Abstract
Pathogenic members of the Yersinia genus require the translocator protein LcrV for proper function of the type III secretion apparatus, which is crucial for virulence. LcrV has also been reported to play an independent immunosuppressive role via the induction of interleukin-10 (IL-10) through stimulation of Toll-like receptor 2 (TLR2). To investigate the LcrV-TLR2 interaction in vitro, His-tagged recombinant LcrV (rLcrV) from Yersinia pestis was cloned and expressed in Escherichia coli and purified through Ni-nitrilotriacetic acid column chromatography. High concentrations (5 μg/ml) of rLcrV stimulated TLR2 in vitro. Fractionation of rLcrV preparations via gel filtration revealed that only a minor component consisting of high-molecular-weight multimers or aggregates has TLR2 stimulating activity. Dimer and tetramer forms of rLcrV, which constitute the bulk of the material, do not have this activity. To investigate the potential role of LcrV/TLR2 in plague pathogenesis, we infected wild-type and TLR2−/− mice with virulent Y. pestis. No discernible difference between the two mouse strains in severity of disease or kinetics of survival after subcutaneous challenge was observed. IL-6, tumor necrosis factor, and IL-10 levels from spleen homogenates; bacterial load; and the extent of inflammation observed in organs from mice infected intravenously were also indistinguishable in both mouse strains. Taken together, our data indicate that the most abundant molecular species of Y. pestis LcrV do not efficiently activate TLR2-signaling and that TLR2-mediated immunomodulation is unlikely to play a significant role in plague.
Three members of the Yersinia genus are pathogenic for humans. Yersinia pseudotuberculosis and Yersinia enterocolitica cause self-limiting mesenteric lymphadenitis or ileitis. In contrast, Yersinia pestis infection results in the highly invasive and often fatal systemic infection known as plague. All three species elaborate a type III contact-dependent secretion system (TTSS) that is essential for virulence and is encoded on closely related plasmids. This apparatus allows delivery of effector molecules directly into host cell cytosol. These intracellular effectors, termed Yops, inhibit phagocytosis, induce apoptosis, and inhibit cytokine induction (4, 29). Three translocator proteins, YopB, YopD, and LcrV, are required for efficient intracellular delivery of the Yops (4). LcrV (V antigen) is a multifunctional protein essential for virulence. It comprises the tip of the injection needle of the TTSS (6, 11, 17) and along with LcrG has a regulatory role in Yop secretion (14). In addition, LcrV has been shown to be one of only two proteins to serve as highly effective vaccine antigens against Yersinia pestis (9, 30, 32).
An immunomodulatory role for LcrV has also been proposed (3). In vivo studies have shown that a recombinant Y. pseudotuberculosis LcrV-protein A fusion, produced in Escherichia coli, suppressed induction of tumor necrosis factor (TNF) and gamma interferon induced by injection of lipopolysaccharide (LPS) into Swiss Webster mice. It also enhanced the severity of disease and the bacterial burden following infection of treated mice with attenuated Y. pestis, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes (18). Further studies utilizing recombinant Y. enterocolitica LcrV (rLcrV), also produced in E. coli, showed suppression of zymosan-induced TNF production in C57BL/6 macrophages (24). It has been proposed that rLcrV interacts with both Toll-like receptor 2 (TLR2) and CD14 to induce interleukin-10 (IL-10) in transfected HEK293 cells (25). Candidate residues responsible for this interaction with TLR2 were identified in the N-terminal region of Y. enterocolitica LcrV based on the activity of cognate synthetic peptides (23, 25). Lower activity was detected in similar peptides based on Y. pestis and Y. pseudotuberculosis LcrV sequences (23). More recently, Overheim et al. (22) showed that His-tagged rLcrV derived from Y. pestis and purified from E. coli induced IL-10 from murine macrophages and human monocytes. It also suppressed induction of TNF from murine macrophages. That group also showed that deletion of either of two domains within LcrV eliminated stimulation of IL-10 secretion but that deletion of only one of these, near the C terminus of the protein, eliminated both induction of IL-10 secretion and suppression of TNF. Neither of these domains corresponds with, or overlaps the sequence of, the active peptide identified by Sing et al. in the LcrV of Y. enterocolitica (23, 25). Overheim et al. (22) also presented evidence that a fragment of LcrV lacking the C terminus, which includes the domain responsible for suppression of TNF, was an effective vaccine against Y. pestis at lower doses than unmodified rLcrV.
TLRs participate in many aspects of the host defense against infections (2). Stimulation of TLR2 results in the induction of proinflammatory cytokines such as TNF and IL-6. Like TLR4, the receptor for LPS, TLR2 is also responsible for the release of anti-inflammatory cytokines such as IL-10 and IL-4, although these are usually detected at a later time than proinflammatory mediators induced simultaneously via the same pathway (1, 21). Curiously, TLR2 has been associated with immunosuppression in microorganisms in addition to Y. enterocolitica (7, 20), suggesting that exploitation of this pathway as a means to evade innate immunity may be a common strategy among pathogens. However, no clear mechanistic basis for a predominantly immunosuppressive effect of TLR2 stimulation has been established.
Here we report that Yersinia pestis-derived rLcrV, purified from E. coli through Ni-nitrilotriacetic acid chromatography, activates TLR2, as has been reported for Yersinia enterocolitica LcrV. However, further purification through gel filtration indicates that only a very high-molecular-weight multimer or aggregate, which comprises a small proportion of total rLcrV, has stimulatory activity. Stimulation with Yersinia pestis LcrV-derived peptides corresponding to stimulatory peptides derived from Yersinia enterocolitica LcrV failed to activate TLR2. In infection experiments, TLR2 deficiency in mice had no significant influence on the course of disease, levels of IL-10, or degree of inflammation in infected tissue. Taken together, these results strongly suggest that TLR2-mediated induction of IL-10 does not contribute significantly to the virulence of Yersinia pestis.
MATERIALS AND METHODS
Bacterial strains, plasmids, cell lines, and reagents.
Virulent Y. pestis strain KIM1001 (27), biotype mediavalis, was grown and quantified on solid medium (TB) containing 10 g Bacto-tryptose, 5 g NaCl, 3 g beef extract (paste form; Difco catalog no. 212610), and 15 g agar per liter and supplemented with 2.5 mM CaCl2. Although this composition is identical to that given for precompounded tryptose blood agar base, we have on occasion encountered significant problems with plating efficiency on the latter preparation and therefore chose to work from the individual components. Unless otherwise indicated, plates were incubated at 25°C for 48 h.
prLcrV was described by Overheim et al. (22) and was the kind gift of Olaf Schneewind. Construction of pLcrVYp is described below.
HEK293 cells stably expressing human TLR2-yellow fluorescent protein (TLR2-YFP) or empty vector pcDNA3 were as described previously (13). HEK293-TLR2-YFP/human CD14 cells were generated by stably expressing human CD14 in the vector pCEP4, selecting in hygromycin, and fluorescence-activated cell sorting. 293 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and 10 μg/ml ciprofloxacin (Biowhittaker), with addition of G418 (0.5 mg/ml) (for 293-pCDNA3 and 293-TLR2-YFP) or G418 plus hygromycin (400 U/ml) (for 293-TLR2-YFP/CD14). Cells were seeded at 3 × 104 per well in 96-well tissue culture plates (Costar) and stimulated for 16 to 18 h before harvest of the supernatant for cytokine analysis. LPS, from E. coli strain 0111:B4, was purchased from Sigma and was subjected to two rounds of phenol reextraction to remove contaminating TLR2-stimulating lipoproteins (10). The synthetic triacylated lipohexapeptide Pam 3-CysSerLys4 (P3C) and macrophage-activating lipopeptide 2 (MALP2) were purchased from EMC Microcollections (Tubingen, Germany). Synthetic LcrV peptides (purified by high-pressure liquid chromatography to >98%) were purchased from Genemed Synthesis, Inc.
Recombinant plasmids expressing LcrV from Yersinia pestis.
lcrV from Yersinia pestis strain KIM (5) amplified by PCR was cloned into vector pBAD/gIIIB (Invitrogen) at NcoI and SalI sites, resulting in pLcrVYp. This vector incorporates a signal sequence to promote export of the cloned protein to the periplasm. The signal sequence was included both to allow extraction by osmotic shock and to reduce inclusion body formation. Because osmotic shock proved to have little advantage, more convenient methods of extraction were used during preparation of recombinant protein (see below). The coding sequence of the rLcrV in this construct is as follows (the cleaved domain of the signal sequence is italicized, nonnative residues of mature protein are in lowercase, and native residues are in uppercase; note the added C-terminal hexahistidyl domain used in purification): mkkllfaiplvvpfyshstmvMIRAYEQNP…DTSGKvdhhhhhh.
A second rLcrV-producing plasmid. prLcrV, was the kind gift of Olaf Schneewind and was described by Overheim et al. (22). This plasmid produces full-length rLcrV with the addition of an amino-terminal decahistidyl domain.
Expression and purification of rLcrV. (i) Expression and purification from plasmid pLcrVYp.
Escherichia coli LMG194 (Invitrogen) carrying pLcrVYp was grown overnight at 37°C in 2× YT broth supplemented with 100 μg/ml ampicillin. Bacteria were then diluted into 4 liters of fresh medium to an optical density at 600 nm (OD600) of 0.1 and grown at 37°C in a New Brunswick Bio Flo 2000 fermentor (with aeration at 5 liters/min, agitation at 300 rpm, and antifoam as needed) to an OD600 of 0.5. Arabinose (0.002%) was then added to induce production of LcrV, and incubation was continued for an additional 3 h. Cells were harvested by centrifugation at 12,000 × g for 10 min, resuspended in 50 ml of 50 mM sodium phosphate buffer (pH 8.0) containing 300 mM NaCl, and sonicated on ice (Branson Sonifier 450 with 0.75-in. solid stepped horn; output power setting, 6; run for 4 min at 50% duty cycle). Alternatively, cells were disrupted via rapid decompression with a prechilled French pressure cell operated at 20,000 lb/in2. The extract was centrifuged at 10,000 × g for 15 min, and the soluble fraction was applied to a nickel nitrilotriacetic acid column (1-ml bed volume) preequilibrated with 30 ml column buffer containing 50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole, pH 8 (QIAGEN). The column was washed with 15 ml wash buffer containing 50 mM NaH2PO4, 300 mM NaCl, and 20 mM imidazole, pH 8. Bound protein was eluted in buffer containing 50 mM NaH2PO4, 300 mM NaCl, and 250 mM imidazole, pH 8. Proteins were subjected to extensive dialysis against endotoxin-free phosphate-buffered saline (PBS) (Cambrex) and immediately frozen at −80°C. Protein concentrations were determined by absorbance at 280 nm, using an extinction coefficient of 0.515 calculated from the rLcrV sequence by the method of Gill and von Hippel (8), and confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). All buffers were prepared using endotoxin-free reagents.
Analysis of purified rLcrV treated with 5 mM dithiothreitol (DTT) by liquid chromatography-mass spectroscopy yielded a mass consistent with removal of the signal sequence; no material corresponding to the full-length precursor form was detected.
(ii) Expression and purification from plasmid prLcrV.
Plasmid prLcrV was transformed into BL21(DE3), protein production was induced, and the rLcrV was extracted and purified as described by Overheim et al. with the following modifications: (i) rather than sonication, a French pressure cell was used to disrupt the bacteria as described above, and (ii) Triton X-114 phase separation and the following size exclusion chromatography on G25 Sephadex to remove Triton were omitted. This material was analyzed identically to rLcrV from pLcrVYp via size exclusion chromatography on Superose 12 resin as described below.
Size exclusion chromatography.
One-milligram samples of rLcrV in a 0.3-ml volume of PBS were loaded on a PBS-equilibrated Superose 12 column (Amersham Biosciences catalog no. 17-5173-01) and eluted with PBS at a flow rate of 0.54 ml/min. Endotoxin-free PBS (Cambrex) was used for column equilibration and elution. Absorbance of the eluate at 280 nm was monitored. Fractions of 330 μl were collected in the wells of microtiter plates, and each was tested for the ability to stimulate TLR2 via induction of IL-8 as described below. Following each use, the column was washed with 1 M NaOH, followed by endotoxin-free water and 0.1 M HCl, and stored in 20% ethanol.
Mice.
Female C57BL/6 mice (6 to 8 weeks of age) were purchased from Jackson Laboratory (Bar Harbor, ME). TLR2−/− mice were originally a generous gift of S. Akira (28) and have now been back-bred to C57BL/6 for 11 generations. All mice were bred under specific-pathogen-free conditions. All animal studies were approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee, and all relevant policies regarding animal care, biosafety, and security were followed.
Experimental infection of mice.
To prepare inocula for experimental infection, Y. pestis KIM1001 was inoculated heavily onto TB plates from suspensions stored at −70°C in TB (no agar) plus 5% glycerol and incubated at 37°C for 24 h. Bacteria were harvested from the plate with a loop and resuspended in sterile distilled water with a vortex mixer to match a turbidity standard of an OD600 of 0.3. It was important to use a low-ionic-strength medium for this initial suspension step to achieve good dispersion of the cells. This initial high-density suspension was subsequently diluted as required using endotoxin-free injection-grade PBS. For survival experiments, 10 age- and sex-matched mice per treatment group were infected with Yersinia pestis KIM1001 by subcutaneous (s.c.) injection of 50 μl on the nape of the neck. For intravenous (i.v.) infection experiments, five mice per group were injected with 500 μl of inoculum containing the indicated doses in the tail vein. Survival was monitored every 12 h for up to 21 days. All TLR2−/− were individually genotyped and bore uniquely numbered ear tags. For collection of organs, mice were sacrificed at 48 h following i.v. infection by pentobarbital overdose followed by cervical dislocation. To determine bacterial load, spleens were homogenized in 1 ml of PBS and titers of bacteria in the resulting suspension were determined by serial dilution and plating. Differences in survival were analyzed by Kaplan-Meyer survival analysis and the log rank test.
Histopathology.
Livers were fixed for in neutral buffered 4% formalin, and sections were stained with hematoxylin-eosin.
Cytokine determination. (i) Cytokine determination from spleens.
Spleens were homogenized in 1 ml of PBS. Following removal of a sample for determination of bacterial titer, ciprofloxacin (100-μg/ml final concentration) and protease inhibitor cocktail (Roche catalog no. 11-873-580; 1× final concentration) were added. The suspension was centrifuged at 10,000 × g for 1 min. The resulting supernatant was filtered to remove bacteria (0.2-μm pore, polyvinylidene difluoride; Pall Acrodisc). Cytokines in the resulting supernatant were measured using enzyme-linked immunosorbent assay (ELISA) kits from BD Pharmingen (moIL-10) or R&D Systems (moTNFa and moIL-6) according to the manufacturer's directions. IL-10 levels were determined twice, each time in triplicate from the same set of tissue samples.
(ii) Cytokine determination from tissue culture.
Culture medium was removed from the wells and diluted as required with fresh medium for use in the assays. IL-8 was determined with a kit from R&D Systems (huIL-8). All cytokine assays were performed in triplicate. The significance of observed differences in median cytokine concentrations were analyzed by the nonparametric Pittman exact test.
NF-κB luciferase reporter assays.
Cells were transfected with the NF-κB luciferase reporter plasmid (a gift of Katherine Fitzgerald, University of Massachusetts Medical School) using Genejuice (Promega) as described by the manufacturer. Following stimulation and incubation as indicated, cells were lysed with passive lysis buffer (Promega), and luciferase activity was measured by luminometry following addition of luciferase substrate.
RESULTS
Preparation of Y. pestis rLcrV.
The lcrV gene from Yersinia pestis strain KIM1001 was cloned into the expression vector pBADgIIIB (Invitrogen), resulting in pLcrVYp. (See Materials and Methods for a detailed description of the protein produced by this construct.) rLcrV was expressed and purified by nickel chelation chromatography as described in Material and Methods. This method of purification yields primarily dimeric and tetrameric rLcrV as shown by native PAGE, SDS-PAGE, Western blotting, and gel filtration chromatography (see below).
Yersinia pestis rLcrV and stimulation of TLR2.
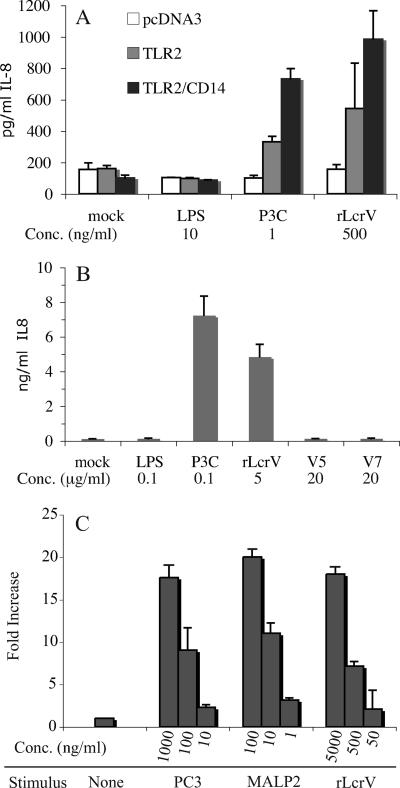
His-tagged rLcrV derived from Y. enterocolitica (rLcrVYe) has been reported to activate TLR2 in a CD14-dependent manner (25). In these reports, LcrV from Y. enterocolitica was cloned and expressed in E. coli with an N-terminal His6 tag in the vector pQE30 (QIAGEN), and purified by Ni2+ chelation chromatography. To determine if similar rLcrV preparations derived from the Yersinia pestis lcrV gene are also able to stimulate TLR2, we transfected HEK293 cells with TLR2 in either the presence or absence of cotransfected CD14 and stimulated them for 18 h with rLcrV purified as described above. As a measure of TLR2-stimulating activity, cell supernatants were assayed for IL-8 by capture ELISA. As shown in Fig. 1A, TLR2 is required for induction of IL-8 by rLcrV, and this induction is enhanced by, but not dependent upon, the coexpression of CD14. These observations are similar to the results reported by Sing et al. (25) for rLcrVYe, except that we find enhancement by, rather than a strict requirement for, CD14.
FIG. 1.
Activation of TLR2 by rLcrV. (A) HEK 293 cells stably transfected with vector alone (pcDNA3), TLR2 alone, or both TLR2 and CD14 were stimulated with 10 ng/ml LPS, 1 ng/ml P3C, or 500 ng/ml rLcrV as indicated for 18 h. Supernatants were assayed by capture ELISA for the presence of IL-8 as an indicator of TLR2 activation. (B) HEK293 cells stably transfected with both TLR2 and CD14 were stimulated with 100 ng/ml LPS, 100 ng/ml P3C, 5 μg/ml rLcrV, or 20 μg/ml of the indicated synthetic peptide for 18 h. Supernatants were assayed by capture ELISA for the presence of IL-8 as an indicator of TLR2 activation. (C) The same cells as for panel B were transfected with an NF-κB luciferase reporter and stimulated with P3C, MALP2, or rLcrV at the indicated concentrations for 18 h. Relative luciferase activity is shown. Data shown are means from triplicate assays with error bars indicating ranges and are representative of at least three experiments.
Because Sing et al. relied primarily on an NF-κB reporter construct to indicate TLR2 activation in the HEK293/TLR2/CD14 system, we also examined activation with a similar reporter construct. The results (Fig. 1C) were consistent with observations made using IL-8 release.
Yersinia pestis LcrV synthetic peptides fail to stimulate TLR2.
Peptides identical to specific domains of Y. enterocolitica LcrV and capable of stimulating TLR2 have been previously described (23, 25). These are derived from the N-terminal globular portion preceding alpha helix 1 in the LcrV structure (6). A peptide comprising residues 31 to 49, designated V7, was most active. We tested the ability of the synthetic cognate peptide based on the Y. pestis sequence, which differs at single residue (K43→N) from the homologous region in Y. enterocolitica, to activate TLR2. This peptide has identical residues at all of the positions established by Sing et al. (23) to be required for TLR2 activation. As shown in Fig. 1B, this peptide is unable to stimulate HEK293 cells stably transfected with both TLR2 and CD14. Sing et al. (23) observed only weak activity with a longer synthetic peptide comprising residues 31 to 66 of Y. pestis LcrV (23). A peptide comprising residues 27 through 43, corresponding to the inactive peptide designated V5 by Sing et al. (25), was also found to be inactive.
Multimers of rLcrV in purified preparations.
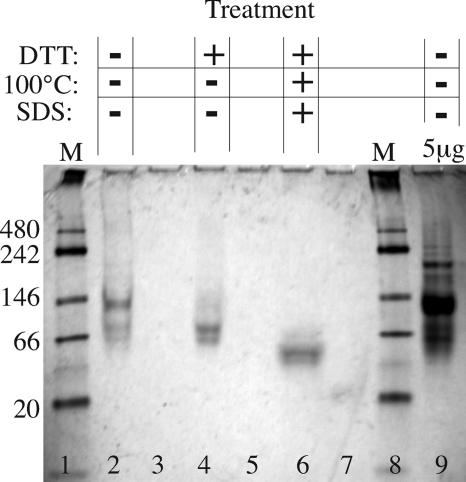
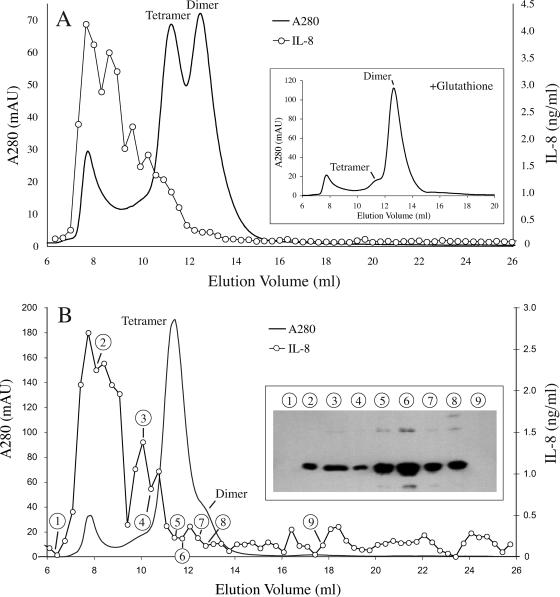
It has been previously observed that in solution, rLcrV is not present in significant amounts in monomer form but instead exists primarily as a mixture of dimers and tetramers (29). Dimers can form through disulfide bonds between the single cysteine residue (C273) in each rLcrV molecule (6). To determine the form(s) present in our rLcrV preparation, we analyzed samples by electrophoresis, gel filtration, and immunoblotting. As shown in Fig. 2, the major band of rLcrV seen by PAGE under native conditions migrates at a rate expected of rLcrV tetramer, and the preparation also contains higher-molecular-weight forms. The addition of a reducing agent results in migration consistent with a dimer. Under combined reducing and denaturing conditions, migration is consistent with a monomer. Analysis by gel filtration on a calibrated Superose 12 column yields similar results (Fig. 3). In the absence of a reducing agent, the elution times of the two primary peaks are consistent with a tetramer and a dimer. In the presence of glutathione, the dimer forms the primary peak and the tetramer peak is dramatically reduced (Fig. 3A, inset). In some preparations, a small peak consistent with monomer was observed under reducing conditions, but in no case did we detect monomer without addition of glutathione to the elution buffer (data not shown). We conclude that disulfide bond formation in necessary for the existence of stable tetramers but not for that of stable dimers.
FIG. 2.
Analysis of rLcrV by PAGE. Lanes 1 and 8, molecular mass markers formulated for use in native gels, with their sizes in kilodaltons indicated at the left. In the absence of SDS, DTT, and thermal denaturation, rLcrV migrates at a rate consistent with a tetramer, with some dimer also present (lane 2). Addition of DTT results in migration consistent with a dimer (lane 4). The combination of DTT and denaturing conditions (lane 6) results in migration consistent with a monomer. Lanes containing rLcrV were separated by an empty lane, as diffusion of DTT into adjacent lanes affected migration. High-molecular-mass species of greater than 200 kDa are visible under native conditions when the gel is loaded with 5 μg rLcrV (lane 9). The bands are less distinct than usual for SDS-PAGE because the buffers do not contain SDS, allowing some loss of detergent from the protein as it migrates.
FIG. 3.
Analysis of rLcrV by gel filtration. (A) rLcrV purified from sonic extracts was separated on a calibrated Superose 12 column. The elution profile (A280) of the primary peaks indicates that rLcrV dimers and tetramers are the major species present. A high-molecular-weight peak, eluting near the void volume of the column, is also present. The profile obtained when 1 mM glutathione is included in the column buffer is shown in the inset. Note conversion of the tetramer peak to dimer but retention of the high-molecular-weight fraction. Samples of fractions were also assayed for TLR2-stimulating activity (○) as for Fig. 1. Note that this activity is associated almost exclusively with material eluting prior to rLcrV tetramer. (B) An independent preparation of rLcrV purified from extracts made via a French pressure cell was analyzed as for panel A. This preparation contained a much higher proportion of tetramers and a similar proportion of high-molecular-weight material. Assay of fractions for TLR2-activating activity (○) again showed the material eluting before the tetramer to be most active. The inset shows an immunoblot of selected fractions prepared from a standard SDS-polyacrylamide gel and probed with an LcrV-specific monoclonal antibody. The selected fractions are indicated by circled numerals. Note that the high-molecular-weight fractions contain rLcrV.
Both native PAGE and gel filtration also revealed high-molecular-weight species (>200 kDa). These eluted near the void volume of the column during gel filtration. The integrated absorbance of this early peak was reduced only slightly in the presence of glutathione, indicating that its integrity is not dependent on disulfide bonds (Fig. 3A, inset). The presence of rLcrV in these high-molecular-weight fractions was confirmed by immunoblotting (Fig. 3B, inset). Silver-stained SDS-polyacrylamide gels showed only a single band consistent with rLcrV in these fractions (data not shown), indicating that rLcrV is the major protein component of this material.
The high-molecular-weight species containing rLcrV is responsible for TLR2-stimulating activity.
To determine which form(s) of rLcrV contains TLR2-stimulating activity, fractions from rLcrV preparations separated on Superose 12 were assayed for TLR2-stimulating activity (Fig. 3). HEK293 cells stably transfected with TLR2 and CD14 were treated with a sample of each fraction, and IL-8 was measured as an indicator of TLR2 activation. Surprisingly, neither the dimer nor tetramer form was able to stimulate TLR2. Virtually all of the TLR2-stimulating activity was associated with the higher-molecular-weight fractions, which contained less than 10% of the total rLcrV protein. The pattern of activity among fractions was remarkably consistent when independent preparations were compared, even when different cell disruption techniques (sonication versus French pressure cell) that yielded different proportion of dimer and tetramer, but a consistent proportion of high-molecular-weight material, were used (compare Fig. 3A and B).
Taken together, these results indicate that the bulk of the TLR2-stimulating activity of our rLcrV resides in stable multimers or aggregates substantially larger than a tetramer. rLcrV is the major, and perhaps the only significant, protein component of this material. However, the presence of nonprotein components, or of highly active protein components in low abundance, cannot be excluded. It is also possible that the tetramer and lower-molecular-weight forms have some stimulatory activity obscured by the tailing peaks of the higher-activity fractions, but if so, this activity must be very weak.
Analysis of an alternative rLcrV construct.
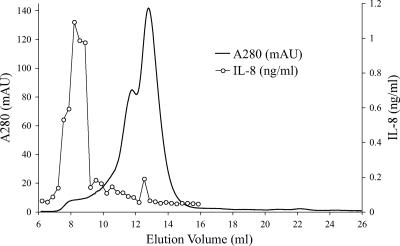
Virtually all studies of the immunosuppressive properties of LcrV are based on recombinant constructs with somewhat different structures. For example, the parent rLcrV fusion protein utilized by Overheim et al. (22) differs from ours in two significant ways: it has an amino-terminal decahistidyl domain as opposed to the carboxy-terminal hexahistidyl domain in our construct, and it includes the native Y. pestis LcrV sequence with no other additions. In contrast, our mature construct has three additional amino-terminal resides (TMV) that precede the native LcrV domain and two additional carboxy-terminal residues (VD) which precede the added polyhistidine sequence. To determine if our finding that TLR2 stimulation is due to high-molecular-weight forms was robust to such differences, we purified and analyzed rLcrV produced by the construct used by Overheim et al. (22). The results of these experiments are shown in Fig. 4. As was found with our construct, all of the TLR2-stimulating activity of this rLcrV form was associated with fractions having much shorter elution times than those for dimers and tetramers. Also, in agreement with results obtained with our construct, almost all of the rLcrV was in the form of dimers and tetramers. No monomer was present. In fact, the preparation analyzed in Fig. 4 contained less high-molecular-weight material than we had observed with our construct, and its TLR2-stimulating activity was correspondingly lower (Compare Fig. 4 and 3, noting the differences in scaling on the IL-8 concentration axis.) Thus, it appears that our basic observations are not sensitive to minor differences in rLcrV primary structure present in the amino- and carboxy-terminal domains.
FIG. 4.
Analysis of an alternative rLcrV construct by gel filtration. rLcrV produced by the prLcrV construct used by Overheim et al. (22) purified as described in Materials and Methods was analyzed in experiments parallel to those of Fig. 3. Note that the results are very similar, although the content of high-molecular-weight material is somewhat lower, as are the levels of TLR2-stimulating activity of the corresponding fractions. Analysis via immunoblotting as in Fig. 3B confirmed the presence of rLcrV in the active fractions (data not shown).
Infection of TLR2−/− mice.
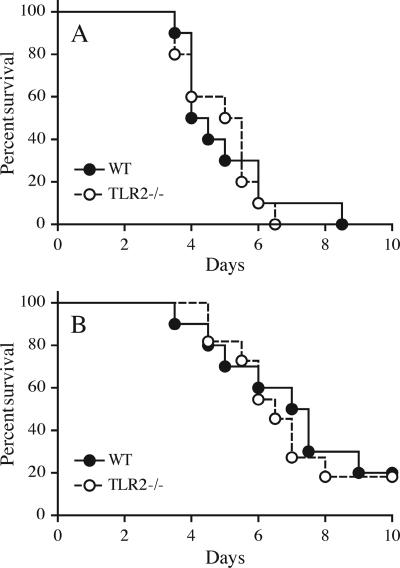
The interaction between Yersinia enterocolitica rLcrV and TLR2 is reported to result in an anti-inflammatory effect, including suppression of TNF and induction of IL-10 (23-25), leading to decreased macrophage activation. During Y. enterocolitica infection, this mechanism is proposed to contribute to evasion of innate immunity. To address the potential relevance of this mechanism to plague, we compared the diseases produced in wild-type and TLR2−/− mice following s.c. infection with virulent Y. pestis. A dose of 1,000 CFU of Y. pestis strain KIM1001 was uniformly lethal for both C57BL/6 mice and C57BL/6 TLR2−/− mice, and no significant difference in mean time of survival was observed (Fig. 5A). Subtle differences in resistance are more readily observed at doses that do not cause complete mortality. Accordingly, we conducted an additional comparison using a dose of 100 CFU, again delivered s.c. (Fig. 5B). This dose yielded 80% mortality in both wild-type and TLR2−/− animals. Again, no significant difference in mean time of survival between mouse genotypes was observed.
FIG. 5.
TLR2 deficiency and survival. Wild-type (WT) and TLR2−/− mice (10 animals per group) were infected s.c. with KIM1001. Survival was monitored for 14 days, and no deaths were observed beyond day 9. (A) Dose of 1,000 CFU. Note that survival times are very similar (means for WT and TLR2−/−, 4.25 and 5.25 days, respectively; P = 0.85). The results shown are representative of three experiments. (B) Dose of 100 CFU. At this lower dose, survival times are also very similar (means for WT and TLR2−/−, 7.25 and 6.5 days, respectively; P = 0.75). The experiment was performed once at this dose. (The TLR−/− group in this experiment contained 11 mice.)
Bacterial load and cytokine levels in mice infected with Y. pestis.
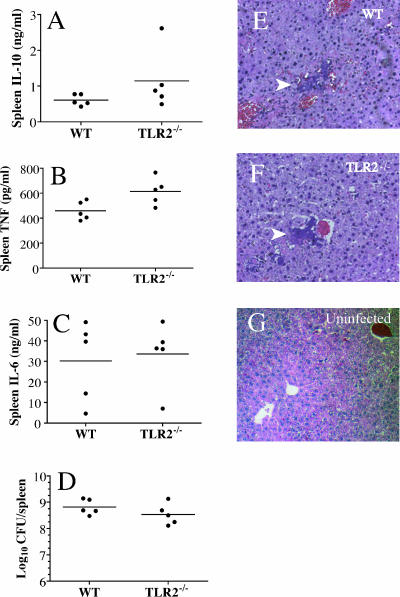
To determine if the bacterial load and/or the induction of selected cytokines during infection was influenced by the TLR2 status of the mice, we measured the numbers of bacteria and the levels of IL-10, TNF, and IL-6 in the spleens of five mice of each genotype at 2 days following i.v. infection with 1,000 CFU of KIM1001. The i.v. route was used in this experiment because it results in essentially simultaneous infection of internal organs; with s.c. infection, the time of dissemination of the bacteria sometimes varies among the animals. The observed differences in mean bacterial load and IL-6 titers were not statistically significant (Fig. 6C and D). IL-10 levels were about twofold greater (1.07 versus 0.58 ng/ml) in TLR2−/− mice (Fig. 6A), a difference that was statistically significant (P = 0.04) but which is inverse to the result expected if interaction of LcrV with TLR2 elevates IL-10 levels. Despite the enhanced IL-10 levels in TLR2−/− mice, their mean TNF levels were also slightly but significantly higher than those observed in the wild-type controls (34% [621 versus 462 pg/ml]; P = 0.02) (Fig. 6B).
FIG. 6.
TLR2 deficiency has little effect during infection with Yersinia pestis. Wild-type (WT) and TLR2−/− mice were infected i.v. with 1,000 CFU KIM1001. (A to C) Spleens and livers were harvested at 2 days postinfection. Spleens were homogenized, and levels of IL-10 (A) TNF (B), and IL-6 (C) were quantified by capture ELISA. No significant differences were detected for any cytokine between genotypes, although the higher TNF levels in TLR2−/− mice were suggestive (P = 0.056). (D) Bacterial loads in the spleens of the two genotypes were also similar (P = 0.2). (E and F) Liver sections from both genotypes stained with hematoxylin and eosin showed a pattern typical of Y. pestis infection in WT mice. Masses of bacteria occupy liver sinusoids, with little sign of local inflammation (white arrows). At least 20 foci in the livers of each of five mice of each genotype were examined by an investigator blind to the sample source. (G) For reference, a liver section from an uninfected WT control is shown. Comparable sections from a Y. pestis derivative that does induce strong local inflammation are presented in reference 15.
Inflammation at foci of infection.
If TLR2-dependent stimulation of IL-10 production makes an important contribution to inhibition of local inflammation, we would expect enhanced inflammation at foci of infection in TLR2−/− mice. This should be readily observable in the livers of infected mice, because there is little inflammatory response to wild-type Y. pestis in this tissue (18, 27) and any enhancement of the inflammation can be readily detected. Accordingly, we harvested livers from the mice at 2 days after infection and examined liver sections to determine the state of inflammation at foci of infection. As shown in Fig. 6E and F, sections from both mouse genotypes show focal bacterial masses largely devoid of inflammatory cells. No evidence of enhanced inflammation in TLR2-deficient animals was observed.
DISCUSSION
The major goal of this work was to examine the hypothesis that the interaction of LcrV and TLR2 contributes significantly to Y. pestis virulence, whether via the induction of IL-10 or by any other means. In the most direct interpretation, this model predicts that TLR2−/− mice will show (i) enhanced resistance to Y. pestis, (ii) enhanced inflammation at foci of infection, and (iii) decreased IL-10 and enhanced proinflammatory cytokine production during Y. pestis infection. The results of our experiments with TLR2−/− mice did not confirm these predictions. We observed no significant differences in survival, mean time to death, or bacterial load; no evidence of enhanced inflammation at foci of infection; and an increase, rather than a reduction, in IL-10 titers. We did observe a slight increase in TNF titer, but this occurred in the presence of enhanced rather than reduced IL-10 levels. The other proinflammatory cytokine measured, IL-6, showed no change. In comparison with survival experiments, small numbers of animals were utilized for bacterial titer and cytokine measurements, and we may have failed to observe modest differences. For example, for bacterial titers and IL-6 titers, the two measurements for which no significant differences were observed, power analysis using a sample size of five and the measured variances of our observations yields an 80% probability of detecting differences of about twofold relative to the mean values, with a correspondingly lower probability of detecting smaller differences. Another limitation of these experiments is that measurements were made at a single time point, 2 days postinfection, and we cannot be certain that our results reflect conditions pertaining at earlier stages of infection. However, both absolute survival and survival kinetics were remarkably consistent at both doses tested, indicating that any influence of TLR2 on the course of infection must be minor. It should be noted that the virulent Y. pestis-mouse infection model is a very sensitive one, in that specific genetic modifications of bacteria or mice often have large effects on virulence. For example, otherwise virulent mutants lacking the Pla protease (27, 31) and mutants with defects in iron acquisition (31) show an increase in 50% lethal dose of several orders of magnitude, as do strains modified to produce highly stimulatory LPS (15). TLR4-deficient mice are highly susceptible to a strain producing stimulatory LPS, while wild-type mice are highly resistant (15). In both of these instances, reduced virulence (or enhanced resistance) was also associated with marked enhancement of inflammation at foci of infection. Thus, it is clear that experimental manipulations in this system do indeed have very large effects when they are related to functional differences in the interaction between the bacteria and host defenses. Our failure to observe any indication of enhanced resistance or improved inflammatory response in TLR2 deficient mice must therefore be regarded as strong evidence that this receptor does not play a significant role in interactions contributing to virulence of Y. pestis during infection. Because we did not carefully examine the progress of infection at multiple time points, it remains possible that there are subtle differences in the development of infection between the genotypes that do not affect survival or time to death.
The literature regarding the induction of IL-10 by the LcrV of the yersiniae presents a somewhat confusing picture. Sing et al. reported that specific residues are required for induction of IL-10 by LcrV of Yersinia enterocolitica and that peptides containing these residues are effective inducers (23, 25). However, they also found that a peptide from the cognate region of Y. pestis and Y. pseudotuberculosis LcrV has little activity (23). On the other hand, Overheim et al. reported that regions of LcrV entirely distinct from that defined by Sing et al. are required for IL-10 induction by Y. pestis LcrV (22). While Sing et al. provided evidence from a well-established in vivo model supporting a role for the TLR2-LcrV interaction during Y. enterocolitica infection (23, 25), these results are unfortunately dependent on the mouse strain employed (26). No similar evidence has been published previously regarding Y. pestis. For this species, currently available in vivo data are indirect in that all the relevant experiments involve injection of mice with various forms of rLcrV, followed by measurement of cytokine levels and/or challenge with LPS, attenuated Y. pestis, or other unrelated pathogens (18, 19). While the differences in IL-10 levels that we observe are not consistent with the LcrV-TLR2 model (levels were not reduced in TLR2-deficient animals), IL-10 is clearly elevated in mice with well-developed Y. pestis infection. Thus, we cannot rule out the possibility that immunosuppression due to elevated IL-10 levels induced by a TLR2-independent mechanism plays an important role in plague. Indeed, it is possible that the unusual combination of proinflammatory stimuli presented by Y. pestis, which as we have shown previously activates TLR4 very poorly (15), results in an aberrant cytokine profile that may compromise innate defenses.
A variety of fusion proteins have been used to demonstrate the immunosuppressive properties of LcrV. For example, Overheim et al. utilized an N-terminal decahistidyl tag (22). Motin et al. fused a 34-kDa fragment of protein A to the N terminus of a truncated LcrV lacking the first 67 residues (16). The three-dimensional structure of LcrV was determined from a fusion protein containing five residues fused to an LcrV N terminus beginning at residue 28 and a C-terminal addition of four residues plus a hexahistidyl tag (6). This structure shows that both the N and C termini are very flexible and are located near each other, external to one of the LcrV globular domains. The flexibility and location of these termini are consistent with tolerance for additions and deletions. Thus, it is unlikely that the three-residue N-terminal and the eight residue C-terminal additions (the latter including a six-residue His tag) present in our LcrV construct are less reflective of the properties of native LcrV than those employed by others. Moreover, we have shown directly that the rLcrV protein of Overheim et al. (22) behaves similarly to our own. It should be noted that the cytokine-inducing properties of native LcrV purified from Y. pestis or any other Yersinia species have not been studied.
Our results with His-tagged Y. pestis rLcrV are consistent with those of others in that we do observe stimulation of TLR2 in vitro. However, we also show that the ability of this material to stimulate TLR2 is unexpectedly complex at the biochemical level. The major forms of rLcrV in our preparations, dimer and tetramer, have no TLR2-stimulating activity. Such activity is detected only in high-molecular-weight multimers or aggregates. Although the data from experimental infections discussed above argue strongly against a role for this activity in Y. pestis virulence, we consider three alternative hypotheses regarding TLR2 stimulation by LcrV, one of which implies physiological significance.
First, the stimulatory activity may result from the presence of a potent TLR2-activating contaminant (e.g., a lipoprotein, lipopeptide, peptidoglycan, etc.) constituting a small proportion of the aggregate and not from rLcrV per se. Such contamination is both common in material purified from whole-cell extracts and notoriously difficult to exclude. TLR2-stimulating activity initially attributed to what were thought to be highly purified materials has later been shown to result from such contamination (for example, see references 10, 12, and 33). There is no general method to ensure freedom from such contaminants in protein preparations. Note that vulnerability to such contaminants is greatly increased when cells expressing a variety of TLRs, such as mouse macrophages, are the targets of stimulation.
A second possibility is that the stimulatory activity is indeed due to rLcrV in the aggregates/multimers but that this material is nonphysiological and is an artifact of overexpression and purification techniques. The tendency of recombinant proteins expressed at high levels in E. coli to form high-molecular-weight aggregates is well established. Exposure of mature rLcrV to cell extracts, as occurs during purification, could also be critical to formation of the stimulatory material. In Y. pestis the level of LcrV expression is much lower, and exposure of mature LcrV to concentrated cell extracts does not occur. In this view, the stimulatory aggregates/multimers are purely an in vitro artifact and have no physiologic relevance.
A third possibility is that the stimulatory fractions contain structures that are, or resemble, physiological multimeric species that are normally detected by TLR2 as an indicator of pathogens with TTSS machinery. Micrographs of LcrV arranged at the tips of secretion needles (17) suggest to us an octamer composed of four dimers. Perhaps such structures are occasionally released from bacteria in vivo and elicit proinflammatory responses via TLR2. However, there is no evidence to support this idea from infection experiments with yersiniae. Also, PcrV, a related protein from Pseudomonas aeruginosa, does not stimulate TLR2 (25).
The association of TLR2 stimulation with aggregates/multimers of rLcrV suggests that individual rLcrV molecules may interact weakly with TLR2 and that activation results from clustering of the receptor. This would explain the failure of low-molecular-weight forms to cause activation. Other groups have not reported the molecular sizes of the active fractions in their rLcrV preparations under nondenaturing conditions. Consequently, the presence of a similar stimulatory high-molecular-weight aggregate in their experiments cannot be excluded. The specific activity of our preparations is similar to that reported by others; if the activity that they observe is not due to aggregates, which we find to constitute a small fraction of total protein, then the activity of rLcrV in their preparations on a per-molecule basis must be correspondingly low. The clustering hypothesis suggests that rather than disrupting a specific TLR2-LcrV interaction, mutations which give rise to inactive rLcrV preparations may interfere with formation of aggregates capable of activating TLR2.
Overheim et al. (22) have presented data suggesting that LcrV deletion mutants failing to stimulate IL-10 production are more effective immunogens, presumably due to the elimination of immunosuppressive activity. Our results show that highly purified LcrV preparations containing only dimers and/or tetramers lack TLR2-stimulating activity and hence may also have improved performance in vaccine applications. However, we have previously shown that with DNA vaccines, production of LcrV multimers in vivo was critical to providing an effective protective response and also biased the response toward TH1 compared with the response produce by constructs producing only rLcrV monomers (30). While we do not know the extent of multimerization occurring in vivo, the association of multimerization with TLR2 stimulation suggests the possibility that large multimers form in vivo and provide adjuvant activity in the context of the live vaccine through stimulation of TLR2, rather than immunosuppression. This adjuvant effect is more consistent with current understanding of TLR2 function than is an immunosuppressive effect.
In summary, our investigation provides no support for the hypothesis that activation of TLR2 by LcrV contributes to the virulence of Y. pestis via immunomodulation. In a sensitive infection model using virulent Y. pestis, elimination of TLR2 has no effect on the course of disease and little on cytokine levels observed in vivo. The bulk of rLcrV protein has no TLR2-stimulating activity in vitro, and such activity is restricted to high-molecular-weight aggregates/multimers which contain LcrV but are of undetermined composition. Given the well-established sensitivity of the Y. pestis mouse infection model, its lack of response to TLR2 deficiency must be regarded as strong evidence that TLR2-induced immunomodulation does not have a significant role in plague. The early observations suggesting a direct immunosuppressive role for LcrV were based on direct injection of rLcrV preparations into mice, resulting in immunosuppression and elevated levels of IL-10 (19). A TLR2-independent mechanism of IL-10 induction would be consistent with these early observations.
Detailed infection experiments have also been conducted with Yersinia enterocolitica and Yersinia pseudotuberculosis by Victoria Auerbuch and Ralph Isberg (2a). They also observed no differences in the course or pattern of disease, or in cytokine levels, between TLR2-sufficient and -deficient mice. The conflicting results of Sing et al. imply that LcrV-TLR2-mediated immunosuppression may operate under certain limited circumstances (i.e., with specific combinations of Y. enterocolitica strains and mouse strains), but, given the present weight of evidence, it is unlikely to be a phenomenon of general importance to virulence in the yersiniae.
Acknowledgments
We thank Nancy Deitemeyer and Chrono Lee for general technical assistance and Neal Silverman and Eicke Latz for advice regarding purification of protein with minimal contamination by TLR-activating substances. We also thank Olaf Schneewind for use of his rLcrV construct and Stephen Baker for help with statistical analysis.
This work was supported by Project 2 of grant AI057159 to J.D.G. and by grant AI057588 to E.L. from the National Institutes of Health.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 2a.Auerbuch, V., and R. R. Isberg. 2007. Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect. Immun. 75:3561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis, G. R. 2002. The Yersinia Ysc-Yop virulence apparatus. Int. J. Med. Microbiol. 291:455-462. [DOI] [PubMed] [Google Scholar]
- 5.Deng, W., V. Burland, G. Plunkett, 3rd, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derewenda, U., A. Mateja, Y. Devedjiev, K. Routzahn, M. A. G. Evdokimov, Z. S. Derewenda, and D. S. Waugh. 2004. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure 12:301-306. [DOI] [PubMed] [Google Scholar]
- 7.Gehring, A. J., R. E. Rojas, D. H. Canaday, D. L. Lakey, C. V. Harding, and W. H. Boom. 2003. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect. Immun. 71:4487-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. [DOI] [PubMed] [Google Scholar]
- 9.Hill, J., S. E. Leary, K. F. Griffin, D. Williamson, and R. W. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 65:4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 11.Holmstrom, A., J. Olsson, P. Cherepanov, E. Maier, R. Nordfelth, J. Pettersson, R. Benz, H. Wolf-Watz, and A. Forsberg. 2001. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39:620-632. [DOI] [PubMed] [Google Scholar]
- 12.Kirschning, C. J., H. Wesche, T. Merrill Ayres, and M. Rothe. 1998. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latz, E., A. Visintin, E. Lien, K. A. Fitzgerald, B. G. Monks, E. A. Kurt-Jones, D. T. Golenbock, and T. Espevik. 2002. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277:47834-47843. [DOI] [PubMed] [Google Scholar]
- 14.Matson, J. S., and M. L. Nilles. 2001. LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J. Bacteriol. 183:5082-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7:1066-1073. [DOI] [PubMed] [Google Scholar]
- 16.Motin, V. L., R. Nakajima, G. B. Smirnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brande, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674-676. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nedialkov, Y. A., V. L. Motin, and R. R. Brubaker. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netea, M. G., R. Sutmuller, C. Hermann, C. A. A. Van der Graaf, J. W. M. Van der Meer, J. H. Van Kreiken, T. Hartung, G. Adema, and B. J. Kullberg. 2004. Toll-like receptor 2 suppressed immunity against Candida albicans through induction of IL-10 and regulatory T-cells. J. Immunol. 3712-3718. [DOI] [PubMed]
- 21.Netea, M. G., J. W. M. Van der Meer, and B. J. Kullberg. 2004. Toll-like receptors as an escape mechanism from the host defense. Trends Microbiol. 12:484-488. [DOI] [PubMed] [Google Scholar]
- 22.Overheim, K. A., W. DePaolo, K. DeBord, L. E. M. Morrin, D. M. Anderson, N. M. Green, R. R. Brubaker, B. Jabri, and O. Schneewind. 2005. LcrV plague vaccine with altered immunomodulary properties. Infect. Immun. 73:5152-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sing, A., D. Reithmeier-Rost, K. Granfors, J. Hill, A. Roggenkamp, and J. Heesemann. 2005. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. USA 102:16049-16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2001. Yersinia enterocolitica evasion of the host innate immune response by V-antigen-induced IL-10 production of macrophages is abrogated in IL-10 deficient mice. J. Immunol. 1315-1321. [DOI] [PubMed]
- 25.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sing, A., N. Tvardovskaia, D. Rost, C. J. Kirschning, H. Wagner, and J. Heesemann. 2003. Contribution of toll-like receptors 2 and 4 in an oral Yersinia enterocolitica mouse infection model. Int. J. Med. Microbiol. 293:341-348. [DOI] [PubMed] [Google Scholar]
- 27.Sodeinde, O. A., Y. V. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 258:1004-1007. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanio, T. H., T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 29.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 30.Wang, S., D. Heilman, F. Liu, T. Giehl, S. Joshi, X. Huang, T. H. Chou, J. Goguen, and S. Lu. 2004. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine 22:3348-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welkos, S. L., A. M. Friedlander, and K. J. Davis. 1997. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain C092. Microb. Pathog. 23:211-223. [DOI] [PubMed] [Google Scholar]
- 32.Williamson, E. D., H. C. Flick-Smith, C. Lebutt, C. A. Rowland, S. M. Jones, E. L. Waters, R. J. Gwyther, J. Miller, P. J. Packer, and M. Irving. 2005. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 73:3598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]