Abstract
We performed Etest, disk diffusion, and broth microdilution susceptibility testing of 2,171 clinical isolates of Candida spp. against posaconazole. By using provisional breakpoints for comparison purposes only, the categorical agreement between the agar-based methods and broth microdilution results ranged from 93 to 98%, with <1% very major errors. The essential agreement (within 2 well dilutions) between the Etest and broth microdilution methods was 94%. These agar-based methods hold promise as simple and reliable methods for determination of the posaconzole susceptibilities of Candida spp.
The Clinical and Laboratory Standards Institute (CLSI) M27-A2 reference broth microdilution method (BMD) requires 48 h of incubation and can be cumbersome to perform for laboratories that test relatively few Candida spp. Therefore, there is interest in the validation of the agar-based methods of disk diffusion (DD) and Etest for the antifungal susceptibility testing of Candida spp. The results of these methods have been demonstrated to have a good correlation with the BMD results for the testing of Candida against fluconazole and voriconazole (4, 5, 7, 8). However, fewer data exist for the newer triazole posaconazole (4, 6, 9). The most recent published data suggest that there is good correlation between both Etest MICs and disk zone diameters with BMD MICs for bloodstream isolates of Candida spp. tested against posaconazole. However, less than 300 Candida bloodstream isolates were tested (9).
The purpose of this study was to broaden and expand the evaluation of agar-based methods (DD and Etest) for the testing of Candida spp. against posaconazole. We tested a large international collection of bloodstream or sterile site isolates of Candida spp. by Etest and DD methods and compared the results obtained by those methods to the results obtained by the reference BMD method, run in parallel.
A total of 2,171 isolates of Candida spp. were obtained from 60 medical centers worldwide during 2004 and 2005. All were incident clinical isolates of Candida obtained from blood or other sterile site cultures. Isolates were identified with the Vitek and API yeast identification systems (bioMerieux, Inc., Hazelwood, MO), and the identification tests with these systems were supplemented by conventional methods as required. The isolates were stored as water suspensions until use. Prior to testing, each isolate was passaged on potato dextrose agar (Remel, Lenexa, KS) and CHROMagar (Hardy Laboratories, Santa Maria, CA) to ensure viability and purity.
All 2,171 Candida were tested in parallel by both DD and BMD, while 1,416 isolates were tested in parallel by Etest and BMD.
Reference antifungal susceptibility testing of Candida against posaconazole was performed by BMD exactly as described by the CLSI (2). A reference powder of posaconazole was obtained from Schering Plough (Kenilworth, NJ).
Posaconazole Etest strips were provided by AB Biodisk (Solna, Sweden). MICs were determined by the Etest as described previously (6) with RPMI 1640 agar supplemented with 2% glucose (Remel), an inoculum suspension adjusted to the turbidity of a 0.5 McFarland standard, and incubation at 35°C for 48 h. The MIC of posaconazole was read as the lowest concentration at which the border of the elliptical inhibition zone intercepted the scale on the strip. Any growth such as microcolonies throughout a discernible inhibition ellipse was ignored.
DD testing of posaconazole was performed as described previously and as outlined by the CLSI (3). Posaconazole disks (5 μg) were obtained from Oxoid (Lenexa, KS). For DD testing, 90-mm-diameter agar plates containing Mueller-Hinton agar supplemented with 2% glucose and methylene blue (0.5 μg/ml) at a depth of 4 mm were used. The agar surface was inoculated by using a swab dipped in a cell suspension adjusted to the turbidity of a 0.5 McFarland standard. After the disks were placed on the agar surface, the plates were incubated in air at 35°C, and the zone diameters surrounding the posaconazole disks were read at 24 h. Zone diameter endpoints were read at 80% growth inhibition by using a BIOMIC image analysis plate reader system (version 5.9; Giles Scientific, Santa Barbara, CA).
Interpretive breakpoints do not exist for posaconazole and Candida spp. For the purpose of method comparison only, we applied the MIC and DD breakpoints recently approved for voriconazole: susceptible: ≤1 μg/ml and ≥17 mm, respectively; susceptible dose dependent, 2 μg/ml and 14 to 16 mm, respectively; and resistant, ≥4 μg/ml and ≤13 mm, respectively.
Quality control was performed for the BMD and Etest methods in accordance with CLSI document M27-A2 by using Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 (2). The quality control determinations made on each day of testing were within the control limits for posaconazole described by Barry et al. (1). The quality control for disk diffusion testing was performed by using Candida albicans ATCC 90028 and C. parapsilosis ATCC 22019 (3).
The Etest MICs of posaconazole were read at 24 and 48 h and were compared to the reference BMD MICs read at 24 and 48 h. The Etest MICs were rounded up to the next even log2 concentration for the analysis.
The diameters of the zones of inhibition (in millimeters) surrounding the posaconazole disks were plotted against their respective BMD MICs. The method of least squares was used to calculate a regression line for this comparison.
The interpretive breakpoints presented above were used to determine the categorical agreement between the Etest, DD, and BMD MIC results. Major errors (MEs) were classified as results of resistance by DD or Etest and susceptible by BMD. Very major errors (VMEs) were classified as results of susceptible by DD or Etest and resistance by BMD. Minor errors occurred when the result of one of the tests was susceptible or resistance and that of the other test was susceptible dose dependent.
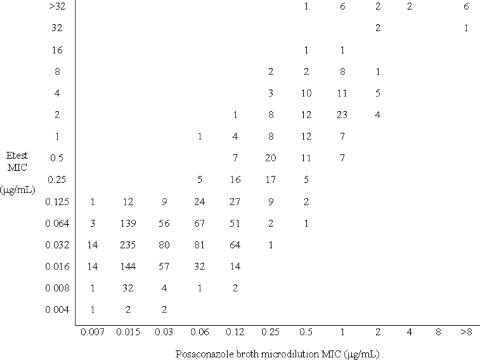
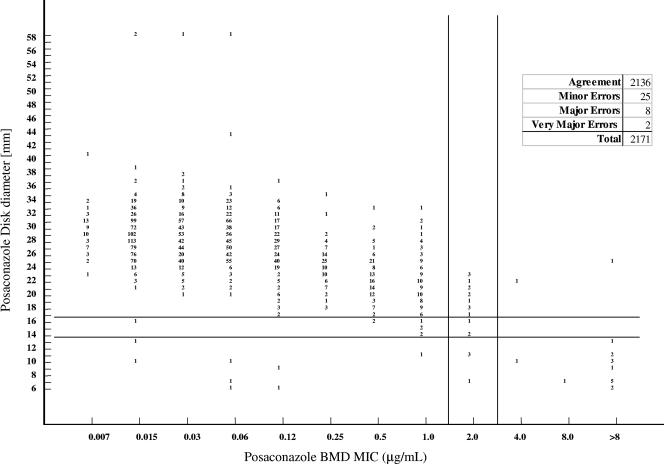
The Figures 1 and 2 and Table 1 demonstrate the agreement between the DD, Etest, and BMD results. A total of 2,171 Candida were tested by both DD and BMD, and 1,416 isolates were tested by both Etest and BMD. Most (98%) of the isolates had a posaconazole MIC <2 μg/ml by BMD, 1% (n = 20) had an MIC of 2 μg/ml (susceptible dose dependent), and 1% (n = 18) had MICs ≥4 μg/ml (resistant). All posaconazole-nonsusceptible isolates were C. glabrata, with the exception of a single posaconazole-resistant Candida pelliculosa isolate.
FIG. 1.
Comparison of Etest MICs at 48 h and BMD MICs at 48 h for 1,416 Candida species isolates.
FIG. 2.
Comparison of DD MICs at 24 h and BMD MICs at 48 h for 2,171 Candida species isolates.
TABLE 1.
Interpretive agreement between BMD results, 24-h DD results, and 48-h Etest results for posaconazole
| Method | No. of isolates tested | No. (%) of results discrepant with respect to BMD results
|
% Agreementa | ||
|---|---|---|---|---|---|
| Minor | Major | Very major | |||
| Etest | 1,416 | 54 (4) | 45 (3) | 0 (0) | 93 |
| DD | 2,171 | 25 (1) | 8 (<1) | 2 (<1) | 98 |
Percent total agreement is the categorical agreement between participant and reference laboratory results. For comparison purposes only, we applied the MIC and DD breakpoints recently approved for voriconazole.
The categorical agreement between Etest and BMD at 48 h was 93%, with no VMEs, 45 (3%) MEs, and 54 (4%) minor errors; and the essential agreement (within 2 well dilutions) was 94%. Categorical agreement between Etest and BMD was even better at 24 h (99%, with <1% MEs and minor errors) due to the much better agreement of the results among the C. glabrata isolates at 24 h (see below). The essential agreement between Etest and BMD at 24 h was 92%, which was not significantly different from the result at 48 h. The categorical agreement between the DD results at 24 h and the BMD results at 48 h was 98%, with 2 (<1%) VMEs, 8 (<1%) MEs, and 25 (1%) minor errors. There was a significant correlation between the DD zone diameter and the BMD MIC (r = 0.55; P < 0.001).
When the results were examined by species, the categorical agreements for both Etest and DD comparisons were excellent (>99%) for all species except C. glabrata. Of the 158 C. glabrata isolates tested by Etest and BMD, the categorical agreement at 48 h was only 54%, with 18% minor errors and 27% MEs, and the essential agreement was 66%. This finding reflected a tendency of Etest to give slightly higher MICs than BMD and the fact that the C. glabrata posaconazole MIC frequency distribution incorporates our breakpoint concentrations of 2 and 4 μg/ml. Interestingly, the agreement between Etest and BMD at 24 h was much better than that at 48 h for C. glabrata (92 and 89% categorical and essential agreements, respectively).
DD and Etest were 89% and 100% sensitive, respectively, for the detection of isolates that were posaconazole resistant (MICs ≥ 4 μg/ml) by BMD testing.
The posaconazole antifungal susceptibility testing results obtained by DD and Etest correlated well with those obtained by the CLSI M27-A2 BMD method, demonstrating overall categorical agreements of 98 and 93%, respectively. Our overall results are very consistent with those of Sims et al. (9), who reported 93% agreement between Etest and BMD at 48 h by using a breakpoint of ≤1 μg/ml. However, with a collection of clinical isolates of Candida much larger than that used by Sims et al., we found better essential agreement at 24 and 48 h (>90%) compared with the essential agreements of 65 and 83%, respectively, that they obtained (9). Agar-based methods hold promise as simple and reliable methods for determining the posaconazole susceptibilities of Candida spp. Future studies should examine more isolates that are “resistant” to posaconazole by the reference BMD method.
Acknowledgments
This study was supported in part by research and educational grants from the Schering Plough Research Institute.
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Barry, A. L., M. A. Pfaller, S. D. Brown, et al. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 2nd ed., M27-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Clinical and Laboratory Standards Institute. 2004. Method for antifungal disk diffusion susceptibility testing of yeasts: approved guidance M44-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Espinel-Ingroff, A., and A. Rezusta. 2002. Etest method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J. Clin. Microbiol. 40:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller, M. A., D. J. Diekema, L. Boyken, et al. 2003. Evaluation of the Etest and disk diffusion methods for determining susceptibilities of 235 bloodstream isolates of Candida glabrata to fluconazole and voriconazole. J. Clin. Microbiol. 41:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller, M. A., S. A. Messer, K. Mills, et al. 2001. 2001. Evaluation of Etest method for determining posaconazole MICs for 314 clinical isolates of Candida species. J. Clin. Microbiol. 39:3952-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller, M. A., D. Diekema, S. Messer, L. Boyken, and R. Hollis. 2003. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 41:1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller, M. A., D. J. Diekema, M. G. Rinaldi, et al. 2005. Results from the ARTEMIS DISK Global Surveillance Study: a 6.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by standardized disk diffusion testing. J. Clin. Microbiol. 43:5848-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims, C. R., V. L. Paetznick, J. R. Rodriguez, et al. 2006. Correlation between microdilution, Etest, and disk diffusion methods for antifungal susceptibility testing of posaconazole against Candida spp. J. Clin. Microbiol. 44:2105-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]