Abstract
In 2005 and 2006, outbreaks of Fusarium keratitis associated with soft contact lens use occurred in multiple U.S. states and Puerto Rico. A case-control study conducted by the Centers for Disease Control and Prevention (CDC) showed a significant association between infections and the use of one particular brand of lens solution. To characterize the full spectrum of the causal agents involved and their potential sources, partial DNA sequences from three loci (RPB2, EF-1α, and nuclear ribosomal rRNA) totaling 3.48 kb were obtained from 91 corneal and 100 isolates from the patient's environment (e.g., contact lens and lens cases). We also sequenced a 1.8-kb region encoding the RNA polymerase II second largest subunit (RPB2) from 126 additional pathogenic isolates to better understand how the keratitis outbreak isolates fit within the full phylogenetic spectrum of clinically important fusaria. These analyses resulted in the most robust phylogenetic framework for Fusarium to date. In addition, RPB2 nucleotide variation within a 72-isolate panel was used to design 34 allele-specific probes to identify representatives of all medically important species complexes and 10 of the most important human pathogenic Fusarium in a single-well diagnostic assay, using flow cytometry and fluorescent microsphere technology. The multilocus data revealed that one haplotype from each of the three most common species comprised 55% of CDC's corneal and environmental isolates and that the corneal isolates comprised 29 haplotypes distributed among 16 species. The high degree of phylogenetic diversity represented among the corneal isolates is consistent with multiple sources of contamination.
Fusarium keratitis (FK) is a rare sight-threatening corneal infection among the many contact lens wearers in the United States and abroad (5, 20). After an increase in FK among contact lens wearers was suspected in Singapore, an investigation in that country suggested a link with the use of ReNu brand contact lens solution (20), based on the disproportionate number of FK cases associated with this product. After an increase in cases of FK was suspected in the United States, the Centers for Disease Control and Prevention (CDC) in conjunction with other public health agencies in the United States conducted a case-control study, which demonstrated that the FK outbreaks in the United States were associated with use of Bausch & Lomb's ReNu with MoistureLoc (Rochester, NY) brand of contact lens solution (5). After Bausch & Lomb recalled and then permanently discontinued worldwide sales of this product, the number of FK cases decreased substantially in Singapore and the United States (5, 20). Since corneal infections caused by Fusarium are typically associated with ocular trauma with soil, organic, or plant matter (5), the outbreaks, which involved cases of FK unassociated with ocular trauma, were unusual.
To date, microbiological assessment of the fusaria associated with the outbreaks has involved DNA typing using single (20) and multilocus DNA sequence data (5, 9), mini- and microsatellite primer probes (9), and phenotypic characterization of cultures obtained from corneal scrapings (1). The latter study also reported on a preliminary assessment of fusaria recovered from patients' contact lenses and lens cases.
Accurate identification of the etiological agents causing mycotic infections is critical to advancing our understanding of the environmental reservoir of each Fusarium species and may provide guidance to the most efficacious regimen of antifungal therapy. This is particularly important for fusaria, for which antifungal MICs are typically higher and more variable than for human pathogenic aspergilli (3). Even though the great majority of fusaria pathogenic to humans and other animals are nested within either the Fusarium solani or the F. oxysporum species complex (38), it has been traditional taxonomic practice to refer to members of these complexes as single species (i.e., F. solani and F. oxysporum). However, recent molecular phylogenetic studies have begun to clarify species boundaries within evolutionary lineages of Fusarium that contain human pathogens (33, 39, 44). Similar investigations of species limits within the Gibberella fujikuroi species complex have also been conducted (30, 32). A clear consensus that has emerged from these studies is that DNA sequence-based methods will be essential for species identification and subtyping of fusaria in clinical laboratories, especially within the pathogen-rich Fusarium solani species complex (FSSC) (5, 44). Indeed, the proactive DNA sequence characterization of human pathogenic isolates from these complexes, derived from major fungal culture collections, greatly assisted the rapid and precise identification of the pathogens involved in the keratitis outbreaks of 2005 and 2006 and subsequent epidemiological inferences (5). Zhang et al. (44) found that all of the 278 F. solani complex isolates from humans were members of a previously defined major clade within the complex (“clade 3”) (29), with approximately three-quarters of them falling into four major groups designated FSSC groups 1 to 4. These researchers also found that isolates from eye infections spanned the phylogenetic breadth of clade 3, which comprises approximately 25 phylogenetic species, with a statistically significant association with FSSC group 3 that was otherwise associated with soil, plants, and plant debris. Fortunately, recent advances in the development of allele-specific assays for identification of human pathogenic fungi (6, 35) and bacterial subtyping (8) clearly demonstrate that fluorescent microsphere array technology offers a powerful platform for rapid and accurate pathogen typing required by the public health community to respond to outbreaks in a timely manner.
Therefore, the objectives of the present study were threefold: (i) to investigate the full spectrum of fusaria associated with the keratitis outbreaks within the United States (including Puerto Rico), we examined the phylogenetic diversity of 91 corneal and 100 environmental isolates associated with the outbreaks, using DNA sequence data from three loci (5); (ii) to assess how the keratitis outbreak isolates fell within the full phylogenetic spectrum of Fusarium, nucleotide sequence data from the RNA polymerase II second largest subunit (RPB2) was used for the first time to identify medically important species and evolutionary lineages (i.e., monophyletic species complexes) and to infer a robust phylogenetic structure for Fusarium; and (iii) to develop and validate a high throughput, microsphere array for the simultaneous detection and identification of human pathogenic fusaria in a single well using flow cytometry, RPB2 nucleotide sequence data was used to design allele-specific probes for the six medically important species complexes and ten of the most important human pathogenic species. In contrast to CDC's published case-control study (5), in which only confirmed case isolates were included, we have analyzed here the full spectrum of isolates from patient corneas and their environment acquired for study by the CDC during the outbreak investigation (Table 1 and Table S1 in the supplemental material).
TABLE 1.
Summary of CDC keratitis investigation isolatesa
| Complex | Speciesb | Haplotypec | No. of isolates (%)d
|
||
|---|---|---|---|---|---|
| Corneal | Environmental | Combined | |||
| FSSC | 54 (59.3) | 64 (64) | 118 (61.8) | ||
| FSSC-1 | 1-a | 13 (18) | 24 (27) | 37 (45) | |
| 1-b | 3 | 2 | 5 | ||
| 1-c | 2 (4) | 2 (3) | 4 (7) | ||
| 1-? | 1 | 0 | 1 | ||
| FSSC-2 | 2-a | 2 (4) | 0 | 2 (4) | |
| 2-b | 2 | 0 | 2 | ||
| 2-d | 9 (13) | 11 (14) | 21 (27) | ||
| 2-f | 0 | 2 (4) | 2 (4) | ||
| 2-g | 0 | 1 | 1 | ||
| 2-h | 1 | 0 | 1 | ||
| 2-i | 0 | 1 | 1 | ||
| 2-j | 1 | 0 | 1 | ||
| FSSC-3 | 3-a | 0 | 2 (4) | 2 (4) | |
| 3-b | 2 | 0 | 2 | ||
| 3-c | 1 | 0 | 1 | ||
| FSSC-4 | 4-a | 1 | 0 | 1 | |
| 4-b | 1 | 0 | 1 | ||
| FSSC-5 | 5-a | 0 | 1 | 1 | |
| 5-b | 0 | 1 | 1 | ||
| 5-c | 1 | 0 | 1 | ||
| 5-d | 0 | 1 (3) | 1 (3) | ||
| FSSC-6 | 6-a | 0 | 1 | 1 | |
| FSSC-7 | 7-a | 0 | 1 | 1 | |
| FSSC-8 | 8-a | 1 | 0 | 1 | |
| FSSC-9 | 9-a | 0 | 1 | 1 | |
| FOSC | 27 (29.7) | 29 (29) | 56 (29.3) | ||
| FOSC-3 | 3-a | 10 (15) | 16 (19) | 26 (34) | |
| 3-b | 0 | 1 | 1 | ||
| 3-c | 1 | 0 | 1 | ||
| 3-d | 0 | 1 | 1 | ||
| 3-e | 1 (2) | 2 | 3 (4) | ||
| 3-f | 1 | 0 | 1 | ||
| 3-g | 2 (4) | 1 | 3 (5) | ||
| FOSC-4 | 4-a | 1 | 0 | 1 | |
| 4-b | 2 (3) | 1 | 3 (4) | ||
| 4-c | 0 | 1 (4) | 1 (4) | ||
| GFSC | 6 (6.6) | 6 (6) | 12 (6.3) | ||
| F. fujikuroi | 0 | 1 | 1 | ||
| F. proliferatum | 1 (2) | 1 | 2 (3) | ||
| F. sacchari | 0 | 1 | 1 | ||
| F. thapsinum | 1 | 0 | 1 | ||
| F. verticillioides | 1 | 1 | 2 | ||
| F. sp. cf. verticillioides | 1 (2) | 2 | 3 (4) | ||
| FIESC | 2 (2.2) | 2 (2%) | 4 (2.1) | ||
| F. sp. cf. incarnatum-1 | 1-a | 0 | 1 | 1 | |
| F. sp. cf. incarnatum-2 | 2-a | 1 | 0 | 1 | |
| F. sp. cf. incarnatum-3 | 3-a | 1 | 0 | 1 | |
| F. sp. cf. incarnatum-4 | 4-a | 0 | 1 | 1 | |
| FDSC | 1 (1.1) | 0 | 1 (0.5) | ||
| F. sp. cf. dimerum | 1-a | 1 | 0 | 1 | |
| Total | 67 (91) | 81 (100) | 148 (191) | ||
See Table S1 in the supplemental material for the histories of all 191 isolates.
Species names can only be applied with confidence to five species with in the GFSC (30, 32). The remaining 17 unnamed species are distributed among the following species complexes: 9 with in the FSSC (1 to 9), 2 with in the FOSC (3 and 4), F. sp. cf. verticillioides with in the GFSC, 4 with in the FIESC (F. sp. cf. incarnatum 1 to 4), and F. sp. cf. dimerum with in the FDSC.
Haplotypes were determined by multilocus DNA sequence-based genotyping as described in Materials and Methods.
The number of isolates in parentheses for each species studied by MLST includes putative clones (see Table S1 in the supplemental material for details). The frequency (%) of isolates recovered from cornea and the patient's environment for each species complex includes putative clones.
MATERIALS AND METHODS
Fungal strains.
The 319 isolates included in the present study were analyzed as the following three groups: a panel of 191 CDC keratitis outbreak isolates (Table 1 and Table S1 in the supplemental material) that were genotyped using multilocus DNA sequence data as described previously (5), a panel of 72 isolates for the design and validation of the microsphere array (design panel [DP]) (Table 2), and an experimental panel (EP) comprising 157 additional isolates (Table 3). In addition, an RPB2 sequence obtained from Lecanicillium lecanii, a member of Clavicipitaceae in the Hypocreales, was chosen as an outgroup for the phylogenetic analysis of the design panel data set (Fig. 1) based on more inclusive analyses (23). The DP and EP contained 36 and 69 outbreak isolates, respectively. All of the outbreak investigation isolates obtained from corneal scrapings, and the environmental assessment were verified as fusaria morphologically at the CDC (12, 27). In addition to the keratitis outbreak isolates, 128 pathogenic isolates were obtained from various sources for genotyping (Tables 2 and 3), including 54 phylogenetically diverse fusaria from The University of Texas Health Science Center's Fungus Testing Laboratory, San Antonio, TX. All strains are stored cryogenically and are available upon request from the Agricultural Research Service (NRRL) Culture Collection, National Center for Agricultural Utilization Research, Peoria, IL.
TABLE 2.
Design panel of fusaria analyzed by allele-specific genotyping and RPB2 sequencing
| NRRL no. | Equivalent no.a | Yr | Isolate source | Origin | Identified by Luminex/RPB2b | IDc (complex/species probe)d |
|---|---|---|---|---|---|---|
| 3211 | Sandoz 484 | 1966 | Switzerland | FIESC | 10.7 (IEC-f) | |
| 5537 | ATCC 28805 | Fescue hay | Missouri | FIESC | 4.6 (IEC-f) | |
| 13335 | FRC R-2138 | FIESC | 11.5 (IEC-f) | |||
| 13379 | FRC R-5198 | FIESC | 14.6 (IEC-f) | |||
| 13448 | CBS 749.97 | 1997 | Sorgum root | New South Wales, Australia | GFSC/F. nygamai | 4.1 (GFC-c) |
| 13566 | IMI 375349 | Rice | Taiwan | F. fujikuroi | 3.9 (GFC-c), 13.7 (F-c) | |
| 13604 | CBS 748.97 | 1997 | Millet | Namibia | GFSC/F. napiforme | 2.9 (GFC-c) |
| 13614 | CBS 258.54 | 1954 | Rice (NRRL 13614) | Vietnam | F. proliferatum | 4.8 (GFC-c), 16.6 (P-a) |
| 13713 | FRC T-428 | Millet | South Africa | FCSC | 10.7 (CC-c) | |
| 20423 | ATCC 42771 | Lizard skin | India | FIESC | 19.7 (IEC-f) | |
| 20425 | CBS 131.73 | 1973 | Banana | Bahamas | FIESC | 11.8 (IEC-f) |
| 22172 | CBS 734.97 | 1997 | Corn | Germany | F. verticillioides | 3.1 (GFC-c), 9.4 (V-a) |
| 25229 | IMI 240460 | Mycetoma | Italy | F. thapsinum | 5.4 (GFC-c), 11.4 (T-e) | |
| 25483 | CBS 217.78 | 1978 | Millet | Namibia | FCSC | 14.8 (CC-c) |
| 25489 | CBS 144.44 | 1944 | FIESC | 5.9 (IEC-f) | ||
| 26360 | FRC O-755 | Cornea | Tennessee | FOSC | 3.7 (OC-a) | |
| 26421 | CBS 140.95 | 1995 | Systemic infIection | Egypt | GFSC/F. nygamai | 7.1 (GFC-c) |
| 28023 | CBS 537.81 | Coccidae | Galapogos Islands | Lecanicillium lecanii | N/A | |
| 28505 | FRC R-8670 | Soil debris | South Africa | FCSC | 8.7 (CC-c) | |
| 28682 | 96-6008 | Germany | F. verticillioides | 4.4 (GFC-c), 14.6 (V-a) | ||
| 31005 | BBA 62211 | Iran | FIESC | 8.4 (IEC-f) | ||
| 31010 | BBA 64316 | Artemisia vulgaris | Germany | FIESC | 16.8 (IEC-f) | |
| 31011 | BBA 69079 | Thuja | Germany | FIESC | 10.9 (IEC-f) | |
| 31160 | MDA #3 | Lung | Texas | FIESC | 3.9 (IEC-f) | |
| 31167 | MDA #10 | Sputum, leukemia | Texas | FIESC | 11.9 (IEC-f) | |
| 32173 | MDA F2 | Arm tissue, leukemic | Delaware | F. sp. cf. dimerum | 11.0 (DC-d), 5.6 (D-c) | |
| 32174 | MDA F8 | Skin, leukemic | Florida | F. proliferatum | 4.1 (GFC-c), 16.2 (P-a) | |
| 32175 | MDA F10 | Sputum | Texas | FIESC | 10.7 (IEC-f) | |
| 32179 | MDA F18 | Sputum, leukemic | Texas | F. thapsinum | 4.6 (GFC-c), 11.3 (T-e) | |
| 32434 | CBS 623.92 | 1992 | Foot lesion | Germany | FSSC | 6.8 (SC-d) |
| 32521 | FRC T-852 | Cancer patient | FCSC | 10.9 (CC-c) | ||
| 32868 | FRC R-8880 | Blood | Ohio | FIESC | 11.0 (IEC-f) | |
| 34032 | UTHSC 98-2172 | 1998 | Mandibular absess | Texas | FIESC | 6.1 (IEC-f) |
| 36064 | FRC O-1747 | FOSC | 6.2 (OC-a) | |||
| 36168 | CBS 110148 | Gymnocalycium damsii | Germany | FDSC | 35.1 (DC-d) | |
| 36511 | CBS 572.94 | 1994 | Pigeon pea | India | GFSC/F. nygamai | 6.1 (GFC-c) |
| 36536 | CBS 673.94 | 1994 | GFSC/F. napiforme | 4.5 (GFC-c) | ||
| 43373 | CDC 2006743215 | 2006 | Contact lens | Malaysia | FSSC 2 | 2.6 (SC-d), 4.5 (S-2) |
| 43374 | CDC 2006743216 | 2006 | Cornea | Nigeria | FSSC 2 | 12.1 (SC-d), 18.4 (S-2) |
| 43375 | CDC 2006011016 | 2006 | Cornea | New Jersey | FSSC 1 | 8.5 (SC-d), 4.2 (S-1) |
| 43431 | CDC 2006011126 | 2006 | Cornea | Connecticut | FOSC | 5.7 (OC-a) |
| 43433 | CDC 2006011214 | 2006 | Cornea | Ohio | FSSC 2 | 20.2 (SC-d), 29.7 (S-2) |
| 43441 | CDC 2006743414 | 2006 | Cornea | Pennsylvania | FSSC 3 | 10.6 (SC-d), 11.9 (S-34) |
| 43442 | CDC 2006743415 | 2006 | Cornea | Pennsylvania | FOSC | 5.0 (OC-a) |
| 43445 | CDC 2006743419 | 2006 | Cornea | Connecticut | FSSC 2 | 15.1 (SC-d), 22.0 (S-2) |
| 43454 | CDC 2006743427 | 2006 | Cornea | Massachusetts | FOSC | 6.0 (OC-a) |
| 43455 | CDC 2006743230 | 2006 | Cornea | Vermont | FOSC | 5.1 (OC-a) |
| 43458 | CDC 2006743233 | 2006 | Cornea | Singapore | FSSC 2 | 14.3 (SC-d), 23.0 (S-2) |
| 43464 | CDC 2006743239 | 2006 | Cornea | Singapore | FSSC 2 | 13.8 (SC-d), 21.8 (S-2) |
| 43466 | CDC 2006743429 | 2006 | Contact lens case | Ohio | FOSC | 4.9 (OC-a) |
| 43468 | CDC 2006743431 | 2006 | Cornea | Iowa | FSSC | 4.8 (SC-d) |
| 43470 | CDC 2006743433 | 2006 | Cornea | Illinois | F. fujikuroi | 2.7 (GFC-c), 10.5 (F-c) |
| 43474 | CDC 2006743441 | 2006 | Cornea | Illinois | FSSC | 8.7 (SC-d) |
| 43489 | CDC 2006743456 | 2006 | Cornea | Maryland | FSSC | 7.4 (SC-d) |
| 43490 | CDC 2006743457 | 2006 | Cornea | Michigan | FSSC 2 | 6.4 (SC-d), 8.7 (S-2) |
| 43503 | CDC 2006743471 | 2006 | Contact lens case | Connecticut | FSSC 4 | 11.3 (SC-d), 11.6 (S-4) |
| 43504 | CDC 2006743483 | 2006 | Cornea | Pennsylvania | FOSC | 5.8 (OC-a) |
| 43514 | CDC 2006743560 | 2006 | Cornea | Florida | FSSC 2 | 11.9 (SC-d), 15.9 (S-2) |
| 43521 | CDC 2006743567 | 2006 | Cornea | Florida | FOSC | 6.3 (OC-a) |
| 43527 | CDC 2006743573 | 2006 | Cornea | Florida | FSSC | 3.8 (SC-d) |
| 43528 | CDC 2006743574 | 2006 | Cornea | Florida | FSSC 2 | 7.1 (SC-d), 12.1 (S-2) |
| 43529 | CDC 2006743575 | 2006 | Cornea | Florida | FSSC 4 | 7.4 (SC-d), 7.4 (S-4) |
| 43532 | CDC 2006743578 | 2006 | Cornea | Florida | FSSC 2 | 15.6 (SC-d), 20.8 (S-2) |
| 43533 | CDC 2006743579 | 2006 | Cornea | Florida | FSSC 1 | 5.9 (SC-d), 3.4 (S-1) |
| 43536 | CDC 2006743582 | 2006 | Cornea | Florida | FSSC 3 | 6.4 (SC-d), 8.1 (S-34) |
| 43537 | CDC 2006743583 | 2006 | Cornea | Florida | FSSC 3 | 9.7 (SC-d), 11.5 (S-34) |
| 43539 | CDC 2006743490 | 2006 | Cornea | Missouri | FOSC | 6.9 (OC-a) |
| 43540 | CDC 2006743491 | 2006 | Cornea | Kansas | FSSC 1 | 6.1 (SC-d), 2.8 (S-1) |
| 43655 | CDC 2006743515 | 2006 | Contact lens case fluid | Ohio | FOSC | 5.9 (OC-a) |
| 43656 | CDC 2006743516 | 2006 | Contact lens | Minnesota | F. verticillioides | 2.8 (GFC-c), 11.4 (V-a) |
| 43665 | CDC 2006743525 | 2006 | Contact lens | North Carolina | F. proliferatum | 3.4 (GFC-c), 12.7 (P-a) |
| 43668 | CDC 2006743529 | 2006 | Cornea | New Jersey | FOSC | 6.1 (OC-a) |
| 43726 | CDC 2006743692 | 2006 | Cornea | Ohio | FDSC | 53.2 (DC-d) |
ATCC, American Type Culture Collection, Manassas, VA; BBA, Biologische Bundesanstalt für Land- und Forstwirtschaft, Institute für Mikrobiologie, Berlin, Germany; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CDC, Centers for Disease Control and Prevention, Atlanta, GA; FRC, Fusarium Research Center, The Pennsylvania State University, State College, PA; IMI, CABI Biosciences, Egham, Surrey, England; MDA, M. D. Anderson Cancer Center, Houston, TX; UTHSC, University of Texas Health Sciences Center, San Antonio, TX.
ID, index of discrimination determined as minimum target fluorescent intensity/maximum nontarget fluorescence intensity.
See Table 4 for a complete listing of the probes. Note that the probes listed here lack the F for Fusarium prefix.
TABLE 3.
Experimental panel of fusaria analyzed by allele-specific genotyping and RPB2 sequencing
| NRRL no. | Equivalent no.a | Yr | Isolate source | Origin | Identified by Luminex/RPB2b | IDc (complex/species probed) |
|---|---|---|---|---|---|---|
| 13459 | CBS 961.87 | 1984 | Plant debris | South Africa | F. proliferatum/F. concolor | 0 (GFC-c, GFCO-c), 6.7 (PF-b) |
| 28000 | CDC B-4536 | 1987 | Blood, leukemic | Georgia | FSSC 3/FSSC | 23.5 (SC-d), 17.0 (S-34) |
| 28001 | CDC B-4572 | 1987 | Skin, leukemic | Colorado | FSSC 3/FSSC | 33.1 (SC-d), 47.1 (S-34) |
| 28002 | CDC B-4581 | 1987 | Skin, leukemic | Georgia | FSSC 3/FSSC | 29.6 (SC-d), 19.6 (S-34) |
| 28003 | CDC B-4680 | 1988 | Toenail | Washington, DC | F. fujikuroi | 4.4 (GFC-a), 14.6 (F-c) |
| 28005 | CDC B-4687 | 1988 | Hospital venilation system | Illinois | F. proliferatum | 8.9 (GFC-c), 17.0 (P-a) |
| 28006 | CDC B-4688 | 1988 | Hospital venilation system | Illinois | F. thapsinum | 3.3 (GFC-c), 6.2 (T-e) |
| 28007 | CDC B-4689 | 1988 | Hospital venilation system | Illinois | F. thapsinum | 3.5 (GFC-c), 8.9 (T-e) |
| 28008 | CDC B-4701 | 1988 | Hospital venilation system | Illinois | FSSC 1/FSSC | 5.9 (SC-d), 7.2 (S-1) |
| 28009 | CDC B-5543 | 1994 | Viterous fluid | Texas | FSSC 1/FSSC | 25.2 (SC-d), 6.5 (S-1) |
| 28010 | CDC B-5546 | 1995 | Viterous fluid | Texas | FSSC 1/FSSC | 36.4 (SC-d), 7.1 (S-1) |
| 28011 | CDC B-5548 | 1994 | Viterous fluid | Texas | FSSC 1/FSSC | 29.3 (SC-d), 7.0 (S-1) |
| 28014 | CDC B-5754 | 1997 | Leg wound, diabetic | California | FSSC 2 | 9.8 (SC-d), 13.5 (S-2) |
| 28016 | CDC B-5779 | 1997 | Bronchial wash, leukemic | Ohio | FSSC | 7.1 (SC-d) |
| 28017 | CDC B-5780 | 1997 | Catheter, lekemic | Ohio | FSSC | 19.1 (SC-d) |
| 28018 | CDC B-5781 | 1997 | Catheter, lekemic | Ohio | FSSC | 8.2 (SC-d) |
| 28019 | CDC B-5782 | 1997 | Skin lesion, leukemic | Ohio | FSSC | 17.5 (SC-d) |
| 28029 | CDC B-3335 | 1980 | Corneal transplant | California | FIESC | 9.6 (IEC-f) |
| 28030 | CDC B-3723 | 1983 | Skin lesion, leukemic | Thailand | FSSC | 4.6 (SC-d) |
| 28031 | CDC B-3882 | 1984 | Toe nail | South Carolina | FOSC | 7.1 (OC-a) |
| 28033 | CDC B-4422 | 1987 | Leukemic | Italy | FSSC 1 | 14.5 (SC-d), 5.9 (S-1) |
| 28035 | CDC B-4809 | 1989 | Lymph node | Arkansas | F. proliferatum | 3.5 (GFC-c), 8.4 (P-a) |
| 37068 | CBS 110192 | Soil | Australia | FDSC | 5.4 (DC-d) | |
| 37071 | CBS 110320 | Toenail | Chile | F. sp. cf. dimerum | 15.9 (DC-d), 7.4 (D-c) | |
| 43544 | CDC 2006743496 | 2006 | Cornea | Ohio | FSSC 1 | 13.9 (SC-d), 7.4 (S-1) |
| 43545 | CDC 2006743497 | 2006 | Cornea | Ohio | F. proliferatum | 4.0 (GFC-c), 15.2 (P-a) |
| 43547 | CDC 2006743499 | 2006 | Contact lens | Minnesota | F. verticillioides | 2.6 (GFC-c), 11.9 (V-a) |
| 43583 | UTHSC 06-989 | 2006 | Cornea | Ohio | FSSC 1 | 13.3 (SC-d), 5.8 (S-1) |
| 43584 | UTHSC 06-743 | 2006 | Catheter tip | Florida | FSSC 3 | 6.2 (SC-d), 6.9 (S-34) |
| 43585 | UTHSC 06-295 | 2006 | Tissue | Utah | FSSC 2 | 3.2 (SC-d), 5.8 (S-2) |
| 43586 | UTHSC 05-3538 | 2005 | Cornea | Texas | FSSC 2 | 11.1 (SC-d), 18.8 (S-2) |
| 43588 | UTHSC 05-3530 | 2005 | Corneal transplant | Illinois | FSSC 1 | 17.1 (SC-d), 16.4 (S-1) |
| 43589 | UTHSC 05-3333 | 2005 | Blood | Ohio | FSSC 1 | 13.2 (SC-d), 5.2 (S-1) |
| 43590 | UTHSC 05-2824 | 2005 | Blood | Maine | FSSC 1 | 14.1 (SC-d), 7.8 (S-1) |
| 43591 | UTHSC 05-2680 | 2005 | Cornea | Florida | FSSC 1 | 15.7 (SC-d), 7.1 (S-1) |
| 43592 | UTHSC 06-689 | 2006 | Cornea | Massachusetts | FOSC | 5.7 (OC-a) |
| 43593 | UTHSC 05-3159 | 2005 | Nail | South Carolina | FOSC | 6.7 (OC-a) |
| 43594 | UTHSC 05-1643 | 2005 | Cornea | Massachusetts | FOSC | 5.7 (OC-a) |
| 43595 | UTHSC 05-721 | 2005 | Blood | California | FOSC | 4.0 (OC-a) |
| 43596 | UTHSC 04-1934 | 2004 | Lung | Arkansas | FOSC | 5.7 (OC-a) |
| 43597 | UTHSC 04-584 | 2004 | Bronchial wash | Utah | FOSC | 4.5 (OC-a) |
| 43598 | UTHSC 03-753 | 2003 | Foot tissue | Florida | FOSC | 5.4 (OC-a) |
| 43599 | UTHSC 06-1103 | 2006 | Toe | Florida | F. verticillioides | 17.2 (GFC-c), 4.7 (V-a) |
| 43600 | UTHSC 05-3141 | 2005 | Cornea | Massachusetts | F. verticillioides | 6.1 (GFC-c), 25.1 (V-a) |
| 43601 | UTHSC 05-1039 | 2005 | Skin | Maryland | F. verticillioides | 5.0 (GFC-c), 29.0 (V-a) |
| 43602 | UTHSC 05-850 | 2005 | Sputum | Wisconsin | F. verticillioides | 7.1 (GFC-c), 26.3 (V-a) |
| 43603 | UTHSC 05-431 | 2005 | Skin | Ohio | F. verticillioides | 6.5 (GFC-c), 22.5 (V-a) |
| 43604 | UTHSC 05-430 | 2005 | Nasal sinus | Ohio | F. verticillioides | 5.6 (GFC-c), 26.3 (V-a) |
| 43605 | UTHSC 04-700 | 2004 | Skin | Massachusetts | F. verticillioides | 6.6 (GFC-c), 25.0 (V-a) |
| 43606 | UTHSC 04-695 | 2004 | Trachea | Lousiana | F. verticillioides | 5.9 (GFC-c), 25.3 (V-a) |
| 43608 | UTHSC 03-2552 | 2003 | Peritoneal fluid | Minnesota | F. verticillioides | 4.5 (GFC-c), 15.4 (V-a) |
| 43609 | UTHSC 06-952 | 2006 | Palate | Florida | F. proliferatum | 5.6 (GFC-c), 19.2 (P-a) |
| 43610 | UTHSC 06-836 | 2006 | Skin | Iowa | F. fujikuroi | 15.9 (GFC-c), 17.0 (F-c) |
| 43611 | UTHSC 06-492 | 2006 | Lacrimal duct | Texas | F. proliferatum | 2.7 (GFC-c), 9.4 (P-a) |
| 43612 | UTHSC 06-432 | 2006 | Nail | Florida | F. fujikuroi | 4.5 (GFC-c), 15.1 (F-c) |
| 43613 | UTHSC 06-197 | 2006 | Cornea | Utah | F. proliferatum | 5.3 (GFC-c), 19.8 (P-a) |
| 43614 | UTHSC 04-1974 | 2004 | Nose | Texas | F. proliferatum | 6.0 (GFC-c), 22.9 (P-a) |
| 43615 | UTHSC 04-1772 | 2004 | Ethmoid sinus | Ohio | F. proliferatum | 6.9 (GFC-c), 23.7 (P-a) |
| 43616 | UTHSC 03-3232 | 2003 | Tissue | Utah | F. proliferatum | 6.0 (GFC-c), 21.8 (P-a) |
| 43617 | UTHSC 03-60 | 2003 | Blood | Colorado | F. proliferatum | 7.2 (GFC-c), 25.0 (P-a) |
| 43618 | UTHSC 02-1631 | 2002 | Blood | Texas | F. proliferatum | 3.8 (GFC-c), 13.0 (P-a) |
| 43619 | UTHSC 05-2847 | 2005 | Index finger | Texas | FIESC | 12.7 (IEC-f) |
| 43622 | UTHSC 03-2501 | 2003 | Lung | Texas | FIESC | 8.5 (IEC-f) |
| 43623 | UTHSC 03-59 | 2003 | Maxillary sinus | Colorado | FIESC | 18.7 (IEC-f) |
| 43624 | UTHSC 02-2060 | 2002 | Sputum | Texas | FIESC | 11.8 (IEC-f) |
| 43625 | UTHSC 02-1698 | 2002 | Maxillary sinus | Texas | FIESC | 12.8 (IEC-f) |
| 43627 | UTHSC 05-3559 | 2005 | Bronchial wash | Texas | FCSC | 15.5 (CC-c) |
| 43628 | UTHSC 05-3396 | 2005 | Finger wound | Florida | FCSC | 16.1 (CC-c) |
| 43629 | UTHSC 05-3200 | 2005 | Blood | Utah | FCSC | 14.7 (CC-c) |
| 43630 | UTHSC 05-2743 | 2005 | Sputum | Texas | FCSC | 5.0 (CC-c) |
| 43631 | UTHSC 05-2441 | 2005 | Leg biopsy | Texas | FCSC | 18.7 (CC-c) |
| 43632 | UTHSC 05-1260 | 2005 | Cornea | Florida | FCSC | 10.0 (CC-c) |
| 43633 | UTHSC 03-3472 | 2003 | Maxillary sinus | Tennessee | FCSC | 9.6 (CC-c) |
| 43634 | UTHSC 02-1276 | 2002 | Horse cornea | Alabama | FCSC | 22.2 (CC-c) |
| 43635 | UTHSC 06-638 | 2006 | Horse | Nebraska | FIESC | 12.9 (IEC-b) |
| 43636 | UTHSC 06-170 | 2006 | Dog | Texas | FIESC | 13.3 (IEC-f) |
| 43637 | UTHSC 05-1729 | 2005 | Dog, cutaneous | Pennsylvania | FIESC | 20.1 (IEC-f) |
| 43638 | UTHSC R-3500 | 2004 | Manatee | Florida | FIESC | 9.0 (IEC-b) |
| 43639 | UTHSC 04-135 | 2004 | Manatee | Florida | FIESC | 13.2 (IEC-b) |
| 43640 | UTHSC 04-123 | 2004 | Dog, nasal | Texas | FIESC | 15.6 (IEC-f) |
| 43641 | UTHSC 06-1377 | 2006 | Horse eye | Missouri | Unknown/Fusarium sp. | 2.9 (5f2-ab), 3.6 (7cf-ab) |
| 43673 | CDC 2006743501 | 2006 | Contact lens case fluid | New Jersey | FSSC 2 | 11.2 (SC-d), 16.0 (S-2) |
| 43674 | CDC 2006743553 | 2006 | Contact lens case | Kentucky | FOSC | 3.7 (OC-a) |
| 43675 | CDC 2006743554 | 2006 | Cornea | Kentucky | FSSC 1 | 14.4 (SC-d), 7.6 (S-1) |
| 43676 | CDC 2006743585 | 2006 | Cornea | Illinois | FSSC 1 | 14.2 (SC-d), 7.9 (S-1) |
| 43677 | CDC 2006743586 | 2006 | Contact lens case | Illinois | FSSC 1 | 3.9 (SC-d), 7.4 (S-1) |
| 43678 | CDC 2006743587 | 2006 | Contact lens case | California | FOSC | 4.0 (OC-a) |
| 43679 | CDC 2006743588 | 2006 | Contact lens | California | FOSC | 3.4 (OC-a) |
| 43680 | CDC 2006743589 | 2006 | Contact lens case fluid | Texas | FIESC | 10.9 (IEC-b) |
| 43681 | CDC 2006743591 | 2006 | Cornea | South Dakota | FSSC | 6.7 (SC-d) |
| 43682 | CDC 2006743592 | 2006 | Cornea | Maine | F. verticillioides | 2.6 (GFC-c), 11.5 (V-a) |
| 43683 | CDC 2006743593 | 2006 | Cornea | California | FSSC 1 | 14.6 (SC-d), 8.4 (S-1) |
| 43684 | CDC 2006743594 | 2006 | Cornea | California | FSSC 1 | 14.2 (SC-d), 8.3 (S-1) |
| 43685 | CDC 2006743595 | 2006 | Cornea | California | FSSC 1 | 15.0 (SC-d), 5.9 (S-1) |
| 43686 | CDC 2006743597 | 2006 | Contact lens case | California | FOSC | 4.6 (OC-a) |
| 43687 | CDC 2006743599 | 2006 | Contact lens solution cap | Kentucky | FOSC | 4.8 (OC-a) |
| 43690 | CDC 2006743602 | 2006 | Contact lens | Puerto Rico | FSSC 2 | 8.1 (SC-d), 13.5 (S-2) |
| 43691 | CDC 2006743603 | 2006 | Contact lens | Puerto Rico | FSSC 2 | 6.4 (SC-d), 11.0 (S-2) |
| 43692 | CDC 2006743604 | 2006 | Cornea | Washington | FOSC | 3.3 (OC-a) |
| 43693 | CDC 2006743606 | 2006 | Contact lens | Texas | F. thapsinum | 4.5 (GFC-c), 12.0 (T-e) |
| 43694 | CDC 2006743607 | 2006 | Cornea | Texas | FIESC | 13.9 (IEC-f) |
| 43695 | CDC 2006743608 | 2006 | Contact lens solution | Texas | FSSC 1 | 15.2 (SC-d), 10.0 (S-1) |
| 43696 | CDC 2006743609 | 2006 | Cornea | Texas | FOSC | 3.3 (OC-a) |
| 43697 | CDC 2006743611 | 2006 | Cornea | Pennsylvania | F. verticillioides | 2.9 (GFC-c), 12.8 (V-a) |
| 43698 | CDC 2006743612 | 2006 | Cornea | Pennsylvania | FOSC | 3.7 (OC-a) |
| 43699 | CDC 2006743615 | 2006 | Contact lens case | Minnesota | FSSC 1 | 9.8 (SC-d), 6.0 (S-1) |
| 43700 | CDC 2006743616 | 2006 | Cornea | Minnesota | FSSC 1 | 14.0 (SC-d), 8.0 (S-1) |
| 43701 | CDC 2006743617 | 2006 | Cornea | Minnesota | FSSC 1 | 14.9 (SC-d), 8.0 (S-1) |
| 43702 | CDC 2006743618 | 2006 | Contact lens case fluid | Ohio | FSSC 2 | 7.7 (SC-d), 13.7 (S-2) |
| 43703 | CDC 2006743619 | 2006 | Contact lens case | South Dakota | FSSC | 7.0 (SC-d) |
| 43704 | CDC 2006743620 | 2006 | Contact lens | South Dakota | FSSC | 5.9 (SC-d) |
| 43705 | CDC 2006743622 | 2006 | Contact lens case fluid | Puerto Rico | FSSC 2 | 8.4 (SC-d), 13.3 (S-2) |
| 43706 | CDC 2006743623 | 2006 | Cornea | Ohio | FOSC | 3.9 (OC-a) |
| 43707 | CDC 2006743596 | 2006 | Cornea | California | FOSC | 4.2 (OC-a) |
| 43708 | CDC 2006743598 | 2006 | Contact lens solution | California | FOSC | 6.5 (OC-a) |
| 43709 | CDC 2006743600 | 2006 | Contact lens case fluid | Kentucky | FOSC | 5.9 (OC-a) |
| 43710 | CDC 2006743601 | 2006 | Contact lens | Kentucky | FOSC | 5.3 (OC-a) |
| 43711 | CDC IFI01-0048 | 2001 | Sinus | Michigan | F. proliferatum | 5.3 (GFC-c), 18.6 (P-a) |
| 43713 | CDC IFI02-0239 | 2002 | Blood | North Carolina | FOSC | 6.7 (OC-a) |
| 43714 | CDC IFI03-0214 | 2003 | Sputum | Maryland | F. verticillioides | 4.5 (GFC-c), 20.8 (V-a) |
| 43715 | CDC IFI04-0065 | 2004 | Skin | Alabama | FSSC 1 | 5.6 (SC-d), 7.5 (S-1) |
| 43716 | CDC IFI04-0194 | 2004 | Blood | - | F. sp. cf. dimerum/FDSC | 10.9 (DC-d), 8.7 (D-b), 6.6 (D-c) |
| 43717 | CDC IFI03-0179 | 2003 | SubQchest | Michigan | FSSC | 9.2 (SC-d) |
| 43724 | CDC 2006743696 | 2006 | Cornea | North Carolina | FSSC 1 | 12.3 (SC-d), 8.0 (S-1) |
| 43725 | CDC 2006743697 | 2006 | Contact lens | North Carolina | FSSC 1 | 10.2 (SC-d), 8.7 (S-1) |
| 43727 | CDC 2006743694 | 2006 | Cornea | Florida | FSSC 2 | 6.7 (SC-d), 12.7 (S-2) |
| 43728 | CDC 2006743691 | 2006 | Cornea | Florida | FSSC 2 | 7.8 (SC-d), 13.1 (S-2) |
| 43729 | CDC 2006743621 | 2006 | Contact lens case fluid | Puerto Rico | FSSC 2 | 8.5 (SC-d), 12.0 (S-2) |
| 43730 | CDC 2006743605 | 2006 | Contact lens | Mississippi | FIESC | 8.9 (IEC-f) |
| 43731 | CDC 2006743689 | 2006 | Contact lens case | Iowa | FSSC 1 | 8.9 (SC-d), 5.7 (S-1) |
| 43732 | CDC 2006743695 | 2006 | Contact lens case | Michigan | FOSC | 3.0 (OC-a) |
| 43733 | CDC 2006743623 | 2006 | Cornea | Ohio | FOSC | 3.0 (OC-a) |
| 43734 | CDC 2006743625 | 2006 | Cornea | New York | FOSC | 2.9 (OC-a) |
| 43735 | CDC 2006743626 | 2006 | Cornea | New York | FOSC | 3.1 (OC-a) |
| 43736 | CDC 2006013784 | 2006 | Cornea | California | FSSC 1 | 11.8 (SC-d), 7.0 (S-1) |
| 43803 | AFR4 | 2006 | Contact lens case | Florida | FSSC 1 | 12.0 (SC-d), 6.8 (S-1) |
| 43804 | AFR9 | 2006 | Contact lens case | Georgia | FOSC | 3.4 (OC-a) |
| 43805 | AFR12 | 2006 | Contact lens case | Georgia | FSSC 1 | 10.2 (SC-d), 8.3 (S-1) |
| 43806 | AFR81036 | 1981 | Cornea | Georgia | FSSC 4 | 8.8 (SC-d), 7.6 (S-4) |
| 43807 | CDC 2006014156 | 2006 | Contact lens solution | Nevada | FOSC | 5.1 (OC-a) |
| 43808 | CDC 2006743699 | 2006 | Unknown | Texas | FOSC | 6.9 (OC-a) |
| 43809 | CDC 2006743700 | 2006 | Cornea | Ohio | FSSC 1 | 14.1 (SC-d), 11.0 (S-1) |
| 43810 | CDC 2006743701 | 2006 | Cornea | Ohio | FOSC | 7.2 (OC-a) |
| 43811 | CDC 2006743702 | 2006 | Cornea | Ohio | FSSC | 11.0 (SC-d) |
| 43812 | CDC 2006743705 | 2006 | Contact lens solution | New York | FSSC 1 | 11.2 (SC-d), 9.9 (S-1) |
| 43813 | CDC 2006743707 | 2006 | Contact lens case | New York | FSSC 1 | 15.9 (SC-d), 11.4 (S-1) |
| 43814 | CDC 2006743708 | 2006 | Contact lens | New York | FSSC 2 | 4.6 (SC-d), 9.9 (S-2) |
| 43815 | CDC 2006743711 | 2006 | Contact lens case | New York | FSSC 1 | 10.0 (SC-d), 7.1 (S-1) |
| 43816 | CDC 2006743713 | 2006 | Contact lens case | New York | FSSC 1 | 8.4 (SC-d), 6.2 (S-1) |
| 43817 | CDC 2006743714 | 2006 | Contact lens | New York | FSSC 1 | 13.5 (SC-d), 10.2 (S-1) |
| 43818 | CDC 2006743716 | 2006 | Contact lens case | New York | FSSC 2 | 11.6 (SC-d), 24.0 (S-2) |
| 43819 | CDC 2006743718 | 2006 | Cornea | Illinois | FSSC 1 | 14.5 (SC-d), 11.0 (S-1) |
| 43873 | CDC 2006743709 | 2006 | Contact lens case | New York | FSSC 1 | 10.1 (SC-d), 9.5 (S-1) |
| 43874 | CDC 2006743710 | 2006 | Contact lens case | New York | FSSC 2 | 8.2 (SC-d), 20.7 (S-2) |
| 43875 | CDC 2006743712 | 2006 | Contact lens case | New York | FSSC 1+FSSC 2/FSSC 1 | 18.8 (SC-d), 17.5 (S-1), 5.1 (S-2) |
| 43876 | CDC 2006743715 | 2006 | Contact lens case | New York | FSSC 2 | 8.8 (SC-d), 16.8 (S-2) |
| 43877 | CDC 2006743727 | 2006 | Cornea | Tennessee | FSSC 1 | 9.9 (SC-d), 9.9 (S-1) |
AFR, Donald G. Ahearn, Georgia State University, Atlanta, GA; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CDC, Centers for Disease Control and Prevention, Atlanta, GA; UTHSC, University of Texas Health Sciences Center, San Antonio, TX.
ID, index of discrimination determined as minimum target fluorescent intensity/maximum non-target fluorescent intensity.
See Table 4 for a complete listing of the probes. Note that the probes listed here lack the F for Fusarium prefix.
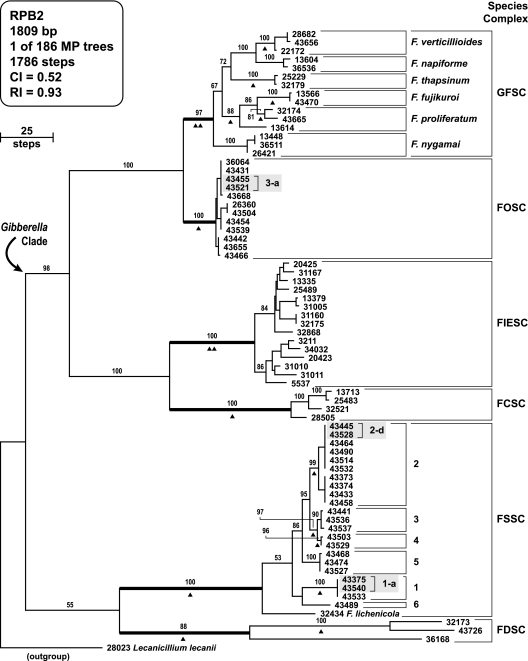
FIG. 1.
One of 186 MPTs inferred from analysis of the design panel RPB2 data set, rooted with the outgroup sequence of Lecanicillium lecanii (consistency index = 0.52, retention index = 0.93). Numbers above nodes represent the frequency (%) with which they were recovered from 1,000 bootstrap pseudoreplicates of the data. The six medically important species complexes are indicated by bold internodes. Six unnamed species within the FSSC are indicated by numbers. Isolate numbers that are shaded represent the three most common haplotypes: FOSC 3-a, FSSC 1-a, and FSSC 2-d. Arrowheads identify each of the 18 allele-specific probes used in the identification of species complexes and species in the design (Table 2) and experimental panels (Table 3).
DNA isolation and PCR amplification for multilocus DNA sequencing.
Fungal mycelium was grown in yeast-malt broth (20 g of dextrose, 5 g peptone, 3 g of yeast extract, and 3 g of malt extract per liter; Difco, Detroit, MI) and freeze-dried, and then a hexadecyltrimethyl-ammonium bromide (CTAB; Sigma, St. Louis, MO) protocol was used to extract total genomic DNA as described previously (30). Portions of the nuclear large rRNA subunit (LSU) and translation elongation factor (EF-1α) genes were amplified by PCR as described previously (5). Two contiguous regions of the RNA polymerase II second largest subunit (RPB2) were amplified with the PCR primers 5F2 (5′-GGGGWGAYCAGAAGAAGGC) and 7cR (5′-CCCATRGCTTGYTTRCCCAT) and the primers 7cF (5′-ATGGGYAARCAAGCYATGGG) and 11aR (5′-GCRTGGATCTTRTCRTCSACC) in separate reactions (36), using Platinum Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA) in an Applied Biosystems 9700 thermocycler (Emeryville, CA), and by following a PCR program of 1 cycle of 90 s at 94°C, followed by 40 cycles of 30 s at 94°C, 90 s at 55°C, and 2 min at 68°C, followed in turn by 1 cycle of 5 min at 68°C and a 4°C soak. After agarose electrophoresis and ethidium bromide staining, the amplicons were visualized over a UV transilluminator, and then they were purified by using Montage96 filter plates (Millipore Corp., Billerica, MA). Amplicons were sequenced as described in O'Donnell et al. (33). Raw sequence data were edited and aligned by using Sequencher version 4.1.2 (Gene Codes, Ann Arbor, MI) and then manipulated manually to establish positional homology.
Phylogenetic analysis.
Initially, the neighbor-joining method, using Kimura's two-parameter model implemented in PAUP* (40), was used to assess the phylogenetic diversity of RPB2 sequences of all 319 isolates included in the present study. Based on these analyses, we constructed a DP (Table 2) data set, which included aligned sequences from 72 fusaria plus the outgroup sequence of Lecanicillium lecanii, to further investigate the phylogenetic diversity of medically important fusaria. One indel, 3 bp in length, was inserted in the RPB2 alignment to accommodate for one additional codon unique to all members of the Fusarium dimerum species complex (FDSC). A second indel, 39 bp in length, was inserted in the alignment to adjust for 13 additional amino acids present in all members of the Gibberella clade. A parsimony search of the DP (Table 2) data set conducted with PAUP* version 4.0b10 (40) was implemented, using tree-bisection and reconnection branch swapping and 1,000 random sequence addition replicates. Only parsimony-informative nucleotide positions were included in the analysis. Nonparametric bootstrapping was used to assess clade stability and relative support for internodes, based on 1,000 pseudoreplicates of the data. One of the 186 most-parsimonious trees (MPTs) found from the maximum-parsimony heuristic search was saved for use in the design of allele-specific probes.
Allele-specific probe design.
Character state changes identified in the DNA sequence were mapped on the MPT in order to identify candidate allele-specific probes, using MacClade's data editor (24). Aligned RPB2 sequences of the DP were used to develop 34 allele-specific probes for all six medically important species complexes (15 probes), ten of the most important species (15 probes), plus 4 probes designed for use as positive controls for the PCR amplification (Table 4 and Fig. 1). Assignment of isolates to one unnamed species within the FSSC, referred to as FSSC group 4 (44), was based on positive and negative genotypes for the S-34 and S-4 probes, respectively. The oligonucleotide probes range in length from 18 to 24 nucleotides (Table 4), and every probe ends with a 3′ single nucleotide difference specific to a species and/or species complex, except for the four positive control probes that represent sequences highly conserved throughout the genus. Once designed, all 34 probes were submitted to the Luminex Corp. website (Austin, TX), where they were assigned a unique 24-bp sequence tag, which was incorporated on the 5′ end of each probe primer (Table 4). During the hybridization step (see below), each unique sequence tag anneals to the complementary anti-tag attached to a unique Luminex xMAP fluorescent polystyrene microsphere, thereby allowing each allele to be scored by flow cytometry. In addition to the panel of 72 phylogenetically diverse fusaria for the design and validation of the microsphere array (DP) (Table 2), an EP comprising 157 medically important fusaria (Table 3) was genotyped by using the microsphere array and also independently by RPB2 sequence analysis (Table 3). In addition to including 69 CDC keratitis outbreak investigation isolates (5), the EP included 88 clinical isolates from several sources to further test the accuracy of the microsphere array (Table 3).
TABLE 4.
Allele-specific primers used in genotyping assay
| Target group | Probea | Luminex microspherec | Sequence (5′-3′)b
|
|
|---|---|---|---|---|
| Luminex probe tagc | Probed | |||
| Positive control for PCR amplification (5F2 × 7cR) | 5f2-aa | 35 | CAATTTCATCATTCATTCATTTCA | GGGYCARGCTTGTGGTYTGG |
| Positive control for PCR amplification (5F2 × 7cR) | 5f2-ab | 37 | CTTTTCATCTTTTCATCTTTCAAT | CARGCTTGTGGTYTGGTC |
| Positive control for PCR amplification (7cF × llaR) | 7cf-aa | 55 | TATATACACTTCTCAATAACTAAC | ATGGARTTCCTCAARTTCC |
| Positive control for PCR amplification (7cF × llaR) | 7cf-ab | 78 | CTATCTATCTAACTATCTATATCA | ATGGARTTCCTCAARTTCCGTG |
| FCSC | CC-c | 7 | CAATTCATTTACCAATTTACCAAT | TTCAGCAGAGAGGGTTTGACC |
| FCSC | CC-d | 44 | TCATTTACCAATCTTTCTTTATAC | ACCGTCGATTCCGTYTCGGAACTC |
| Fusarium sp. cf. dimerum | D-b | 23 | TTCAATCATTCAAATCTCAACTTT | TGTTCAGGATGCCAAGCAATTCTG |
| Fusarium sp. cf. dimerum | D-c | 51 | TCATTTCAATCAATCATCAACAAT | AAGCAATTCTGTAATTCGGTAGTC |
| FDSC | DC-a | 59 | TCATCAATCAATCTTTTTCACTTT | AGGCTTGTGGTYTGGTCAAGAACC |
| FDSC | DC-d | 9 | TAATCTTCTATATCAACATCTTAC | GAGTGTCTTCTYAGCAAAGTCTCC |
| Fusarium fujikuroi | F-b | 3 | TACACTTTATCAAATCTTACAATC | ACCAAGGGACAACTAGTGTTGACG |
| Fusarium fujikuroi | F-c | 77 | CAATTAACTACATACAATACATAC | ACACTCACAGAAGAAGAGAAACGA |
| Fusarium fujikuroi + F. proliferatum | PF-a | 18 | TCAAAATCTCAAATACTCAAATCA | GAGGTCATGTACAATGGTCACACG |
| Fusarium fujikuroi + F. proliferatum | PF-b | 30 | TTACCTTTATACCTTTCTTTTTAC | GATGTCACAGTCGATTCAGTCTCT |
| Fusarium sp. cf. incarnatum | I-a | 26 | TTACTCAAAATCTACACTTTTTCA | TGTGAGATTCATCCCAGTATGATC |
| Fusarium sp. cf. incarnatum | I-b | 10 | ATCATACATACATACAAATCTACA | CCTGCCGAGACACCAGAAGGC |
| FIESC | IEC-b | 17 | CTTTAATCCTTTATCACTTTATCA | TTCGTCAACGGAAGTTGGGTCGGC |
| FIESC | IEC-f | 5 | CAATTCAAATCACAATAATCAATC | AAGCATGGAACATACGACAAGCTC |
| FOSC | OC-a | 69 | CTATAAACATATTACATTCACATC | AACTACACTGAGATCTTYGAGAAA |
| Fusarium proliferatum | P-a | 28 | CTACAAACAAACAAACATTATCAA | GAAGAGGAGAAACGGGCCAAGGCT |
| FSSC group 1 | S-1 | 40 | CTTTCTACATTATTCACAACATTA | GGTGATGCCACGCCTTTCACCGAC |
| FSSC group 2 | S-2 | 94 | CTTTCTATCTTTCTACTCAATAAT | GGTATCGTGGCYCCCGGTGTGCGC |
| FSSC groups 3 and 4 | S-34 | 14 | CTACTATACATCTTACTATACTTT | CGCTTGCTACTCTGGTTACAACCAA |
| FSSC group 4 | S-4 | 6 | TCAACAATCTTTTACAATCAAATC | ACGCCGCTGCGAAGTACCGAGAAT |
| FSSC | SC-d | 95 | TACACTTTAAACTTACTACACTAA | ACAATTGCMCATTTGATTGAATGC |
| FSSC | SC-f | 76 | AATCTAACAAACTCATCTAAATAC | ATTGAATGCCTCCTCAGTAAGGTG |
| Fusarium thapsinum | T-b | 29 | AATCTTACTACAAATCCTTTCTTT | ATGTGTTATGTCAGTGTCGGCTCC |
| Fusarium thapsinum | T-e | 100 | CTATCTTTAAACTACAAATCTAAC | CAAGTCATTCTGACAGTCAACGCG |
| Fusarium verticillioides | V-a | 33 | TCAATTACTTCACTTTAATCCTTT | CTTGTTCGTGATATCCGAGACCGC |
| Fusarium verticillioides | V-d | 12 | TACACTTTCTTTCTTTCTTTCTTT | CTTGATGAGGATGGTATCGTGGCA |
| GFSC | GFC-a | 65 | CTTTTCATCAATAATCTTACCTTT | GTYCACTTCGAAGTATCRCTTGTT |
| GFSC | GFC-c | 85 | ATACTACATCATAATCAAACATCA | CRGAAGARGAGAARCGRGCYAAGG |
| GFSC + FOSC | GFCO-c | 80 | CTAACTAACAATAATCTAACTAAC | YCGTGARCARAAGAATGATGARGC |
| GFSC + FOSC | GFCO-d | 24 | TCAATTACCTTTTCAATACAATAC | GACAGGAGGATATGCCTTTCAGCC |
Minimum fluorescence intensity values for the 12 probes in boldface were not used to calculate the ID, because they did not perform as well as the other probes. The V-d probe frequently gave high background non-target fluorescence values and was therefore excluded from the ID scores.
M, AC; R, AG; Y, CT.
Microspheres and tag sequences were assigned at the Luminex website.
Probe sequences used to identify the various target groups within Fusarium spp.
PCR amplification for multiplex primer extension.
RPB2 template for the multiplex primer extension was obtained using the PCR conditions outlined above, with the exception that the 5F2-7cR and 7cF-11aR RPB2 amplicons of each isolate were pooled at the Montage96 filter plate purification step. Multiplex primer extension reactions were performed in a total volume of 20 μl that included the following: 1× PCR buffer, 1.25 mM MgCl2, 5 μM dATP/dGTP/dTTP, 5 μM biotin-dCTP, 25 nM concentrations of each probe primer, 0.75 U of Platinum GenoTYPE Tsp DNA polymerase (Invitrogen Life Technologies), and 5 μl of purified RPB2 amplicon. Cycling parameters for the multiplex primer extension reactions included 120 s at 96°C, followed by 40 cycles of 30 s at 94°C, 60 s at 55°C, and 120 s at 74°C, followed by a 4°C soak.
Hybridization and detection.
Hybridization reactions were conducted in a total volume of 50 μl in 1× TM buffer (0.1 M Tris, 0.2 M NaCl, 0.08% Triton X-100; Sigma Chemical Co., St. Louis, MO), which included 5 μl of multiplex primer extension product, and ∼1,250 microspheres of each of the 34 bead sets. Hybridizations were conducted in a MJ PTC-100 thermocycler (MJ Research, Waltham, MA), using the following cycling parameters: 1 cycle of 90 s at 96°C and 45 min at 37°C, followed by a 4°C soak. Hybridization reactions were purified as described previously (8), after which they were resuspended in 70 μl of 1× TM buffer containing 2 μg of streptavidin-R-phycoerythrin conjugate (Invitrogen Molecular Probes, Eugene, OR)/ml. Reactions were incubated for 10 min at 37°C in a Luminex 100 flow cytometer, and then the samples were sorted and scored by measuring the fluorescence intensity of biotinylated extension products conjugated to 100 microspheres of each of the 34 probes. Each individual microsphere set used in the assay was labeled with a specific mixture of fluorescent dyes (Luminex Corp.), thereby creating a unique spectral address that enabled the extension products from the 34 different allele-specific probes to be sorted and evaluated individually. Indices of discrimination (ID) (8) for the microsphere array-based identifications were determined by dividing the minimum target fluorescence intensity by the maximum nontarget fluorescence intensity based on duplicate independent runs (Tables 2 and 3).
Validation of the Luminex assay using a DP.
Duplicate microsphere array-based identifications of all 72 fusaria in the DP, including 36 strains from the CDC's keratitis outbreak investigation (5), were compared to independent RPB2 sequence-based identifications to assess the accuracy and reproducibility of the microsphere array (Table 2).
Nucleotide sequence accession numbers.
All DNA sequence data generated in the present study has been deposited in GenBank under accession numbers EF452919 to EF453221 and EF469958 to EF470237. RPB2 sequences previously published (5) are available under GenBank accession numbers DQ790471 to DQ790602.
RESULTS
Fusarium phylogeny.
Neighbor-joining analyses of RPB2 sequences of all 318 fusaria included in the present study were conducted to provide an initial assessment of how the keratitis outbreak isolates fell within the full phylogenetic spectrum of Fusarium. The results of these analyses were used to construct a DP data set that included representatives of all medically important species complexes and the most important species. The 73 aligned RPB2 sequences (1,809 bp) of the DP were analyzed by maximum parsimony to assess the phylogenetic diversity of medically and veterinary important fusaria. Thirty-six of the isolates were associated with the contact lens solution keratitis outbreaks of 2005 and 2006 in the United States, including 20 strains that were included in the only detailed microbiological account of these outbreaks (5) (Table 2).
Unweighted maximum-parsimony analysis of the RPB2 data set, which included 608 parsimony informative characters, yielded 186 MPTs (Fig. 1). The outgroup-rooted MPT provided a nearly completely resolved phylogeny of the medically important fusaria sampled, with strong support throughout the tree, as indicated by bootstrap values ranging from 88 to 100% for the six human pathogen containing species complexes (each indicated by a bold internode in Fig. 1), and ten of the most important species. The root of the outgroup species connected to the tree with the F. dimerum (FDSC, 88% bootstrap) and F. solani (FSSC, 100% bootstrap) species complexes always forming the basal most branches within the phylogeny. The branching order of the FDSC and FSSC, however, was unresolved (bootstrap 55%). Phylogenetic relationships of the most important human pathogenic members of the species-rich FSSC were nearly completely resolved (Fig. 1), as reflected by bootstrap values of 90 to 100% for the four most important species groups (i.e., groups 1 to 4 [44]). The remaining ingroup taxa, comprising the Gibberella clade, were strongly supported as monophyletic (98% bootstrap). Phylogenetic results support a sister group relationship between the F. chlamydosporum (FCSC, 100% bootstrap) and F. incarnatum-equiseti species complexes (FIESC, 100% bootstrap), and the terminal sister groups, the Gibberella fujikuroi (GFSC, 97% bootstrap) and F. oxysporum species complexes (FOSC, 100% bootstrap).
Multilocus DNA sequence-based genotyping.
All 191 CDC keratitis investigation isolates from cornea or patient environments were genotyped by using multilocus DNA sequence typing (MLST) (5) (Table 1 and Table S1 in the supplemental material). Included among these were replicate isolates from the cornea of 15 separate patients and from the environments of 14 separate patients, which appeared to be putative clones based on the MLST data (Table 1 and Table S1 in the supplemental material). Although 13 MLSTs were represented, three haplotypes accounted for about two-thirds of the putative clones (i.e., FSSC 1-a, FSSC 2-d, and FOSC 3-a; Fig. 1). Of the 10 matched corneal-environment isolates, 6 were from confirmed cases, and each of the 10 paired isolates shared the same MLST. In addition, all of the replicate isolates from each patient's environment (n = 14) shared the same MLST (see Table S1 in the supplemental material). In all, the corneal isolates comprised 29 multilocus haplotypes distributed among 16 species, whereas the environmental isolates included 27 haplotypes and 15 species (Table 1). Although isolates from five species complexes were represented among the corneal isolates (Table 1), members of the FSSC comprised 61.8% of the isolates. FSSC haplotypes 1-a and 2-d, representing two phylogenetically distinct species, accounted for the majority of FSSC isolates from patients and their environment. Collectively, the FSSC was represented by 24 multilocus haplotypes, including 14 from corneal isolates and 14 from the environmental assessment, and these were distributed among nine phylogenetically distinct species (i.e., FSSC 1 to 9). The only other complex represented by significant numbers was the FOSC, which accounted for 29.7% of the corneal and 29% of the environmental isolates (Table 1). Overall, the FOSC was represented by 10 unique multilocus haplotypes, with the widespread FOSC 3-a clonal lineage (33) accounting for the majority of all isolates within this complex. The single most common haplotype from each of the three most common species (i.e., FSSC 1-a, FSSC 2-d, and FOSC 3-a) collectively comprised 55% of the corneal and environmental isolates (Table 1 and Table S1 in the supplemental material). Moreover, members of the FSSC and FOSC accounted for 91.1% of the CDC keratitis outbreak isolates and 34 of the 45 multilocus haplotypes. The 17 remaining outbreak investigation isolates were distributed among three species complexes (Table 1; GFSC, FIESC, and FDSC) and 11 phylogenetically distinct species; however, they accounted for only 11% of the corneal and 7% of the environmental isolates.
Allele-specific genotyping.
Independent RPB2-based identifications of the 72 isolates in the DP revealed that all were correctly identified to species and/or species complex by the microsphere array (Table 2). ID values for the species and/or species complex probes for the 72 DP isolates ranged from 2.6 to 53.2 with a mean fluorescence of 10.1. Moreover, 96 of 101 positives in the DP had fluorescence intensity values of at least three times above background (95%; Table 2). Of the 34 probes, 23 accounted for 100% of the ID scores for the DP (Table 2) and the EP (see below; Table 3). However, only the ID scores for the F. verticillioides V-d probe were excluded from the analysis because it frequently gave false-positive results.
An EP consisting of 157 clinical isolates was genotyped by using the validated microsphere array and also independently by RPB2 sequence analysis (Table 3). The EP included 69 CDC keratitis outbreak investigation isolates (5), 27 additional clinical isolates from the CDC unrelated to the keratitis investigation, 54 clinical isolates from the Fungus Testing Laboratory at the University of Texas, San Antonio, together with 7 isolates from other sources (Table 3). All 69 CDC keratitis outbreak isolates genotyped by the microsphere array were correctly assigned to species and/or species complex (Tables 2 and 3), as confirmed by the independent RPB2 sequence analysis. Of the 105 CDC keratitis outbreak isolates genotyped in the combined DP and EP, 87.5% of the FSSC (56 of 64) and all 8 GFSC isolates were identified to species by using the single locus assay. The eight isolates for which species identity could not be determined using the microsphere assay were all correctly assigned to the FSSC. However, the RPB2 and MLST data (Table 1 and Table S1 in the supplemental material) indicated that they represented relatively uncommon taxa that were not included in the DP. Therefore, probes for these rare species were not represented in the microsphere array.
All of the EP isolates were accurately assigned to one of six species complexes by the microsphere array, as confirmed by the RPB2 sequence data (Table 3), with the exception of NRRL 13459 F. concolor (later synonym = F. polyphialidicum) and NRRL 43641 Fusarium sp. (ex horse eye), each of which represents an additional evolutionary lineage within Fusarium. However, in contrast to the DP test results, in which all of the isolates were correctly assigned to species and/or species complex, nine EP isolates not involved in the keratitis outbreaks were misidentified at the species level by the microsphere array (i.e., 94.3% accuracy). Seven of the nine misidentifications involved CDC isolates that were incorrectly identified as FSSC group 1 (NRRL 28008 to 28011) or FSSC group 3 (NRRL 28000 to 28002) by the S-1 and S-34 probes, respectively. Analysis of the RPB2 sequence data indicated that the FSSC isolates that were misidentified represent three species that are phylogenetically distinct from FSSC groups 1 to 4 (44). It is also noteworthy that the F. proliferatum-F. fujikuroi PF-b group probe incorrectly indicated that F. concolor was a member of the GFSC. However, the GFSC-specific GFC-c probe for this species complex was negative, indicating the result obtained with the PF-b group probe was in error. Subsequently, this finding was confirmed independently by RPB2 sequence analysis. The only other isolate misidentified at the species level by the microsphere array was FDSC isolate NRRL 43716 Fusarium sp. (ex human blood), which was typed as F. sp. (cf. F. dimerum) (F. sp. cf. dimerum) with the D-b and D-c species probes. Although the RPB2 sequence data determined this species identification was incorrect, the microsphere array correctly assigned this isolate to the FDSC (Table 3). We determined that all nine species level misidentifications were not false positives by comparing the five probe sequences with the respective nontarget sequence. These comparisons revealed that each probe sequence was either an exact match (S-34, D-b, and D-c) or differed by a single nucleotide in the middle of the probe (S-1 and PF-b).
ID values for the target species and/or species complex probes for the 157 EP isolates ranged from 2.6 to 47.1, with a mean of 10.9. Also, 245 of the 250 positives in the EP had fluorescence intensity values at least three times above background (98%; Table 3). In the combined DP and EP data, 341 of the 351 positives had fluorescence intensity values at least three times above background (97.2%; Table 3), with a mean ID of 10.7. Lastly, NRRL 43875 (CDC 2006743712 ex contact lens case in Kentucky) was identified as FSSC group 1 by the RPB2 sequence data; however, the microsphere array indicated that it was a mixed culture containing FSSC groups 1 and 2.
DISCUSSION
Multilocus and allele-specific genotyping.
The present study extends CDC's keratitis outbreak investigation that established use of the since discontinued Bausch & Lomb's ReNu with MoistureLoc contact lens solution as a significant risk factor for acquiring FK among soft contact lens wearers (5). Moreover, we provide here the first detailed genotypic comparison of the fusaria causing keratitis with those recovered from patient's environments (i.e., contact lens and lens case). In addition to facilitating an early diagnosis of FK and prompt antifungal therapy (4), the availability of cultures from corneal scrapings and environmental isolates played a central role in developing objective epidemiological data and molecular diagnostic tools in the present study.
In contrast to a phenotype-based report that members of the FOSC were the most common fusaria associated with the outbreaks in 2005 and 2006 (1), the molecular phylogenetic data presented herein and by Chang et al. (5) clearly demonstrate that FSSC outbreak isolates from the United States (including Puerto Rico) collectively accounted for twice as many corneal isolates as those from the FOSC (Table 1). When this comparison was restricted to the keratitis outbreak isolates from Florida (15 corneal and 6 environmental; see Table S1 in the supplemental material), all of these isolates were identified as members of the FSSC, except for two isolates of the widespread FOSC 3-a clonal lineage (33). Similar findings that all of the outbreak isolates of 2005 and 2006 from Singapore and Hong Kong were members of the FSSC (5, 20) are consistent with the results of previous studies that have documented the predominance of FSSC fusaria in causing ocular mycoses worldwide (13, 26, 44). The results of Chang et al. (5) and the present study, however, have established that phylogenetically diverse members of the FOSC can infect the human cornea (Table 1). This finding is one of the surprising results to emerge from the present study, in that the only comprehensive molecular phylogenetic analysis of medically important FOSC concluded that members of this complex might only rarely cause eye infections (33). Another surprise was our finding that corneal infections were most frequently associated with FSSC group 1 and least frequently associated with FSSC group 3, whereas Zhang et al. (44) reported the converse. To reconcile these seemingly conflicting reports, Chang et al. (5) hypothesized that FSSC group 3 isolates may be most commonly associated with ocular trauma, given they are commonly found in soil and on vegetation; however, patients with a recent history of ocular trauma were excluded from the keratitis outbreak investigation by CDC's case definition. It is also noteworthy that the initial report by Chang et al. (5) and the present study are the first to conclusively document F. fujikuroi as a human pathogen, and these involved four separate patients with infections of either their cornea, skin, or nails (Tables 2 and 3).
Our finding that the three haplotypes most frequently recovered from the patient's environment (FSSC 1-a, FSSC 2-d, and FOSC 3-a) were also responsible for the greatest numbers of ocular infections strongly suggests, as originally proposed by Chang et al. (5), that the majority of the keratitis outbreak infections were likely caused by the most prevalent fusaria in the patient's environment at the point of contact lens and lens solution use. This conclusion is supported by the fact that each of the 10 matched corneal-environment isolates we genotyped shared the same MLST, suggesting that extrinsic contamination of the patient's contact lens and lens cases played a major role in the keratitis outbreaks. The high-degree of matching between corneal and environmental isolates also supports the common ophthalmologic practice of culturing contact lens supplies when contact lens-associated corneal infection is suspected (7). By way of contrast, Fusarium was not recovered by the CDC from unopened contact lens solution bottles from relevant lots of ReNu with MoistureLoc nor from environmental samples taken from Bausch & Lomb's production facility in Greenville, SC, providing additional evidence that the outbreaks were not due to a common point-source (5). Although genetically matching isolates were obtained from contact lens and bottles of opened MoistureLoc lens solution from patients in Georgia (FOSC 3-a) and Pennsylvania (FSSC 3-a) in the present study (see Table S1 in the supplemental material) and from a patient's lens case and opened bottle of MoistureLoc in New York state (9), all of the available evidence supports the conclusion that unopened bottles of ReNu with MoistureLoc are sterile microbiologically. We hypothesize that the patient's water system (e.g., bathroom sink drain) may represent the primary reservoir of infection, largely because the three predominant haplotypes we recovered from cornea and the patient's environment appear to be common and widespread inhabitants of sink and shower drains (33, 44), including those in hospitals where they may potentially contribute to life-threatening nosocomial infections among immunocompromised patients (2, 22). Assuming that the three prevalent haplotypes are particularly well adapted to water systems, such as sink drains, it seems probable that any shared adaptations predate the evolution of these phylogenetic lineages, given that these haplotypes exhibit a polyphyletic distribution in the Fusarium phylogeny (Fig. 1).
Reproducibility of the Fusarium microsphere array was confirmed in the present study by duplicate runs of all 229 isolates we typed. Minimum fluorescence scores for isolates with a positive genotype were always at least two and one-half times greater than the maximum fluorescent intensity scores for those with a negative genotype. However, based on independent comparisons of the microsphere array-based identifications with those obtained via RPB2 sequencing and/or MLST, the current array has several noteworthy limitations that need to be addressed before clinical laboratories can benefit from its full potential. The present allele-specific genotyping scheme, which is based exclusively on RPB2 nucleotide variation, incorrectly identified a small number of isolates at the species level within the species-rich FSSC (Table 3), and it lacked the power to resolve the multilocus genotypes identified by MLST within the FSSC, FIESC, and FOSC (Table 1 and Table S1 in the supplemental material). Because the current assay only detects a relatively small number of RPB2 genotypes, ongoing efforts are directed at expanding the database to facilitate MLST-based estimation of haplotype-disease associations in the future.
Fusarium phylogeny.
The present study extends our growing knowledge of the evolutionary relationships and phylogenetic diversity of medically and veterinary important fusaria, focusing on isolates from the keratitis outbreaks of 2005 and 2006 associated with the use of ReNu with MoistureLoc contact lens solution within the United States (5). Prior to the present study, published phylogenies that spanned the breath of the genus were based exclusively on analyses of domains D1+D2 of the nuclear large subunit (LSU) ribosomal DNA (rDNA) (17, 39), primarily because this region is conserved enough such that a genus-wide alignment reflects positional homology and secondarily because of the ready availability of diverse fusarial LSU rDNA sequences from GenBank. However, because the LSU molecule is so highly conserved and the region sequenced in these studies is so small (440 to 500 bp), it is not surprising that this locus contains relatively little phylogenetic signal within Fusarium. As a result, the bootstrap values recovered were much too low for the LSU-based phylogenies to represent meaningful hypotheses of evolutionary relationships within Fusarium. For example, the FOSC, FSSC, and GFSC received bootstrap support of 68%, 35 to 67%, and 56 to 79%, respectively, in the two aforementioned LSU rDNA-based phylogenies. By way of contrast, the monophyly of these three clades was strongly supported in the present RPB2 phylogeny as reflected by bootstrap values of 97 to 100% (Fig. 1). Not surprisingly, the multilocus species level studies published to date, that included nuclear LSU and/or ITS rDNA sequence data, clearly demonstrate that molecular diagnostic assays targeting either the LSU (15, 28) or ITS rDNA (18, 43) would fail to differentiate medically important species within several species complexes within Fusarium.
Although frequently analyzed as protein coding sequence to help elucidate deep-level evolutionary relationships within the fungi (19, 23), several studies have demonstrated the utility of RPB2 nucleotide variation at the species level in diverse fungi (10, 16). The present study highlighted two attractive features of RPB2 nucleotide sequence data for phylogeny reconstruction within Fusarium: the region sequenced (1.8 kb) was easily aligned across the entire genus, and the wealth of phylogenetic signal contained within this locus provided strong bootstrap support for many clades for the first time. However, given the poor bootstrap support for the branching order of the two basal-most clades (i.e., FSSC and FDSC), data from additional phylogenetically informative loci will be required to fully resolve the phylogeny for this medically and agriculturally important genus. Nucleotide sequence data from translation elongation factor 1α have been extraordinarily useful in Fusarium phylogenetics within species complexes and at the species level (reference 11 and references therein); however, the large phylogenetically informative intronic regions within this gene are much too variable to align across the genus.
A noteworthy finding to emerge from the present study is that 99.4% of the clinical fusaria genotyped were nested in one of six monophyletic species complexes. Members of two other evolutionary lineages were identified, but they are both represented by a single known keratitis isolate, Fusarium sp. strain NRRL 43641 from a horse in Missouri and F. concolor from a human corneal ulcer in Spain (14). Although we genotyped several isolates from the FCSC, including human and equine corneal isolates from Florida (NRRL 43632) and Texas (NRRL 43636), respectively, there is only one published report of a member of this species complex causing keratitis (26). No member of this complex, however, was encountered in the present keratitis outbreak investigation. Unfortunately, conventional taxonomic practice has been to treat members of the FSSC, FOSC, FCSC, and FDSC each as a single morphospecies (27; i.e., F. solani, F. oxysporum, F. chlamydosporum, and F. dimerum, respectively) and members of the FIESC as only two morphospecies (i.e., F. incarnatum and F. equiseti; the former morphospecies is also reported as F. semitectum). The present study is the first to highlight the need for multilocus species level studies within the FCSC, FDSC, and FIESC, each of which appears to contain multiple morphologically cryptic species pathogenic to humans based on the present phylogenic results. Although multilocus phylogenetic hypotheses of evolutionary relationships have been developed for the FSSC (29, 44), FOSC (31, 33), and GFSC (30, 32), additional species level studies that use multilocus phylogenetic species recognition (42) are needed within the pathogen-rich FSSC to clarify the limits of the approximately 18 phylogenetically distinct and medically important species that are nested within clade 3 of this complex (44). Fortunately, there is a growing recognition within the medical community that isolates identified as F. solani typically represent multiple genetically diverse species (5, 13, 26). However, at present species names can only be confidently applied to two of the human pathogenic members of the FSSC, and these species were only recently transferred to Fusarium from Acremonium and Cylindrocarpon where they had been misplaced (39).
Future directions.
Because MLST typing schemes are so labor-intensive and expensive and because they are currently considerably less efficient than allele-specific assays such as the one developed herein, the present study should be viewed as just the first step in the transition from a multilocus DNA sequence-based to a single nucleotide polymorphisms (SNP)-based platform for genotyping medically important fusaria and their epidemiologically relevant haplotypes. It is noteworthy that an SNP-based assay has been developed recently for toxigenic fusaria (21). Because it is relatively easy to design SNP or allele-specific probes from a comprehensive set of aligned, phylogenetically informative DNA sequences, improvements in the present assay could easily take advantage of allelic variation identified in the present and future multilocus phylogenetic analyses. Given the electronic portability of multilocus DNA sequence data (25), future improvements of the Fusarium-ID database (11) will also incorporate MLST data on clinically important fusaria to help facilitate strain typing via the internet. However, irrespective of what genotyping platform is used in the future, the utility of the MLST data is expected to increase as the database expands. Ongoing whole-genome sequencing projects of four phylogenetically diverse fusaria (http://www.broad.mit.edu/annotation/fungi/fgi/and http://www.jgi.doe.gov/) should greatly accelerate the discovery of phylogenetically informative loci and population level molecular markers. Additional multilocus molecular phylogenetic analyses that use dense taxon sampling should prove to be invaluable in improving our understanding of species limits and their reproductive mode and in identifying nucleotide variation necessary for increasing the discriminatory power of the Fusarium database so that robust typing and subtyping assignments can be achieved. In addition, pathogen control programs and future outbreak investigations could greatly benefit from microsatellite (41) and variable numbers of tandem repeat-based typing schemes (37) for elucidating the population structure and reproductive mode of the most common and geographically widespread human pathogenic fusarial haplotypes, such as FSSC 1-a, FSSC 2-d, and FOSC 3-a. Such studies will make robust clone or clonal lineage assignments possible for the first time, which are essential for objective epidemiological assessments of their host range, virulence (34), geographic distribution, and population of origin.
Supplementary Material
Acknowledgments
We thank Alison Strom and Jean Juba for excellent technical assistance, Thomas Usgaard for invaluable discussions regarding genotyping with the Luminex 100 flow cytometer, Don Fraser for preparation of the tree figure, Jennifer Steele for running all of the DNA sequences in the NCAUR DNA core facility, François Lutzoni for advice on RPB2 primers, and the individuals and culture collections that supplied isolates used in this study.
The mention of trade products or firm names does not imply that they are recommended or endorsed by the U.S. Department of Agriculture over similar products or other firms not mentioned. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 16 May 2007.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Alfonso, E. C., J. Cantu-Dibildox, W. M. Munir, D. Miller, T. P. O'Brien, C. L. Karp, S. H. Yoo, R. K. Forster, W. W. Culbertson, K. Donaldson, J. Rodila, and Y. Lee. 2006. Insurgence of Fusarium keratitis associated with contact lens wear. Arch. Ophthalmol. 124:941-947. [DOI] [PubMed] [Google Scholar]
- 2.Anaissie, E. J., R. T. Kuchar, J. H. Rex, A. Francesconi, M. Kasai, F.-M. Müller, M. Lozano-Chiu, R. C. Summerbell, M. C. Dignani, S. J. Chanock, and T. J. Walsh. 2001. Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin. Infect. Dis. 33:1871-1878. [DOI] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, S. Nangia, and J. H. Rex. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal, M. D., N. R. Acharya, T. M. Lietman, E. C. Strauss, S. D. McLeod, and D. G. Hwang. 2006. Outbreak of Fusarium keratitis in soft contact lens wearers in San Francisco. Arch. Ophthalmol. 124:1051-1053. [DOI] [PubMed] [Google Scholar]
- 5.Chang, D. C., G. B. Grant, K. O'Donnell, K. A. Wannemuehler, J. Noble-Wang, C. Y. Rao, L. M. Jacobson, C. S. Crowell, R. Sneed, F. M. T. Lewis, J. K. Schaffzin, M. Kainer, C. A. Genese, E. C. Alfonso, D. B. Jones, A. Srinivasan, S. K. Fridkin, and B. J. Park. 2006. A multistate outbreak of Fusarium keratitis associated with use of a new contact lens solution. JAMA 296:953-963. [DOI] [PubMed] [Google Scholar]
- 6.Das, S., T. M. Brown, K. L. Kellar, B. P. Holloway, and C. J. Morrison. 2006. DNA probes for the rapid identification of medically important Candida species using a multianalyte profiling system. FEMS Immunol. Med. Microbiol. 46:244-250. [DOI] [PubMed] [Google Scholar]
- 7.Driebe, W. T. 2003. Present status of contact lens-induced corneal infections. Ophthalmol. Clin. N. Am. 16:485-494. [DOI] [PubMed] [Google Scholar]
- 8.Ducey, T. F., B. Page, T. Usgaard, M. K. Borucki, K. Pupedis, and T. J. Ward. 2006. A SNP-based multilocus genotyping assay for subtyping Lineage 1 isolates of Listeria monocytogenes. Appl. Environ. Microbiol. 73:133-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyavaiah, M., R. Ramani, D. S. Chu, D. C. Ritterband, M. K. Shah, W. A. Samsonoff, S. Chaturvedi, and V. Chaturvedi. 2007. Molecular characterization, biofilm analysis and experimental biofouling study of Fusarium isolates from recent cases of fungal keratitis in New York State. BMC Ophthalmol. 7:1. [Online.] doi: 10.1186/1471-2415-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froslev, T. G., P. B. Matheny, and D. S. Hibbett. 2005. Lower level relationships in the mushroom genus Cortinarius (Basidiomycota, Agaricales): a comparison of RPB1, RPB2, and ITS phylogenies. Mol. Phylogenet. Evol. 37:602-618. [DOI] [PubMed] [Google Scholar]
- 11.Geiser, D. M., M. del M. Jiménez-Gasco, S. Kang, I. Makalowska, N. Veeraraghavan, T. J. Ward, N. Zang, G. A. Kuldau, and K. O'Donnell. 2004. FUSARIUM-ID v.1.0: a DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 110:1-7. [Google Scholar]
- 12.Gerlach, W., and H. Nirenberg. 1982. The genus Fusarium: a pictorial atlas. Mitt. Biol. Bundesanst. Land-u. Forstwirtsch. 209:1-406. [Google Scholar]
- 13.Godoy, P., J. Cano, J. Gené, J. Guarro, A. L. Hőfling-Lima, and A. L. Colombo. 2004. Genotyping of 44 isolates of Fusarium solani, the main agent of fungal keratitis in Brazil. J. Clin. Microbiol. 42:4494-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarro, J., C. Rubio, J. Gené, J. Cano, J. Gil, R. Benito, M. J. Moranderia, and E. Miguez. 2003. Case of keratitis caused by an uncommon Fusarium species. J. Clin. Microbiol. 41:5823-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, L., S. Wohlfiel, and G. D. Roberts. 2004. Experience with the Microseq D2 large-subunit ribosomal DNA sequencing kit for identification of filamentous fungi encountered in the clinical laboratory. J. Clin. Microbiol. 42:622-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen, K., K. F. Lobuglio, and D. H. Pfister. 2005. Evolutionary relationships of the cup-fungus genus Peziza and Pezizaceae inferred from multiple nuclear genes: RPB2, beta-tubulin, and LSU rDNA. Mol. Phylogenet. Evol. 36:1-23. [DOI] [PubMed] [Google Scholar]
- 17.Hennequin, C., E. Abachin, F. Symoens, V. Lavarde, G. Reboux, N. Nolard, and P. Berche. 1999. Identification of Fusarium species involved in human infections by 28S rRNA gene sequencing. J. Clin. Microbiol. 37:3586-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao, C. R., L. Huang, J.-P. Bouchara, R. Barton, H. C. Li, and T. C. Chang. 2005. Identification of medically important molds by an oligonucleotide array. J. Clin. Microbiol. 32:3760-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James, T. Y., F. Kauff, C. L. Schoch, P. B. Matheny, V. Hofstetter, C. J. Cox, G. Celio, C. Gueidan, E. Fraker, J. Miadlikowska, H. T. Lumbsch, A. Rauhut, V. Reeb, A. E. Arnold, A. Amtoft, J. E. Stajich, K. Hosaka, G. H. Sung, D. Johnson, B. O'Rourke, M. Crockett, M. Binder, J. M. Curtis, J. C. Slot, Z. Wang, A. W. Wilson, A. Schussler, J. E. Longcore, K. O'Donnell, S. Mozley-Standridge, D. Porter, P. M. Letcher, M. J. Powell, J. W. Taylor, M. M. White, G. W. Griffith, D. R. Davies, R. A. Humber, J. B. Morton, J. Sugiyama, A. Y. Rossman, J. D. Rogers, D. H. Pfister, D. Hewitt, K. Hansen, S. Hambleton, R. A. Shoemaker, J. Kohlmeyer, B. Volkmann-Kohlmeyer, R. A. Spotts, M. Serdani, P. W. Crous, K. W. Hughes, K. Matsuura, E. Langer, G. Langer, W. A. Untereiner, R. Lucking, B. Budel, D. M. Geiser, A. Aptroot, P. Diederich, I. Schmitt, M. Schultz, R. Yahr, D. S. Hibbett, F. Lutzoni, D. J. McLaughlin, J. W. Spatafora, and R. Vilgalys. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818-822. [DOI] [PubMed] [Google Scholar]
- 20.Khor, W.-B., T. Aung, S.-M. Saw, T.-Y. Wong, P. A. Tambyah, A.-L. Tan, R. Beuerman, L. Lim, W.-K. Chan, W.-J. Heng, J. Lim, R. S. K. Loh, S.-B. Lee, and D. T. Tan. 2006. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA 295:2867-2873. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen, R., K. G. Berdal, and A. Holst-Jensen. 2007. Simultaneous detection and identification of trichothecene- and moniliformin-producing Fusarium species based on multiplex SNP analysis. J. Appl. Microbiol. 02:1071-1081. [DOI] [PubMed] [Google Scholar]
- 22.Kuchar, R. T. 1996. Isolation of Fusarium from hospital plumbing fixtures: implications for environmental health and patient care. M.S. thesis. The University of Texas Health Science Center, Houston, TX.
- 23.Lutzoni, F., F. Kauff, C. J. Cox, D. McLaughlin, G. Celio, B. Dentinger, M. Padamsee, D. Hibbett, T. James, E. Baloch, M. Grube, V. Reeb, V. Hofstetter, C. Schoch, A. E. Arnold, J. Spatafora, G.-H. Sung, S. Hambleton, J. Miadlikowska, R. Lücking, R. Shoemaker, K. O'Donnell, M. Binder, M. Crockett, P. Diederich, D. Ertz, C. Gueidan, B. Hall, K. Hansen, R. C. Harris, K. Hosaka, D. Johnson, Y.-W. Lim, Y. Liu, T. Lumbsch, B. Matheny, H. Nishida, D. Pfister, J. Rogers, A. Rossman, I. Schmitt, H. Sipman, J. Stone, J. Sugiyama, R. Yahr, and R. Vilgalys. 2004. Where are we in assembling the fungal tree of life, classifying the Fungi, and understanding the evolution of their subcellular traits. Am. J. Bot. 91:1446-1480. [DOI] [PubMed] [Google Scholar]
- 24.Maddison, W. P., and D. R. Maddison. 2002. MacClade 4. Analysis of phylogeny and evolution, version 4.06. Sinauer Associates, Sunderland, MA.
- 25.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 5:140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naiker, S., and B. Odhav. 2003. Mycotic keratitis: profile of Fusarium species and their mycotoxins. Mycoses 47:50-56. [DOI] [PubMed] [Google Scholar]
- 27.Nelson, P. E., T. A. Toussoun, and W. F. O. Marasas. 1983. Fusarium species: an illustrated manual for identification. Pennsylvania State University Press, University Park.
- 28.Ninet, B., I. Jan, O. Bontems, B. Léchenne, O. Jousson, D. Lew, J. Schrenzel, R. G. Panizzon, and M. Monod. 2005. Molecular identification of Fusarium species in onychomycoses. Dermatology 210:21-25. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell, K. 2000. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92:919-938. [Google Scholar]
- 30.O'Donnell, K., E. Cigelnik, and H. I. Nirenberg. 1998. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465-493. [Google Scholar]
- 31.O'Donnell, K., H. C. Kistler, E. Cigelnik, and R. C. Ploetz. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 95:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell, K., H. I. Nirenberg, T. Aoki, and E. Cigelnik. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41:61-78. [Google Scholar]
- 33.O'Donnell, K., D. A. Sutton, M. G. Rinaldi, K. C. Magnon, P. A. Cox, S. G. Revankar, S. Sanche, D. M. Geiser, J. H. Juba, J.-A. H. van Burik, A. A. Padhye, E. J. Anaissie, A. Francesconi, T. J. Walsh, and J. S. Robinson. 2004. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J. Clin. Microbiol. 42:5109-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortoneda, M., J. Guarro, M. P. Madrid, Z. Caracuel, M. I. G. Roncero, E. Mayayo, and A. Di Pietro. 2004. Fusarium oxysporum as multihost model for the genetic dissection of fungal virulence in plants and animals. Infect. Immun. 72:1760-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page, B. T., C. E. Shields, W. G. Merz, and C. P. Kurtzman. 2006. Rapid identification of ascomycetous yeasts from clinical specimens by a molecular method based on flow cytometry and comparison with identifications from phenotypic assays. J. Clin. Microbiol. 44:3167-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeb, V., F. Lutzoni, and C. Roux. 2004. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet. Evol. 32:1036-1060. [DOI] [PubMed] [Google Scholar]
- 37.Suga, H., L. R. Gale, and H. C. Kistler. 2004. Development of VNTR markers for two Fusarium graminearum clade species. Mol. Ecol. Notes 4:468-470. [Google Scholar]
- 38.Summerbell, R. C. 2003. Aspergillus, Fusarium, Sporothrix, Piedraia, and their relatives, p. 237-498. In D. H. Howard (ed.), Pathogenic fungi in humans and animals. Marcel Dekker, Inc., New York, NY.
- 39.Summerbell, R. C., and H.-J. Schroers. 2002. Analysis of phylogenetic relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. J. Clin. Microbiol. 40:2866-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA.
- 41.Taylor, J. W., and M. C. Fisher. 2003. Fungal multilocus sequence typing: it's not just for bacteria. Curr. Opin. Microbiol. 6:351-356. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, J. W., D. J. Jacobson, S. Kroken, T. Kasuga, D. M. Geiser, D. S. Hibbett, and M. C. Fisher. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31:21-31. [DOI] [PubMed] [Google Scholar]
- 43.Turenne, C. Y., S. E. Sanche, D. J. Hoban, J. A. Karlowsky, and A. M. Kabani. 1999. Rapid identification of fungi by using the ITS2 genetic region and an automatic fluorescent capillary electrophoresis system. J. Clin. Microbiol. 37:1846-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, N., K. O'Donnell, D. A. Sutton, F. A. Nalim, R. C. Summerbell, A. A. Padhye, and D. M. Geiser. 2006. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 44:2186-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.