaford, DE) were implanted s.c. at the base of the neck, which reported the animal number, group assignment, and body temperature. The body temperatures were monitored manually at intervals twice daily with a wireless probe pointed at the animal's transponder. The PBS controls consisted of Dutch-belted rabbits that remained untreated (no antibody administration) but were challenged with B. anthracis Ames spores, while the other rabbits were dosed with a single dose of AVP-21D9 (2 mg/kg) by the s.c. route at the time of challenge with B. anthracis Ames spores.
A delayed-treatment study with AVP-21D9 was also performed with Dutch-belted rabbits (1.1 kg) that were challenged by aerosol with approximately 102 to 108 LD50 B. anthracis Ames spores (Dp = 1.15 × 107 to 1.21 × 107 CFU). The PBS controls consisted of Dutch-belted rabbits that were not treated with antibody but were infected with spores, while the other rabbits were dosed either with 0.5 or 2.0 mg/kg of AVP-21D9 s.c. at the time of challenge, 24 h postchallenge, or 36 h postchallenge with B. anthracis Ames spores.
Passive immunization with AVP-21D9.
AVP-21D9 is a purified, fully human MAb preparation. In the protection study described above, 24 of the 48 rabbits were dosed with a single s.c. injection of AVP-21D9 (10 mg/kg), while the remainder served as unimmunized controls. Both were subsequently challenged with approximately 100 LD50 of B. anthracis Ames spores. In the pathology and microbiology time course study, three adult Dutch-belted dwarf rabbits from each of the seven groups of rabbits were dosed with AVP-21D9 (2 mg/kg) at the time of challenge, while the remainder served as unimmunized controls that were challenged with B. anthracis Ames spores.
Collection of specimens.
In the pathology time course study, specimens were collected at specified time intervals postchallenge (0, 12, 24, 36, 48, 60, and 72 h); the rabbits were anesthetized with a mixture of ketamine and xylazine prior to collection of samples of blood and tissue specimens. These specimens were analyzed by blood culture, blood chemistry, hematology, and tissue pathology. Death of the animal was ensured by cutting the diaphragm.
Tissue specimens were weighed, and a 0.1-g portion was stored frozen for homogenization and bacterial plate counts. Another piece of each tissue specimen was fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin (H&E), as well as with the tissue Gram stain, for light microscopic evaluation.
Blood samples were collected into microisolator blood collection tubes (Wampole Laboratories, Princeton, NJ) to prevent coagulation, and then a 100-μl aliquot of the blood sample was used to inoculate 30 ml of sterile Difco brain heart infusion broth (Becton Dickinson and Company, Franklin Lakes, NJ). The enrichment broth culture was observed for turbidity for up to 14 days. Subculture onto 5% sheep blood agar was used to isolate the bacterial colonies, and γ phage typing confirmed the identity of bacteria as B. anthracis.
Pathology sections.
Tissue specimens were collected and placed in 10% buffered formalin immediately following euthanasia of control and experimental rabbits (n = 3 animals/time point) at 0, 12, 24, 36, 48, 60, and 72 h postchallenge with 100 LD50 of B. anthracis Ames by nasal instillation. Tissues examined from all rabbits included the lung, spleen, liver, brain, heart, kidney, and small intestine. The thymus and mediastinal lymph node were examined for at least one animal from each group. After fixation and dehydration, sections from each animal were stained with either H&E or a tissue Gram stain. All slides were read unblinded by light microscopy by a veterinary pathologist (W. B. Blaze). Lesions were scored according to the parameters detailed in a paper by Zaucha et al. (31) Each lesion (e.g., edema, hemorrhage, or necrosis) was graded individually on a severity scale of 1 (minimal) to 5 (severe) based on estimates of distribution and extent of involvement in the examined microscopic sections (1, <10%; 2, 10 to 20%; 3, >20 to 50%; 4, >50 to 75%; 5, >75 to 100%).
Statistics.
All of the animal data were analyzed with Fisher's exact test using the SigmaStat statistical package (SYSTAT, Richmond, CA).
RESULTS
AVP-21D9 protects rabbits against inhalation anthrax.
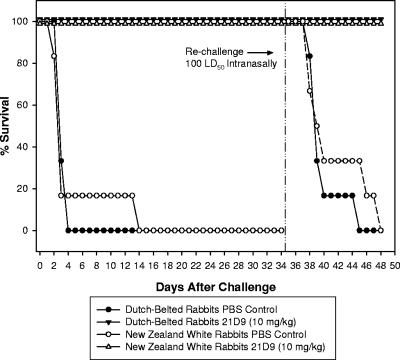
All rabbits used in this study were housed in an AAALAC International-approved facility. Prior to the study, none showed any clinical symptoms of disease. Figures 1 and 2 illustrate that the AVP-21D9 human anti-PA MAb (10 mg/kg) conferred complete protection for 5 weeks to Dutch-belted dwarf and New Zealand White rabbits against an initial lethal challenge with B. anthracis Ames spores administered by either nasal instillation or aerosol. Virtually all PBS control rabbits (infected with B. anthracis spores but unimmunized) died of anthrax before day 4 following challenge, regardless of whether the spores were delivered by nasal instillation or by aerosol. The exception can be seen in Fig. 1, in which one New Zealand White rabbit (PBS control), inoculated with B. anthracis Ames spores by nasal instillation, did not die until day 13.
FIG. 1.
Protection afforded to Dutch-belted dwarf and New Zealand White rabbits by AVP-21D9 (10 mg/kg) administered by s.c. injection at the time of challenge with 1 × 107 CFU (100 LD50) B. anthracis Ames spores by nasal instillation. The LD50 was previously determined in the nasal instillation model with Dutch-belted rabbits to be 1 × 105 CFU B. anthracis Ames spores (21). At 3 to 4 days after initial nasal instillation of B. anthracis Ames spores, the survival of both Dutch-belted and New Zealand White rabbits, dosed with 10 mg/kg AVP-21D9, was significantly different (P < 0.05) from that of untreated control animals by Fisher's exact test. Similarly, at 5 and 11 days following secondary B. anthracis Ames spore challenge (100 LD50) by nasal instillation, the same Dutch-belted and New Zealand White rabbits were still significantly protected (P < 0.05).
FIG. 2.
Protection afforded to Dutch-belted dwarf and New Zealand White rabbits by AVP-21D9 (10 mg/kg) administered by s.c. injection at the time of initial challenge with B. anthracis Ames spores by aerosol administration. The Dp for the Dutch-belted dwarf rabbits was calculated to be 1.13 × 107 CFU (100 LD50), while that for New Zealand rabbits was 9.78 × 106 CFU (87 LD50). At 3 or 4 days after initial aerosolization of B. anthracis Ames spores, the survival of both Dutch-belted and New Zealand White rabbits, dosed with AVP-21D9, was significantly different (P < 0.05) from that of untreated control animals by Fisher's exact test. Upon rechallenge 5 weeks later, the Dp for the Dutch-belted dwarf rabbits was calculated to be 1.19 × 107 CFU (106 LD50), while that for New Zealand rabbits was 1.42 × 107 CFU (126 LD50). At 4 days following secondary B. anthracis Ames spore challenge by aerosolization, Dutch-belted, but not New Zealand White, rabbits were still significantly protected (P < 0.05). In this experiment, one of the New Zealand rabbits initially dosed with AVP-21D9 died 7 days after secondary challenge (41 days after initial challenge), and two of the six positive control animals failed to die of inhalation anthrax.
As is typical for this bacterial infection in the animal model, no clinical symptoms were apparent prior to death; that is, the appearance and behavior of the rabbits remained normal. All of the animals passively immunized with a single dose of AVP-21D9 (10 mg/kg) were completely protected against lethal infection for 5 weeks, at which time the animals were rechallenged with B. anthracis Ames spores by nasal instillation (100 LD50) or aerosol (106 to 126 LD50). All but one of the animals dosed with AVP-21D9 5 weeks earlier were again completely protected against rechallenge. Among the PBS control rabbits purchased for rechallenge, 2 of 12 PBS control rabbits exposed to an aerosol of B. anthracis Ames spores survived until the experiment was terminated (Fig. 1 and 2); however, all the PBS control animals inoculated by nasal instillation (12/12) died of anthrax as expected.
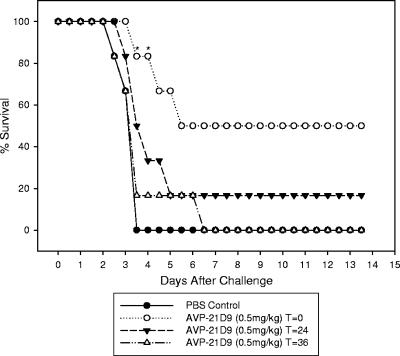
Figure 3 illustrates the importance of prompt administration of the protective antibody as soon as possible following exposure of the respiratory tract to B. anthracis Ames spores. While there was complete protection against an aerosol dose of 102 to 108 LD50 (Dp = 1.15 × 107 to 1.21 × 107 CFU) of B. anthracis Ames spores when antibody administration was at the same time as challenge, delays of 24 and 36 h in administering AVP-21D9 (2 mg/kg) reduced protection to 66% and 33%, respectively. In an attempt to determine the lower limit of protection by this antibody preparation, Fig. 4 shows that a minimum dose of 0.5 mg/kg of AVP-21D9 was only slightly protective when given at the time of challenge and was only transiently protective at 3 days postchallenge. Further delay in administering this low dose of the antibody preparation caused it to be totally ineffective. These data demonstrate the dose- and time-dependent protection by AVP-21D9.
FIG. 3.
Effect of time of administration of AVP-21D9 (2 mg/kg) on protection conferred to Dutch-belted rabbits challenged with a Dp of 1.15 × 107 CFU (102 LD50) B. anthracis Ames spores by aerosol. Protection was progressively less when the antibody preparation was administered 24 h or later postchallenge. Asterisks indicate a significant difference from the positive control group by Fisher's exact test (P < 0.05).
FIG. 4.
Effect of time of administration of AVP-21D9 (0.5 mg/kg) on protection conferred to Dutch-belted rabbits challenged with 1.21 × 107 CFU (108 LD50) B. anthracis Ames spores by aerosol. Some protection was noted when 0.5 mg/kg was administered at the time of challenge, indicating the lower limit of protection by this antibody. Asterisks indicate a significant difference from the positive control group by Fisher's exact test. (P < 0.05).
Culture of rabbit blood.
The results of blood cultures of Dutch-belted dwarf rabbits challenged with B. anthracis by nasal instillation are summarized in Table 1. The earliest confirmed positive blood culture with B. anthracis was observed in the PBS control group (infected but unimmunized) at 24 h postchallenge, and only 1/3 rabbits was positive. By 36 h, all three rabbits had positive blood cultures for B. anthracis as confirmed by γ phage typing. After 48 h, 1/3 rabbits had positive blood cultures, while at 60 and 72 h, 3/5 and 1/1 rabbits had positive blood cultures, respectively. In total, 9/18 PBS control animals (50%) developed positive blood cultures within 72 h after challenge, while in striking contrast, rabbits dosed with AVP-21D9 (2 mg/kg) at the time of B. anthracis spore challenge were virtually all protected against positive blood cultures, except for 1/18 rabbits (5.5%). Further, despite positive cultures of B. anthracis from the blood of one-half of the PBS control animals after 24 h, there was no detectable persistent change in body temperature when the temperatures were monitored twice each day. The average temperature of the 18 PBS control rabbits (infected with B. anthracis Ames spores) at all time points was 39.7 ± 0.6°C, and the 18 infected rabbits that were dosed with AVP-21D9 had a similar temperature of 39.7 ± 0.7°C.
TABLE 1.
Blood culture results after challenge of rabbits with B. anthracis Ames spores by nasal instillationa
| Time of sacrifice postinfection (h) | PBS control rabbitsb
|
AVP-21D9 (2 mg/kg)-immunized rabbitsc
|
||||
|---|---|---|---|---|---|---|
| Temp (°C)d | No. with positive blood culture (no. dead)/total | No. phage susceptible/ total | Temp (°C)e | No. with positive blood culture/total | No. phage susceptible/ total | |
| 0 (uninfected) | ||||||
| 12 | 39.5 | 1/3 | 0/1 | 39.9 | 1/3 | 0/1 |
| 24 | 39.6 | 1/3 | 1/1 | 40.9 | 0/3 | 0/0 |
| 36 | 41.0 | 3/3 | 3/3 | 40.1 | 0/3 | 0/0 |
| 48 | 39.4 | 1/3 | 1/1 | 39.5 | 0/3 | 0/0 |
| 60 | 39.2 | 3 (2)/5 | 3/3 | 39.0 | 1/3 | 1/1 |
| 72 | 1 (1)/1 | 1/1 | 38.5 | 0/3 | 0/0 | |
AVP-21D9 was given by the s.c. route at the time of spore challenge; n = 3 rabbits/group. Blood samples were cultured by enrichment for up to 14 days in Luria-Bertani broth prior to streaking onto 5% sheep blood agar. Confirmation of B. anthracis was by typing with γ phage.
Rabbits infected with B. anthracis but unimmunized.
Rabbits infected with B. anthracis and immunized.
Average temperature, 39.7 ± 0.6°C.
Average temperature, 39.7 ± 0.7°C.
Culture of rabbit organs.
Lung specimens taken at 12 h after nasal instillation of B. anthracis Ames spores yielded large numbers of B. anthracis colonies (Table 2). Earlier studies have indicated that germination occurs after phagocytosis and transport of the spores to lymph nodes in the mediastinum (8, 14). The spleens of the PBS control animals became positive for B. anthracis within 36 h and remained positive through 60 h. In contrast, the spleen of only 1/3 infected rabbits dosed with AVP-21D9 was positive, and the colony count was very low. Kidney cultures of PBS control rabbits at 36 to 60 h were similar to spleen cultures, but none of the infected rabbits dosed with AVP-21D9 were positive. Cultures of heart, liver, and brain of the infected PBS control rabbits became positive at 36 h, but counts rapidly decreased by 48 h. None of the latter tissues were culture positive in infected rabbits dosed with AVP-21D9.
TABLE 2.
Quantitative culture of selected organs from rabbits challenged with B. anthracis
| Time (h) after infection and treatment groupa | Plate count (CFU/0.1 g)b
|
|||||
|---|---|---|---|---|---|---|
| Lung | Spleen | Kidney | Heart | Liver | Brain | |
| 12 | ||||||
| PBS | 423 ± 270 (3/3) | 0 | 0 | 0 | 0 | 0 |
| 21D9 | 537 ± 510 (3/3) | 0 | 0 | 0 | 0 | 0 |
| 24 | ||||||
| PBS | 130 ± 102 (2/3) | 0 | 0 | 0 | 0.67 ± 0.94 (1/3) | 0 |
| 21D9 | 141 ± 76 (3/3) | 0 | 0 | 0 | 0 | 0 |
| 36 | ||||||
| PBS | 6,040 ± 8,038 (3/3) | 220 ± 269 (3/3) | 91 ± 113 (3/3) | 71 ± 99 (2/3) | 4 ± 3 (3/3) | 0.33 ± 0.47 (1/3) |
| 21D9 | 218 ± 77 (3/3) | 1.3 ± 1.9 (1/3) | 0 | 0 | 0 | 0 |
| 48 | ||||||
| PBS | 301 ± 332 (3/3) | 10 ± 14 (1/3) | 8.3 ± 12 (1/3) | 0.33 ± 0.48 (1/3) | 3.3 ± 4 (2/3) | 0 |
| 21D9 | 115 ± 115 (3/3) | 0 | 0 | 0 | 0 | 0 |
| 60 | ||||||
| PBS | 4,944 ± 6,828 (3/3) | 43 ± 61 (1/3) | 2.7 ± 3.8 (1/3) | 0 | 0 | 0 |
| 21D9 | 62 ± 84 (2/3) | 0 | 0 | 0 | 0 | 0 |
| 72 | ||||||
| PBS | —c | — | — | — | — | — |
| 21D9 | 133 ± 124 (3/3) | 0 | 0 | 0 | 0 | 0 |
PBS, animals infected with B. anthracis Ames spores alone; 21D9, animals that were passively immunized and infected with anthrax.
An aliquot (0.1 g) of the tissue was used for homogenization. The values shown represent the mean CFU (± standard deviation) of B. anthracis from plate counts per 0.1 g of tissue from three rabbits. The values in parentheses indicate the number of rabbits with positive organ culture/number of rabbits per group.
—, no sample taken from dead animals.
Tissue pathology.
Tissues were collected from three uninfected and unimmunized Dutch-belted control rabbits (T = 0 h). While no significant lesions were observed, each of the rabbits had minimal to mild peribronchiolar and/or perivascular lymphocytic infiltrates with subacute inflammation (mononuclear cells and heterophils) in the lung. The latter were considered background lesions, and similar histological findings were observed in most animals at all study time points. After 12 h (T = 12 h), tissues were collected from three positive control rabbits (PBS, infected with B. anthracis but unimmunized) and three rabbits dosed with AVP-21D9 (infected but passively immunized). One PBS control animal had minimal acute inflammation in the liver; another had minimal edema in a mediastinal lymph node. Two of three AVP-21D9 rabbits had minimal edema in the mediastinal lymph node. In addition to edema, one of these AVP-21D9-immunized and infected rabbits had minimal acute inflammation in the node and similar inflammation and mild lymphoid depletion in the thymus. At 24 h (T = 24 h), 3/3 PBS control animals had minimal to mild lung edema. One of these B. anthracis-infected animals also had minimal edema in the mediastinal lymph node and acute inflammation in the liver. Two of the AVP-21D9 rabbits had similar lung changes, while the third animal in this group had minimal edema and inflammation in a mediastinal lymph node. At 36 h (T = 36 h), 3/3 PBS rabbits had lung edema, and two had bacilli in the lung. Both of the latter two rabbits at 36 h had significant necrosis, inflammation, edema/fibrin, and bacilli in a mediastinal lymph node. There were minimal acute inflammatory changes visible in the spleen of one PBS rabbit and in the livers of all three animals. Lesions in these untreated, infected rabbits were consistent with anthrax (21). Of the 36-h AVP-21D9-dosed animals, one had changes in the lung and mediastinal lymph node similar to those in the PBS rabbits; however, the changes in this rabbit may not have been anthrax related, as there was abscess formation in the lung associated with the background lesion observed in many of the animals. Also, no bacilli were observed. Another 36-h AVP-21D9-dosed rabbit had mild inflammation in the mediastinal lymph node. At 48 h, 2/3 PBS control (infected) rabbits had minimal edema in the lung and mediastinal lymph nodes, and one had minimal acute inflammation in the node and thymus. Two of the AVP-21D9-dosed and infected rabbits had edema in the mediastinal lymph nodes, and one had a significant acute inflammatory reaction and necrosis in the node. The latter rabbit and the remaining AVP-21D9-dosed animal had minimal lung edema. At 60 h, 5/5 of the PBS positive control animals had minimal to severe lung edema. Two of the 60-h rabbits were dead, with one having a few bacilli in a larger airway, while the other dead animal had severe edema with fibrin and bacilli in the lung. Changes were observed in other tissues in most of these rabbits. Bacilli were present in the meninges of one of these positive control rabbits, the mediastinal lymph node of another had necrosis and inflammation, and the spleen and mediastinal lymph node of another was necrotic and inflamed. Lesions in 4/5 positive control rabbits were consistent with anthrax at 60 h. The AVP-21D9-dosed and infected animals at 60 h had minimal edema in the mediastinal lymph node; two of these rabbits also had minimal acute inflammation in the node. Mild hemorrhage was present in the spleens of two AVP-21D9-dosed and infected animals at 60 h, and one had minimal acute inflammation in the spleen. One AVP-21D9-dosed and infected animal had minimal lung edema. The remaining PBS control animal was dead by 72 h and had anthrax-like lesions in several tissues (e.g., lung, lymph node, and spleen), with the splenic lesions being particularly noteworthy. Bacilli were seen in almost every tissue of this PBS control animal; Fig. 5B and C illustrate H&E-stained and Gram-stained sections of lung, respectively, from a positive PBS control rabbit soon after death at 72 h postchallenge. These photomicrographs show the numerous bacilli in a blood vessel and lung parenchyma. Large masses of bacilli were also seen in the mediastinal lymph nodes, with significant loss of normal lymph node architecture (Fig. 5E and F). As illustrated in Fig. 5A, the lungs of AVP-21D9-dosed and infected animals at 72 h were essentially normal, but 2/3 animals had minimal lung edema. Further, the mediastinal lymph nodes in these rabbits (72 h) showed edema (3/3) and inflammation (2/3), as illustrated in Fig. 5D, and acute inflammation in the spleen (1/3).
FIG. 5.
Lung and mediastinal lymph node tissues from Dutch-belted dwarf rabbits 72 h after nasal instillation with 100 LD50 B. anthracis Ames spores (1 × 107 CFU). (A) H&E-stained section of lung from an AVP-21D9-dosed and infected rabbit, showing essentially normal tissue. Bar, 50 μm. (B) H&E-stained section of lung tissue from a positive PBS control rabbit infected with B. anthracis Ames spores. The arrow points to numerous bacilli within a blood vessel and the lung parenchyma; the arrowhead points to an area of alveolar edema. Bar, 50 μm. (C) Gram-stained section of lung tissue from same animal as for panel B. The arrow points to numerous bacilli. Bar, 50 μm. (D) H&E-stained section of mediastinal lymph node from an AVP-21D9-dosed and infected rabbit. Asterisks indicate areas of mild edema; the arrowhead points to a small clump of heterophils. Bar, 50 μm. (E) H&E-stained section of mediastinal lymph node from a positive PBS control rabbit infected with B. anthracis Ames spores, showing masses of bacilli with loss of normal lymph node architecture. Bar, 50 μm. (F) Gram-stained section of mediastinal lymph node from the same animal as for panel E, showing masses of bacilli as in panel E, with loss of normal architecture. Bar, 50 μm.
Serum antibody analysis.
Blood samples from the Dutch-belted and New Zealand White rabbits dosed with AVP-21D9 at the time of challenge were collected at 1, 5, and 7 weeks following initial challenge with B. anthracis Ames spores. The resulting sera were analyzed for residual PA-specific human antibodies using a PA ELISA and AVP-21D9 as a standard, and the serum concentration of antibodies was expressed as μg/ml of AVP-21D9. Table 3 summarizes the residual AVP-21D9 human anti-PA antibody concentrations at 1, 5, and 7 weeks after primary challenge with B. anthracis Ames spores. Although all rabbits were given a 10-mg/kg dose of AVP-21D9 s.c. based on body weight, the Dutch-belted rabbits were approximately 21% larger than the New Zealand White rabbits. The mean serum level of residual AVP-21D9 in Dutch-belted rabbits at 1 week after challenge was 108 ± 11 μg/ml, while that in New Zealand rabbits was 78 ± 15 μg/ml. By 5 weeks postchallenge, the levels of AVP-21D9 in the Dutch-belted rabbits were approximately 16% of the levels seen at 1 week postchallenge. Similarly, the levels of AVP-21D9 in the New Zealand White rabbits were approximately 4% of the levels seen at 1 week postchallenge. By 7 weeks postchallenge, AVP-21D9 levels in Dutch-belted and New Zealand White rabbits had been reduced to 5% and 1% of the levels at 1 week postchallenge, respectively. The MTT neutralization titers of the rabbit sera correlated roughly with the residual AVP-21D9 concentrations in the rabbit sera (Table 3).
TABLE 3.
Residual human anti-PA AVP-21D9 levels and MTT neutralization titers in sera of rabbits
| Rabbit strain and challenge routea | Wk 1
|
Wk 5
|
Wk 7
|
|||
|---|---|---|---|---|---|---|
| Human PA ELISA result, μg/mlb | MTT neutralization titerc | Human PA ELISA result, μg/ml | MTT neutralization titer | Human PA ELISA result, μg/ml | MTT neutralization titer | |
| DB | ||||||
| Aerosol | 109.7 ± 14.6 | 65,000 ± 24,759 | 13.7 ± 13.3 (12.5)d | 4,450 ± 3,112 (6.9) | 3.6 ± 4.3 (3.3) | 6,933 ± 5,340 (10.7) |
| Intranasal | 106.2 ± 7.8 | 40,000 ± 12,220 | 20.4 ± 6.6 (19.2) | 7,100 ± 2,300 (17.8) | 7.3 ± 3.6 (6.9) | 1,108 ± 217 (2.8) |
| NZ | ||||||
| Aerosol | 82.0 ± 10.4 | 29,333 ± 3,197 | 3.9 ± 4.3 (4.8) | 1,500 ± 891 (5.1) | 0.9 ± 1.2 (1.1) | 920 ± 591 (3.1) |
| Intranasal | 73.1 ± 18.7 | 25,333 ± 5,850 | 2.7 ± 3.2 (3.7) | 1,850 ± 2,114 (7.3) | 1.0 ± 1.4 (1.4) | 1,583 ± 1,423 (6.3) |
DB, Dutch-belted dwarf rabbits; NZ, New Zealand White rabbits.
Mean residual human anti-PA (AVP-21D9) levels (± standard deviation) in the sera of rabbits at various times postchallenge and dosing.
Mean MTT neutralization titers (± standard deviation) of the same sera in protecting J774 cells from lethal toxin challenge.
The numbers in parentheses indicate the percentage of the residual serum level of human anti-PA remaining relative to the serum level at 1 week postchallenge.
The levels of residual human antibody remaining after 5 weeks had diminished and likely approached the lower limit of protection afforded by AVP-21D9 in rabbits challenged with B. anthracis Ames spores. Earlier, we established that the elimination half-life of a single 10-mg/kg dose of AVP-21D9 in the rabbit was 8.9 ± 2.7 days, with a maximum concentration in serum (Cmax) of 218 ± 43 μg/ml (21). Similarly, a 1-mg/kg dose of AVP-21D9 yielded an elimination half-life of 5.0 ± 2.7 days and a Cmax of 23 ± 6 μg/ml (21). We also determined that 1 mg/kg of the antibody was the lowest dose of AVP-21D9 given at the time of challenge that would confer 100% protection against 100 LD50 (by nasal instillation) of B. anthracis Ames spores (21). We then analyzed the sera using a PA ELISA specific for rabbit IgG. The results shown in Table 4 indicate that rabbit-specific anti-PA titers were low and gradually decreasing rather than rising. By 2 weeks following rechallenge, the rabbit anti-PA titers increased only in one of the four animal groups.
TABLE 4.
Rabbit-specific anti-PA ELISA titers of rabbits dosed with AVP-21D9 human anti-PA MAb
| Rabbit strain and challenge routea | Rabbit-specific anti-PA titer (reciprocal dilution)b at:
|
||
|---|---|---|---|
| 1 wk after primary spore challenge | 5 wk after primary spore challenge | 7 wk after primary spore challenge and 2 wk after secondary spore challenge | |
| DB | |||
| Aerosol | 1,158 ± 781 | 375 ± 122 | 1,644 ± 1,165 |
| Intranasal | 400 ± 100 | 454 ± 120 | 192 ± 132 |
| NZ | |||
| Aerosol | 583 ± 461 | 279 ± 55 | 244 ± 138 |
| Intranasal | 342 ± 51 | 345 ± 60 | 223 ± 158 |
DB, Dutch-belted dwarf rabbits; NZ, New Zealand White rabbits.
Mean (± standard deviation) reciprocal dilution yielding 50% of the maximum reading at 405 nm. There were six rabbits in each group.
DISCUSSION
Following primary challenge, a single dose of AVP-21D9 human MAb to PA conferred 100% protection to both Dutch-belted and New Zealand White rabbits for 5 weeks against lethal infection with B. anthracis spores administered by nasal instillation or aerosolization. In this study, all Dutch-belted and New Zealand White rabbits challenged by nasal instillation received 100 LD50 of B. anthracis Ames spores (1 × 107 CFU). Rechallenge of all the AVP-21D9-dosed rabbits at 5 weeks after primary challenge showed that the rabbits were still highly resistant to B. anthracis infection, with only 1 of the 24 AVP-21D9-vaccinated rabbits dying of anthrax. We noted that 2 of the 12 control rabbits purchased for aerosol rechallenge (Fig. 2) failed to die as expected, but we attributed this to technical difficulties during the experiment execution. We did not observe that animal size was correlated with challenge dose; however, the large size (1.75 to 2.0 kg) of the rabbits generating the results shown in Fig. 1 and 2 was thought to be a contributory cause of two PBS control rabbits not dying as expected. Because of their size, the animals fit tightly into the perforated stainless steel restrainer boxes, such that their challenge dose could have been reduced by their nares being placed tightly against the fur of the adjoining animal, which was facing the opposite direction. This situation could have created a filter-like effect, reducing the aerosol dose inhaled by some animals during rechallenge. In the case of 1 of the 12 intranasally challenged control rabbits that died late postchallenge (Fig. 1), occasionally the challenge droplet was not inspired properly due to the level of anesthesia. Nevertheless, the results showed that there was little difference in susceptibility of Dutch-belted rabbits versus New Zealand White rabbits, and there appeared to be little difference in mortality following nasal instillation compared to aerosol challenge with comparable LD50 of B. anthracis Ames spores. There was no known difference in the B. anthracis Ames spore challenge dose or technique between the initial challenge and rechallenge 5 weeks later. Any perceived change in time to death in Fig. 1 and 2 could have been due to experimental variation. Alternatively, the short delay in time to death could have resulted from the formation of antibodies to B. anthracis or its toxins following spore challenge (see, e.g., Table 4).
The pathology results obtained in this study indicated that the intranasal Dutch-belted dwarf rabbit model described herein yielded pathogenic effects similar to those reported previously for New Zealand White rabbits challenged with B. anthracis spores by aerosol delivery (18, 28, 31). Based on the lesions described by Zaucha et al. (31), six of the PBS control rabbits had lesions consistent with anthrax. These rabbits included two T = 36 h rabbits, three T = 60 h rabbits, and one T = 72 h rabbit. The principal histologic findings occurred in the order mediastinal lymph node > lung > spleen > thymus. Lesions included edema/fibrin, necrosis/depletion, hemorrhage, and a variable leukocytic infiltration, with bacilli in various numbers. Confounding the evaluation of the lung, and to a certain extent other lymphoid tissues, was the presence of a background lesion in the lungs of uninfected animals. That is, lung sections from all the uninfected control rabbits had low-grade peribronchiolar and/or perivascular lymphocytic infiltrates with variable subacute inflammation (mononuclear cells and heterophils). The latter results were consistent with prior exposure of the entire rabbit population to antigens unrelated to that of B. anthracis. Investigation of the online health certificate of our SPF rabbit supplier indicated that nasopharyngeal swabs from their rabbits were positive for Bordetella bronchiseptica, which commonly colonizes the upper respiratory tract of rabbits. Indeed, the health certificate published online by a second SPF supplier of rabbits also indicated that the nasopharnyxes of 100% of their rabbits were also positive for this bacterium. Therefore, we suspect that the inflammatory response observed in the lungs of our uninfected control rabbits may have resulted from exposure of the animals’ respiratory tract to antigens from this common bacterial agent. Despite this finding, none of the rabbits in this study displayed any clinical symptoms of illness, such as wheezing, nasal discharge, weight loss, ocular discharge, or respiratory distress.
We also monitored an extensive battery of quantitative blood chemistry and hematology tests using the Hemavet 950 hematology system (Drew Scientific Inc., Oxford, CT); however, we saw no significant change in any measurable parameter in multiple blood samples taken during the 72-h observation period following challenge with B. anthracis spores or dosing with AVP-21D9 (data not shown). The data were consistent with the absence of clinical symptoms and normal body temperature taken twice each day by telemetry using s.c. implanted transponders prior to the sudden death of the animals. It is peculiar, if not unique, during a bacterial infection for bacteria to be cultured from the blood and tissues and for the body temperature to remain normal. This represents the ultimate control that a bacterium can have over its host. The reason for the absence of fever could have been related to the rapid course of disease in this very susceptible species. The lack of fever might also have been due to the inhibitory effect of lethal toxin on polymorphonuclear neutrophils (20), which normally release endogenous pyrogens that evoke the febrile response. Comer et al. (5) reported that the release of virtually all cytokines from lymphocytes was blocked by exposure to lethal toxin in vitro and in vivo in a murine model. This effect on lymphocytes may help explain the apparent paralysis of the normal host response to infection with B. anthracis. The anthrax toxins constitute a powerful immunoavoidance mechanism, facilitating the dissemination of the bacteria throughout the body.
In conclusion, the rabbit model inoculated by nasal instillation, as well as aerosol inoculation, appears to mimic many of the symptoms of inhalation anthrax in humans. The pathological and microbiological results with Dutch-belted dwarf rabbits are also consistent with earlier reports obtained with New Zealand White rabbits challenged with aerosols of B. anthracis. The advantage of the Dutch-belted dwarf rabbits obviously is usually in their small size, enabling the housing of three times as many animals, of the same gender, in the same amount of animal caging space, consistent with NIH recommendations. In some experiments reported here, we challenged rabbits larger than normal, either because they grew substantially during the 7-week study or because they were size matched between the two rabbit varieties.
From earlier studies, we were aware that human anti-PA MAb AVP-21D9 effectively neutralized the cytotoxic effects of lethal toxin and the capacity of edema toxin to increase cyclic AMP formation in a murine macrophage cell line, RAW264.7 (21). It was not clear whether this antibody might exert other protective effects. In this study, we demonstrated that AVP-21D9 reduced the dissemination of B. anthracis from the lungs to the bloodstream and various organ tissues. The exact mechanism by which the anti-PA limited bacterial dissemination remains unclear, but the most likely possibilities include the following: (i) immunoavoidance, i.e., neutralization of the anthrax toxins, thereby preventing them from functioning as virulence factors; (ii) opsonization, i.e., increased phagocytic uptake of spores, as previous studies reported the presence of PA on their surface (7); (iii) antiphagocytic activity, reducing the detrimental effect of the anthrax toxins on the macrophage and other phagocytic cells; and (iv) that the anthrax toxins may also exert an immunosuppressive effect on lymphocytes (5) and dendritic cells (2), thereby enabling the bacterial cells to migrate freely without release of cytokines by lymphocytes or macrophages. In the presence of anti-PA, the bacteria appear to have a more limited focus of infection, with the innate immune system effectively controlling the infection (8).
The human IgG-specific PA ELISA data shown in Table 3 clearly indicated that the passively administered human MAb diminished substantially by the time of rechallenge. The levels of anti-PA actually measured after 1, 5, and 7 weeks by a human PA ELISA were of the same order of magnitude that one would have expected to see with an elimination half-life established for this antibody of 5 to 9 days (21). The Cmax for AVP-21D9 after s.c. injection of 10 mg/kg was 218 μg/ml (21), and 1 week after challenge, the residual levels of AVP-21D9 were approximately one-half the Cmax. During the 5-week period after primary challenge, the AVP-21D9 would have gone through five half-lives. If one extrapolated five half-lives from a single 10-mg/kg dose of AVP-21D9 administered to the rabbits, the serum concentration would be estimated to be 7.5 μg/ml, which corresponds reasonably well with the residual levels of human IgG (AVP-21D9) actually measured (Table 3) after 5 weeks. This premise is supported by our observations that intranasally challenged Dutch-belted rabbits at 5 weeks had 13 to 20 μg/ml residual AVP-21D9 at the time of rechallenge (Table 3). This level of residual human anti-PA could have provided protection, since a single dose of 1 mg/kg was completely protective for rabbits when given at the time of challenge, and the Cmax for a 1-mg/kg dose of AVP-21D9 is 23 ± 6 μg/ml) (21). In contrast, in New Zealand White rabbits, the 5-week residual human anti-PA levels were in the 3- to 4-μg/ml range (Table 3), and most animals had less than 5 μg/ml at that time. Based on the waning protection from the earlier 10-mg/kg dose, one could question whether the level of human anti-PA antibodies would be sufficient to provide protection from lethal infection. Perhaps the elimination half-lives of AVP-21D9 in New Zealand and Dutch-belted rabbits behave slightly differently; however, it could be argued that the New Zealand White rabbits could have produced some of their own antibodies to protect themselves against the second challenge (Table 4). We know that the blood level of PA following spore challenge by nasal instillation is closely correlated with the number of B. anthracis organisms in the blood, reaching low microgram-per-ml levels as early as 36 to 60 h postchallenge (17). The low levels of rabbit anti-PA antibodies in Table 4 indicate that the infected rabbits dosed with AVP-21D9 formed some antibodies against PA released during the infection; however, the low level of the rabbit anti-PA response could reflect down regulation of the immune response by the anthrax toxins (5). Alternatively, AVP-21D9 (human anti-PA) in the sera of the rabbits may have interfered with the development of an adaptive immune response to PA in the animals.
The results of testing the rabbit sera with a rabbit IgG-specific PA ELISA (Table 4) showed low and gradually falling titers of rabbit antibodies to PA. Because the rabbit sera contained various levels of residual AVP-21D9 (human anti-PA), we used a goat anti-rabbit IgG conjugate that had been adsorbed with human Ig to render it specific for the rabbit. Further, we analyzed the specificity of the conjugate for rabbit IgG and demonstrated no cross-reactivity (optical density at 405 of <0.06) with AVP-21D9 (1 to 200 μg/ml) (data not shown). Thus, we are left to draw either of two conclusions from the antibody titrations. The first possibility is that the level of AVP-21D9 could have diminished below the protective level, and the low levels of rabbit anti-PA measured in the sera contributed to the protection observed at 5 weeks. Other, uncharacterized immunogens formed following initial spore challenge could also have been a factor. The second possibility is that the rabbit anti-PA response was very low and an unlikely factor in providing protection from rechallenge. The residual level of AVP-21D9 had decreased substantially by 5 weeks postchallenge, but the passively administered human anti-PA might have been at the critical concentration capable of providing virtually complete protection (to all except 1 of 24 rabbits). We think that the data favors the latter possibility; however, it would be difficult, based on the present results, to eliminate the possibility that both residual human anti-PA (AVP-21D9) and low levels of newly formed rabbit anti-PA (or other antibodies) contributed to protection from rechallenge. Future studies are planned to determine whether AVP-21D9 will interfere with or possibly enhance the adaptive immune responses of rabbits to vaccination with recombinant PA vaccines.
Current medical practice for the prophylactic treatment of individuals exposed to aerosols of B. anthracis spores is to prescribe long-term antibiotic treatment with ciprofloxacin (60 days) and, when appropriate, switch patients to alternate antibiotics such as tetracycline (30). In this study, we evaluated the protective capacity of AVP-21D9, a human MAb to PA (21). With further development and evaluation, AVP-21D9 could offer an adjunct or alternative clinical treatment regimen to provide extended protection against inhalation anthrax. Such a strategy could reduce the clinical side effects (e.g., gastrointestinal effects) experienced by patients being treated with either ciprofloxacin or other antibiotics (4, 30).
Acknowledgments
We thank Judy Hewitt, Kristin DeBord, Ed Nuzum, and Anthony Macaluso of the NIAID for advice on experimental design and encouragement during the progression of these studies. We also thank Fei Wang and Shu Man Sun for expert production and purification of AVP-21D9. We appreciate the expert advice on aerosol challenge procedures generously supplied by Chad Roy (Tulane Primate Center, New Orleans, LA).
We acknowledge financial support from NIAID contract N01-AI-30065, NIAID grant U01 AI5385802, and Army grant DAMD170210699. The production and purification of AVP-21D9 was supported by grant R43 AI058458-02 from NIH/NIAID (to W.W.S.).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Abramova, F. A., L. M. Grinberg, O. V. Yampolskaya, and D. H. Walker. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. USA 90:2291-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329-334. [DOI] [PubMed] [Google Scholar]
- 3.Brey, R. N. 2005. Molecular basis for improved anthrax vaccines. Adv. Drug Deliv. Rev. 57:1266-1292. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2001. CDC update: adverse events associated with anthrax prophylaxis among postal employees—New Jersey, New York City, and the District of Columbia metropolitan area.Morb. Mortal. Wkly. Rep. 50:1051-1054. [PubMed] [Google Scholar]
- 5.Comer, J. E., A. K. Chopra, J. W. Peterson, and R. Konig. 2005. Direct inhibition of T-lymphocyte activation by anthrax toxins in vivo. Infect. Immun. 73:8275-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comer, J. E., D. M. Noffsinger, D. J. McHenry, D. M. Weisbaum, B. M. Chatuev, A. K. Chopra, and J. W. Peterson. 2006. Evaluation of the protective effects of quinacrine against Bacillus anthracis Ames. J. Toxicol. Environ. Health A 69:1083-1095. [DOI] [PubMed] [Google Scholar]
- 7.Cote, C. K., C. A. Rossi, A. S. Kang, P. R. Morrow, J. S. Lee, and S. L. Welkos. 2005. The detection of protective antigen (PA) associated with spores of Bacillus anthracis and the effects of anti-PA antibodies on spore germination and macrophage interactions. Microb. Pathog. 38:209-225. [DOI] [PubMed] [Google Scholar]
- 8.Cote, C. K., N. Van Rooijen, and S. L. Welkos. 2006. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect. Immun. 74:469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flick-Smith, H. C., N. J. Walker, P. Gibson, H. Bullifent, S. Hayward, J. Miller, R. W. Titball, and E. D. Williamson. 2002. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect. Immun. 70:1653-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garmory, H. S., R. W. Titball, K. F. Griffin, U. Hahn, R. Bohm, and W. Beyer. 2003. Salmonella enterica serovar Typhimurium expressing a chromosomally integrated copy of the Bacillus anthracis protective antigen gene protects mice against an anthrax spore challenge. Infect. Immun. 71:3831-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinberg, L. M., F. A. Abramova, O. V. Yampolskaya, D. H. Walker, and J. H. Smith. 2001. Quantitative pathology of inhalational anthrax. I. Quantitative microscopic findings. Mod. Pathol. 14:482-495. [DOI] [PubMed] [Google Scholar]
- 12.Guarner, J., J. A. Jernigan, W. J. Shieh, K. Tatti, L. M. Flannagan, D. S. Stephens, T. Popovic, D. A. Ashford, B. A. Perkins, and S. R. Zaki. 2003. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 163:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamouda, T., A. Y. Shih, and J. R. Baker. 2002. A rapid staining technique for the detection of the initiation of germination of bacterial spores. Lett. Microbiol. 34:86-90. [DOI] [PubMed] [Google Scholar]
- 14.Heninger, S., M. Drysdale, J. Lovchik, J. Hutt, M. F. Lipscomb, T. M. Koehler, and C. R. Lyons. 2006. Toxin-deficient mutants of Bacillus anthracis are lethal in a murine model for pulmonary anthrax. Infect. Immun. 74:6067-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacono-Connors, L. C., S. L. Welkos, B. E. Ivins, and J. M. Dalrymple. 1991. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect. Immun. 59:1961-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivins, B. E., S. L. Welkos, S. F. Little, M. H. Crumrine, and G. O. Nelson. 1992. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect. Immun. 60:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobiler, D., S. Weiss, H. Levy, M. Fisher, A. Mechaly, A. Pass, and Z. Altboum. 2006. Protective antigen as a correlative marker for anthrax in animal models. Infect. Immun. 74:5871-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little, S. F., B. E. Ivins, W. M. Webster, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2006. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine 24:2530-2536. [DOI] [PubMed] [Google Scholar]
- 19.Melnyk, R. A., and R. J. Collier. 2006. A loop network within the anthrax toxin pore positions the phenylalanine clamp in an active conformation. Proc. Natl. Acad. Sci. USA 103:9802-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien, J. A. Friedlander, T. Dreier, J. Ezzell, and S. Leppla. 1985. Effects of anthrax toxin components on human neutrophils. Infect. Immun. 47:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson, J. W., J. E. Comer, D. M. Noffsinger, A. Wenglikowski, K. G. Walberg, B. M. Chatuev, A. K. Chopra, L. R. Stanberry, A. S. Kang, W. W. Scholz, and J. Sircar. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitt, M. L. M., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 23.Rainey, G. J., D. J. Wigelsworth, P. L. Ryan, H. M. Scobie, R. J. Collier, and J. A. Young. 2005. Receptor-specific requirements for anthrax toxin delivery into cells. Proc. Natl. Acad. Sci. USA 102:13278-13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy, C. J., and L. M. L. Pitt. 2005. Infectious disease aerobiology: aerosol challenge methods. In J. L. Swearengen (ed.), Biodefense: research methodology and animal models. CRC Press, Boca Raton, FL.
- 25.Sawada-Hirai, R., I. Jiang, F. Wang, S. M. Sun, R. Nedellec, P. Ruther, A. Alvarez, D. Millis, P. R. Morrow, and A. S. Kang. 2004. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune Based. Ther. Vaccines 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varughese, M., A. V. Teixeira, S. Liu, and S. H. Leppla. 1999. Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect. Immun. 67:1860-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, F., P. Ruther, I. Jiang, R. Sawada-Hirai, S. M. Sun, R. Nedellec, P. R. Morrow, and A. S. Kang. 2004. Human monoclonal antibodies that neutralize anthrax toxin by inhibiting heptamer assembly. Hum. Antibodies 13:105-110. [PubMed] [Google Scholar]
- 28.Weiss, S., D. Kobiler, H. Levy, H. Marcus, A. Pass, N. Rothschild, and Z. Altboum. 2006. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect. Immun. 74:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welkos, S. L., and A. M. Friedlander. 1988. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb. Pathog. 5:127-139. [DOI] [PubMed] [Google Scholar]
- 30.Williams, J. L., S. S. Noviello, K. S. Griffith, H. Wurtzel, J. Hamborsky, J. F. Perz, I. T. Williams, J. L. Hadler, D. L. Swerdlow, and R. Ridzon. 2002. Anthrax postexposure prophylaxis in postal workers, Connecticut, 2001. Emerg. Infect. Dis. 8:1133-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaucha, G. M., L. M. Pitt, J. Estep, B. E. Ivins, and A. M. Friedlander. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982-992. [PubMed] [Google Scholar]