Abstract
Mycobacterium bovis BCG is widely used as a vaccine against tuberculosis (TB), despite its variable protective efficacy. Relatively little is known about the immune response profiles following BCG vaccination in relation to protection against TB. Here we tested whether BCG vaccination results in immune responses to DosR (Rv3133c) regulon-encoded proteins. These so-called TB latency antigens are targeted by the immune system during persistent Mycobacterium tuberculosis infection and have been associated with immunity against latent M. tuberculosis infection. In silico analysis of the DosR regulon in BCG and M. tuberculosis showed at least 97% amino acid sequence homology, with 41 out of 48 genes being identical. Transcriptional profiling of 14 different BCG strains, under hypoxia and nitric oxide exposure in vitro, revealed a functional DosR regulon similar to that observed in M. tuberculosis. Next, we assessed human immune responses to a series of immunodominant TB latency antigens and found that BCG vaccination fails to induce significant responses to latency antigens. Similar results were obtained with BCG-vaccinated BALB/c mice. In contrast, responses to latency antigens were observed in individuals with suspected exposure to TB (as indicated by positive gamma interferon responses to TB-specific antigens ESAT-6 and CFP-10) and in mice vaccinated with plasmid DNA encoding selected latency antigens. Since immune responses to TB latency antigens have been associated with control of latent M. tuberculosis infection, our findings support the development of vaccination strategies incorporating DosR regulon antigens to complement and improve the current BCG vaccine.
Tuberculosis (TB) remains a major global health threat. Each year, about eight million new TB cases occur and two million people die from TB. It is estimated that one-third of the world population is latently infected with Mycobacterium tuberculosis. From this vast latent reservoir, about 10% of infected people are expected to develop overt TB disease during their lifetimes. However, with the expanding human immunodeficiency virus type 1-AIDS pandemic, this number is expected to soar in the next few decades (11, http://www.who.int/mediacentre/factsheets/fs104/en/).
The current TB vaccine is the live attenuated bacterium Mycobacterium bovis Bacillus Calmette-Guérin (BCG). BCG is known to protect against severe forms of TB in young children and against leprosy. However, it does not efficiently and consistently protect against pulmonary TB in adults, the most prevalent and contagious form of TB; BCG also does not offer protection from reactivation of latent TB infection. This partly explains why BCG has little impact on the global TB epidemic despite its widespread use as a prophylactic TB vaccine (http://www.who.int/wer/2004/en/wer7904.pdf). Over the years, many hypotheses have been put forward to explain the apparent variability in the protective efficacy of BCG, which varies from 0 to 80% (16). Explanations for this inconsistency include differences in trial methodology, host population genetics, use of different BCG vaccine strains (2), and heterogeneous immunity to a variety of environmental mycobacteria that may interfere with or mask the protection provided by BCG (7, 26).
Immune response profiles following BCG vaccination comprise myriad effector mechanisms, multiple T-cell subsets, and many targeted antigens. BCG is capable of inducing Th1 responses (38), which are critical in mycobacterial infections (17). In addition, BCG is also capable of inducing both CD4+ and CD8+ T-cell responses to antigens shared with M. tuberculosis, such as secreted antigens of the mycolyl transferase family (Ag85) (19, 20, 33) and nondeleted members of the ESAT-6 family (e.g., TB10.4) (32), but also heat shock proteins like Hsp65 and Hsp70 (15). However, it is still not completely known how these and other antigen-specific immune responses contribute to protection against TB.
Recently we studied human T-cell responses to DosR (Rv3133c) regulon-encoded antigens (referred to as TB latency antigens) of M. tuberculosis (24). We observed preferential recognition of latency antigens by Mantoux skin test-positive individuals with latent TB compared to patients with TB disease, suggesting that these immune responses are associated with latent TB disease (13, 24). The DosR regulon is expressed by tubercle bacilli under in vitro conditions of hypoxia and low-dose nitric oxide exposure (40), and in gamma interferon (IFN-γ)-activated macrophages (30). These conditions are thought to mimic the environment encountered by tubercle bacilli in vivo when persisting in immunocompetent hosts (31).
One of the most abundantly produced proteins during hypoxia is the 16-kDa α-crystallin homolog HspX (Rv2031c) (12). HspX is one of the DosR-regulated genes and is targeted by both CD4+ T cells (9, 41) and CD8+ T cells (10). Interestingly, infants vaccinated with BCG do not mount immune responses to HspX, whereas they are capable of generating immune responses to other TB antigens (39). The absence of immune responses to this particular latency antigen led us to the formulation of our hypothesis. In this study, we tested the hypothesis that BCG vaccination in humans and in a mouse model fails to induce immune responses not only to HspX but generally to TB latency antigens that are targeted by the immune system during latent M. tuberculosis infection.
MATERIALS AND METHODS
In vitro expression of the BCG DosR regulon.
Expression of the DosR regulon by M. tuberculosis strain H37Rv and 14 different BCG strains (Connaught JPG, Pasteur 140, Sweden, Connaught, Japan, Canada, Vietnam, Danish SSI 1331, Russian, Brazil, Tice, Moscow, Pasteur 133A, and Pasteur 1173; kindly provided by Angelo Izzo, Colorado State University) was determined by using standard methods of microarray RNA expression analysis as previously described (40). Briefly, mycobacteria were grown in 7H9 medium (supplemented with bovine serum albumin, NaCl, glucose, and glycerol) in 250-ml vented tissue culture flasks with shaking at 90 rpm. At a culture optical density of 0.15, 30-ml cultures were placed in an anaerobic GasPak chamber (Becton Dickinson & Company) and shaking was continued. Reference cultures were treated in the same manner but not placed in the anaerobic chamber. After 4 h, RNA was isolated and microarray analysis was conducted as previously reported (40), with TB oligonucleotide microarrays provided by Colorado State University through the Tuberculosis Vaccine Testing and Research Materials contract.
Study subjects.
A cross-sectional study (The Netherlands) comprised 43 healthy adult persons (10 male, 33 female) who were vaccinated with BCG between 1 and 37 years before this study (median time after vaccination, 25 years). The ages at the time of blood sampling ranged from 20 to 60 years. Thirty-eight individuals were of Dutch origin; the remaining five individuals were of German, British, Netherlands Antillean, Philippine, and South Korean descent. After written informed consent was obtained, blood samples were obtained from all study subjects by standard venous puncture and blood collection in heparinized tubes. Subsequently, peripheral blood mononuclear cells (PBMC) were isolated with a Ficoll density gradient and stored in liquid nitrogen as described previously (24). The study protocol used (P207/99) was approved by the ethical review board of the Leiden University Medical Center.
A longitudinal BCG vaccination study (United Kingdom) comprised adolescents who were recruited through the United Kingdom schools' BCG vaccination program (4). Year 8 pupils (ages, 12 to 13 years) were informed of the study verbally and by an information sheet, and recruitment proceeded after written informed consent had been obtained from a parent or guardian and verbal consent had been obtained from the child. Exclusion criteria were evidence of previous BCG vaccination (BCG scar or vaccination records) or serious illness. The ethnic backgrounds of participants were as follows: White British, 30%; White Irish, 3%; Black Caribbean, 22%; Black African, 13%; Black other, 7%; Asian Pakistan, 12%; Asian other, 5%; and mixed or other, 8%.
Ethical approval for this study was given by the Local Research Ethics Committee of Redbridge and Waltham Forest Health Authority and by the Ethics Committee of the London School of Hygiene & Tropical Medicine.
Baseline blood samples were collected from participants at the time of tuberculin skin testing. Skin testing was carried out on the volar surface of the forearm by the Heaf technique with tuberculin. A baseline 10 ml of intravenous blood was taken and transferred into tubes containing 100 U of preservative-free sodium heparin (Monoparin; CP Pharmaceuticals Ltd., Wrexham, United Kingdom). Blood samples were set up in the whole-blood assay as soon as possible on the same day as venipuncture. The Heaf test induration was inspected after 7 days and graded appropriately by experienced nurses. Those due to receive BCG following a negative Heaf test received BCG immediately (n = 22). Two to 4 weeks following vaccination, depending upon school availability, recruits were revisited for a follow-up 10-ml blood sample.
M. tuberculosis antigens.
Recombinant proteins were produced as previously described (18). Briefly, nucleotide sequences of selected M. tuberculosis H37Rv genes were obtained from http://genolist.pasteur.fr/TubercuList. Genes were amplified by PCR from genomic DNA of M. tuberculosis H37Rv and cloned by Gateway Technology (Invitrogen, San Diego, CA) in pDEST17, a bacterial expression vector containing an N-terminal hexahistidine tag for rapid purification with nickel-chelating resin. The proteins were overexpressed in Escherichia coli BL21(DE3) and purified as previously described (18). Sequencing was performed to confirm the identities of the cloned DNA fragments. Size and purity were checked by gel electrophoresis and Western blotting with anti-His antibodies (Invitrogen). Residual endotoxin levels were determined with a Limulus amebocyte lysate assay (Cambrex) and were found to be below 50 IU/mg recombinant protein. Protein batches were subsequently tested for nonspecific T-cell stimulation and for potential cellular toxicity in lymphocyte stimulation assays with PBMC of non-M. tuberculosis-exposed, non-BCG-vaccinated, Mantoux skin test-negative, healthy donors.
Lysate of M. tuberculosis grown under low-oxygen conditions was obtained from M. tuberculosis H37Rv growing for 24 h in tubes with tightly screwed-on caps as previously described (29). The low-oxygen-derived M. tuberculosis lysate was kindly provided by Karen Weldingh and Peter Andersen (Statens Serum Institute [SSI], Copenhagen, Denmark).
In vitro proliferation assays.
Lymphocyte stimulation assays (cross-sectional BCG vaccination study) were performed with isolated PBMC as previously described (24). Briefly, PBMC (1.5 × 105/well) were cultured in Iscove's modified Dulbecco modified Eagle medium (Gibco, Paisley, United Kingdom) supplemented with 10% pooled human serum, 40 U/ml penicillin, and 40 μg/ml streptomycin in 96-well round-bottom microtiter plates (Nunc, Roskilde, Denmark) at 37°C in 5% CO2 in the absence or presence of stimulant. Antigens were tested at the following concentrations: latency antigens, ESAT-6, CFP-10, and Ag85B at 0.33 μM; M. tuberculosis hypoxic lysate and purified protein derivative (PPD) of M. tuberculosis (batch RT49 SSI, Denmark) at 5 μg/ml; and positive control phytohemagglutinin (PHA; Remel, United Kingdom) at 2 μg/ml. The total volume was 200 μl/well. All stimulations were performed in triplicate. At day 6, supernatants were harvested (75 μl/well, pooled per triplicate) and stored at maximally −20°C until use in the IFN-γ detection assay.
The diluted-whole-blood assay (longitudinal BCG vaccination study) was performed as previously described (3). In summary, whole blood was diluted 1:5 with serum-free medium (RPMI 1640 medium supplemented with 2 mM l-glutamine; Invitrogen) and 100 μl was plated in 96-well, round-bottom tissue culture plates (Nunc, Roskilde, Denmark). Antigen was also added in 100 μl to give a final whole blood dilution of 1:10 and a culture volume of 200 μl. Medium- and PHA (5 μg/ml; Sigma)-containing wells were included as negative and positive controls, respectively, in parallel with the following M. tuberculosis antigens at 5 μg/ml: PPD of M. tuberculosis (batch RT49, lot 210; SSI, Copenhagen, Denmark), Rv1733c, Rv2029c, Rv2623, Rv2627c, Rv2628, Ag85A, and the ESAT-6/CFP10 fusion protein. Cell cultures were incubated on the day of blood collection at 37°C with 5% CO2. Supernatants were harvested on day 6 and stored at maximally −20°C prior to enzyme-linked immunosorbent assay (ELISA).
Detection of IFN-γ by ELISA. (i) Cross-sectional study.
IFN-γ concentrations in supernatants were measured by ELISA (U-CyTech, Utrecht, The Netherlands). The detection limit of the assay was 20 pg/ml. ELISA samples were tested in duplicate. The mean value of unstimulated cultures was subtracted from the mean value of the stimulated cultures. A positive IFN-γ response was predefined as ≥100 pg/m (24) in the cross-sectional study (The Netherlands). Positive in vitro responses of the study subjects to the TB-specific antigens ESAT-6 and/or CFP-10 were used as a marker of previous TB exposure (21, 37).
(ii) Longitudinal study.
Quantitative IFN-γ ELISAs were done in single wells (100 μl) with commercially available antibody pairs (BD Pharmingen) as previously described (3). Recombinant IFN-γ (BD Pharmingen) was used for the standard curve with a lower detection limit of 31 pg/ml. Negative control (medium) values were subtracted from all results. A positive IFN-γ response was predefined as >62 pg/ml (3) in this longitudinal study (United Kingdom). A positive control supernatant was included in duplicate on each ELISA plate to control for interplate and intraplate variations.
In vivo studies.
BALB/c (H-2d) mice were bred at the Animal Facilities of the WIV-Pasteur Institute of Brussels, from breeding couples originally obtained from Bantin & Kingman (United Kingdom). All animals were 8 to 10 weeks old at the start of the experiments. Experiments were performed in accordance with the Ethical Committee of CODA-PIB-WIV regulations (permit no. 060202-02).
Male BALB/c mice were vaccinated with 0.2 mg (106 CFU) of a freshly prepared solution of M. bovis BCG vaccine (strain GL2) grown as a surface pellicle on synthetic Sauton medium for 14 days and homogenized by ball mill (22). Mice were immunized subcutaneously with 0.2 ml. Female BALB/c mice were anesthetized with ketamine-xylazine and injected intramuscularly in both quadriceps muscles with 2 × 50 μg of the V1J.ns-tPA vector encoding the latency antigen Rv1733c, Rv1738, Rv2029c, HspX (Rv2031c), Rv2032, Rv2626c, Rv2627c, Rv2628, ESAT-6/CFP-10 fusion protein (21), or Ag85A (14, 28). Mice were vaccinated three times at 3-week intervals, and immune responses were analyzed 4 weeks after the third immunization.
BCG-vaccinated mice were sacrificed at 1 or 3 months after vaccination, and their spleens were removed aseptically and homogenized with a loosely fitting Dounce homogenizer. Plasmid DNA-vaccinated mice were sacrificed 4 weeks after the third DNA immunization. Splenocytes (4 × 106 cells/ml) from three mice per group were tested as a pool (month 1 after BCG) or individually (three or four mice) (month 3 after BCG or after the last DNA vaccination) for a cytokine response to purified recombinant His-tagged latency antigens, Ag85A, or ESAT-6/CFP-10 (all at 5 μg/ml). Pooled supernatants from at least three wells were harvested after 72 h, when peak IFN-γ values can be measured, and stored at −20°C until assayed. IFN-γ activity was quantified by sandwich ELISA with coating antibody R4-6A2 and biotinylated detection antibody XMG1.2 (both from Pharmingen). The assay sensitivity was 5 pg/ml. Samples were tested undiluted and at 1:10 and 1:100 dilutions. Cytokine content was calculated for the dilution with an optical density value in the linear part of the standard curve.
Statistical analysis.
For comparison of the proportion of responders between test groups in the cross-sectional study, the chi-square (χ2) test for independent samples was used. However, Fisher's exact test for independent samples was applied if expected values were lower then or equal to 5 (E ≤ 5). Comparison of the proportion of responders in the longitudinal study was performed by McNemar's test for paired samples. For all assays, P < 0.05 was considered statistically significant. For statistical analysis, SPSS 11.0 for Windows was used.
RESULTS
In silico sequence analysis and in vitro expression of the DosR regulon of BCG.
The DosR regulon of M. tuberculosis is expressed in vitro under conditions of hypoxia and low-dose nitric oxide stimulation (40). To evaluate whether BCG is similarly capable of expressing the DosR regulon, we first assessed available genome sequences of M. tuberculosis, M. bovis, and BCG for the level of conservation and then performed in vitro transcription profiling of DosR regulon-encoded genes. Blast searches (http://ncbi.nlm.nih.gov/BLAST/) of DosR regulon-encoded sequences showed that all 48 DosR regulon-encoded genes were highly conserved between M. tuberculosis strains H37Rv and CDC1551 and M. bovis strains AF2122/97 and BCG Pasteur 1173P2. The homology of amino acid sequences was at least 97%, with 85% (41 of the 48) of the genes being identical (data not shown).
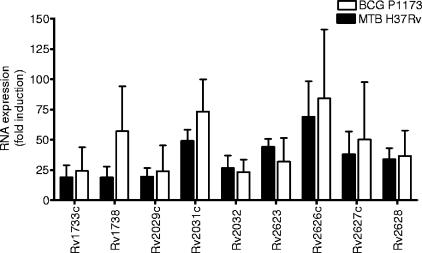
Subsequently, we assessed the RNA expression profiles of 14 different BCG vaccine strains and M. tuberculosis H37Rv under in vitro conditions of hypoxia and nitric oxide stimulation. The results showed that all of the BCG strains tested have a functional DosR regulon with an expression profile similar to that of M. tuberculosis (40). Figure 1 shows the RNA profiles of nine DosR-regulated genes of M. tuberculosis H37Rv and BCG Pasteur strain 1173 when exposed to low oxygen levels. Selection of these nine genes was based on results from our previous study (24), and the four most recognized latency antigens in humans were included. These genes were further analyzed in this study.
FIG. 1.
Expression of DosR genes (RNA). RNA transcript expression profiles (fold induction) of nine DosR-encoding genes induced under low-oxygen conditions in M. tuberculosis H37Rv (n = 4) and BCG Pasteur 1173 (n = 4). The RNA expression profiles of both strains are highly similar, except for that of Rv1738. Data are shown as the mean plus the standard deviation.
Immune responses to DosR regulon-encoded antigens in human BCG vaccinees.
Next we addressed the question of whether BCG vaccination in human beings leads to the induction of immune responses to DosR regulon-encoded latency antigens that are targeted during natural M. tuberculosis infection.
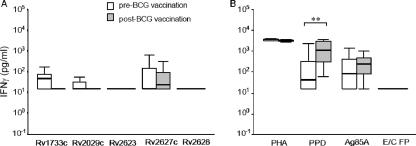
IFN-γ responses to immunodominant latency antigens were assessed in a longitudinal BCG vaccination study of young adolescents in the United Kingdom. Twenty-two tuberculin skin test-negative adolescents were included in the vaccination group; blood was taken before and 2 to 4 weeks after BCG vaccination. Results showed that responses to the latency antigens of the participants before BCG vaccination were minimal; the median responses to the latency antigens Rv1733c Rv2029c, Rv2623, Rv2627c, and Rv2628 were below the predefined cutoff level of 62 pg/ml IFN-γ, as used in this study (Fig. 2A). BCG vaccination did not alter the response profiles of the adolescents to the latency antigens; the proportion of responders in the prevaccination group ranged from 4.5% to 33.3%, compared to 0% to 31.8% postvaccination with BCG. Statistical analysis of the proportion of responders to TB latency antigens showed no significant difference between pre- and postvaccination with BCG (McNemar's test, P = 0.500 to P = 1.000).
FIG. 2.
IFN-γ responses to M. tuberculosis latency antigens in a longitudinal BCG study. Box-and-whisker plots of IFN-γ responses to the latency antigens in schoolchildren pre- and postvaccination with BCG are shown. The horizontal black line in the box represents the median, the lower boundary of the box represents the 25th percentile, and upper boundary represents the 75th percentile. Whiskers extend from the box to the highest and lowest values with exclusion of the extreme values and the outlier values. Supernatants were taken after 6 days for measurement of IFN-γ production. (A) IFN-γ responses of the pre-BCG-vaccination group to the latency antigens tested. (B) Responses to PHA and control antigens PPD, Ag85A, and the ESAT-6/CFP-10 fusion protein (E/C FP). Vaccinated adolescents, n = 22, except for antigen Rv1733c in the prevaccinated group, where 12 individuals were tested. An IFN-γ response of ≥62.5 pg/ml was considered positive. The McNemar test for paired samples was used to compare the proportions of responders per group (**, P = 0.001).
Following BCG vaccination, there was a significant increase in the IFN-γ response to PPD, from 46% to 96% responders (P = 0.001). There was no significant difference for responses to Ag85A, only a trend toward an increase in response, from 64% to 77% responders (P = 0.375). Although all of the eligible subjects tested negative in the tuberculin skin test (Heaf test) prior to BCG vaccination, in vitro stimulation with PPD or Ag85A showed that a considerable proportion of the adolescents tested gave positive whole-blood assays (46% and 64%, respectively), most likely as a result of exposure to cross-reactive environmental mycobacteria. As expected, none of the participants responded to the M. tuberculosis-specific ESAT-6/CFP-10 fusion protein (Fig. 2B). Thus, these results show that in adolescents following BCG vaccination virtually no increases in responses to M. tuberculosis latency antigens are observed.
Next, we carried out a cross-sectional study (The Netherlands) including BCG-vaccinated adults without exposure to TB (n = 27) compared to subjects with likely exposure to TB (n = 16). For this study, individuals with an IFN-γ response of >100 pg/ml to M. tuberculosis-specific antigens ESAT-6 and CFP-10 (21, 37) were considered previously exposed to TB.
Figure 3 shows the immune recognition profiles of both BCG-vaccinated groups to the latency antigens. Overall, BCG-vaccinated individuals without evidence of exposure to TB showed significantly lower production of IFN-γ in response to the latency antigens than did the group that had positive responses to ESAT-6 and/or CFP-10; the median IFN-γ responses to all of the latency antigens tested in the unexposed group were all below the cutoff level of 100 pg/ml, whereas the opposite was true of the TB-exposed group. In the latter group, the median IFN-γ values in response to all of the latency antigens tested, except protein Rv2628, were above 100 pg/ml, indicating that more than half of the individuals strongly recognized the antigens (Fig. 3A). The proportions of responders (i.e., >100 pg/ml IFN-γ) per antigen were as follows (BCG vaccinated without TB exposure versus BCG vaccinated with TB exposure): Rv1733c, 44.4% versus 93.8% (P = 0.001); Rv2029c, 37.0% versus 81.3% (P = 0.005); HspX, 22.2% versus 56.3% (P = 0.024); Rv2032, 29.6% versus 68.8% (P = 0.013); Rv2626c, 20.0% versus 68.8% (P = 0.003); Rv2627c, 25.9% versus 75% (P = 0.002); Rv2628, 25.9% versus 43.8% (P = 0.228). Furthermore, the levels of IFN-γ production in the BCG-vaccinated group with suspected TB exposure were similar to those we previously found in Mantoux-positive individuals (24). In addition to the responses to latency antigens, exposure to TB was also associated with higher IFN-γ production in response to Ag85B (P = 0.005), which is shared between BCG and M. tuberculosis. The data also suggest that the responses to mycobacterial complex antigens like PPD (P = 0.139) and M. tuberculosis lysate (from bacteria cultured under hypoxic conditions) (P = 0.279) are higher, but no significant difference was detected (Fig. 3B).
FIG. 3.
IFN-γ responses to M. tuberculosis latency antigens in a cross-sectional BCG study. Box-and-whisker plots of IFN-γ responses to the latency antigens by BCG-vaccinated individuals are shown. The horizontal black line in the box represents the median, the lower boundary of the box represents the 25th percentile, and the upper boundary represents the 75th percentile. Whiskers extend from the box to the highest and lowest values, with exclusion of the extreme values and the outlier values. Supernatants were taken after 6 days for measurement of IFN-γ production. (A) IFN-γ responses to the latency antigens of the group that was BCG vaccinated without TB exposure (no IFN-γ production in response to ESAT-6 or CFP-10) or with exposure to TB (positive IFN-γ production in response to ESAT-6 or CFP-10). (B) IFN-γ responses to control stimuli PHA, PPD, MTB, Ag85B, ESAT-6, and CFP-10. MTB, lysate of M. tuberculosis cultured under low-oxygen conditions. BCG-vaccinated group without TB exposure, n = 23, BCG-vaccinated group with TB exposure, n = 16. An IFN-γ response of ≥100 pg/ml was considered positive. χ2 test: *, P < 0.05; **, P < 0.01. †, Fisher's exact test.
Time after BCG vaccination and moment of sampling varied considerably in our study population. We therefore attempted to seek a correlation between the level of IFN-γ production and time since vaccination. However, no time-dependent association was observed (data not shown).
Taken together, BCG vaccination of the skin, in general, appears to induce poor responses not only to HspX but also to other latency antigens of M. tuberculosis. In contrast, following exposure to TB, significant responses to latency antigens are seen.
Immune responses to DosR regulon antigens in mice.
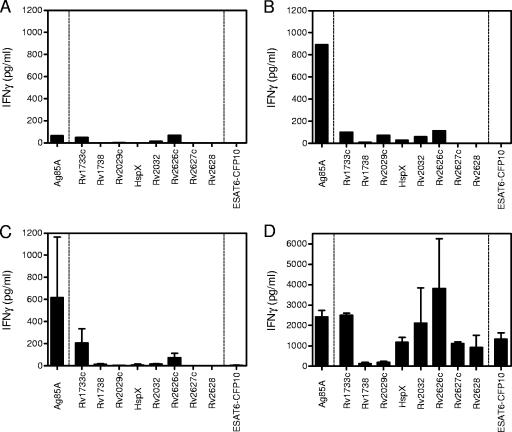
In order to determine whether BCG vaccination also fails to induce adequate T-cell responses to M. tuberculosis latency antigens in mice, BALB/c mice were vaccinated subcutaneously with BCG (n = 3). The mice were sacrificed 4 weeks and 3 months after BCG vaccination in order to test splenocytes for IFN-γ production in response to latency antigens, the homologous or shared mycobacterial antigen Ag85A, and the ESAT-6/CFP-10 fusion protein of M. tuberculosis (21). Four weeks postvaccination with BCG, splenocytes showed very low to no production of IFN-γ when stimulated with latency antigens (Fig. 4B). The levels of IFN-γ were low, similar to those of naive control mice (Fig. 4A). At this same time point, cells produced significant amounts of IFN-γ in response to Ag85A (Fig. 4B) and PPD (data not shown). As expected, responses to the fusion protein ESAT-6/CFP-10 were absent. When responses were assessed at 3 months postimmunization, similar results were obtained: IFN-γ responses of splenocytes of vaccinated mice to latency antigens remained at levels comparable to those of naive mice (Fig. 4A), and significant IFN-γ production was found only in response to Ag85A (Fig. 4C). To rule out the possibility that the absence of IFN-γ response to latency antigens was due to an inherent inability of BALB/c mice to respond to these latency antigens, mice were vaccinated with DNA. BALB/c mice (n = 4) were vaccinated three times at 3-week intervals with naked plasmid DNA encoding one of eight different latency antigens, Ag85A, or ESAT-6/CFP-10. Four weeks after the last immunization, spleen cell IFN-γ production was analyzed. Vaccination with six out of eight of these plasmids induced significant antigen-specific IFN-γ production (Fig. 4D). Robust levels of IFN-γ were produced after in vitro restimulation with Rv1733c, HspX, Rv2032c, Rv2626c, Rv2627c, and Rv2628. Antigens Rv1738 and Rv2029c appeared to be not very immunogenic for T cells in BALB/c mice, although significant antibody responses to the latter protein could be induced (29a). Vaccination with plasmid DNA encoding Ag85A or the ESAT-6/CFP-10 fusion protein was also very effective at inducing a strong IFN-γ response. The results thus demonstrate that mice are able to generate strong Th1-type immune responses to latency antigens after immunization with plasmid DNA but that subcutaneous BCG administration fails to induce immune responses to M. tuberculosis latency antigens in BALB/c mice.
FIG. 4.
IFN-γ responses to M. tuberculosis latency antigens in BALB/c mice following subcutaneous or intramuscular BCG vaccination with plasmid DNA. Supernatants were taken after 3 days (72 h) for measurement of IFN-γ production. (A) Naive, non-BCG-vaccinated mice tested as a pool (n = 3). (B) Mice at 4 weeks postvaccination with BCG, tested as a pool (n = 3). (C) Mice at 3 months postvaccination with BCG (n = 3). (D) Mice were immunized three times with plasmid DNA encoding one of the eight latency antigens, Ag85A, or ESAT-6/CFP-10. Splenocytes were restimulated in vitro with their respective antigens (n = 4).
DISCUSSION
Relatively little is known about the antigen specificity of immune response profiles following BCG vaccination in relation to protection against TB. In this study, we addressed the question of whether BCG vaccination induces immune responses to DosR regulon-encoded antigens, in particular, to those TB latency antigens that are targeted during persistent M. tuberculosis infection (24).
Comparison of available genome sequences of M. tuberculosis, M. bovis, and BCG showed that the entire set of DosR regulon coding sequences is conserved in BCG; except for a few minor point mutations, which are not expected to have a major impact on the expression or immunologic recognition of these antigens. Subsequently, transcriptional analysis of 14 different BCG vaccine strains under in vitro conditions of low oxygen and nitric oxide exposure were studied. Our results show that RNA expression profiles of the DosR regulon latency antigens reported in this study were highly similar to those previously reported for M. tuberculosis (40). In addition, BCG is reported to be capable of adapting to anaerobiosis in vitro by shifting down to a nonreplicating persistent state, similar to M. tuberculosis (25). When tested in the so-called Wayne model or in standing (i.e., hypoxic) cultures, it was previously shown that BCG is capable of producing at least four DosR regulon-encoded proteins, Rv2623, Rv2626c, HspX (Rv2031c, acr), and DosR (Rv3133c) (5, 6). We conclude from these in vitro observations that BCG has a functional DosR regulon, although no complete proteomic data are available to demonstrate that every single protein encoded by the regulon is indeed expressed.
Next we monitored immune responses to a series of immunodominant TB latency antigens following BCG vaccination, in both longitudinal and cross-sectional studies with two different human cohorts, and corroborated our findings in a BALB/c mouse model.
As mentioned before, neonates vaccinated with BCG at birth do not develop an immune response to the latency antigen HspX, whereas they are capable of mounting responses to other non-dormancy-associated TB antigens (including PPD) (39). BCG-vaccinated adolescents (longitudinal study) and BCG-vaccinated adults who had not been exposed to TB (i.e., in vitro negative for production of IFN-γ in response to ESAT-6 and/or CFP-10) (cross-sectional study) mounted immune responses to secreted antigens of the Ag85 complex and PPD but did not develop a response to HspX or any of the other latency antigens tested (Fig. 2 and 3).
In contrast to the results described above, BCG-vaccinated adults with previous exposure to TB (i.e., in vitro positive production of IFN-γ in response to ESAT-6 and/or CFP-10) in the cross-sectional study showed significantly higher IFN-γ responses to selected TB latency antigens next to increased and significant (only Ag85B) responses to common TB antigens. These results agree with our recent findings that T cells from individuals with latent TB infection preferentially recognize a set of TB latency antigens (i.e., Rv1733c, Rv2029c, and Rv2627c) while patients with (past or active) TB disease preferentially recognize secreted antigens such as CFP-10 and ESAT-6 (13, 24).
The absence of detectable in vitro responses to M. tuberculosis latency antigens in BCG-vaccinated adults may, in part, be explained by the common belief that BCG-induced protection against TB wanes over a period of 10 to 15 years (34). Since time after BCG vaccination in our study group varied between 1 and 37 years, we attempted to correlate the level of responses to latency antigens with the time elapsed since vaccination but found no significant association.
Somewhat unexpectedly, 46% of the adolescents who tested negative in the Heaf skin test prior to BCG vaccination showed in vitro responses to PPD. Our own observations (unpublished) and those of others (4) show that in vitro detectable immune responses to PPD can be observed in a significant group of Mantoux-negative, non-BCG-vaccinated, non-TB-exposed, healthy individuals. This phenomenon is likely caused by previous exposure to cross-reactive environmental mycobacteria (4, 7, 16, 26). In addition, previous exposure to environmental mycobacteria might also have induced low-level responses (4.5% to 33% responders) in the prevaccinated BCG group in the longitudinal study (Fig. 2) since DosR regulon-encoded antigens have not been reported to be solely expressed in M. tuberculosis.
Our findings on humans were corroborated with BCG-vaccinated BALB/c mice. Upon subcutaneous vaccination with BCG, the mice generated immune responses to the common M. tuberculosis antigens Ag85A and PPD. In contrast, poor responses were detectable against M. tuberculosis latency antigens both 1 and 3 months postvaccination. Yet, vaccination with recombinant plasmid DNAs induced a strong T-cell response to several latency antigens, including Rv1733c, HspX, Rv2032, Rv2626c, Rv2627c, and Rv2628, as well as Ag85A and the ESAT-6/CFP-10 fusion protein. Plasmid DNAs encoding Rv1738 and Rv2029c were not immunogenic in mice. This demonstrated that the mice were able to generate strong Th1-type immune responses to M. tuberculosis latency antigens when immunized with plasmid DNAs but that subcutaneous BCG administration failed to induce immune responses to such antigens in BALB/c mice. With HLA-transgenic mice (19, 20), a similar observation concerning HspX was recently made in our laboratory; mice vaccinated with BCG did not develop immune responses to HspX, whereas immunization with HspX did induce responses to HspX and its predefined T-cell epitopes (10, 20a).
Of interest is our observation that the recognition patterns of latency antigens seem to be different between humans and BALB/c mice. It appears that humans responded most strongly to antigens Rv1733c, Rv2029c, and Rv2627c, whereas mice responded most strongly to Rv1733c, Rv2032, and Rv2626c (although both were not statistically significant). However, this may be related to the different modes of antigen exposure, i.e., natural infection of humans versus DNA vaccination of mice. This observation might have implications for proof-of-concept studies which involve TB latency antigens in mouse models and their extrapolation to latent TB in humans.
Finally, two other animal models confirmed the lack of T-cell responses to M. tuberculosis latency antigens following BCG vaccination. First, adult male rhesus monkeys were vaccinated with BCG (23) and in vitro immune responses to M. tuberculosis latency antigens were monitored at 9 weeks postvaccination. Similar to observations on humans and mice, significant immune responses to PPD were seen following BCG vaccination but responses to M. tuberculosis latency antigens were not (F. Verreck, personal communication). Second, cattle were vaccinated with BCG or infected with M. bovis (8) and immune responses to a series of latency antigens, (bovine) PPD, and the ESAT-6/CFP-10 fusion protein were measured. Significant responses to PPD were observed, and animals infected with virulent M. bovis also responded significantly to ESAT-6 and CFP-10, but no significant responses to any of the latency antigens tested were observed (M. Denis and B. Buddle, personal communication). Although it is difficult to establish positive controls for responses to TB latency antigens in these two animal models, the results are likely accounted for by the same mechanisms underlying the effects observed in humans and mice following BCG vaccination.
The studies summarized above were performed with different strains of BCG, and this may potentially confound the interpretation and comparison of results. However, microarray expression profiling of 14 different BCG vaccine strains showed highly similar regulation of the DosR regulon. This rather suggests that lack of immune responses to TB latency antigens following BCG vaccination is not a strain-specific phenomenon but an intrinsic property of dermal vaccination with BCG.
Upon administration to the skin, BCG is ordinarily not expected to persist in immunocompetent humans, although rare cases of persistence and reactivation of BCG have been reported in individuals who developed immune deficiencies many years after vaccination (1, 27, 35, 36). It therefore seems unlikely that BCG in the skin will encounter the necessary environmental triggers needed to express the latency antigens and subsequently to trigger immune responses. Moreover, the absence of T-cell responses is not due to restrictions in the T-cell repertoire since mice respond to these antigens when immunized with plasmid DNAs and BCG-vaccinated individuals exposed to TB also respond to latency antigens by IFN-γ production. Further studies will be aimed at the identification of the exact mechanisms responsible for the regulation and expression of the DosR regulon and immunity to latency antigens following BCG vaccination.
Since the start of its first use in the 1920s, three billion doses of BCG have been delivered throughout the world, unfortunately with relatively little impact on the global TB epidemic, underlining the need for a better vaccine regimen. Our findings suggest future studies evaluating the protective efficacy of latency antigens against (latent) TB and potential use of latency antigens as vaccine candidates, either alone, with BCG, or as postexposure TB vaccines.
Acknowledgments
This research project was funded by grants from the Bill & Melinda Gates Foundation through the Grand Challenges in Global Health Initiative (http://www.grandchallengesgh.org) (grants 37885 [Preclinical and Clinical Evaluation of Post-Exposure TB Vaccine] and 37772 [Biomarkers of Protective Immunity and Surrogate Markers of TB Disease in the Context of HIV/AIDS in Africa]). This work was supported by the European Commission, within the 6th Framework Program, contract LSHP-CT-2003-503367. M.Y.L. is supported by a grant from the Foundation Microbiology Leiden, The Netherlands. M.I.V. is supported by NIH grant RO1AI061505. TB microarrays were provided as part of NIH NIAID contract HHSN266200400091C (Tuberculosis Vaccine Testing and Research Materials), awarded to Colorado State University. K.H. is partially supported by grants G.0376.05 and 1.5.026.07 from the FWO-Vlaanderen and by the Damiaanaktie Belgium.
This report represents our views and does not necessarily represent a position of the European Commission, which will not be liable for the use made of such information.
We thank Christine Sloczynska, Judy Flanagan, Brid O'Halloran, and the school nurses of Redbridge and Waltham Forest Health Authority for help with the United Kingdom school study; the staff and students of The Lammas School and Aveling Park School for cooperation and participation in this project; and Rose Blitz and Shakuntala Patel for help on school study days. We thank Corine Prins for help in obtaining blood samples for the cross-sectional BCG study. The excellent technical assistance of Fabienne Jurion and Pierre-Yves Adnet, WIV-Pasteur Institute, Brussels, Belgium, is gratefully acknowledged. In addition, we thank Astrid van Halteren and Cees Melief for critically reading the manuscript.
We have no financial conflict of interest.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Armbruster, C., W. Junker, N. Vetter, and G. Jaksch. 1990. Disseminated bacille Calmette-Guerin infection in an AIDS patient 30 years after BCG vaccination. J. Infect. Dis. 162:1216. [DOI] [PubMed] [Google Scholar]
- 2.Behr, M. A., and P. M. Small. 1997. Has BCG attenuated to impotence? Nature 389:133-134. [DOI] [PubMed] [Google Scholar]
- 3.Black, G. F., P. E. M. Fine, D. K. Warndorff, S. Floyd, R. E. Weir, J. M. Blackwell, L. Bliss, L. Sichali, L. Mwaungulu, S. Chaguluka, E. Jarman, B. Ngwira, and H. M. Dockrell. 2001. Relationship between IFN-γ and skin test responsiveness to Mycobacterium tuberculosis PPD in healthy, non-BCG-vaccinated young adults in Northern Malawi. Int. J. Tuber. Lung Dis. 5:664-672. [PubMed] [Google Scholar]
- 4.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 5.Boon, C., and T. Dick. 2002. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 184:6760-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boon, C., R. Li, R. Qi, and T. Dick. 2001. Proteins of Mycobacterium bovis BCG induced in the Wayne dormancy model. J. Bacteriol. 183:2672-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt, L., C. J. Feino, O. A. Weinreich, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buddle, B. M., F. E. Aldwell, M. A. Skinner, G. W. de Lisle, M. Denis, H. M. Vordermeier, R. G. Hewinson, and D. N. Wedlock. 2005. Effect of oral vaccination of cattle with lipid-formulated BCG on immune responses and protection against bovine tuberculosis. Vaccine 23:3581-3589. [DOI] [PubMed] [Google Scholar]
- 9.Caccamo, N., A. Barera, C. Di Sano, S. Meraviglia, J. Ivanyi, F. Hudecz, S. Bosze, F. Dieli, and A. Salerno. 2003. Cytokine profile, HLA restriction and TCR sequence analysis of human CD4+ T clones specific for an immunodominant epitope of Mycobacterium tuberculosis 16-kDa protein. Clin. Exp. Immunol. 133:260-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caccamo, N., S. Milano, C. Di Sano, D. Cigna, J. Ivanyi, A. M. Krensky, F. Dieli, and A. Salerno. 2002. Identification of epitopes of Mycobacterium tuberculosis 16-kDa protein recognized by human leukocyte antigen-A*0201 CD8+ T lymphocytes. J. Infect. Dis. 186:991-998. [DOI] [PubMed] [Google Scholar]
- 11.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demissie, A., E. M. Leyten, M. Abebe, L. Wassie, A. Aseffa, G. Abate, H. Fletcher, P. Owiafe, P. C. Hill, R. Brookes, G. Rook, A. Zumla, S. M. Arend, M. Klein, T. H. Ottenhoff, P. Andersen, and T. M. Doherty. 2006. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 13:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Souza, S., V. Rosseels, M. Romano, A. Tanghe, O. Denis, F. Jurion, N. Castiglione, A. Vanonckelen, K. Palfliet, and K. Huygen. 2003. Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect. Immun. 71:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraz, J. C., E. Stavropoulos, M. Yang, S. Coade, C. Espitia, D. B. Lowrie, M. J. Colston, and R. E. Tascon. 2004. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infect. Immun. 72:6945-6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 17.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 18.Franken, K. L., H. S. Hiemstra, K. E. van Meijgaarden, Y. Subronto, J. den Hartigh, T. H. Ottenhoff, and J. W. Drijfhout. 2000. Purification of His-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr. Purif. 18:95-99. [DOI] [PubMed] [Google Scholar]
- 19.Geluk, A., V. Taneja, K. E. van Meijgaarden, E. Zanelli, C. Abou-Zeid, J. E. Thole, R. R. de Vries, C. S. David, and T. H. Ottenhoff. 1998. Identification of HLA class II-restricted determinants of Mycobacterium tuberculosis-derived proteins by using HLA-transgenic, class II-deficient mice. Proc. Natl. Acad. Sci. USA 95:10797-10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geluk, A., K. E. van Meijgaarden, K. L. Franken, J. W. Drijfhout, S. D'Souza, A. Necker, K. Huygen, and T. H. Ottenhoff. 2000. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8+ T cells in HLA-transgenic mice and humans. J. Immunol. 165:6463-6471. [DOI] [PubMed] [Google Scholar]
- 20a.Geluk, A., M. Y. Lin, K. E. van Meijgaarden, E. M. S. Leyten, K. L. M. C. Franken, T. H. M. Ottenhoff, and M. R. Klein. 2007. T-cell recognition of the HspX protein of Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination. Infect. Immun. 75:2914-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill, P. C., D. Jackson-Sillah, A. Fox, K. L. Franken, M. D. Lugos, D. J. Jeffries, S. A. Donkor, A. S. Hammond, R. A. Adegbola, T. H. Ottenhoff, M. R. Klein, and R. H. Brookes. 2005. ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of Mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J. Clin. Microbiol. 43:2070-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huygen, K., D. Abramowicz, P. Vandenbussche, F. Jacobs, J. De Bruyn, A. Kentos, A. Drowart, J. P. Van Vooren, and M. Goldman. 1992. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect. Immun. 60:2880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langermans, J. A., P. Andersen, D. van Soolingen, R. A. Vervenne, P. A. Frost, T. van der Laan, L. A. van Pinxteren, J. van den Hombergh, S. Kroon, I. Peekel, S. Florquin, and A. W. Thomas. 2001. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc. Natl. Acad. Sci. USA 98:11497-11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leyten, E. M. S., M. Y. Lin, K. L. M. C. Franken, A. H. Friggen, C. Prins, K. E. Meijgaarden, M. I. Voskuil, K. Weldingh, P. Andersen, G. K. Schoolnik, S. Arend, T. H. M. Ottenhoff, and M. R. Klein. 2006. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect. 8:2052-2060. [DOI] [PubMed] [Google Scholar]
- 25.Lim, A., M. Eleuterio, B. Hutter, B. Murugasu-Oei, and T. Dick. 1999. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J. Bacteriol. 181:2252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer, C. E., and M. W. Long. 1966. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am. Rev. Respir. Dis. 94:553-568. [DOI] [PubMed] [Google Scholar]
- 27.Reynes, J., C. Perez, I. Lamaury, F. Janbon, and A. Bertrand. 1989. Bacille Calmette-Guerin adenitis 30 years after immunization in a patient with AIDS. J. Infect. Dis. 160:727. [DOI] [PubMed] [Google Scholar]
- 28.Romano, M., S. D'Souza, P. Y. Adnet, R. Laali, F. Jurion, K. Palfliet, and K. Huygen. 2006. Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine 24:3353-3364. [DOI] [PubMed] [Google Scholar]
- 29.Rosenkrands, I., R. A. Slayden, J. Crawford, C. Aagaard, C. E. Barry III, and P. Andersen. 2002. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J. Bacteriol. 184:3485-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Roupie, V., M. Romano, L. Zhang, H. Korf, M. Y. Lin, K. L. M. C. Franken, T. H. M. Ottenhoff, M. R. Klein, and K. Huygen. 2007. Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice. Infect. Immun. 75:941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi, L., Y. J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skjøt, R. L., I. Brock, S. M. Arend, M. E. Munk, M. Theisen, T. H. Ottenhoff, and P. Andersen. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 70:5446-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, S. M., M. R. Klein, A. S. Malin, J. Sillah, K. Huygen, P. Andersen, K. P. McAdam, and H. M. Dockrell. 2000. Human CD8+ T cells specific for Mycobacterium tuberculosis secreted antigens in tuberculosis patients and healthy BCG-vaccinated controls in The Gambia. Infect. Immun. 68:7144-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne, J. A., L. C. Rodrigues, and I. N. Guedes. 1998. Does the efficacy of BCG decline with time since vaccination? Int. J. Tuber. Lung Dis. 2:200-207. [PubMed] [Google Scholar]
- 35.Talbot, E. A., M. D. Perkins, S. F. Silva, and R. Frothingham. 1997. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin. Infect. Dis. 24:1139-1146. [DOI] [PubMed] [Google Scholar]
- 36.van Deutekom, H., Y. M. Smulders, K. J. Roozendaal, and van D. Soolingen. 1996. Bacille Calmette-Guerin (BCG) meningitis in an AIDS patient 12 years after vaccination with BCG. Clin. Infect. Dis. 22:870-871. [DOI] [PubMed] [Google Scholar]
- 37.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-γ production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 39.Vekemans, J., M. O. Ota, J. Sillah, K. Fielding, M. R. Alderson, Y. A. Skeiky, W. Dalemans, K. P. McAdam, C. Lienhardt, and A. Marchant. 2004. Immune responses to mycobacterial antigens in the Gambian population: implications for vaccines and immunodiagnostic test design. Infect. Immun. 72:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson, R. J., K. A. Wilkinson, K. A. De Smet, K. Haslov, G. Pasvol, M. Singh, I. Svarcova, and J. Ivanyi. 1998. Human T- and B-cell reactivity to the 16kDa alpha-crystallin protein of Mycobacterium tuberculosis. Scand. J. Immunol. 48:403-409. [DOI] [PubMed] [Google Scholar]