Abstract
The cytotoxic necrotizing factors CNF1 and CNF2 produced by pathogenic Escherichia coli strains and CNFY of Yersinia pseudotuberculosis constitutively activate small GTPases of the Rho family. They deamidate a glutamine (Gln63 in RhoA), which is crucial for GTP hydrolysis. CNF1 and CNFY exhibit 61% identity on the amino acid level, with equal distribution over the whole molecule. Although the two toxins are homologous in the receptor binding domain, we show that they bind to different cellular receptors. CNFY does not enter Caco-2 and CHO-K1 cells, which are responsive to CNF1. In contrast, HeLa, Hep-2, and HEK 293 cells do respond to both toxins. Competition studies with catalytically inactive mutants of the toxins revealed that binding of CNF1 has no influence on the uptake of CNFY into HeLa cells. In contrast, uptake of CNF1 is retarded after preincubation of HeLa cells with the catalytically inactive mutant of CNFY, suggesting that the toxin receptors overlap. Moreover, we compared the pathways of the toxins from receptor binding into the cytosol and showed that both toxins are taken up independent of the presence of clathrin or lipid rafts and are released into the cytosol from acidified endosomes.
Cytotoxic necrotizing factor 1 (CNF1) is an AB-type toxin expressed by pathogenic Escherichia coli strains which cause urinary tract infections and neonatal meningitis. CNF1 is a 115-kDa single-chain molecule comprising an N-terminal receptor binding domain and a C-terminal catalytic domain, which contains deamidase activity (for a review, see reference 9). These two domains are separated by a putative translocation domain, which contains two hydrophobic helices involved in membrane translocation (16). CNF1 deamidates small GTP-binding proteins of the Rho family at glutamine 63/61, leading to constitutive activation of the small GTPases by blocking their intrinsic and GTPase activating protein-stimulated GTP hydrolysis (5, 17).
CNF1 has been shown to enter cells by receptor-mediated endocytosis independent of clathrin and independent of sphingolipid-cholesterol-rich membrane microdomains (lipid rafts), including caveolae (4). Based on interaction studies, using a yeast two-hybrid system, it has been suggested that cell binding of the toxin is mediated by the laminin receptor precursor p37, a subunit of the mature nonintegrin 67-kDa laminin receptor (3, 11). Moreover, it was shown that CNF1 contributes to E. coli K1 invasion of human brain microvascular endothelial cells via the 67-kDa laminin receptor. Following internalization, CNF1 is delivered to late endosomes by microtubule-dependent transport. From there, it is released into the cytosol in an acidic pH-dependent manner. Specific acidic residues located in the hydrophilic loop, connecting the two hydrophobic helices of the translocation domain, appear to be involved in the translocation process (16).
More recently, a cytotoxic necrotizing factor (CNF) produced by Yersinia pseudotuberculosis (CNFY) was discovered, which catalyzes the same deamidation reaction as CNF1 (8, 14). The amino acid sequence of CNFY is about 61% identical to that of CNF1 from E. coli, with equal distribution over the whole molecule. The amino acid residues which are essential for the catalytic activity in CNF1 (e.g., Cys866 and His881) are also conserved in CNFY with the same spacing. Based on the high sequence homology, the overall structure of CNFY is considered to resemble the AB-type toxin structure of CNF1. Besides all the similarities, striking differences between the two toxins have been reported. In contrast to the vesicle-associated CNF1 (12), CNFY is thought to be secreted by Y. pseudotuberculosis (14). In addition, neither an anti-CNF1 polyclonal antibody nor an anti-CNF1 monoclonal antibody could neutralize the biological activity of CNFY-containing bacterial extracts (14). However, the most notable difference was reported for the substrate specificity of the toxins. Whereas CNF1 modifies RhoA, Rac, and Cdc42 in HeLa cells, recombinant CNFY selectively activates RhoA (8). Rac and Cdc42 are not activated in vivo at least 6 h after toxin treatment.
The uptake of CNF1 has been analyzed previously, but nothing is known about the cellular entry of CNFY and its subsequent delivery into the cytosol. Here, we show that the two toxins use different cellular receptors. They are then internalized by clathrin-independent and lipid raft-independent endocytosis and translocate from late endosomes into the cytosol in an acidification-dependent step. Direct translocation of CNFY through the plasma membrane is possible at low pH but requires more stringent conditions than translocation of CNF1.
MATERIALS AND METHODS
Cell culture.
All cell lines used were obtained from Cell Lines Service, Heidelberg, Germany. HeLa, HEp-2, and HEK293 cells were grown in Dulbecco's modified Eagle's medium (DMEM) (12 mM l-glutamine) supplemented with 10% fetal calf serum, sodium pyruvate (1 mM), penicillin (4 mM), and streptomycin (4 mM). Caco-2 cells were grown in DMEM (12 mM l-glutamine) supplemented with 10% fetal calf serum, sodium pyruvate (1 mM), 1% nonessential amino acids, penicillin (4 mM), and streptomycin (4 mM). CHO-K1 cells were grown in alpha modified Eagle's medium supplemented with 5% fetal calf serum, penicillin (4 mM), and streptomycin (4 mM). All cell lines were cultivated in a humidified atmosphere containing 5% CO2 at 37°C.
Mutagenesis and cloning.
The glutathione S-transferase (GST)-CNF1-C866S mutant and the GST-CNFY-C866S mutant were generated by QuickChange site-directed mutagenesis as previously reported (8). pGEX2TGL+2-CNF1 and pGEX2TGL-CNFY were used as template plasmids. Constructs were verified by DNA cycle sequencing using a BigDye Terminator cycle sequencing kit (Applied Biosystems, United States).
For expression in mammalian cells RhoA was cloned into the vector pcDNA3.1/HisC (Invitrogen, Germany) in frame with the vector His tag, generating pcDNA3.1 His-RhoA.
Toxins.
GST-CNF1, GST-CNFY, and the mutants based on these toxins were purified as previously described (8). Bacterial supernatants from Helicobacter pylori containing active VacA were provided by S. Backert (Universität Magdeburg, Germany). Diphtheria toxin was obtained from Calbiochem (Darmstadt, Germany).
Rho shift assay.
Cells were incubated with GST-CNF1, GST-CNFY, and CNF1- or CNFY-derived mutants at 37°C using the concentrations indicated below for the time periods indicated below. Following toxin treatment, cells were washed twice with cold phosphate-buffered saline (PBS) and lysed on ice using lysis buffer containing 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 2 mM MgCl2, 10% glycerol, 1% Igepal, and 0.5 mM phenylmethylsulfonyl fluoride. After clearance of the lysates by centrifugation (20 min, 20,000 × g, 4°C) sodium dodecyl sulfate (SDS) sample buffer was added to the supernatant and samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (1 M urea) and subsequent Western blotting against RhoA.
Cell staining.
For rhodamine-phalloidin staining, cells on coverslips were treated with GST-CNF1 (1 nM) or GST-CNFY (1 nM) in cell culture medium at 37°C for the times indicated below. After washing with PBS, cells were fixed in 3% formaldehyde and 0.05% Triton X-100 in PBS for 10 min. Then cells were washed three times and incubated in 30 μg of rhodamine-phalloidin per ml in PBS for 30 min. Following additional washing steps with PBS, cells were subjected to fluorescence microscopy. For heparansulfate proteoglycan (HSPG) staining, cells on coverslips were washed with PBS and fixed in 3% formaldehyde and 0.05% Triton X-100 in PBS for 10 min. Thereafter, cells were washed three times and incubated with 1 μg of fluorescein isothiocyanate-coupled anti-human heparan sulfate antibody (10E4 epitope; United States Biological) per 100 μl in PBS for 60 min. Following additional washing steps with PBS, cells were analyzed by fluorescence microscopy (Kaiser's glycerol gelatin [Merck] was used as a bleaching preservative).
Rhotekin pull-down.
Cells were intoxicated with GST-CNF1 or GST-CNFY (400 ng per ml each) at 37°C for the time periods indicated below. Cells were then washed twice with cold PBS and incubated for 5 min at 4°C in lysis buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 2 mM MgCl2, 10% glycerol, 1% Igepal, and 0.5 mM phenylmethylsulfonyl fluoride). After centrifugation (20 min, 20,000 × g, 4°C), supernatants were further subjected to pull-down experiments. Rhotekin pull-down was performed as previously reported (8).
Measurement of TER.
Transepithelial resistance (TER) of Caco-2 cells was determined by use of a volt-ohm meter (World Precision Instruments, United States) equipped with a chamber for filter inserts. Filters with confluent cell monolayers with an initial resistance of ≥200 Ω per cm2 were used. Toxins or toxin mutants were applied from the apical or basolateral side, using a concentration of 1 nM of the proteins in culture medium. Thereafter, cells were incubated at 37°C, and TER values were determined every hour. The results are expressed as percentages of the initial values.
Inhibitor assays for investigation of CNF uptake.
Cells were preincubated for 1 h with the following drugs: chlorpromazine (15 μM), nystatin (25 μM), bafilomycin A1 (100 nM), and nocodazole (30 μM). All compounds were purchased from Sigma-Aldrich (Deisenhofen, Germany). Preincubation was performed at 37°C except for nocodazole treatment. Nocodazole treatment was carried out on ice to enhance microtubule depolymerization. Cells were then incubated at 37°C for 60 min with GST-CNF1 (1 nM) or for 150 min with GST-CNFY (1 nM) in the presence of the drugs. Afterwards, a Rho shift assay was performed as described above.
Treatment of cells with sodium chlorate (80 mM) was carried out at 37°C for 48 h. Following incubation of the cells for 60 min with GST-CNF1 (1 nM) or for 150 min with GST-CNFY (1 nM), intoxication was analyzed by the Rho shift assay.
Transfection studies for investigation of CNF uptake.
The dominant negative green fluorescent protein-tagged plasmid construct pEΔ95/295 (Eps15mut), which encodes an Eps15 deletion mutant lacking the second and third EH domains, and the control plasmid pD3Δ2, expressing a C-terminally truncated fragment of Eps15 (Eps15ctrl), were generous gifts from A. Benmerah, INSERM, Paris, France (1).
The plasmids mentioned above were cotransfected with pcDNA3.1 His-RhoA at a ratio of 2:1 by using metafectene (Biontex, Germany) according to the manufacturer's protocols. Usually, cells were transfected 48 h before morphological studies or Rho shift assays were performed.
pH shift assay.
Cells were preincubated with bafilomycin A1 (100 nM) at 37°C for 45 min and then washed with cold Hanks' balanced salt solution (HBSS) containing 1.25 mM CaCl2, 0.4 mM MgSO4, 5 mM KCl, 0.4 mM KH2PO4, 0.14 M NaCl, 0.3 mM Na2HPO4, 5 mM glucose, and 10 mM HEPES (pH 7.5). Afterwards, GST-CNF1 or GST-CNFY (1 μg/ml) was bound to the cells for 2 h at 4°C in the presence of bafilomycin A1. Thereafter, cells were washed with cold HBSS (pH 7.5), HBSS (pH 5.2), or HBSS (pH 4.8) and incubated at 37°C in these buffers for the times indicated below. Then cells were washed with DMEM and further incubated overnight at 37°C in the presence of bafilomycin A1 (100 nM). Translocation of CNF molecules into the cytosol by a short acidic pulse was proven by phase-contrast microscopy and the Rho shift assay.
Western blot analysis.
For Western blot analysis proteins were separated by SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. RhoA was detected using a monoclonal anti-RhoA mouse antibody (clone 26C4; Santa Cruz Biotechnology) recognizing an epitope between amino acids 120 and 150 of the RhoA insert region. His-tagged proteins were detected using a monoclonal anti-His tag mouse antibody (clone 4D11) from Upstate (NY).
RESULTS
Responsiveness of different cell lines.
Two CNFs, namely, CNF1 from E. coli and CNFY produced by Y. pseudotuberculosis, exhibit a sequence identity of 61% on the amino acid level. Because the region of this high similarity also comprises the receptor binding domain, we compared the cellular uptake mechanisms of the toxins.
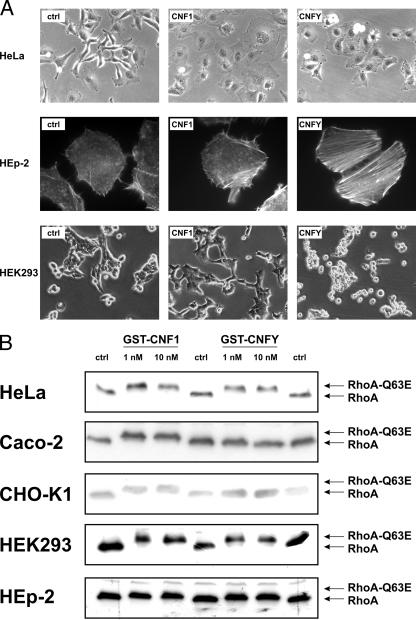
First, different cell lines were tested for their responsiveness to the toxins. For this purpose, we treated several cell types with CNF1 or CNFY and analyzed the cell morphology, as well as the upward shift of RhoA from lysates of toxin-treated cells in SDS-PAGE, which indicates deamidation of the GTPase by the toxins. Moreover, activation of RhoA was determined by precipitation of the GTP-bound form of the GTPase with its effector rhotekin coupled to Sepharose beads. In the case of Caco-2 cells, the influence of the toxins on TER was additionally investigated as described previously (7). Because of the high homology within the receptor binding domains, we expected that CNF1-responsive cell lines, like human cervix epithelial carcinoma cells (HeLa), human laryngeal carcinoma cells (HEp-2), human embryonic kidney cells (HEK293), Chinese hamster ovary cells (CHO-K1), or human colon carcinoma cells (Caco-2), would also respond to CNFY. But unexpectedly, we found that some cell lines showed responsiveness to both toxins, whereas others were sensitive exclusively to CNF1. In HeLa cells CNF1 as well as CNFY induced morphological changes, which is in line with previous reports (8). CNF1 and CNFY caused, for example, cell flattening and polynucleation (Fig. 1A), indicating that both toxins entered the cells. This observation was supported by the upward shift of RhoA from lysates of intoxicated HeLa cells to an apparently higher molecular weight in SDS-PAGE (Fig. 1B). Moreover, more activated RhoA from lysates of cells treated with CNF1 and much more activated RhoA from lysates of CNFY-treated HeLa cells were precipitated in our pull-down experiments compared to the amounts observed with lysates of untreated control cells (Fig. 1C). Morphological changes induced by CNF1 and CNFY were also observed in HEp-2 cells and HEK293 cells (Fig. 1A). After treatment of HEp-2 cells with CNF1 or CNFY, we observed formation of stress fibers in the intoxicated cells by actin staining. In HEK293 cells we found membrane blebbing following incubation with CNFY, which indicates strong RhoA activation (18). Incubation of these cells with CNF1 led to cell flattening (Fig. 1A). Consistent with these findings, RhoA from lysates of toxin-treated HEp-2 and HEK293 cells shifted in SDS-PAGE, indicating deamidation (Fig. 1B).
FIG. 1.
Cellular responsiveness to CNF1 and CNFY. (A) HeLa cells, HEK293 cells, and HEp-2 cells were treated with 1 nM GST-CNF1 or 1 nM GST-CNFY at 37°C overnight. Toxin-induced morphological changes were studied by phase-contrast microscopy or fluorescence microscopy of actin-stained cells. (B) HeLa cells, CHO-K1 cells, Caco-2 cells, HEK293 cells, and HEp-2 were treated with GST-CNF1 and GST-CNFY at 37°C for 3 h at the concentrations indicated. Cells were lysed, and a Rho shift assay was performed. (C) HeLa cells, CHO-K1 cells, and Caco-2 cells were incubated with GST-CNF1 or GST-CNFY for 4 h at 37°C using the concentrations indicated. After lysis of the cells, 1/10 of each sample was subjected to Western blot analysis. The main sample volume (9/10 of the sample) was incubated with the GTPase-binding domain of GST-rhotekin coupled to beads to examine the RhoA activation status by a pull-down assay. For detection of total RhoA and activated RhoA an anti-RhoA antibody was used for Western blotting. (D) Effect of GST-CNF1 and GST-CNFY on the TER of Caco-2 cells. GST-CNF1 (1 nM) and GST-CNFY (1 nM) were applied apically as well as basolaterally to cellular monolayers growing on filter inserts; untreated cells (ctrl) were also included. Cells were incubated at 37°C, and the TER was measured at the times indicated. The graph presents data from one typical experiment representative of three independent experiments performed.
In contrast to HeLa cells, HEp-2 cells, and HEK293 cells, which respond to both toxins, colon carcinoma cells (Caco-2) took up the E. coli toxin but surprisingly were not responsive to the Yersinia toxin. Caco-2 cells were incubated in the absence and presence of CNF1 and CNFY. After 3 h the cells were lysed and tested for deamidated RhoA by SDS-PAGE and subsequent Western blotting (Fig. 1B). Moreover, the amount of activated RhoA in lysates of toxin-treated Caco-2 cells was detected by a pull-down assay with recombinant rhotekin, which binds exclusively GTP-bound RhoA (Fig. 1C). Whereas RhoA from CNF1-treated cells was deamidated as well as activated, CNFY had no effect on RhoA, suggesting that the toxin was not internalized by Caco-2 cells. In line with this, RhoA from Caco-2 lysates treated with CNFY in vitro shifted to a higher molecular weight in SDS-PAGE, indicating that RhoA from these cells can be modified by the toxin (data not shown). Furthermore, we analyzed the effect of CNF1 and CNFY intoxication on epithelial permeability (7). Thus, Caco-2 cells were grown on filter inserts as monolayers and incubated with the toxins from the apical side or from the basolateral side. TER was measured in a time course, as shown in Fig. 1D. In contrast to CNF1 intoxication, which led to a decrease in epithelial resistance, no change was observed when Caco-2 monolayers were treated with the Yersinia toxin.
There are yet other cell lines that do not respond to CNFY. Another colon carcinoma cell line, HT29, was not sensitive to CNFY (data not shown). Also, CHO-K1 cells were not responsive to CNFY. The toxin induced no morphological changes in these cells (data not shown), no upward shift of RhoA in SDS-PAGE (Fig. 1B), and no activation of the GTPase (Fig. 1C). In contrast, these cell lines did respond to E. coli CNF1.
Competition studies.
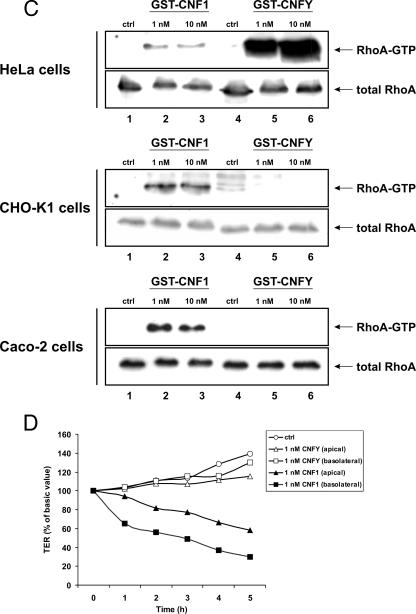
The lack of responsiveness of some cell lines could be due to defects in the uptake machinery of the Yersinia toxin or due to the absence of the appropriate cellular receptor. If a different cellular receptor is responsible for the varying responsiveness of cell lines to CNF1 and CNFY, one should expect no competition with catalytically inactive mutants of both toxins. Therefore, we tested whether preincubation of Caco-2 cells or HeLa cells with an excess of a catalytically inactive mutant of either toxin (GST-CNF1-C866S or GST-CNFY-C866S) resulted in competition with the wild-type toxin. Cellular entry of the active toxin was checked by examining the induced shift of RhoA in SDS-PAGE, by examining morphological changes of the cells (data not shown), and in the case of Caco-2 cells by examining the CNF1-induced increase in epithelial permeability (7). Caco-2 cells were preincubated with a 100-fold excess of GST-CNFY-C866S or GST-CNF1-C866S for 30 min at 4°C and subsequently treated with GST-CNF1. Cell lysis was performed 60 min after intoxication to determine differences in the velocity of the toxin uptake. As expected, incubation of Caco-2 cells with the catalytically inactive mutant of CNF1 completely blocked the cell entry of the wild-type toxin, as indicated by the lack of the upward shift of RhoA in SDS-PAGE (Fig. 2A). In contrast, treatment of the cells with the catalytically inactive mutant of CNFY had no influence on CNF1 internalization. The same amount of RhoA was shifted with and without preincubation with GST-CNFY-C866S. No retardation of CNF1 uptake occurred (Fig. 2A). We supported this finding by analysis of the CNF1-induced decrease in TER as described previously (7). Thus, Caco-2 cells were preincubated with the catalytically inactive proteins for 30 min at 4°C and then treated with GST-CNF1. TER was measured in a time course as indicated in Fig. 2B. Consistent with the Rho shift assay, the same decrease in resistance over time that was observed for GST-CNF1 alone was measured. In contrast, preincubation with the inactive mutant of CNF1 (GST-CNF1-C866S) completely blocked the uptake of CNF1. Because the Yersinia toxin did not enter Caco-2 cells, the same competition study was performed with HeLa cells. As expected, preincubation of HeLa cells with a 100-fold excess of GST-CNF1-C866S completely blocked intoxication of the cells with GST-CNF1. Unexpectedly, pretreatment of the cells with the same amount of GST-CNFY-C866S did not completely block but delayed the intoxication by wild-type CNF1, suggesting that the two toxins bind to different but overlapping cellular structures (Fig. 2C). The experiment was also performed the other way around. HeLa cells were preincubated with GST-CNF1-C866S for 30 min at 4°C and then intoxicated with GST-CNFY. Cell lysis was performed 150 min after intoxication. In this experiment we observed the same time-dependent intoxication of cells preincubated with GST-CNF1-C866S that was observed for controls, indicating that CNF1 binding had no influence on the uptake of CNFY in HeLa cells.
FIG. 2.
Competition studies with GST-CNF1 and GST-CNFY and their inactive mutants. (A and B) Caco-2 cells were pretreated with the inactive CNF mutants GST-CNF1-C866S (100 nM) and GST-CNFY-C866S (100 nM) for 30 min at 4°C. Cells were then incubated with GST-CNF1 (1 nM) at 37°C. Competition was studied by both a Rho shift assay (A) and TER measurement (B). For the Rho shift assay incubation lasted for 1 h, and for TER measurement incubation lasted for 4 h. Upon intoxication, TER was determined every hour. For the TER assay, the open circles represent untreated control cells (ctrl). The other symbols are as follows: solid circles, positive control with CNF1; open triangles, cells preincubated with CNFY-C866S and treated with CNF1; solid triangles, cells preincubated with CNF1-C866S and treated with CNF1; open squares, cells only treated with CNFY-C866S; solid squares, cells only treated with CNF1-C866S. The graph presents data from one typical experiment representative of more than three independent experiments performed. (C) For the Rho shift assay with HeLa cells preincubation with GST-CNF1-C866S (100 nM) and GST-CNFY-C866S (100 nM) was performed for 30 min at 4°C. Thereafter, GST-CNF1 (1 nM) or GST-CNFY (1 nM) was added, and cells were incubated at 37°C. The incubation time for CNF1 was 1 h, and the incubation time for CNFY was 2.5 h.
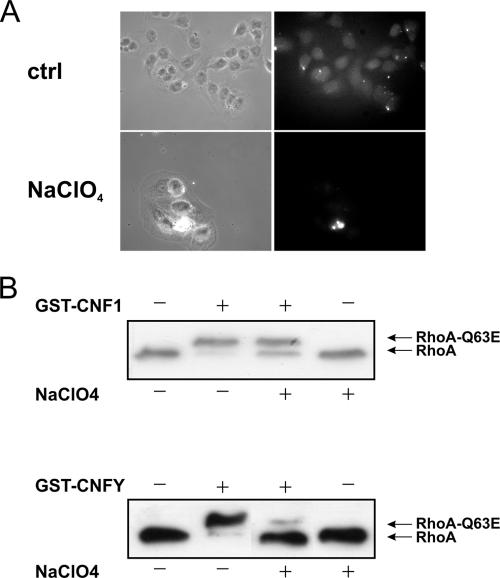
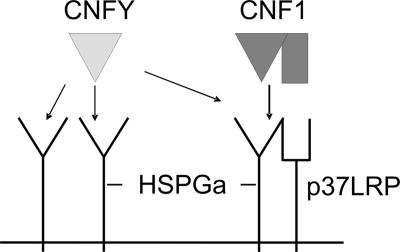
Recently, it has been suggested that HSPGs are required for the binding of prion proteins to the laminin receptor precursor p37 (10). Because CNF1 cell binding is thought to be mediated by this receptor (3), we tested whether HSPGs are involved in the internalization of CNF1 or CNFY. We cultivated HeLa cells with sodium chlorate, which is known to block the synthesis of HSPGs (2). Incubation with this inhibitory substance reduced the amount of HSPG molecules on the cell surface (Fig. 3A). Moreover, this treatment retarded the cell entry of CNFY and inhibited to a minor extent the uptake of CNF1 (Fig. 3B). Therefore, it is feasible that an HSPG represents the receptor for CNFY and is part of the receptor for CNF1, as suggested in the model shown in Fig. 8 (see Discussion). In summary, the data presented here indicate that CNF1 and CNFY use different cellular receptors with partially overlapping structures.
FIG. 3.
Involvement of HSPGs in CNF1 and CNFY uptake into HeLa cells. HeLa cells were preincubated with sodium chlorate (80 mM) for 48 h at 37°C. Cells were then stained for surface HSPGs and analyzed by fluorescence microscopy (A), or they were treated with GST-CNF1 (1 nM) for 1 h or with GST-CNFY (1 nM) for 2.5 h at 37°C in the presence of sodium chlorate. Following cell lysis, a Rho shift assay was performed to investigate the influence of NaClO4 on intoxication (B). Note that a lot of cells were lost during sodium chlorate treatment.
FIG. 8.
Model for CNF receptor binding. In this model, HSPG is part of the receptor for CNF1 and additionally acts as the receptor for CNFY. CNFY binds to a subtype of HSPGs (called HSPGa) present on HeLa cells, thereby blocking HSPG-dependent binding of CNF1 to the laminin receptor precursor p37 (p37LRP). Because HSPGs are found not only in complex with p37, binding of CNF1 has no influence on the uptake of CNFY. CNFY may not bind to a different subtype of HSPGs (HSPGb), expressed by nonresponsive cell lines like Caco-2 cells.
Endocytosis of CNF1 and CNFY.
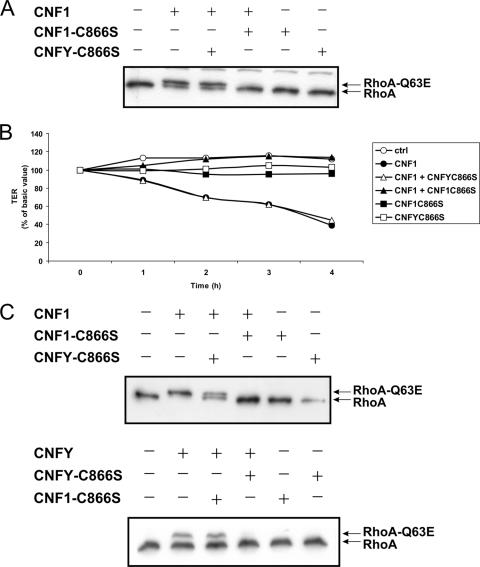
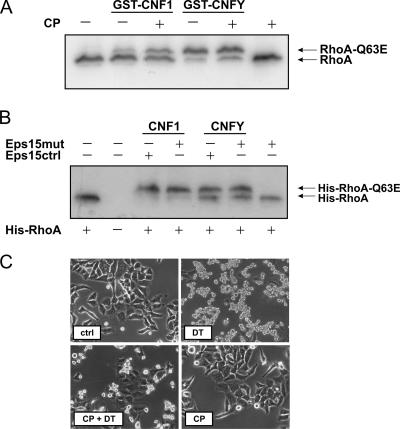
For characterization of the endocytic pathways used by CNF1 and CNFY, toxin internalization into HeLa cells was studied. To block clathrin-dependent endocytosis, we used two different tools: the drug chlorpromazine, which disrupts the assembly of clathrin-coated pits at the cell surface (4), and, as a more specific inhibitor, a construct for a dominant negative mutant of Eps15 (Eps15mut), which encodes an Eps15 deletion mutant known to block the clathrin-dependent endocytic pathway (1). HeLa cells were preincubated with chlorpromazine and thereafter intoxicated with CNF1 or CNFY. As shown in Fig. 4A, both toxins shifted the same amount of RhoA to a higher molecular mass irrespective of the presence or absence of chlorpromazine. This finding was in line with the results of the transfection study using the dominant negative Eps15 mutant. This mutant was coexpressed with His-tagged RhoA in HeLa cells, and an antibody against the His tag was used for Western blotting to analyze exclusively transfected cells upon intoxication with CNF1 or CNFY. We found that expression of the dominant negative Eps15 mutant did not affect Rho deamidation by both toxins as shown by the Rho shift assay (Fig. 4B). As an inhibitor control we used diphtheria toxin, which is taken up by clathrin-mediated endocytosis (13). Diphtheria toxin-induced cell rounding of HeLa cells was blocked in the presence of chlorpromazine (Fig. 4C) and was inhibited upon expression of the dominant negative form of Eps15 (data not shown). Our data suggest that clathrin is not necessary for the cellular uptake of CNF1 or CNFY.
FIG. 4.
Role of clathrin in endocytosis of CNF1 and CNFY. HeLa cells were preincubated with chlorpromazine (CP) (15 μM) for 1 h at 37°C. Cells were then treated with GST-CNF1 (1 nM) for 1 h, with GST-CNFY (1 nM) for 2.5 h, or with diphtheria toxin (10 nM) for 5 h at 37°C in the presence of chlorpromazine. To analyze the effect of chlorpromazine on intoxication after cell lysis, a Rho shift assay was performed (A). Additionally, HeLa cells were cotransfected with a dominant negative form of Eps15 (Eps15mut) or a control plasmid (Eps15ctrl) and a His-tagged form of RhoA. After 48 h, cells were intoxicated with GST-CNF1 (1 nM) for 1 h or with GST-CNFY (1 nM) for 2.5 h at 37°C. Cells were then lysed, and a Rho shift assay was performed (B). (C) Cell rounding of HeLa cells by diphtheria toxin (DT) and inhibition of rounding in the presence of chlorpromazine as an inhibitor control. ctrl, control.
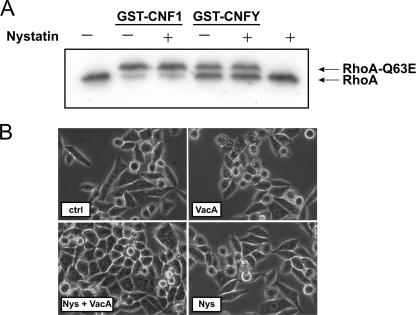
To determine whether the toxins are internalized by lipid raft-dependent endocytic mechanisms, HeLa cells were incubated with the sterol-binding drug nystatin prior to toxin treatment. Nystatin is known to inhibit formation of lipid rafts and therefore also impairs caveola-mediated endocytosis. As an inhibitor control we used H. pylori VacA, which requires the presence of lipid rafts at the plasma membrane to be taken up into cells (6). Whereas VacA-induced vacuolation of HeLa cells was inhibited by nystatin (Fig. 5B), there was no influence of the inhibitor on the modification of RhoA by CNF1 or CNFY as shown by the Rho shift assay (Fig. 5A). Therefore, the data indicate that the presence of lipid rafts is not required for the cellular uptake of CNF1 and also is not necessary for the uptake of the Yersinia CNF.
FIG. 5.
Role of lipid rafts in endocytosis of CNF1 and CNFY. HeLa cells were pretreated with nystatin (25 μM) for 1 h at 37°C. Cells were then intoxicated with bacterial supernatant containing VacA for 2 h, GST-CNF1 (1 nM) for 1 h, or GST-CNFY (1 nM) for 2.5 h at 37°C in the presence of nystatin. To study the effect of nystatin on CNF intoxication, a Rho shift assay was performed (A). For VacA intoxication, cells were analyzed morphologically by phase-contrast microcopy (B). Nys, nystatin.
CNF translocation into the cytosol.
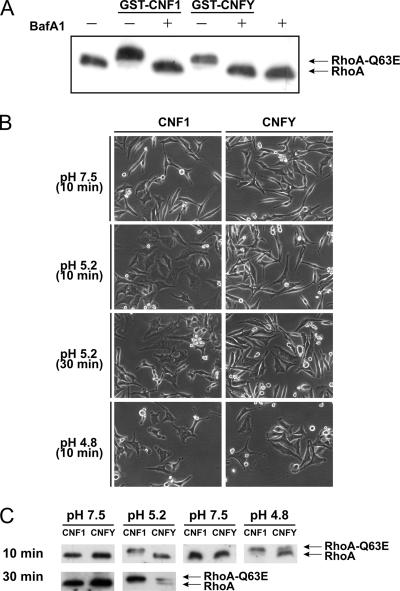
CNF1 has been shown to enter the cytosol by an acidic pH-dependent translocation step from late endosomes (4). Toxin translocation is blocked by the use of bafilomycin A1, which specifically inhibits the endosomal proton pump and thereby blocks acidification of endosomes (19). To investigate the dependency of CNFY translocation on endosomal acidification, HeLa cells were preincubated with bafilomycin A1 and then intoxicated with CNF1 or CNFY. Bafilomycin A1 blocked the morphological changes of HeLa cells induced by CNF1 and by CNFY (data not shown). Moreover, no shift of modified RhoA was detectable in the presence of the proton pump inhibitor upon toxin treatment (Fig. 6A). The data suggest that not only the delivery of CNF1 but also the delivery of CNFY into the cytosol is dependent on acidification of endosomes.
FIG. 6.
CNF entry is dependent on endosomal acidification. (A) HeLa cells were preincubated with bafilomycin A1 (BafA1) (100 nM) for 1 h at 37°C. Afterwards, cells were intoxicated with GST-CNF1 (1 nM) and GST-CNFY (1 nM) overnight at 37°C in the presence of bafilomycin A1. A Rho shift assay was performed after lysis of the treated cells. (B and C) To investigate CNFY translocation through the plasma membrane by an acidic pulse, HeLa cells were preincubated with bafilomycin A1 (100 nM) at 37°C for 45 min. Cells were then washed with HBSS (pH 7.5), and 1 μg/ml GST-CNF1 or GST-CNFY was bound to the cells for 2 h at 4°C. Thereafter, cells were washed with HBSS (pH 7.5), HBSS (pH 5.2), or HBSS (pH 4.8) and incubated at 37°C for the times indicated using these different pH values. Afterwards, cells were further incubated overnight at 37°C in DMEM in the presence of bafilomycin A1 (100 nM). Translocation of CNF molecules through the plasma membrane into the cytosol was monitored by morphological changes of the cells (B) or by the Rho shift assay (C).
After cell binding, direct translocation of CNF1 from the plasma membrane into the cytosol is made possible by a short-term pH shift in the medium, which mimics the low pH of endosomes (4). We studied whether the Yersinia toxin can be delivered directly into the cytosolic compartment by an acidic pulse. For this purpose, CNF1 and CNFY were bound to HeLa cells at 4°C in the presence of bafilomycin A1 to block the usual endocytic uptake. Then a shift to pH 5.2 was performed for 10 min at 37°C at the plasma membrane. To allow full formation of morphological changes induced by translocated toxin molecules, cells were further incubated overnight in DMEM in the presence of bafilomycin A1. Under the conditions used, import of CNF1 but not import of CNFY by external acidification was detectable in the presence of bafilomycin A1 (not shown). After prolongation of the acidification phase up to 30 min, however, CNFY was delivered through the plasma membrane into the cytosol in the presence of the proton pump inhibitor (Fig. 6A). Also, a more acidic pH (pH 4.8) was sufficient to pulse the Yersinia toxin through the plasma membrane in only 10 min (Fig. 6B). These observations were confirmed by an additional analysis of deamidated RhoA by SDS-PAGE (Fig. 6C). In summary, the results indicate that the times necessary for CNF1 and CNFY to cross a biological membrane differ.
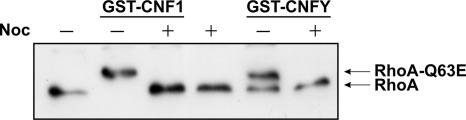
Maturation of endosomes requires the transport of the organelles along microtubules. Microtubule polymerization can be inhibited by the drug nocodazole (15). Incubation of HeLa cells with CNF1 or CNFY in the presence of nocodazole delayed the toxin-induced upward shift of RhoA as determined by SDS-PAGE compared to controls, indicating that endosomal maturation is required for the delivery of both toxins into the cytosol (Fig. 7).
FIG. 7.
Role of microtubules in CNF1 and CNFY uptake into HeLa cells. HeLa cells were pretreated with nocodazole (Noc) (30 μM) for 1 h at 4°C. Cells were then treated with GST-CNF1 (1 nM) for 1 h or with GST-CNFY (1 nM) for 2.5 h at 37°C in the presence of nocodazole. To analyze the effect of microtubule inhibition on intoxication, a Rho shift assay was performed.
Taken together, our data show that CNF1 and CNFY are internalized by cells via receptor-mediated endocytosis independent of the presence of clathrin or lipid rafts. Following cell entry, both toxins appear to be delivered to late endosomes by microtubule-dependent transport, where they translocate into the cytosol in an acidic pH-dependent manner.
DISCUSSION
CNF1 and CNF2 produced by pathogenic E. coli strains and Y. pseudotuberculosis CNFY differ in their type of delivery from the bacteria (12, 14) and in substrate specificity (8). On the amino acid level, however, CNF1 and CNFY share a similarity of 61%, including the receptor binding domain. Cell binding and subsequent uptake of CNF1 into mammalian cells have been studied in detail (for a review, see reference 9). Nothing is known so far about the cellular entry of CNFY and its subsequent delivery into the cytosol. Since CNFY and CNF1 exhibit high homology, we compared the two toxins for uptake into different cell lines and with respect to the entry pathway into the cytosol. Surprisingly, CNF1 and CNFY use different cellular receptors, as concluded from the fact that CNFY does not enter cell lines which are responsive to CNF1.
To obtain further insight into receptor binding, we performed competition studies with catalytically inactive mutants of the toxins. Binding of a catalytically inactive CNF1 mutant (CNF1-C866S) had no influence on the entry of CNFY into HeLa cells. Interestingly, uptake of CNF1 was retarded after preincubation of HeLa cells with the catalytically inactive mutant CNFY-C866S, suggesting that there is an overlapping structure which allows binding of CNFY and inhibits binding of CNF1.
It has been shown that the laminin receptor precursor p37 binds to CNF1 (11). The mature receptor in the plasma membrane is the nonintegrin 67-kDa laminin receptor p67. It is not entirely clear whether p67 is a dimer of p37 or whether a second component, which has not been identified yet, is necessary for receptor maturation.
HSPGs are required for the binding of prion proteins to the laminin receptor precursor p37 (10). Treatment of HeLa cells with sodium chlorate, which blocks the synthesis of HSPGs, retarded the uptake of CNF1 as well as CNFY. This supports the hypothesis that HSPGs may be involved in the internalization of both toxins, as depicted in our model (Fig. 8). In line with binding of CNF1 to p37 and HSPGs, sodium chlorate only weakly inhibited CNF1 uptake. We suggest that CNFY binds to HSPGs but not to p37. The tissue-specific expression of HSPGs explains the cell line-specific uptake of CNFY under the assumption that CNFY binds to a specific HSPG. CNFY may bind to one subtype of HSPGs (HSPGa) present on HeLa cells and block HSPG-dependent binding of CNF1 to the laminin receptor precursor. In contrast, CNFY does not bind to a different subtype (HSPGb), which is expressed by Caco-2 cells and therefore does not inhibit uptake of CNF1. Because HSPGs are not necessarily in a complex with p37, binding of CNF1 has no influence on the uptake of CNFY.
Following binding to the membrane receptors, the toxins are taken up into the cytosol, where they meet their substrates. For CNF1, it is known that the toxin enters the cytosol independent of clathrin- or lipid raft-mediated endocytosis (4). We studied whether the same is true for the uptake of CNFY. Treatment with chlorpromazine or expression of the dominant negative form of Eps15 inhibited diphtheria toxin-induced cell rounding. However, CNFY uptake was not affected, indicating a clathrin-independent endocytic mechanism for the toxin. Moreover, we found no difference in the velocity of CNFY action in the absence or presence of nystatin. The data show that CNFY uptake is not dependent on lipid rafts or on caveolin-mediated endocytosis, which requires intact lipid rafts. Our data do not exclude the possibility that CNF1 and CNFY may be taken up by different endocytosis pathways, but the experiments suggest that clathrin and lipid rafts are not required.
CNF1 is known to be released from acidified late endosomes into the cytosol (4). We used bafilomycin A1, as well as monensin (data not shown), to inhibit acidification of endosomes. Additionally, we incubated the cells with nocodazole to block microtubule-dependent maturation of endosomes. All inhibitors blocked uptake of CNF1 as well as uptake of CNFY, indicating that the Yersinia toxin also translocates from late endosomes in an acidic pH-dependent manner. Both CNFs can be directly pulsed from the culture medium through the plasma membrane into the cytosol by external acidification. Compared to CNF1, CNFY needs a more acidic pH (pH 4.8) or a longer incubation time (30 min) to enter the cytosol. One possible explanation is that the times required for the two toxins to cross a biological membrane differ. In analogy to the model of diphtheria toxin delivery, it was suggested that CNF1 is taken up at low pH due to the loss of charge of acidic residues located in a short peptidic loop, which separates two hydrophobic helices (16). To study whether glutamate 374, an additional negative charge in the loop of CNFY, is responsible for the retarded uptake of CNFY through the plasma membrane, we mutated it to glutamine, which is the corresponding amino acid present in CNF1. The mutant behaved like wild-type CNFY (data not shown). Thus, the difference in pH or time required for the pulse through the plasma membrane cannot be explained by the additional acidic residue in CNFY. One reason could be that the isoelectric point of CNFY (pI 4.77) is lower than the pI of CNF1 (pI 5.16). The same holds true for the helix-loop-helix motif (amino acids 350 to 412) with isoelectric points of 4.38 for CNF1 and 4.13 for CNFY. An important issue, which remains to be studied, is the question whether the holotoxins or only parts of the toxins enter the cytosol.
Altogether, our data show that the CNF1 and CNFY toxins, which have a sequence identity of about 61%, bind to different cellular receptors. We suggest that HSPGs are possible candidates for overlapping regions of the receptors. Both toxins are then taken up independent of the presence of clathrin or lipid rafts and are released into the cytosol from acidified endosomes.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Sonderforschungsbereich (SFB) 388.
We thank Jürgen Dumbach for excellent technical assistance and Alexandre Benmerah for the Eps15 constructs.
Editor: D. L. Burns
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 2.Chen, N., C. C. Chen, and L. F. Lau. 2000. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin alpha 6beta 1 and cell surface heparan sulfate proteoglycans. J. Biol. Chem. 275:24953-24961. [DOI] [PubMed] [Google Scholar]
- 3.Chung, J. W., S. J. Hong, K. J. Kim, D. Goti, M. F. Stins, S. Shin, V. L. Dawson, T. M. Dawson, and K. S. Kim. 2003. 37 kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 278:16857-16862. [DOI] [PubMed] [Google Scholar]
- 4.Contamin, S., A. Galmiche, A. Doye, G. Flatau, A. Benmerah, and P. Boquet. 2000. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell 11:1775-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 6.Gauthier, N. C., P. Monzo, V. Kaddai, A. Doye, V. Ricci, and P. Boquet. 2005. Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes. Mol. Biol. Cell 16:4852-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhard, R., G. Schmidt, F. Hofmann, and K. Aktories. 1998. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect. Immun. 66:5125-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann, C., M. Pop, J. Leemhuis, J. Schirmer, K. Aktories, and G. Schmidt. 2004. The Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFY) selectively activates RhoA. J. Biol. Chem. 279:16026-16032. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann, C., and G. Schmidt. 2004. CNF and DNT. Rev. Physiol. Biochem. Pharmacol. 152:49-63. [DOI] [PubMed] [Google Scholar]
- 10.Hundt, C., J.-M. Peyrin, S. Haik, S. Gauczynski, C. Leucht, R. Rieger, M. L. Riley, J.-P. Deslys, D. Dormont, C. I. Lasmézas, and S. Weiss. 2001. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 20:5876-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, K. J., J. W. Chung, and K. S. Kim. 2005. 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J. Biol. Chem. 280:1360-1368. [DOI] [PubMed] [Google Scholar]
- 12.Kouokam, J. C., S. N. Wai, M. Fallman, U. Dobrindt, J. Hacker, and B. E. Uhlin. 2006. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli. Infect. Immun. 74:2022-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzrein, M., O. Garred, S. Olsnes, and K. Sandvig. 1995. Diphtheria toxin endocytosis and membrane translocation are dependent on the intact membrane-anchored receptor (HB-EGF precursor): studies on the cell-associated receptor cleaved by a metalloprotease in phorbol-ester-treated cells. Biochem. J. 310:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockman, H. A., R. A. Gillespie, B. D. Baker, and E. Shakhnovich. 2002. Yersinia pseudotuberculosis produces a cytotoxic necrotizing factor. Infect. Immun. 70:2708-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer, D. K., C. Olenik, F. Hofmann, H. Barth, J. Leemhuis, I. Brünig, K. Aktories, and W. Nörenberg. 2000. Regulation of somatodendritic GABAA receptor channels in rat hippocampal neurons: evidence for a role of the small GTPase Rac1. J. Neurosci. 20:6743-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pei, S., A. Doye, and P. Boquet. 2001. Mutation of specific acidic residues of the CNF1 T domain into lysine alters cell membrane translocation of the toxin. Mol. Microbiol. 41:1237-1247. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature 387:725-729.9192900 [Google Scholar]
- 18.Verhoef, P. A., M. Estacion, W. Schilling, and G. R. Dubyak. 2003. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J. Immunol. 170:5728-5738. [DOI] [PubMed] [Google Scholar]
- 19.Werner, G., H. Hagenmaier, H. Drautz, A. Baumgartner, and H. Zahner. 1984. Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity. J. Antibiot. 37:110-117. [DOI] [PubMed] [Google Scholar]