Abstract
Pneumocystis carinii is an opportunistic fungal pathogen that causes life-threatening pneumonia in immunocompromised individuals. Infants appear to be particularly susceptible to Pneumocystis pulmonary infections. We have previously demonstrated that there is approximately a 3-week delay in the clearance of Pneumocystis organisms from pup mouse lungs compared to that in adults. We have further shown that there is approximately a 1-week delay in alveolar macrophage activation in pups versus adult mice. Alveolar macrophages are the primary effector cells responsible for the killing and clearance of Pneumocystis, suggesting that pup alveolar macrophages may be involved in the delayed clearance of this organism. Alveolar macrophages cultured in vitro with Pneumocystis alone demonstrate little to no activation, as indicated by a lack of cytokine production. However, when cultured with lipopolysaccharide (LPS) or zymosan, cytokine production was markedly increased, suggesting that pup alveolar macrophages are specifically unresponsive to Pneumocystis organisms rather than being intrinsically unable to become activated. Furthermore, pup mice treated with aerosolized, heat-killed Escherichia coli in vivo were able to clear Pneumocystis more efficiently than were control mice. Together, these data suggest that while pup alveolar macrophages are unresponsive to P. carinii f. sp. muris organisms, they are capable of activation by heat-killed E. coli in vivo, as well as LPS and zymosan in vitro. The lack of response of pup mice to P. carinii f. sp. muris may reflect protective mechanisms specific to the developing pup lung, but ultimately it results in insufficient clearance of Pneumocystis organisms.
Pneumocystis carinii f. sp. hominis is an opportunistic fungal pathogen that is found to cause life-threatening pneumonia in immunocompromised individuals. More recently, Pneumocystis colonization and infections have been identified in immunocompetent individuals, particularly in young children (11, 34-36). Infants appear to be particularly susceptible to primary Pneumocystis infection, likely because of an immature immune system and a lung environment that protects against damaging inflammation early after birth. In a murine infection model, we have found that the clearance of P. carinii f. sp. muris (hereafter referred to as P. carinii) from the lungs of neonates is delayed compared to that in adults (9, 10, 22). Furthermore, this delayed clearance is correlated with a delay in the infiltration of the lungs of young mice by inflammatory cells (9, 10, 22). Pup T cells isolated from draining lymph nodes, however, were able to both produce gamma interferon (IFN-γ) and proliferate in vitro in response to anti-CD3 stimulation. These data suggest that the pup lung innate defenses are less efficient than in the adult lung in response to P. carinii (10, 22).
Alveolar macrophages (AMs) are the first known line of defense against inhaled substances and play a key role in maintaining lung homeostasis (6, 40). This is achieved primarily through the phagocytosis of foreign materials and the secretion of a wide range of cytokines and chemokines. For P. carinii infection, AMs are the key effector cells involved in the clearance of the organism (15). Neonates are known to be more susceptible to lung infections, including P. carinii. This may be due in part to a deficiency in the function of AMs. There are several functions that are carried out by AMs that may be deficient in neonates, including phagocytosis, antigen presentation, cytokine production, and chemokine production. The inability of pup AMs to clear P. carinii may be associated with one or a combination of all of these functions.
In the respiratory tract, AMs demonstrate two effective means of responding to pathogens. First, AMs may directly bind, phagocytose, and kill pathogens. Some studies have demonstrated that pup, compared to adult, AMs are deficient in phagocytic ability (16, 26), whereas other studies have reported that pup and adult AMs are equivalent in the ability to phagocytose microorganisms (5, 28). A likely explanation for these contradictory findings may be that pup AMs respond differently depending on the nature of the invading pathogen. Certain bacterial pathogens may trigger the phagocytic functions of pup AMs, whereas fungal pathogens like P. carinii are more efficient at evading an AM response. A second AM function is the secretion of a large range of mediators, including cytokines and chemokines, which act to recruit and activate inflammatory cells. These AM functions are influenced by the type of invading pathogen which may interact with different surface receptors. The β-glucan receptors commonly interact with cell walls of fungal pathogens, whereas Toll-like receptors (TLRs) interact with bacterial cell wall components. TLR4, for example, has been identified in binding gram-negative bacterial components; TLR2 is more commonly associated with binding gram-positive bacterial components. There has also been evidence to suggest that TLRs are important in the activation of AMs in the presence of fungal pathogens such as P. carinii (2, 23, 24). The goals of this study were to examine the function of pup AMs in response to P. carinii challenge and determine whether stimulation with exogenous agents can stimulate pup AMs and facilitate clearance of P. carinii. In our research, we found that treatment with exogenous heat-killed Escherichia coli (HKEC) stimulated the activation of resident AMs and promoted the clearance of P. carinii from pup lungs.
MATERIALS AND METHODS
Mice.
Five- to 6-week-old BALB/c or B6D2F1/J mice were purchased from the National Cancer Institute (Bethesda, MD) or Jackson Laboratory (Bar Harbor, ME) and bred and maintained at the Veterinary Medical Unit of the Veterans Administration Medical Center under specific-pathogen-free conditions. C.B-17 severe combined immune deficient (SCID) mice were used to maintain a source of P. carinii and were also bred at the Veterans Administration Medical Center Veterinary Medical Unit in microisolator cages containing sterilized food and water. Protocols for the use of mice were approved by the Veterinary Medical Unit Institutional Animal Care and Use Committee. Mice referred to as neonates are 2 to 3 days old, mice referred to as pups are ≥3 and ≤14 days old, neonates and pups are collectively referred to as infant mice, and mice referred to as adults are ≥8 weeks old.
Pneumocystis infection.
Lungs were excised from P. carinii-infected SCID mice and pushed through stainless steel mesh in Hanks' balanced salt solution (HBSS). P. carinii organisms were isolated and enumerated by microscopy as previously described (9). Neonatal (2- to 3-day-old) mice were lightly anesthetized with halothane and inoculated intranasally with 1 × 106 P. carinii organisms (approximately 5 ×105 P. carinii organisms/g body weight). In some experiments, mice were infected with 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO)-labeled P. carinii. Live P. carinii organisms were stained with DiO (Invitrogen Molecular Probes, Carlsbad, CA) in accordance with the manufacturer's instructions.
E. coli aerosolization.
Lyophilized cells of strain W (9637; American Type Culture Collection) E. coli were purchased from Sigma Chemical Co. One gram of E. coli cells was suspended in 100 ml of water and boiled for 45 min to remove the capsular antigens and expose the O antigens. The cells were cooled, spun at 3,500 × g for 15 min, and resuspended in 80 ml of water. This process was performed a total of three times. After the last spin, the cells were resuspended in 20 ml of sterile, deionized water. This E. coli solution was aliquoted into 1-ml cryotubes under sterile conditions and frozen at −80°C. To aerosolize the E. coli, a 1-ml aliquot of HKEC was thawed and added to 4 ml of sterile deionized water. This was placed in the reservoir of a T Updraft Nebulizer and aerosolized into a Plexiglas chamber (20 by 20 by 40 cm). P. carinii-infected pups were treated with aerosolized HKEC three times a week beginning at 48 h following P. carinii infection and ending at day 20 postinfection. Control animals were exposed to aerosolized sterile filtered water.
IFN-γ administration.
Murine IFN-γ (Peprotech, Rocky Hill, NJ) was administered intranasally to pups 24 h following P. carinii infection and readministered every 48 h for the remainder of the experiment. The doses of IFN-γ were escalated among different groups of mice as follows: phosphate-buffered saline (PBS) only, 16 ng/g, 80 ng/g, and 160 ng/g. PBS was used as the diluent for each dose of IFN-γ given to each group of mice on their respective dosing day.
Isolation of lung interstitial and alveolar cells.
Lung cells were prepared as previously described (22). Adult and pup lungs were lavaged with five washes (for final volumes of 5 and 1.5 to 3 ml, respectively) of cold HBSS-EDTA (3 mM). Right lung lobes were excised, minced, and enzyme treated at 37°C for 1 h in RPMI 1640 medium containing 3% fetal calf serum, 50 U/ml DNase (Sigma-Aldrich), and 1 mg/ml collagenase A (Sigma-Aldrich). Digested lung tissues were pushed through mesh screens to obtain a single-cell suspension. Red blood cells (RBCs) were lysed by treatment with a hypotonic buffer, and the cells were then resuspended in HBSS for enumeration and phenotypic analysis by flow cytometry.
Enumeration of Pneumocystis organisms.
Aliquots of digested lung tissue were diluted, spun onto glass slides, fixed in methanol, and stained with Diff-Quik (Dade International, Deerfield, IL). P. carinii nuclei were enumerated microscopically as previously described (9). The number of P. carinii organisms was expressed as log10 nuclei/right lung lobe. The limit of detection of P. carinii was log10 3.23 per lung.
Differential cell counts.
Aliquots of lung lavage fluid were diluted, spun onto glass slides, fixed in methanol, and stained with Diff-Quik. A minimum of 100 cells per slide were counted, and the percentages of macrophages, monocytes, lymphocytes, and (polymorphonuclear) neutrophils were determined.
Preparation of cells for flow cytometry analysis.
Cells isolated from lungs and lung lavage fluid (∼1 × 106 cells) were used for staining with fluorochrome-conjugated antibodies specific for murine CD4, CD8, CD44, CD62L, CD80, CD86, CD11c, CD11b, major histocompatibility complex (MHC) class II, TLR4 (BD Pharmingen), or F4/80 and TLR2 (eBioscience, San Diego, CA) or dectin-1 (Serotec, Oxford, United Kingdom). Multiparameter analysis was performed with a FACScalibur cytofluorimeter (BD Biosciences, San Jose, CA). Greater than 50,000 events were routinely acquired.
AM isolation, culture, and stimulation.
Mouse pup (between 10 and 13 days old) and adult lungs were lavaged with HBSS plus 0.3 mM EDTA under sterile conditions. All of the lavage fluid cells from the pups were pooled, as were the lavage fluids from the adults, and each was spun at 1,200 rpm for 10 min. The RBCs were lysed with a hypotonic salt solution, and cells were washed twice with HBSS under sterile conditions. We routinely obtained greater than 95% macrophages, as assessed by morphological examination after staining an aliquot with Diff-Quik. The cells were then resuspended in sterile medium containing RPMI medium plus 5% heat-inactivated fetal calf serum (Atlanta Biologicals, Norcross, GA), penicillin-streptomycin (1%), 2-mercaptoethanol (0.05%), and gentamicin (0.1%) (Gibco [Invitrogen Corp.], Carlsbad, CA). The cells were adjusted to 1 × 106 to 2 × 106 cells/ml of medium, pipetted into sterile 96-well tissue culture plates in 100-μl aliquots, and allowed to rest overnight at 37°C in 5% CO2. After the macrophages had adhered to the tissue culture plates, fresh medium was added and the cells were treated with lipopolysaccharide (LPS; 100 ng/ml; Sigma, St. Louis, MO), P. carinii (1:50), zymosan A (250 μg/ml; Sigma, St. Louis, MO), combinations thereof, or medium alone and cultured for 48 to 72 h. The medium was removed and frozen at −80°C for later cytometric bead array (CBA; BD Pharmingen) analysis according to the manufacturer's protocol.
Phagocytosis assay.
For phagocytosis experiments, P. carinii organisms were labeled with DiO at a ratio of 5 × 106 P. carinii cells to 5 μl DiO in 1 ml PBS for 30 min at 37°C prior to inoculating the mice. Seven-day-old BALB/c or SCID mice were treated with aerosolized HKEC on days 4 and 2 prior to infection and once more on the day of infection with DiO-labeled P. carinii. On day 1 postinfection, the mice were lavaged. Cells were processed for flow cytometry as previously described and stained with fluorochrome-conjugated antibodies specific for CD11c and CD11b. Cells that were large in size and granular on the basis of forward and side scatter and were also positive for CD11c were considered to be lung macrophages. Cells that were also DiO positive were assumed to have phagocytosed at least one P. carinii organism. This assay does not account for P. carinii that was bound to the outside of the cell versus P. carinii that was ingested.
Analysis of cytokine levels in BALF by CBA.
The quantitation of multiple cytokines in each sample of bronchial alveolar lavage fluid (BALF) or culture supernatant was performed by using CBA kits purchased from Pharmingen. An inflammation CBA kit was used to quantitate murine interleukin-6 (IL-6), IL-10, monocyte chemoattractant protein 1 (MCP-1), IFN-γ, tumor necrosis factor alpha (TNF-α), and IL-12p70. Assays were performed according to the manufacturer's instructions.
Statistical analysis.
Data are expressed as the means ± standard deviations (SD) for three to five mice per group. Differences between experimental groups were determined by using Student's t test or analysis of variance, followed by the Student-Newman-Keuls post-hoc test where appropriate. Differences were considered statistically significant when P was <0.05. SigmaStat statistical software (SPSS, Inc., Chicago, IL) was used for all analyses.
RESULTS
IFN-γ administration to neonates did not improve Pneumocystis clearance.
We have previously shown that infant mice have a significant delay in mounting an immune response to P. carinii compared to that of adult mice (9, 10). Delayed clearance is accompanied by a delay in the infiltration of T cells and activation of AMs, as well as delayed cytokine upregulation, including that of IFN-γ (9, 10, 22). It has been shown, moreover, that the delivery of IFN-γ to the lungs of P. carinii-infected adult SCID mice can stimulate clearance of the organism (14, 31). We therefore hypothesized that infected pup mice would clear the infection faster when treated with exogenous IFN-γ.
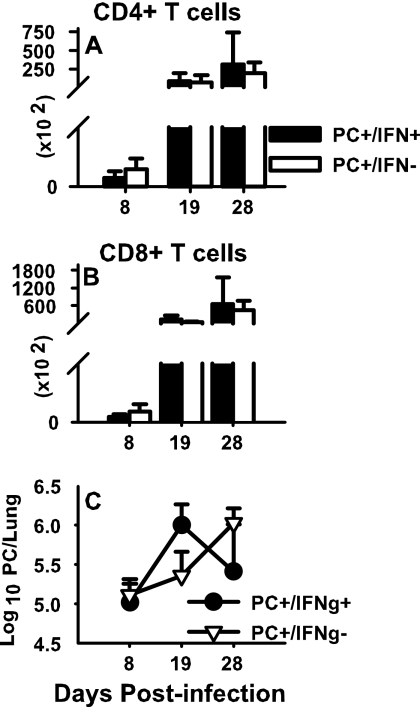
Murine IFN-γ (16 ng/g) or vehicle (PBS) was administered intranasally to P. carinii-infected pup mice every 48 h beginning at 24 h postinfection and continuing throughout the course of the experiment. Mice from each group were killed at days 8, 19, and 28 postinfection. Whole lungs and lung lavage fluid were collected, and flow cytometry was performed to examine the infiltration of lymphocytes in response to P. carinii infection. Exogenous IFN-γ had no effect on the infiltration of the lungs of P. carinii-infected neonates by either CD4 T cells (Fig. 1A) or CD8 T cells (Fig. 1B). Similarly, no statistically significant differences were found in the lung burden of P. carinii organisms between the two groups by day 28 (Fig. 1C). However, there appeared to be a shift in clearance kinetics between the two groups. The IFN-γ-treated group actually had a greater P. carinii burden than the control group at day 19 but by day 28 the burden diverged in opposite directions between the two groups, with the IFN-γ-treated group having fewer P. carinii organisms than the control group. Ultimately, no statistically significant difference in P. carinii clearance was observed between the two groups; furthermore, no mice from either group were able to clear the P. carinii completely by day 28 postinfection.
FIG. 1.
Effects of exogenous IFN-γ on neonatal lung defenses against P. carinii. Mice were infected with P. carinii (PC) as neonates (24 to 72 h after birth) and then treated with either IFN-γ or PBS intranasally every 48 h beginning at 24 h postinfection. Lungs were lavaged and cells were collected at the indicated times postinfection. Cells from BALF were stained with antibodies specific for CD4 or CD8 and then analyzed by flow cytometry. Total CD4 (A) and CD8 (B) T-cell numbers were determined. (C) Whole lungs were digested, and aliquots were stained with Diff-Quik stain; Pneumocystis nuclei were enumerated microscopically. Results represent the mean ± SD of four or five mice per group and are representative of three separate experiments. No statistically significant differences were found between groups.
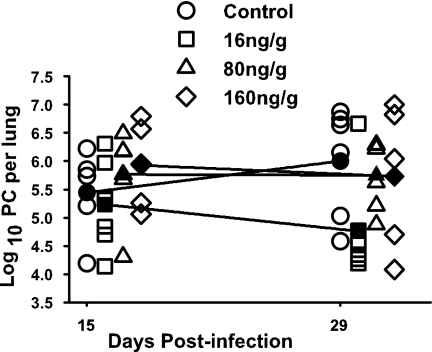
To determine if the IFN-γ dosing strategy had an impact on initiating the immune response and P. carinii clearance, this experiment was repeated with a dose escalation. P. carinii-infected pup mice were divided into four groups. Three groups received IFN-γ intranasally at 16, 80, or 160 ng/g. The fourth group served as a control and received PBS only. Mice from each group were killed at days 15 and 29 postinfection. Despite increased doses of IFN-γ, no differences in either T-cell or macrophage infiltration of pup P. carinii-infected lungs were observed, compared to the control group (data not shown). Additionally, cytokine production in the BALF of each group was determined by CBA analysis and no statistically significant differences were observed between any of the groups receiving IFN-γ and the group receiving PBS, suggesting that the resident AMs were not stimulated by the presence of P. carinii plus IFN-γ (data not shown). Lastly, P. carinii clearance was assessed microscopically by counting the P. carinii nuclei in each group treated with IFN-γ, as well as the control group (Fig. 2). While the group receiving 16 ng/g had more mice clearing the organism than mice in the control group, the results were highly variable and, as with the previous IFN-γ experiment, no significant differences were detected. Furthermore, increased doses of IFN-γ did not improve P. carinii clearance. These data indicate that none of the intranasally administered doses of IFN-γ tested significantly improved the clearance of P. carinii in pup mice. Furthermore, no dose of IFN-γ tested was able to stimulate the infiltration or activation of immune cells in the infected lung or increase the production of cytokines in P. carinii-infected pup lungs.
FIG. 2.
Low levels of exogenous IFN-γ reduce P. carinii lung burdens in pups. Mice were infected with P. carinii (PC) as neonates (24 to 72 h after birth) and then treated with 16, 80, or 160 ng/g IFN-γ or PBS intranasally every 48 h beginning at 24 h postinfection. Whole lungs were collected and digested at days 15 and 29 postinfection. P. carinii nuclei were enumerated microscopically. Filled symbols represent the means ± SD for five or six mice per group.
LPS increases cytokine production from pup AMs in vitro.
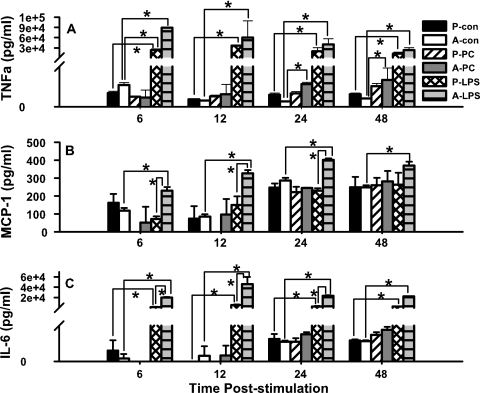
We have previously reported that AM activation is delayed in pups, compared to adult mice, in response to P. carinii infection. This finding was based on a delay in the upregulation of activation markers, including CD11b, MHC class II, CD40, and CD80, on nonlymphocytes in the alveolar spaces of P. carinii-infected neonates through 2 weeks postinfection. In contrast, these markers were upregulated on alveolar cells of P. carinii-infected adult mice by day 6 postinfection (6). To determine if pup AMs could be stimulated exogenously to improve their cytokine response, an in vitro model was designed comparing pup AM cytokine production following either P. carinii or LPS stimulation. AMs from uninfected pups and adult mice were isolated and cultured with P. carinii alone, LPS alone, or medium alone. The activation of AMs was assessed by quantifying their cytokine responses at 6, 12, 24, and 48 h poststimulation. At all time points, pup AMs treated with LPS produced significantly more TNF-α than did the corresponding cells stimulated with P. carinii (Fig. 3A). These data suggest that pup AMs are capable of a robust cytokine response when given an adequate stimulus such as LPS. Interestingly, the level of cytokine production from pup AMs following stimulation with LPS reached those produced by adult cells by 12 h poststimulation. In addition, we consistently see that adult AMs tend to produce more TNF-α than do pup AMs in response to P. carinii; however, the levels are always considerably lower than when using LPS as a stimulus. This is consistent with our previously published in vivo observations that show that adult AMs become activated sooner after P. carinii infection than do pup AMs (6).
FIG. 3.
Pup and adult AMs produce significant amounts of TNF-α upon stimulation with LPS. Macrophages from 2-week-old and adult mice were isolated from BALF, placed in 96-well tissue culture plates, and allowed to rest for 24 h. Subsequently, the cells were treated with 100 ng/ml LPS, 4 × 105 P. carinii (PC) organisms, or medium alone for 6, 12, 24, or 48 h. Supernatant concentrations of LPS- and P. carinii-stimulated cells were determined by CBA. P, pups; A, adults; con, unstimulated control. Data represent the means ± SD for three wells per group. *, P < 0.05 compared to control cells at the same time point. Comparisons are representative of two different experiments.
To reveal potential differences in the production of other cytokines in pup AMs stimulated with LPS or P. carinii, MCP-1, IL-6, IL-12p70, and IL-10 were also analyzed. Unlike the pattern observed with TNF-α, pup AMs did not produce more MCP-1 following LPS stimulation than following P. carinii stimulation (Fig. 3B). While pup AMs fail to respond to MCP-1, adult cells maintain a rather robust MCP-1 response to LPS, suggesting that pup AMs specifically fail to produce MCP-1 following LPS stimulation in vitro.
The production of IL-6 closely resembled the pattern observed with TNF-α in that LPS stimulated significant IL-6 production in pup AMs compared to the respective P. carinii-stimulated cells (Fig. 3C). At 6 and 12 h poststimulation with P. carinii, the production of IL-6 was so minimal among the pup AMs that it fell below the limit of detection. Thus, LPS is a much stronger stimulus for IL-6 production among pup AMs than is P. carinii, reinforcing the observation that pup AMs are capable of cytokine production, given the appropriate stimulus.
The production of IL-12p70 was found to be no different in LPS-treated cells than that in P. carinii-treated pup and adult AMs (data not shown). Likewise, no differences in the production of IL-10, an antiinflammatory cytokine, occurred following stimulation with LPS or P. carinii in pup or adult AMs. Rather, IL-10 production was constitutively elevated among all AMs, including those that received LPS and P. carinii, as well as the unstimulated control cells (data not shown). These data show that LPS, but not P. carinii, stimulates TNF-α and IL-6 in vitro in pup AMs.
To evaluate the possibility that neonatal AMs have a delayed cytokine response compared to that of adults, this experiment was repeated with a 72-h stimulation period. The cytokine response at 72 h, however, was similar to that seen at the earlier time points, with adult cells producing more TNF-α, IL-6, and MCP-1 than pup AMs. The one notable difference observed after 72 h of stimulation, compared to the shorter simulation periods, was that IL-10 production was greater in pup AMs than in adult AMs when the cells were LPS treated (P < 0.05; data not shown). This observation suggests that with continuous stimulation, pup AMs respond by producing the antiinflammatory cytokine IL-10.
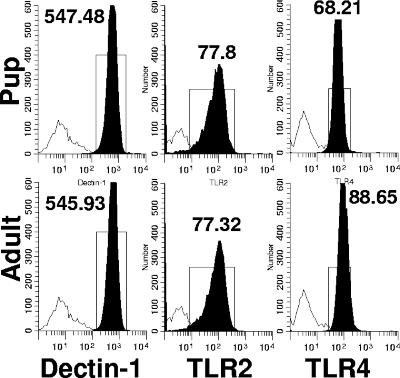
The in vitro and in vivo data described above demonstrate that pup macrophages are capable of a robust cytokine response following stimulation with LPS compared to P. carinii. LPS can even produce pup AM cytokine levels similar to those of adult macrophages treated with LPS, suggesting that pup AMs do not lack the functional capacity to produce strong proinflammatory cytokines such as TNF-α and IL-6. These findings begged the question of whether or not differences exist in the expression level of the following three important receptors: dectin-1, a beta-glucan receptor shown to bind beta-glucans found in fungal cell walls and cyst forms of P. carinii (29, 39); TLR4, a receptor for LPS binding protein found on LPS (27, 39); and TLR2, a receptor for ligands such as zymosan, peptidoglycan, and lipoprotein and more recently thought to be a receptor for the major surface glycoprotein found on P. carinii (17, 30, 33, 37, 39). To answer this question, the levels of dectin-1, TLR2, and TLR4 expression on pup and adult AMs were determined. As illustrated in Fig. 4, the expression of dectin-1 and TLR2 was the same on pup and adult AMs isolated from uninfected mice (Fig. 4A to D). These data suggest that the difference in production of TNF-α between P. carinii-stimulated pup and adult macrophages is not due to differences in specific pattern recognition receptor expression. Small differences were detected in TLR4 expression between pup and adult AMs (Fig. 4E to F), which may account for the reduced cytokine production observed in pup versus adult AMs stimulated with LPS. However, the TLR4 receptors present were clearly functional since LPS induced significant TNF-α and IL-6 in AMs from pups, albeit at lower levels than in those from adults.
FIG. 4.
Adult AMs express significantly higher baseline TLR4 levels than do pup AMs (P < 0.05). AMs were isolated from uninfected 14-day-old pup and adult (≥8 weeks old) BALF. Cells were stained with antibodies specific for CD11c, dectin-1, TLR2, and TLR4 and analyzed by flow cytometry. Representative histograms of dectin-1-, TLR2-, and TLR4-positive cells (gated on CD11c+ cells) are shown. Cells were gated for large nonlymphoid cells by using forward and side light scatter. Data are representative of five mice per group and two separate experiments.
As described above, both pup and adult AMs were shown to be relatively less responsive to P. carinii than to LPS. However, as shown in Fig. 3A, adult macrophages stimulated with P. carinii produced TNF-α above the baseline whereas pup cells did not. We next asked the question of whether pup AMs have a global unresponsiveness to other fungal ligands. To investigate this question, an in vitro experiment was performed comparing the responses of pup and adult AMs to the Saccharomyces cerevisiae cell wall polysaccharide zymosan. This yeast cell wall preparation is known to express substrates for several pattern recognition receptors present on AMs, including beta-glucan and mannose receptors, as well as TLR2 and TLR6 ligands (3, 8).
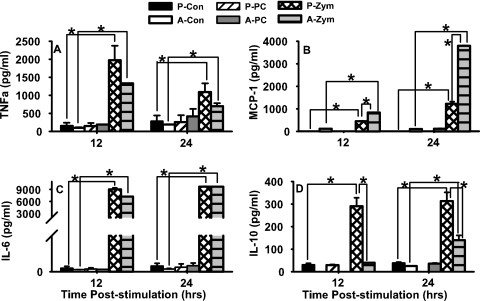
After 12 and 24 h of stimulation with zymosan, pup AMs produced significantly more TNF-α than did the respective P. carinii-stimulated and control cells (Fig. 5A), suggesting that pup AMs, like adult AMs, have functional beta-glucan receptors and TLR2. Interestingly, pup AMs stimulated with zymosan also produced significantly more TNF-α than their adult counterparts after 12 h of stimulation. Consistent with the results shown in Fig. 3, stimulation of the AMs with P. carinii failed to induce the production of TNF-α in pup AMs after 12 and 24 h of stimulation. Although both P. carinii cysts and zymosan are known to contain beta-1,3-glucans (13, 37), only zymosan stimulates an increase in pup AM cytokine production, suggesting that other zymosan ligands may be binding and activating pup AMs to produce cytokines.
FIG. 5.
Zymosan stimulated significantly more AM cytokine production than did P. carinii. AMs were isolated from the pooled BALF of adult (A) (≥8 weeks) and 12- to 14-day-old pup (P) mice and cultured in a 96-well plate with P. carinii (PC), zymosan (Zym), or medium alone (Con). Supernatants were removed at 12 and 24 h poststimulation, and TNF (A), MCP-1 (B), IL-6 (C), and IL-10 (D) were analyzed by CBA analysis and flow cytometry. Data represent three or four wells per group. *, Cytokine production was significantly greater than that of the respective controls; P < 0.05.
Production of the other proinflammatory cytokines tested, IL-6 and MCP-1, was significantly increased in both pup and adult AMs secondary to stimulation with zymosan (Fig. 5B and C). The levels of IL-6 and MCP-1 following P. carinii stimulation in pup AMs were minimal or undetectable compared to those measured after zymosan stimulation, further suggesting that AMs are specifically less responsive to P. carinii organisms. Interestingly, IL-10, an inhibitory cytokine, was significantly increased in pup AMs, compared to adult AMs, following 12 and 24 h of stimulation with zymosan, but not after stimulation with P. carinii (Fig. 5D). These data indicate that pup AMs respond differently than adult AMs to fungal antigens; however, pup AMs are capable of producing significant amounts of proinflammatory cytokines when stimulated appropriately. Overall, P. carinii is a significantly weaker stimulus than either zymosan or LPS. This may be because we used the same mixture of P. carinii life forms for stimulation as are found in the lungs, only about 10% of which are cysts that have β-glucans in their cell walls.
Aerosolized HKEC improves Pneumocystis clearance in pup mice.
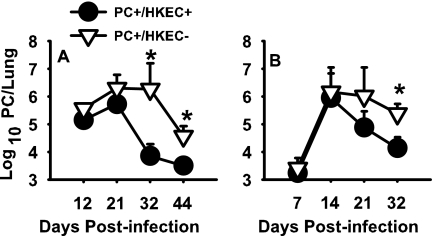
The resolution of P. carinii in immunocompetent adult mice is associated with upregulation of the proinflammatory cytokines IFN-γ and TNF-α (10, 22). Since our in vitro studies demonstrated that LPS, but not P. carinii, stimulates pup AMs to produce significant levels of TNF-α, we wanted to evaluate whether the TNF-α produced secondary to LPS administration in vivo would improve P. carinii clearance in pup mice. Reports in the literature further support the hypothesis that exogenously increasing the level of TNF-α can improve P. carinii clearance (19). Harmsen and Chen have reported that treatment of adult thymectomized and CD4-depleted mice with aerosolized HKEC also expedites the clearance of P. carinii (12). They further showed that pretreatment of the animals with anti-TNF immunoglobulin G minimizes the benefit imparted by the aerosolized HKEC, suggesting that stimulation of TNF-α plays a significant role in the clearance of P. carinii from immunocompromised mice (12). On the basis of this information, we administered aerosolized HKEC or distilled, ionized water to P. carinii-infected pup mice. By day 12 postinfection, a divergence between HKEC-treated and control mice could already be identified (Fig. 6). By day 21, there was a sharp decline in the P. carinii burden in the HKEC-treated group, and by day 32 postinfection, there was a significant difference in the P. carinii burden in the HKEC-treated group compared to the control group. These data suggest that pup mice, as was found with CD4-depleted adult mice (12), can clear a P. carinii infection more efficiently when treated with aerosolized HKEC.
FIG. 6.
Mice treated with HKEC demonstrated a faster rate of P. carinii (PC) clearance than control mice. Mice were infected as neonates (24 to 72 h after birth) and treated with aerosolized HKEC or sterile water three times per week. Lungs were collected at days 12, 21, 32, and 44 postinfection (A) or days 7, 14, 21, and 32 postinfection (B); digested; and spun onto glass slides. Slides were Diff-Quik stained and enumerated microscopically. Data are representative of results of four or five mice per group and three separate experiments. *, P < 0.05.
Aerosolized HKEC influences the infiltration of immune cells in pup mice.
We were able to induce more efficient clearance of P. carinii organisms from the lungs of infected pup mice by the administration of aerosolized HKEC. In order to determine if this improved P. carinii clearance was associated with an increase in cellular lung infiltration, BALFs were collected from mice treated with aerosolized HKEC and control mice treated with aerosolized sterile water. Cell differential counts were performed to determine macrophage, lymphocyte, and neutrophil infiltration of the lungs of both the HKEC-treated mice and control mice. Overall, minimal differences were noted, but most importantly, a divergence between the treated and untreated groups was observed among macrophages infiltrating the lung by day 32 postinfection (data not shown). This correlates with the significant decrease in the P. carinii burden observed in HKEC-treated mice by day 32 postinfection (Fig. 6). Neutrophil numbers appeared to follow HKEC treatment rather than P. carinii infection, as the neutrophil numbers rose early on and declined upon discontinuation of the HKEC treatment by day 21 (data not shown).
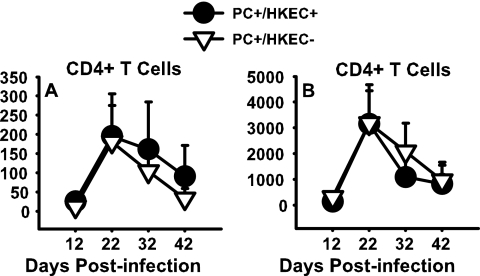
For both the HKEC-treated and untreated P. carinii-infected mice, the infiltration of the lung space by lymphocytes, as determined by cell differential counts, sharply increased on day 21 postinfection and rapidly declined by day 32 postinfection (data not shown). The evaluation of lymphocytes, namely, CD4+ T cells, by flow cytometry showed similar results, with a rise in CD4+ T cells by day 21 postinfection and a subsequent drop thereafter (Fig. 7A). By comparing the timing of CD4+ T-cell infiltration of the lungs and P. carinii clearance, one can see that the increase in CD4+ T cells in the alveolar spaces, as well as the lung tissue, immediately precedes the subsequent decline in the P. carinii burden in the HKEC-treated mice (Fig. 6 and 7A and B). The infiltration by CD8+ T cells followed the same pattern as that by CD4+ T cells (data not shown).
FIG. 7.
Treatment with HKEC did not affect the infiltration of the lungs of P. carinii (PC)-infected pups by CD4+ T cells. Mice were infected with P. carinii as neonates (24 to 72 h after birth) and then treated with either aerosolized HKEC or sterile water. BALF and whole lungs were collected on days 12, 21, 32, and 44 postinfection. No statistically significant difference in CD4+ T cells in the BALF (A) or lung (B) digest was observed between the groups treated with HKEC and the control group. Data represent the means ± SD for five mice per group and are representative of three separate experiments.
Aerosolized HKEC influences the activation of pup AMs.
The activation of pup AMs in response to P. carinii infection is poorly understood. The cell surface markers typically used to define an activated AM in adult mice are not straightforward. We have found that all AMs express CD11c and that CD11b may be a useful marker of activation or infiltration from the periphery on adult and pup macrophages infected with P. carinii (6). We used flow cytometry to assess the activation status of P. carinii-infected pup AMs treated with aerosolized HKEC compared to those treated with aerosolized vehicle. Several different combinations of surface markers known to be signs of macrophage activation in adults were used. For all experiments involving flow cytometry, the cells were gated on nonlymphocytes by using forward and side scatter.
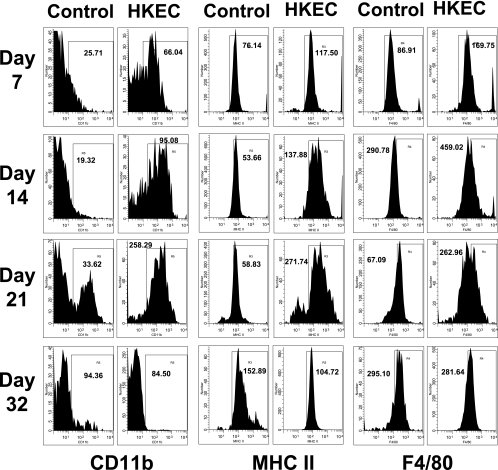
Figure 8 shows the expression of the AM activation markers CD11b, MHC class II, and F4/80 at four time points after P. carinii infection. The P. carinii-infected group receiving HKEC showed an increase in activated AMs, as indicated by a rightward shift in the CD11b cell populations, compared to the infected group receiving aerosolized water only (Fig. 8). This rightward shift could be seen in the HKEC group as early as day 7 postinfection and persisted through day 21 postinfection. These data correlated well with the decreased P. carinii lung burden beginning at day 21 postinfection, suggesting that the activation of pup AMs by HKEC contributed to the expedited P. carinii clearance seen (Fig. 6B). The group that was P. carinii infected but received aerosolized water only did not start to increase in CD11b expression until day 21 postinfection, and both groups showed a return to baseline expression levels by day 32 postinfection. Similarly, the group receiving HKEC had increased expression of both MHC class II and F4/80 molecules compared to untreated mice by day 7 postinfection. The difference in expression was most notable at days 14 and 21 postinfection and correlated with P. carinii clearance (Fig. 6B). Overall, these data suggest that the treatment of P. carinii-infected mice with HKEC increases the activation of AMs in pup lungs, likely leading to T-cell activation, thereby contributing to the reduced P. carinii lung burden.
FIG. 8.
Expression levels of CD11b, MHC class II, and F4/80 on macrophages from alveolar spaces of P. carinii-infected, HKEC-treated neonatal mice were increased at days 14 and 21 postinfection. Mice were infected with P. carinii as neonates (24 to 72 h after birth) and then treated with either aerosolized HKEC or sterile, deionized water. BALF was collected at days 7, 14, 21, and 32 postinfection. Cells were stained with antibodies specific for CD11c and CD11b, MHC class II, or F4/80, and phenotypes were examined by flow cytometry. Representative histograms of CD11b-, MHC class II-, or F4/80-positive cells and the mean fluorescence intensity (MFI) for each (gated on CD11c+ cells) are shown. Cells were gated for large nonlymphoid cells by using forward and side light scatter. Data are representative of four or five mice per group and two separate experiments.
AM phagocytosis is less efficient in the absence of lymphocytes.
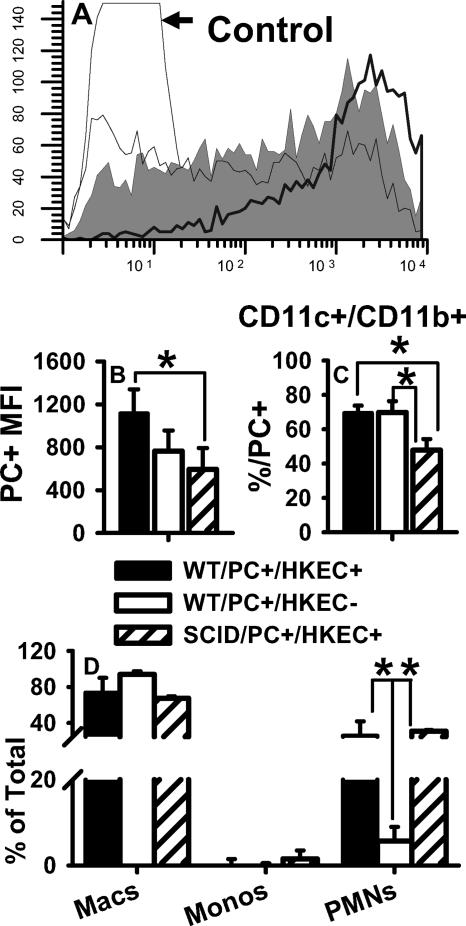
To determine if T lymphocytes are playing a role in the increased AM activation and P. carinii clearance observed in HKEC-treated pups, HKEC-treated SCID and WT pups were compared for the ability to phagocytose DiO-labeled P. carinii organisms. The 6- to 7-day-old BALB/c or SCID mice were treated with aerosolized HKEC (as previously described) on days 4 and 2 prior to infection and once more on the day of P. carinii infection (DiO labeled). On day 1 postinfection, the mice were lavaged. Some cells were reserved for differential counts, and the rest were processed for analysis via flow cytometry to assess the association of P. carinii with AMs. The total number of AMs associated with fluorescent P. carinii organisms was the same in SCID and WT pups (data not shown). However, treatment with HKEC drove more efficient phagocytosis of P. carinii in AMs from treated than from untreated WT pups, as shown by the increased mean fluorescence intensity of DiO (Fig. 9B). AMs from WT pups were significantly more efficient at phagocytosing P. carinii than were SCID pups, despite the HKEC treatment of both groups (Fig. 9A and B). The overall MHC class II expression was similar between the WT and SCID pups, suggesting that the difference in phagocytosis was not likely due to less activation in the SCID pups (data not shown). Although neutrophils are not thought to be important in P. carinii clearance (32), we show that neutrophil infiltration in SCID pups was equal to infiltration in WT pups but they were less capable of phagocytosing P. carinii organisms than were WT pups, despite treatment with HKEC (Fig. 9C and D). These data suggest that in the absence of T lymphocytes, HKEC-treated pup AMs are still capable of phagocytosing P. carinii but are less efficient.
FIG. 9.
SCID pups treated with HKEC did not phagocytose DiO-labeled P. carinii as efficiently as did WT pups. SCID and WT pups were treated with aerosolized HKEC on days 4 and 2 previous to DiO-labeled P. carinii (PC) infection and on the day of infection. BALF was collected on day 1 postinfection. Cells were stained with antibodies specific for CD11c, CD11b, and MHC class II and examined for coexpression with DiO-labeled P. carinii. (A) Representative histograms showing DiO fluorescence of CD11c+ cells from HKEC-treated WT pups (thick black line), HKEC-treated SCID pups (medium line), and untreated WT pups (gray fill). (B) Mean fluorescence intensity (MFI) of DiO in CD11c+ cells. *, P < 0.05 compared to untreated WT and HKEC-treated SCID pups. (C) Percentage of CD11c+ CD11b+ cells positive for DiO; *, P < 0.05 compared to HKEC-treated SCID pups. (D) Differential counts of BALF cells; *, P < 0.05 compared to untreated WT pups. Data represent the means ± SD of four mice per group. Macs, macrophages; Monos, monocytes; PMNs, polymorphonuclear neutrophils.
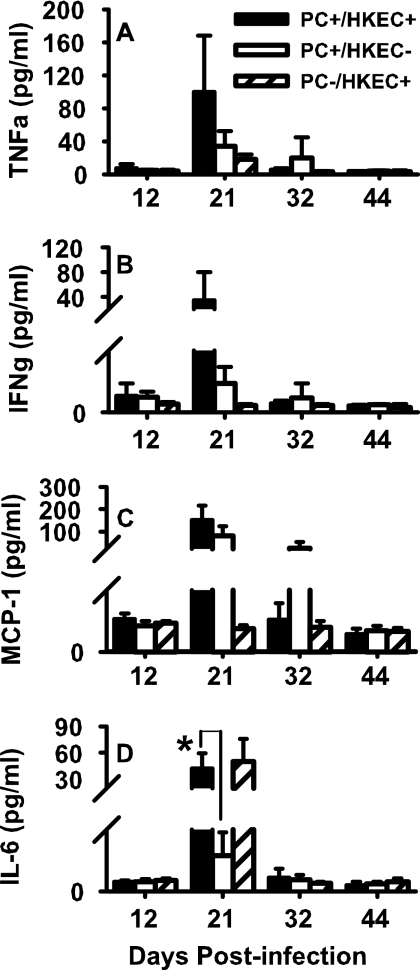
Cytokine production was increased secondary to aerosolized HKEC.
We have previously reported that cytokines such as TNF-α and IFN-γ appear in the lungs of P. carinii-infected pup mice much later than in the lungs of infected adult mice (10). We next examined cytokine production in vivo following treatment of P. carinii-infected mice with aerosolized HKEC by CBA analysis. Similar to the results observed in vitro with LPS, neither IL-12p70 nor IL-10 levels were elevated in the HKEC group compared to the control group at any time point following infection with P. carinii (data not shown). While no statistically significant differences were found between groups in the concentrations of TNF-α, IFN-γ, or IL-6 found in the BALFs, the group treated with HKEC tended to have greater levels of cytokines than the untreated group (Fig. 10A to C). Additionally, AMs from the HKEC-treated group did produce significantly more MCP-1 than those from the uninfected group (Fig. 10C). The production of IL-6 throughout this experiment was interesting in that it appeared to follow the HKEC treatment rather than the P. carinii treatment (Fig. 10D). At day 21 postinfection, only those groups receiving HKEC had increased production of IL-6, regardless of P. carinii infection. Furthermore, the production of IL-6 was minimal following the day 21 time point, which can likely be explained by the fact that the final HKEC treatment occurred on day 20 postinfection. Overall, these cytokine data reiterate the findings that AM-produced cytokines, particularly TNF-α, are involved in the expedited clearance of P. carinii observed in the HKEC-treated groups. Although IFN-γ is not produced by AMs in significant quantities, AMs are known to stimulate IFN-γ-producing cells such as T lymphocytes, which would explain the increase seen herein (Fig. 10B).
FIG. 10.
P. carinii (PC)-infected mice treated with HKEC had elevated cytokine levels by day 21 postinfection. Mice were infected as neonates (24 to 72 h after birth) and then treated with either aerosolized HKEC or sterile, deionized water. BALF was collected on days 12, 21, 32, and 44 postinfection. Production of TNF-α (A), IFN-γ (B), MCP-1 (C), and IL-6 (D) was determined by CBA. There were no significant differences between the group receiving HKEC and the group receiving only sterile water. Data represent the means ± SD of five mice per group. *, P < 0.05.
DISCUSSION
We have demonstrated that IFN-γ alone is not sufficient to expedite clearance of P. carinii organisms in pup mice. We also showed that pup AMs are capable of responding to exogenous stimuli, specifically, LPS and zymosan. Furthermore, we demonstrated that pup mice treated with aerosolized HKEC were able to clear P. carinii infection faster than untreated mice. The goal of this research was to determine if stimulation of pup AMs with exogenous stimuli would improve the activation of these cells and expedite the clearance of P. carinii. In the present studies, we chose IFN-γ and HKEC because both IFN-γ and TNF-α have been shown to be important cytokines in clearing P. carinii in adult mice (4, 25). Since direct administration of TNF-α can cause detrimental hyperimmune stimulation, we chose to use LPS in vitro and HKEC in vivo, which are known to stimulate the production of TNF-α from macrophages. Additionally, we found supportive literature which demonstrated the effective use of aerosolized HKEC in CD4-depleted mice in clearing P. carinii organisms more efficiently (12).
Intranasal administration of IFN-γ did not activate pup AMs, as determined by both markers of activation and cytokine responses. While low-dose IFN-γ (16 ng/g) did improve P. carinii clearance compared to that in the control, this was not accompanied by any changes in AM activation. This reduction in the P. carinii burden is similar to the findings by Beck et al., who reported that aerosol IFN-γ administration resulted in a reduced intensity of P. carinii organisms in adult CD4+ lymphocyte-depleted mice (1). The difference between adult and pup AMs in their response to IFN-γ has not been elucidated but may be partly due to a difference in AM characteristics. In our laboratory, we have shown that in mice infected with P. carinii, adult AMs have a significant increase in the expression of activation markers earlier after infection than pup AMs (6). This apparent delay in pup AM activation may be attributed to a protective mechanism intrinsic to lung macrophages designed to minimize the inflammatory response in the developing pup lung. We saw some evidence of this in the large IL-10 response to zymosan by pup AMs. Alternatively, the lung environment may contribute to macrophage unresponsiveness. Fernandez has shown that lung epithelial cells constitutively express IL-10, and we have reported that transforming growth factor β mRNA is elevated in neonatal mouse lungs (7, 10). The data generated herein suggest that IFN-γ alone is incapable of stimulating pup AMs in regard to cytokine production and the expression of activation markers in vivo. While the administration of IFN-γ did improve P. carinii clearance to a small degree, it did not significantly affect AM stimulation; thus, we considered other ways to stimulate AM function in our pup mouse model.
Previously published data from our laboratory showed that locally administered TNF-α induces the infiltration of pup lungs by T cells, as well as upregulates cytokines and adhesion molecules; however, there was only a trend toward increased P. carinii clearance, with no statistically significant differences (21). We hypothesized that stronger stimulation of pup AMs would facilitate greater cytokine production and clearance of P. carinii. To validate this hypothesis in pup mice, we first performed some in vitro experiments comparing pup AMs treated with LPS to those treated with P. carinii. LPS is a bacterial cell wall component known to stimulate the production of TNF-α through TLR4. The addition of LPS to the cultured pup AMs induced significant cytokine production, as indicated by increases in MCP-1, TNF-α, and IL-6. Pup cell TNF-α and IL-6 production secondary to LPS mimicked that of adult cells over time, with these cytokines increasing rapidly and maintaining high levels through 48 h. TNF-α is a potent proinflammatory cytokine produced by AMs, and AMs can subsequently become activated by TNF-α in an autocrine fashion. Activation of AMs is important in the clearance of P. carinii, making TNF-α a crucial cytokine in the host response to this organism (18). We have demonstrated that pup AMs, unlike adult AMs, are specifically incapable of producing TNF-α in response to P. carinii (Fig. 3A), thereby suggesting one possible mechanism behind the delayed clearance of P. carinii from pup versus adult lungs.
Importantly, the cytokine levels induced by P. carinii alone in adult cells were still significantly lower than those produced by cells treated with LPS. Overall, this suggests that P. carinii organisms provide an inefficient antigenic stimulus compared to LPS and, together with reduced responsiveness in pup AMs, may contribute to the neonates' inability to clear the organism as efficiently as adults. Our data suggest that while pup AMs are less responsive to P. carinii, they are still capable of mounting a response to other exogenous stimuli, as reflected by their production of cytokines in response to LPS and zymosan.
Moreover, this may explain the delayed infiltration by immune cells of the lungs of pup mice infected with P. carinii. The relative unresponsiveness of pup AMs to P. carinii compared to LPS or zymosan may be due to potential differences in the expression of beta-glucan on P. carinii organisms. P. carinii organisms express beta-glucans in their cell walls in the cyst form of their life cycle but not in their trophic form, thus minimizing their ability to ligate with dectin-1 compared to zymosan. We did not separate cysts from trophic forms of the organisms for our in vitro assays in order to mimic in vivo conditions, and trophic forms normally outnumber cysts by about 10:1. Thus, the encounter of pup or adult AMs with the trophic forms of the organism may yield a much milder response, if any response at all (20). It has been proposed that TLR2 mediates the AM response to P. carinii in mice (39) and that TLR2 colocalizes with dectin-1 to initiate macrophages' proinflammatory response (38). We have shown that the expression of TLR2 and that of dectin-1 are present in equal amounts on both pup and adult AMs; however, it is possible that the colocalization of these molecules is impaired on pup cells following stimulation with P. carinii organisms. Additionally, zymosan is a complex yeast extract that is known to ligate TLR2 and dectin-1 but may also ligate many other macrophage receptors as well. Thus, while we know that the levels of TLR2 and dectin-1 on pup and adult AMs are equivalent, it is possible that zymosan provides a stronger stimulus because of either more abundant ligands for dectin-1 and TLR2 or the presence of other ligands that are binding the macrophages that have yet to be described.
We have previously shown that upregulation of TNF-α is delayed in pup mice infected with P. carinii compared to that in adult mice (10, 22). Direct treatment with TNF-α, however, is problematic as prolonged treatment with exogenous TNF-α can be fatal (unpublished observations). We therefore considered other methods to increase TNF-α in the lungs of neonates without the harmful side effects of direct exogenous administration of TNF-α. We chose to use HKEC (which expresses LPS) in our in vivo experiments because it is known to be a strong inducer of TNF-α production and because it is a complex antigen that has been shown to have efficacy in adult thymectomized, CD4-depleted P. carinii-infected mice (4, 12). Our goal was to stimulate the production of TNF-α indirectly by the administration of aerosolized HKEC to determine if the stimulation of pup AMs through CD14 or TLR4 is more effective at improving P. carinii clearance than P. carinii alone. Our data showed that stimulation of TLR4 by an exogenous antigen resulted in infiltration and activation of macrophages and CD4+ T cells coincident with elevated lung TNF-α levels. Moreover, the aerosolized administration of HKEC was capable of increasing TNF-α, MCP-1, and IL-6 levels in the BALF simultaneous to lymphocyte infiltration, suggesting that the cognate interactions between AMs and CD4+ T cells are critical to mounting a response to P. carinii in both pups and adults. Interestingly, in vitro cytokine studies demonstrated no increase in MCP-1 production from pup macrophages stimulated with LPS; however, in vivo studies show a significant increase in MCP-1 production following HKEC treatment. These findings emphasize the importance of the complex interactions found in the lung environment which affect the response to antigenic stimuli. It is possible that cellular interactions within the pup lung environment promote a more fulminant MCP-1 cytokine response, which was lacking in our in vitro experiments, which used isolated AMs only. It is possible that alveolar epithelial cells are responsible for the production of macrophage chemoattractants, including MCP-1. The increased MCP-1 response observed in vivo following treatment with HKEC correlated with the increased infiltration of P. carinii-infected pup mice by macrophages.
Harmsen and Chen (12) performed similar experiments with adult mice that were thymectomized and depleted of CD4+ lymphocytes. They discovered that 10 days of HKEC treatment caused a slight reduction in P. carinii nuclei in the lungs of mice and that treatment for 22 days resulted in almost complete eradication of P. carinii organism. Our findings obtained with pup mice treated with HKEC were similar, with a reduction in P. carinii organisms beginning on day 21 postinfection. Unlike the findings obtained with adult mice, however, nearly complete eradication of P. carinii organisms did not occur until day 32 postinfection. Despite treatment with aerosolized HKEC, our study demonstrated that pup mice still showed a delay in the clearance of P. carinii organisms compared to that in the adult, CD4+ lymphocyte-depleted mice treated with HKEC used by Harmsen and Chen. Additionally, Harmsen and Chen demonstrated that large numbers of macrophages, polymorphonuclear leukocytes, and lymphocytes accumulated in the lungs of adult CD4+ lymphocyte-depleted mice (12). In our study, we demonstrated that while there was some increased infiltration by immune cells of HKEC-treated mice that correlated with P. carinii clearance, the increase was not statistically significant. The ability of HKEC to stimulate the activation of AMs in P. carinii-infected pup mice further supports our hypothesis that pup AMs may be intrinsically functional but are located in an antiinflammatory environment. This renders them less efficient than adult cells in mounting an adequate response to select invading pathogens. The mechanism by which HKEC increases AM activation and P. carinii clearance is not fully understood. However, the similar increase in MHC class II molecules in SCID pups treated with HKEC compared to WT pups, along with the continued ability to phagocytose P. carinii, suggests that AMs are being activated. The less efficient phagocytosis observed in the SCID pups, however, suggests that lymphocytes are contributing to an improved AM response. Since the activation of the AMs is similar in the SCID and WT pups, perhaps the lymphocytes' contribution is in the form of cytokine production. Our in vitro data suggest that the costimulation of both TLR4 and dectin-1 receptors provides a stronger intracellular signal with more cytokine production.
In conclusion, we found that administration of aerosolized HKEC resulted in a statistically significant reduction in P. carinii organisms and increased cytokine concentrations in pup alveolar spaces, along with an upregulation of AM activation markers. In future experiments, we will focus on using immune stimuli that are more clinically relevant, as administration of E. coli is not a realistic clinical option for the treatment of neonatal pulmonary infections. Together, our data suggest that neonatal AMs differ from adults in the ability to respond to different stimuli but that it may be possible to boost lung immune function in the face of life-threatening infection.
Acknowledgments
This work was supported by Public Health Service grant HL062053 from the National Heart, Lung, and Blood Institute to B.A.G.
Special thanks to Kevin Schuer for expert technical assistance.
Editor: A. Casadevall
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Beck, J. M., H. D. Liggitt, E. N. Brunette, H. J. Fuchs, J. E. Shellito, and R. J. Debs. 1991. Reduction in intensity of Pneumocystis carinii pneumonia in mice by aerosol administration of gamma interferon. Infect. Immun. 59:3859-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172:3059-3069. [DOI] [PubMed] [Google Scholar]
- 3.Brown, G. D., P. R. Taylor, D. M. Reid, J. A. Willment, D. L. Williams, L. Martinez-Pomares, S. Y. Wong, and S. Gordon. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conly, M. E., and D. P. Speert. 1991. Human neonatal monocyte-derived macrophages and neutrophils exhibit normal nonopsonic and opsonic receptor-mediated phagocytosis and superoxide anion production. Biol. Neonate 60:361-366. [DOI] [PubMed] [Google Scholar]
- 6.Empey, K. M., M. Hollifield, K. Schuer, F. Gigliotti, and B. A. Garvy. 2004. Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation. Infect. Immun. 72:6211-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez, S., P. Jose, M. G. Avdiushko, A. M. Kaplan, and D. A. Cohen. 2004. Inhibition of IL-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J. Immunol. 172:2613-2620. [DOI] [PubMed] [Google Scholar]
- 8.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvy, B. A., and A. G. Harmsen. 1996. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect. Immun. 64:3987-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvy, B. A., and M. H. Qureshi. 2000. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J. Immunol. 165:6480-6486. [DOI] [PubMed] [Google Scholar]
- 11.Gigliotti, F., A. G. Harmsen, and T. W. Wright. 2003. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect. Immun. 71:3852-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmsen, A. G., and W. Chen. 1992. Resolution of Pneumocystis carinii pneumonia in CD4+ lymphocyte-depleted mice given aerosols of heat-treated Escherichia coli. J. Exp. Med. 176:881-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman, O. A., J. E. Standing, and A. H. Limper. 1993. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J. Immunol. 150:3932-3940. [PubMed] [Google Scholar]
- 14.Kolls, J. K., S. Habetz, M. K. Shean, C. Vazquez, J. A. Brown, D. Lei, P. Schwarzenberger, P. Ye, S. Nelson, W. R. Summer, and J. E. Shellito. 1999. IFN-γ and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J. Immunol. 162:2890-2894. [PubMed] [Google Scholar]
- 15.Koziel, H., D. O'Riordan, A. Warner, and R. M. Rose. 1994. Alveolar macrophage interaction with Pneumocystis carinii. Immunol. Ser. 60:417-436. [PubMed] [Google Scholar]
- 16.Kurland, G., A. T. Cheung, M. E. Miller, S. A. Ayin, M. M. Cho, and E. W. Ford. 1988. The ontogeny of pulmonary defenses: alveolar macrophage function in neonatal and juvenile rhesus monkeys. Pediatr. Res. 23:293-297. [DOI] [PubMed] [Google Scholar]
- 17.Lebron, F., R. Vassallo, V. Puri, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-κB activation. J. Biol. Chem. 278:25001-25008. [DOI] [PubMed] [Google Scholar]
- 18.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pesanti, E. L. 1982. Effects of bacterial pneumonitis on development of pneumocystosis in rats. Am. Rev. Respir. Dis. 125:723-726. [DOI] [PubMed] [Google Scholar]
- 20.Powles, M. A., P. Liberator, J. Anderson, Y. Karkhanis, J. F. Dropinski, F. A. Bouffard, J. M. Balkovec, H. Fujioka, M. Aikawa, D. McFadden, and D. Schmatz. 1998. Efficacy of MK-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob. Agents Chemother. 42:1985-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi, M. H., J. Cook-Mills, D. E. Doherty, and B. A. Garvy. 2003. TNF-α-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J. Immunol. 171:4700-4707. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi, M. H., and B. A. Garvy. 2001. Neonatal T cells in an adult lung environment are competent to resolve Pneumocystis carinii pneumonia. J. Immunol. 166:5704-5711. [DOI] [PubMed] [Google Scholar]
- 23.Roeder, A., C. J. Kirschning, R. A. Rupec, M. Schaller, and H. C. Korting. 2004. Toll-like receptors and innate antifungal responses. Trends Microbiol. 12:44-49. [DOI] [PubMed] [Google Scholar]
- 24.Roeder, A., C. J. Kirschning, R. A. Rupec, M. Schaller, G. Weindl, and H. C. Korting. 2004. Toll-like receptors as key mediators in innate antifungal immunity. Med. Mycol. 42:485-498. [DOI] [PubMed] [Google Scholar]
- 25.Rudmann, D. G., A. M. Preston, M. W. Moore, and J. M. Beck. 1998. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-γ and type 1 and 2 TNF receptor genes. J. Immunol. 161:360-366. [PubMed] [Google Scholar]
- 26.Sherman, M., E. Goldstein, W. Lippert, and R. Wennberg. 1977. Neonatal lung defense mechanisms: a study of the alveolar macrophage system in neonatal rabbits. Am. Rev. Respir. Dis. 116:433-440. [DOI] [PubMed] [Google Scholar]
- 27.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 28.Speer, C. P., M. Wieland, R. Ulbrich, and M. Gahr. 1986. Phagocytic activities in neonatal monocytes. Eur. J. Pediatr. 145:418-421. [DOI] [PubMed] [Google Scholar]
- 29.Steele, C., L. Marrero, S. Swain, A. G. Harmsen, M. Zheng, G. D. Brown, S. Gordon, J. E. Shellito, and J. K. Kolls. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the dectin-1 beta-glucan receptor. J. Exp. Med. 198:1677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steele, C., J. E. Shellito, and J. K. Kolls. 2005. Immunity against the opportunistic fungal pathogen Pneumocystis. Med. Mycol. 43:1-19. [DOI] [PubMed] [Google Scholar]
- 31.Steele, C., M. Zheng, E. Young, L. Marrero, J. E. Shellito, and J. K. Kolls. 2002. Increased host resistance against Pneumocystis carinii pneumonia in γδ T-cell-deficient mice: protective role of gamma interferon and CD8+ T cells. Infect. Immun. 70:5208-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain, S. D., T. W. Wright, P. M. Degel, F. Gigliotti, and A. G. Harmsen. 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect. Immun. 72:5722-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 34.Totet, A., N. Respaldiza, J. C. Pautard, C. Raccurt, and G. Nevez. 2003. Pneumocystis jiroveci genotypes and primary infection. Clin. Infect. Dis. 36:1340-1342. [DOI] [PubMed] [Google Scholar]
- 35.Vargas, S. L., W. T. Hughes, M. E. Santolaya, A. V. Ulloa, C. A. Ponce, C. E. Cabrera, F. Cumsille, and F. Gigliotti. 2001. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 32:855-861. [DOI] [PubMed] [Google Scholar]
- 36.Vargas, S. L., C. A. Ponce, W. T. Hughes, A. E. Wakefield, J. C. Weitz, S. Donoso, A. V. Ulloa, P. Madrid, S. Gould, J. J. Latorre, R. Avila, S. Benveniste, M. Gallo, J. Belletti, and R. Lopez. 1999. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin. Infect. Dis. 29:1489-1493. [DOI] [PubMed] [Google Scholar]
- 37.Vassallo, R., J. E. Standing, and A. H. Limper. 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol. 164:3755-3763. [DOI] [PubMed] [Google Scholar]
- 38.Yadav, M., and J. S. Schorey. 2006. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108:3168-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, C., S.-H. Wang, M. E. Lasbury, D. Tschang, C.-P. Liao, P. J. Durant, and C.-H. Lee. 2006. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect. Immun. 74:1857-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, J., J. Zhu, A. Imrich, M. Cushion, T. B. Kinane, and H. Koziel. 2004. Pneumocystis activates human alveolar macrophage NF-κB signaling through mannose receptors. Infect. Immun. 72:3147-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]