Abstract
Human enterovirus 71 and coxsackievirus A16 are important causes of hand-foot-and-mouth disease (HFMD). Like other enteroviruses, they can be isolated from a range of sterile and nonsterile sites, but which clinical sample, or combination of samples, is the most useful for laboratory diagnosis of HFMD is not clear. We attempted virus culture for 2,916 samples from 628 of 725 children with HFMD studied over a 3 1/2-year period, which included two large outbreaks. Overall, throat swabs were the single most useful specimen, being positive for any enterovirus for 288 (49%) of 592 patients with a full set of samples. Vesicle swabs were positive for 169 (48%) of 333 patients with vesicles, the yield being greater if two or more vesicles were swabbed. The combination of throat plus vesicle swabs enabled the identification of virus for 224 (67%) of the 333 patients with vesicles; for this patient group, just 27 (8%) extra patients were diagnosed when rectal and ulcer swabs were added. Of 259 patients without vesicles, use of the combination of throat plus rectal swab identified virus for 138 (53%). For 60 patients, virus was isolated from both vesicle and rectal swabs, but for 12 (20%) of these, the isolates differed. Such discordance occurred for just 11 (10%) of 112 patients with virus isolated from vesicle and throat swabs. During large HFMD outbreaks, we suggest collecting swabs from the throat plus one other site: vesicles, if these are present (at least two should be swabbed), or the rectum if there are no vesicles. Vesicle swabs give a high diagnostic yield, with the added advantage of being from a sterile site.
Hand-foot-and-mouth disease (HFMD) is a common febrile illness in young children and is characterized by lesions on the skin and oral mucosa. The skin rash, which may be maculopapular or vesicular, typically occurs on the palms and soles but can also involve the buttocks, elbows, and knees. Mouth ulcers are the most common enanthema, but some patients have herpangina (multiple oral ulcers affecting predominantly the posterior part of the oral cavity), and others have no oral lesions (16, 20).
Many human enteroviruses (family Picornaviridae, genus Enterovirus) can cause HFMD, but human enterovirus 71 (HEV71) and the closely related coxsackievirus A16 (CVA16) are the most important (16, 20). Since the late 1990s, HEV71 has caused a series of large HFMD epidemics in the Asia-Pacific region, associated with a rapid fulminant course, severe neurological complications, and a large number of fatalities (1-4, 8-11, 14, 17, 18, 21). CVA16 causes a similar clinical illness initially, but neurological and other severe complications are extremely rare (5). In much of Asia, there is now epidemiological and virological surveillance for HFMD so that effective public health measures, such as closing nurseries and schools, can be instituted early. However, because of the similar clinical presentations of the viruses, establishing the actual cause of HFMD cases relies on laboratory identification of the virus. Diagnostic techniques include isolating the virus in susceptible continuous cell lines or detecting viral RNA by PCR (12, 28). Though laborious and time consuming, virus isolation remains the gold standard for enterovirus diagnosis; it is cheaper than PCR and is the most widely used method, particularly in developing countries.
There is a wide range of samples from which virus isolation can be attempted, including rectal and throat swabs, serum, and cerebrospinal fluid (CSF) (when taken) and vesicles and ulcers when they are present. However, for HEV71-associated HFMD outbreaks, there has been relatively little work examining which sample, or combination of samples, is the most useful. This question becomes especially important in the context of large outbreaks with many thousands of patients. Rectal and throat swabs are available for all patients and do not require the presence of mucocutaneous stigmata. However, they have the disadvantage that, because they are not sterile sites, isolation of virus there may represent coincidental asymptomatic carriage rather than the causative agent (24): many enterovirus infections are asymptomatic, and viral shedding may persist for up to 2 weeks from the throat and up to 11 weeks from the rectum (7, 20, 24). In the absence of virus isolation from a sterile site, isolates from nonsterile sites are usually accepted as surrogate markers for enterovirus infections (20, 22, 24), but there is little data available on the validity of this approach for HEV71-associated HFMD. We therefore set out to answer three important clinical microbiological questions during a 3 1/2-year prospective clinical and diagnostic study of HFMD, which included two large outbreaks: first, which single specimen is most often positive for the different HFMD patient groups; second, which combination of samples is the most efficient in terms of diagnostic yield; and third, how reliable samples from nonsterile sites are compared with those from sterile sites.
MATERIALS AND METHODS
Clinical and laboratory methods.
Between January 2000 and July 2003, we studied all children with HFMD in the pediatric ward and intensive care unit at Sibu Hospital, Sarawak, Malaysia. Children were defined as having HFMD if they had new onset of at least one of the following: maculopapular and/or vesicular rash on the palms and/or soles, vesicles or ulcers in the oral cavity, or herpangina (defined as multiple oral ulcers affecting predominantly the posterior parts of the oral cavity). All children with HFMD admitted into the hospital were assessed by the pediatricians of the study team. A detailed history and results of a clinical examination, including an examination for mucocutaneous lesions, were recorded on standardized forms. A rectal swab and a throat swab were taken for each child. The skin was examined carefully for vesicles, and the oral cavity for ulcers; if they were present, swabs were taken from at least one of each (usually the largest and most accessible lesions). Rectal swabs were taken with a gentle circular motion on the rectal wall. Throat swabs were taken with the aid of a tongue depressor, by carefully swabbing the lateral and posterior pharynx without touching the tongue or buccal mucosa. For vesicle swabs, the skin was cleaned gently with 0.9% sterile normal saline, but not with alcohol, which kills viruses. A sterile 24-gauge needle was used to rupture the vesicle, and a swab was used to absorb the fluid. Alternatively, the swab was gently rolled over the vesicle to squeeze out fluid. Mouth ulcers were sampled by rolling the swab over the floor of the ulcer. When more than one vesicle or ulcer was swabbed, a fresh swab was used for each lesion and put into a separate tube of viral transport material, because we were interested in the yield from each swab. Swab samples were collected by study team members or by nursing staff, after training.
CSF and serum were collected from children with suspected central nervous system (CNS) involvement if they had a history of fever, or fever on examination (≥38°C), and at least one of the following: toxic and ill in appearance, recurrent vomiting (at least twice), tachycardia (heart rate, >150/min), breathlessness, poor perfusion (cold, clammy skin), reduced consciousness (irritability, lethargy, drowsiness, coma), limb weakness, meningism (neck stiffness or positive Kernig's sign), or seizures.
The clinical samples were stored immediately in a −70°C freezer until further testing. Out of hours, when immediate storage at −70°C was not possible, clinical samples were stored at 4°C overnight and were transferred to a −70°C freezer the following morning. Five percent of samples were handled in this way, but their isolation rates did not differ significantly from those of other samples. Virus isolation was attempted for all swabs and for CSF and any serum which had adequate volume. Specimens were inoculated into rhabdomyosarcoma (RD) and 293 cells as described previously (1, 19). Enteroviruses isolated were typed by nucleotide sequencing of the VP1 or the VP4 genes (2, 13).
For the purposes of analysis, swabs from herpangina lesions were grouped with those from other mouth ulcers. Vesicles, serum, and CSF were considered sterile sites, and the throat, mouth ulcers, and the rectum were considered nonsterile sites. All samples were investigated, irrespective of the results for other samples from the same patient.
Analytical approach.
The patients were divided into four groups according to their presenting mucocutaneous lesions and, thus, the availability of samples: those with a papulovesicular rash and mouth ulcers (referred to hereafter as HFMD with vesicles and ulcers), those with a papulovesicular rash only (HFMD with vesicles), those with a maculopapular rash and mouth ulcers only (HFMD with ulcers), and those with maculopapular rash only (HFMD with maculopapular rash). As we were interested in which combination of samples gives the best diagnostic yield, we adopted a stepwise approach to the analysis for each patient group. First, we determined which sample type gave the most positive results. Then we looked at the remaining undiagnosed patients and determined which of the remaining samples gave the most positive results. We continued in this manner until all sample types had been assessed. We decided to use data from the first outbreak to determine the usefulness of different samples and combinations of samples. We then applied these findings to the second outbreak to see if the predicted samples remained useful. However, the sample analysis was not begun until the end of the study, to avoid any bias in sample collection.
Statistical analysis.
Statistical analysis was performed by using the statistical software Statview 4.02 (Abacus Concepts, Inc.). Sensitivity, specificity, positive predictive value, and negative predictive value were calculated from a 2 × 2 table.
Ethical approval.
The study was approved by the Director of Health for Sarawak (Malaysia) and the Ethics Committee of the Liverpool School of Tropical Medicine (Liverpool, United Kingdom). Informed consent was obtained from each child's accompanying parent or guardian.
RESULTS
Seven hundred twenty-five patients were entered into the study: 471 (299 [63%] males, with a median age of 28 months [range, 4 to 120 months]) were enrolled in the first half (the majority from an outbreak between January 2000 and March 2001) and 254 (158 [62%] males, with a median age of 28 months [range, 2 to 153 months]) were enrolled in the second half (mostly during an outbreak between January and July 2003). The 471 patients in the first half of the study included 110 patients with vesicles and ulcers, 112 patients with vesicles only, 78 patients with ulcers only, and 171 patients with a maculopapular rash only. Of the 254 patients in the second half of the study, 98 had vesicles and ulcers, 29 had vesicles only, 87 had ulcers only, and 40 had a maculopapular rash only. The median duration of illness before admission was 2 days (range, 0 to 8 days) and did not differ significantly between patient groups.
Virology results.
We attempted viral isolation for 2,916 samples: 1,666 samples from 389 (83%) of the 471 patients in the first half of the study and 1,250 samples from 239 (94%) of the 254 patients in the second half of the study. For most patients, a single throat swab and single rectal swab were cultured. In addition, for 127 patients with vesicles, at least one (median, 2; range, 1 to 10) vesicle was investigated, and for 185 patients with ulcers, at least one (median, 2; range, 1 to 6) ulcer sample was investigated. For a single swab, 35% of vesicle and 17% of ulcer samples were positive (Table 1), but the percentages increased as more swabs were taken.
TABLE 1.
Relationship between the number of vesicle and ulcer swabs collected and the number positive
| Sample type | No. of swabs per patient | No. of patients with at least one swab positive/no. of patients tested (%) |
|---|---|---|
| Vesicle | 1 | 62/177 (35) |
| 2 | 40/81 (49) | |
| 3 | 18/30 (60) | |
| ≥4 | 46/61 (75) | |
| Ulcer | 1 | 26/152 (17) |
| 2 | 21/103 (20) | |
| 3 | 37/77 (48) | |
| ≥4 | 13/40 (33) |
The number of patients tested for each sample type and the number that tested positive for any enterovirus, for HEV71, and for CVA16 are shown in Table 2. During the first half of the study, a throat swab was most likely to be positive (being positive for any enterovirus for 191 [52%] of 367 patients), followed by vesicle, ulcer, and then rectal swabs. During the second half of the study, vesicle swabs were most likely to be positive (positive for any enterovirus for 63 [50%] of 127 patients), followed by throat, rectal, and then ulcer swabs. Most viruses (>95%) were isolated following a single passage.
TABLE 2.
Positive isolation rates for different viruses according to sample typea
| Sample type | No. of patients with positive results/total no. of patients tested (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| First half of study (n = 471)
|
Second half of study (n = 254)
|
|||||||
| HEV71 (n = 167) | CVA16 (n = 80) | Any HEV (n = 255) | All (n = 389) | HEV71 (n = 106) | CVA16 (n = 19) | Any HEV (n = 153) | All (n = 239) | |
| Rectal | 65/166 (39) | 22/78 (28) | 100/252 (40) | 100/378 (27) | 38/103 (37) | 7/16 (44) | 68/147 (46) | 68/229 (30) |
| Throat | 127/164 (77) | 50/76 (66) | 191/248 (77) | 191/367 (52) | 66/104 (64) | 9/15 (60) | 101/148 (68) | 101/231 (44) |
| Vesicle | 66/111 (60) | 36/57 (63) | 106/167 (63) | 106/222 (47) | 54/81 (67) | 10/13 (77) | 63/97 (65) | 63/127 (50) |
| Ulcer | 36/85 (42) | 11/42 (26) | 53/129 (41) | 53/188 (28) | 28/87 (32) | 7/15 (47) | 44/117 (38) | 44/185 (24) |
| Serum | 0/9 (0) | 1/3 (33) | 1/10 (10) | 1/20 (5) | 2/26 (8) | 1/11 (9) | 6/43 (14) | 6/61 (10) |
| CSF | 2/43 (5) | 0/11 (0) | 2/58 (3) | 2/96 (2) | 0/29 (0) | 0/0 (0) | 1/42 (2) | 1/60 (2) |
The number of patients with positive results for each sample type is shown as a proportion of the HEV71-positive patients, the CVA16-positive patients, all HEV-positive patients, and all patients (whether their samples tested positive or negative). Eleven patients in the first half of the study and three in the second half of the study had co-isolation of HEV71 and CVA16.
In the first half of the study, 167 (65%) of 255 patients with positive viral isolation were HEV71 positive (11 of whom were also infected with CVA16, 2 with CVA4, 2 with CVA24, and 1 with adenovirus 7 [Ad-7]); CVA16 was isolated from a further 69 patients (2 of whom were also infected with another virus). In addition, 19 patients were infected with other enteroviruses, Ads, or unidentified viruses (6 of whom had multiple viruses isolated). In the second half of the study, 106 (44%) of 239 patients had HEV71 isolated, 10 of whom had a second virus isolated: there were 3 with CVA16, 2 with CVA5, and 1 each with CVA10, poliovirus 1 Sabin vaccine strain, Ad-1, an untypeable enterovirus, and an unidentified virus. CVA16 was isolated from 16 further patients (2 of whom were also infected with a second virus, Ad2 or Ad4). In addition, 31 patients were infected with other enteroviruses, Ads, or unidentified viruses (4 of whom had multiple viruses isolated). For most patients with multiple isolates, the viruses came from different clinical samples; however, in 20 cases, two viruses were isolated from the same clinical sample. These comprised nine rectal swabs, five throat swabs, and two ulcer swabs, which gave different isolates in different cell lines; in addition, four patients with mild HFMD had two different viruses grown from different vesicles (three with HEV71 and CVA16 and one with HEV71 and P1S). The serotyping of enterovirus isolates was repeated and verified.
Across the whole study, 79 (51%) of 156 patients who required CSF examination had elevated CSF cell counts (>5/mm3), but only 3 had virus cultured (two HEV71 and one other). Enteroviruses were isolated from the serum of 7 (9%) of 81 patients: two of these were identified as HEV71, two as CVA16, and one (each) as CVA6, CVA9, and CVA10.
Analysis of sample combinations during the first outbreak.
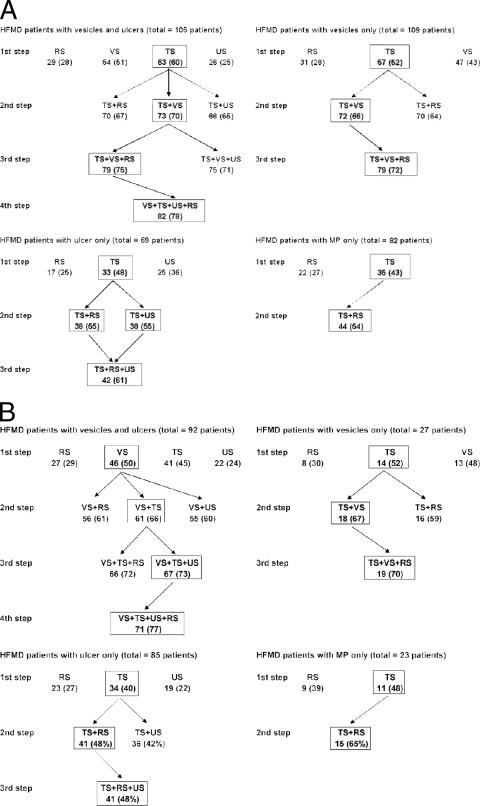
Figure 1A shows, for each patient group, the possible incremental increases in the numbers of patients diagnosed virologically, by different combinations of samples assessed stepwise according to the analytical plan. For this part of the analysis, only patients with full sets of samples were studied. For vesicles and ulcer swabs, the results for multiple swabs of a single type were treated as a single result, so that at least one swab testing positive was taken as a positive result for that sample type. Figure 1B shows similar data for a later outbreak.
FIG. 1.
Analysis of which combination of samples gave the greatest diagnostic yield for the four groups of HFMD patients, assessed stepwise according to the analytical plan during the first half of the study (A) and second half of the study (B). The number (%) of positive patients for each sample type at each step is shown; the boxed sample gave the greatest diagnostic yield and thus was the one used for the next step. Only patients with complete sets of samples were analyzed. For the first half of the study, this comprised 105 (95%) of 110 HFMD patients with vesicles and ulcers, 109 (97%) of 112 with vesicles only, 69 (88%) of 78 with ulcers only, and 82 (48%) of 171 with maculopapular rash. For the second half, this comprised 92 (94%) of 98 HFMD patients with vesicles and ulcers, 27 (93%) of 29 with vesicles, 85 (98%) of 87 with ulcers, and 23 (58%) of 40 with maculopapular rash. RS, rectal swab; TS, throat swab; US, ulcer swab; VS, vesicle swab.
For the 105 patients with vesicles and ulcers in the first half of the study, use of the throat swab alone diagnosed 63 patients (60%), whereas use of the vesicle swabs alone would have diagnosed 54 patients, the rectal swab alone 29 patients, and the ulcer swab alone 26 patients. Throat swabs were therefore taken as the first sample (Fig. 1A). The number of patients with a virological diagnosis (the diagnostic yield) would increase to 73 patients if the results for vesicle swabs were added next, 70 if results for rectal swabs were added, or 68 if results for ulcer swabs were added. The vesicle swab results were therefore added as a next step. From here, the addition of the rectal swab results would increase the yield to 79 patients, whereas ulcer swab results would increase it to 75 patients; the rectal swab was therefore added next, and the ulcer swab was added last, increasing the yield to 82 patients (78%).
For patients with HFMD and a vesicular rash, a throat swab was again the most useful single sample, allowing for the diagnosis of 57 (52%) of 109 patients. The addition of the rectal swab result would increase the diagnostic yield to 70 (64%), whereas the addition of the vesicle swab result would increase it to 72 (66%). The vesicle swab result was therefore used as the second investigation; finally, the addition of the rectal swab result increased the number of patients diagnosed to 79 (72%). For patients with ulcers only, a throat swab result diagnosed virologically 33 (48%) of 69 patients; the addition of either the rectal or vesicle swab result increased the diagnostic yield to 38 patients (55%), and results from the combination of all three samples diagnosed 42 patients (61%). Finally, for patients with a maculopapular rash only, results from a throat swab alone diagnosed 35 (43%) of 82 patients, whereas results from a rectal swab alone diagnosed 22 patients. A throat swab was therefore used as the first sample, followed by the rectal swab, which increased the number of patients diagnosed to 44 (54%) of 82 patients.
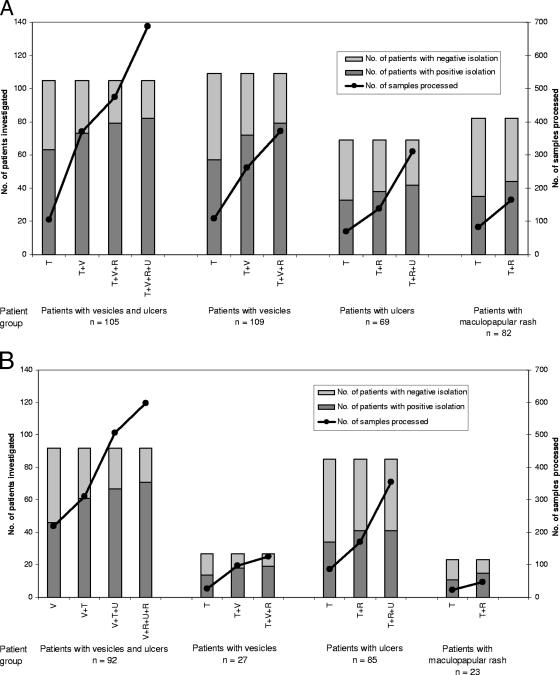
In Fig. 2A, the proportion of patients diagnosed at each step,by use of the best combination of samples as determined above, is compared with the total number of samples analyzed at each step. It is clear that although the detection rate increased as more clinical sample types were included, the number of samples analyzed increased to a much greater extent. For example, for the patients with vesicles and ulcers, 63 patients were diagnosed by throat swab samples alone (105 samples; 1.7 samples per patient diagnosed). The addition of the vesicle swab results enabled the diagnosis of a further 10 patients but required the processing of a further 264 samples (26.4 samples per patient), the addition of the rectal swab results allowed the diagnosis of 6 more patients with a further 105 samples (17.5 per patient) processed, and the addition of ulcer swab allowed the diagnosis of 3 more patients with 213 additional samples (71 per patient) analyzed. Thus, the total number of samples needed to be analyzed to diagnose each additional patient increased dramatically for each additional sample type included.
FIG. 2.
Histograms showing the proportion of patients diagnosed at each step for the different patient groups, using the optimum combination of samples as determined in Fig. 1, and the total number of samples analyzed at each step. Panel A shows the first half of the study, and panel B shows the second half. T, throat swab; V, vesicle swab; R, rectal swab; U, ulcer swab.
Recommendation based on the first outbreak.
Based on these observations, it was decided that during a large outbreak, if one wanted to limit the number of samples, the following could be recommended. For patients with both vesicles and ulcers, most patients (70%) could be diagnosed by investigating throat and vesicle swabs, and the addition of rectal or ulcer swabs added little value for the extra work and cost involved. Similarly, for patients with vesicles only, the combination of throat swabs and vesicles gave a good diagnostic yield, and the addition of rectal swabs only increased the yield marginally (6%). For patients with ulcers only, throat and ulcer swabs or throat and rectal swabs were equally useful, but combining all three swabs increased the diagnostic yield by only 6%. For patients with a maculopapular rash only, both throat and rectal swabs should be tested.
Application of findings to the second outbreak.
The validity of these recommendations was tested with data from the second outbreak. The same analytic process was applied to determine which combination of samples gave the best diagnostic yield and to see if the recommended combinations would prove to be the most useful. Figures 1B and 2B show that, for the most part, the approach remained valid. For patients with vesicles plus ulcers, and for patients with vesicles only, the combination of throat and vesicle swabs gave a good diagnostic yield (66 and 67%, respectively), with further samples not improving the yield greatly. Interestingly, though, for the first patient group, vesicles, rather than throat swabs, were the single most useful sample. For patients with a maculopapular rash only, throat swabs and then rectal swabs were most useful. However, for those with ulcers only, the addition of rectal swabs to throat swabs proved more useful than the addition of ulcer swabs, increasing the yield to 41 (48%) of 85 patients compared to 36 (42%).
Thus, to summarize the data from both outbreaks together, the combination of throat swabs plus vesicle swabs was the most useful approach for patients with vesicles (whether or not they also had ulcers), identifying virus for 134 (64%) of 208 patients with vesicles and ulcers and 90 (66%) of 136 patients with vesicles only; the combination of throat swab and rectal swab was most useful for patients without vesicles (whether or not they had ulcers), identifying virus for 79 (51%) of 154 patients with ulcers only and 59 (56%) of 105 patients with maculopapular rash only.
Concordance of viral diagnosis between samples.
To examine the concordance of virus isolates from nonsterile sites (rectal, throat, and ulcer swabs) with those from a sterile site (vesicle swabs), all HFMD patients with swabs taken from vesicles plus another site were studied (Table 3). The isolation results from 212 (63%) of 337 patients with throat swabs, 187 (55%) of 342 with rectal swabs, and 112 (54%) of 208 with ulcer swabs were in agreement with the results for vesicle swabs from the same patients (either the same virus was isolated or no virus was isolated). However, a different virus was isolated for 11 (10%) of 112 patients with positive throat and vesicle swabs, 4 (12%) of 33 patients with positive ulcer and vesicle swabs, and 12 (20%) of 60 patients with positive rectal and vesicle swabs. Overall, by taking the vesicle swab as a reference, the sensitivity of the throat swabs for isolating the same virus was 67%, the specificity was 63%, and the positive and negative predictive values were 61% and 69%, respectively. Equivalent values were 31%, 79%, 56%, and 57% for the rectal swabs and 28%, 81%, 60%, and 53% for the ulcer swabs. The detailed viral isolation results of these patients with different viruses isolated from vesicle and nonsterile sites are shown in Table 4.
TABLE 3.
Pairwise comparison of virus isolates grown from a sterile site with isolates grown from nonsterile sites in the same patienta
| Result | Finding | No. (%) of swabs testing positive from:
|
||
|---|---|---|---|---|
| Throat (n = 337) | Rectum (n = 342) | Ulcer (n = 208) | ||
| Concordance | Same virus isolated as vesicle swab | 101 (30) | 48 (14) | 29 (14) |
| No virus isolated from either swab | 111 (33) | 139 (41) | 83 (40) | |
| Total | 212 (63) | 187 (55) | 112 (54) | |
| Discordance | Different virus isolated compared with vesicle swab | 11 (3) | 12 (4) | 4 (2) |
| Vesicle swab negative but sample positive | 65 (19) | 37 (11) | 19 (9) | |
| Vesicle swab positive but sample negative | 49 (15) | 106 (31) | 73 (35) | |
| Total | 125 (37) | 155 (45) | 96 (46) | |
Vesicles were the sterile site; throat, rectum, and ulcers were the nonsterile sites. Many patients had swabs from multiple sites. The total number of patients represented here is 349.
TABLE 4.
Virus isolation results for patients with different viruses grown from sterile and nonsterile sitesa
| Study no. | Age (mo) | Gender | Clinical severity | Virus isolated from:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Sterile site
|
Nonsterile site
|
||||||||
| Vesicle | Serum | CSF | Rectum | Throat | Ulcer | ||||
| HFM-4 | 46 | Male | Severe HFMD with CNS | HEV71 | — | NEG | HEV71, Ad-7b | HEV71 | HEV71 |
| HFM-9 | 52 | Female | Mild HFMD | HEV71 | CA16 | — | NEG | NEG | NEG |
| HFM-90 | 37 | Female | Mild HFMD | CVA16 | — | — | NEG | CVA16 | HEV71 |
| HFM-112 | 16 | Male | Mild HFMD | HEV71 | — | — | CVA16 | HEV71 | NEG |
| HFM-152 | 45 | Male | Mild HFMD | HEV71, CVA16c | — | — | NEG | HEV71 | NEG |
| HFM-174 | 73 | Male | Mild HFMD | CVA16 | — | — | HEV71 | HEV71 | NEG |
| HFM-178 | 14 | Male | Severe HFMD with CNS | CVA16 | — | NEG | CVA16 | CVB1 | — |
| HFM-193 | 36 | Female | Severe HFMD, no CNS | HEV71 | — | NEG | CVA17 | HEV71 | — |
| HFM-198 | 16 | Female | Mild HFMD | HEV71 | — | — | HEV71, CVA17d | HEV71 | — |
| HFM-228 | 11 | Female | Mild HFMD | CVA10 | — | — | CVA6 | CVA6, CVB5e | NEG |
| HFM-298 | 22 | Female | Mild HFMD | CVA10 | — | — | NEG | CVB1, CVA10f | NEG |
| HFM-302 | 15 | Female | Mild HFMD | HEV71 | — | — | CVA16 | HEV71 | HEV71 |
| HFM-328 | 19 | Male | Severe HFMD, no CNS | HEV71 | NEG | HEV71 | HEV71, CVA24g | HEV71 | |
| HFM-337 | 36 | Male | Mild HFMD | CVA16 | — | — | HEV71 | HEV71 | NEG |
| HFM-338 | 27 | Male | Mild HFMD | Unidentified virus | — | — | HEV71 | CVA16 | NEG |
| HFM-435 | 11 | Male | Mild HFMD | CVA16 | — | — | NEG | NEG | HEV71 |
| HFM-489 | 13 | Male | Mild HFMD | CVA16 | — | — | Ad-4 | CVA16 | NEG |
| HFM3-7 | 38 | Male | Mild HFMD | HEV71, P1Sh | NEG | — | NEG | HEV71 | NEG |
| HFM3-35 | 20 | Female | Mild HFMD | HEV71 | NEG | — | CVA5 | CVA5 | |
| HFM3-63 | 17 | Female | Mild HFMD | HEV71, CVA16i | — | — | CVA16 | NEG | CVA16 |
| HFM3-97 | 61 | Male | Severe HFMD, no CNS | HEV71 | — | — | NEG | NEG | HEV71, Untyped EVj |
| HFM3-161 | 32 | Male | Mild HFMD | HEV71 | — | — | NEG | CVA16 | NEG |
| HFM3-164 | 5 | Male | Severe HFMD, no CNS | NEG | — | Unidentified virus | NEG | HEV71 | HEV71 |
Details of dual viruses isolated from a single site by using different cell lines are given below. NEG, negative; P1S, poliovirus 1 Sabin strain; with CNS, with CNS involvement; no CNS, no CNS involvement; —, no sample or insufficient sample for testing.
HEV71 isolated using RD cells; Ad-7 isolated using 293 cells.
HEV71 isolated from right palm vesicle using RD cells; CVA16 isolated from left sole vesicle using RD cells.
HEV71 isolated using RD cells; CVA17 isolated using 293 cells.
CVA6 isolated using RD cells; CVB5 isolated using 293 cells.
CVB1 isolated using RD cells; CVA10 isolated using 293 cells.
HEV71 isolated using RD cells; CVA24 isolated using 293 cells.
HEV71 isolated from right and left palm vesicles using RD cells; P1S isolated from right and left sole vesicles using RD cells.
HEV71 isolated from right sole vesicles using RD cells; CVA16 isolated from right knee and left sole vesicles using RD cells.
HEV71 isolated from lip and tongue ulcers using RD cells; untyped EV isolated from right buccal ulcer using using RD cells.
DISCUSSION
The outbreaks of HEV71-associated HFMD which have swept across the Asia-Pacific region since 1997 have posed a great economic and social burden, not least on the health-care facilities and laboratories that have to diagnose and manage such patients. To diagnose HEV71 infection, clinicians are faced with a wide range of samples to choose from, but there has been relatively little work comparing them.
Most of the recent studies of HEV71 infection have relied on stool culture and throat swabs and have found the latter to have greater sensitivity, with throat swabs being positive for 90 to 95% and stool culture being positive for 40 to 65% of patients tested (6, 26, 27). Few studies have looked systematically at all samples from a large patient group. One report of 175 patients with HFMD during the 2000 outbreak in Singapore found that rectal swabs most often yielded virus, followed by stool samples, vesicle swabs, and then throat swabs (3). However, our study found that in most patient groups, a throat swab was the single specimen most likely to be positive, being positive for 288 (49%) of the 592 patients with a full set of samples and 292 (49%) of all 598 patients studied. Approximately half of our patients had skin vesicles, and we showed that vesicle swabs were also very useful. In patients with vesicles, they gave the second highest yield, being positive for 169 (48%) of 333 patients studied; in one patient group (during the second outbreak), they were the single most sensitive specimen (positive for 50%). Vesicle swabs have not been widely used to diagnose HFMD previously. One study reported virus isolation from four children for whom vesicle swabs were investigated (5), and another reported 50% positive vesicles for 62 HFMD patients with vesicles (3). But for most outbreaks, vesicle fluid was not investigated (8, 11, 18, 21, 27).
Because we wanted to examine the optimal number of lesions to sample, in our study we took separate swabs from each vesicle. We found a single vesicle was positive 35% of the time, but this increased to 49% with two vesicles and 60% with three vesicles. Our recommended practice now is to apply a single swab to two or more vesicles. This maximizes the chance of isolating virus without doubling the number of samples to be processed. In our study, approximately half the patients had vesicles. They usually appeared early in the illness and resolved after a few days, so that their presence may depend on the time of presentation. Like previous reports for HEV71 (3, 6, 8, 10, 15, 18, 27) and other HEVs (20, 24), our study reports a low isolation rate of CSF (3 of 102 HFMD patients with aseptic meningitis). The yield would likely have been higher if PCR had been used (23), but this investigation is not available in most developing countries. We found that the isolation rate for rectal swabs was not as high as that for throat and vesicle swabs; the median time of presentation (and thus sampling) was just 2 days, which may be before viral shedding in the stool has become fully established.
In addition to looking at individual samples, we examined which combinations of samples were the most useful. This was achieved by determining the extent to which the addition of a sample type increased the number of patients diagnosed; this is different from asking how frequently a sample is positive. Thus, for example, in the first half of the study, although ulcer swabs were positive more often than rectal swabs, they were less useful diagnostically, because most of the patients in whom they were positive had already been diagnosed by a throat swab. Determining the “added diagnostic value” of a sample type allowed us to produce predictions about which combinations of samples should prove useful, which we were then able to test in the second outbreak. We found that our predictions were basically sound. Results from the combination of throat swabs plus vesicle swabs were the most useful for patients with vesicles whether or not they also had ulcers, identifying virus for 224 (67%) of 333 such patients across the whole study. For these patients, the addition of rectal and ulcer swabs enabled the diagnosis of just 27 extra patients (8%). For patients without vesicles (whether or not they had mouth ulcers), the combination of throat swab and rectal swab was most useful, identifying virus for 138 of 259 such patients (53%). Thus, during large outbreaks, we suggest that a throat swab plus one other sample type be taken for each patient. If no vesicles are present, the second sample type should be a rectal swab, but if there are vesicles, a swab should be applied to as many of these as possible (but at least two). Although we advocate limited sampling, particularly for community surveillance and during large outbreaks, for individual patients, especially those that are critically ill, physicians will want to maximize the chance of obtaining a diagnosis. One approach might be to collect all sample types but to investigate them in a stepwise manner, starting with the most useful samples, until a diagnosis has been made.
During our study, we were also able to examine the concordance of virus isolates from nonsterile sites (rectal, throat, and ulcer swabs) with those from a sterile site (vesicle swabs). We found that 20% of rectal isolates differed from vesicle isolates (in individual patients with isolates from both sites), whereas such discordance with vesicle isolates occurred for only 10% of throat isolates. While most would accept the virus isolated from a vesicle as the causative pathogen, the significance of other viruses concomitantly detected from throat and/or rectum is not always clear. One plausible explanation is that the isolation of a virus from throat or rectum may be only a confounding factor related to asymptomatic carriage or ongoing shedding from recent enterovirus infection. Our data show that in the majority of patients with HFMD, throat and rectal isolates do reflect viruses isolated from sterile sites, but in a minority, they may be coincidental infections. However, it may not be correct to assume that virus isolated from vesicle is always the most important pathogen. For example, one patient (HFM-178) (Table 4) with severe HFMD and CNS disease had CVA16 isolated from a vesicle swab but CVB1 isolated from a throat swab. CVA16, a common cause of HFMD, is not known to cause CNS disease; in contrast, CVB1 is not known to cause HFMD and, being a species B HEV, is a more likely neuropathogen (25). So in this patient with dual infection, the two virus isolates may have been responsible for two clinical syndromes: the throat isolate CVB1 causing CNS disease and the vesicle isolate CA16 causing HFMD. For four patients, we also isolated different viruses from different vesicles: three with HEV71 and CVA16 and one (with mild HFMD) with HEV71 and poliovirus 1 Sabin strain. These patients clearly demonstrate that systemic infection with two viruses can occur simultaneously. In addition, the latter case suggests the possibility that occasionally during dual infection, viruses may cross tissue barriers that they would not normally be able to (poliovirus Sabin strain type 1 does not itself cause HFMD and thus is not normally found in vesicles) (20). Our findings on dual infection underscore the need to look for a possible second pathogen before attributing pathogenesis to a virus rarely associated with a severe disease phenotype.
In summary, we have shown that the throat swab is the single most useful sample from patients with HFMD during an HEV71 outbreak. Vesicle swabs, which have been relatively neglected until now, can also be extremely useful. Although they are not as easy to obtain as throat and rectal swabs and are not available for approximately half the patients, the viral yield is almost as good as that of throat swabs, with the added advantage that they come from sterile sites.
Acknowledgments
We thank the Former State Health Director of Sarawak, Yao Sik Chi, for his approval and support for this work and support during his tenure as Director. We are grateful to the Director, Abdul Rahim Abdullah, and the doctors, nurses, and medical records officers of Sibu Hospital, Peng Chin Pek, Guloi Selingau, for administrative, clinical, and laboratory assistance and to David Chadwick and Anthony Hart for their support.
This work was funded by grant 06-02-09-002 BTK/ER/003 from the Ministry of Science, Technology and Innovation, Government of Malaysia; the Walton Centre for Neurology and Neurosurgery Research Fund; and the Wellcome Trust of Great Britain. M.H.O. is a Wellcome Trust Clinical training Fellow, and T.S. is a United Kingdom Medical Research Council Senior Clinical Fellow.
M. H. Ooi, S. C. Wong, J. Cardosa, and T. Solomon conceived of the study; they were assisted by S. del Sel, D. Clear, C. H. Chieng, A. Mohan, B. F. Lai, K. M. Kontol, and E. Blake in the planning, design, and execution of the clinical aspects and by D. Perera, W. Akin, M. A. Yusuf, and Y. Podin in the analysis and interpretation of the virological samples; all authors contributed to writing the manuscript. We declare no conflicts of interest.
Footnotes
Published ahead of print on 19 April 2007.
REFERENCES
- 1.Cardosa, M. J., S. Krishnan, P. H. Tio, D. Perera, and S. C. Wong. 1999. Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet 354:987-991. [DOI] [PubMed] [Google Scholar]
- 2.Cardosa, M. J., D. Perera, B. A. Brown, D. Cheon, H. M. Chan, K. P. Chan, H. Cho, and P. McMinn. 2003. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: comparative analysis of the VP1 and VP4 genes. Emerg. Infect. Dis. 9:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, K. P., K. T. Goh, C. Y. Chong, E. S. Teo, G. Lau, and A. E. Ling. 2003. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg. Infect. Dis. 9:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, L. G., U. D. Parashar, M. S. Lye, F. G. Ong, S. R. Zaki, J. P. Alexander, K. K. Ho, L. L. Han, M. A. Pallansch, A. B. Suleiman, M. Jegathesan, and L. J. Anderson. 2000. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin. Infect. Dis. 31:678-683. [DOI] [PubMed] [Google Scholar]
- 5.Chang, L. Y., T. Y. Lin, K. H. Hsu, Y. C. Huang, K. L. Lin, C. Hsueh, S. R. Shih, H. C. Ning, M. S. Hwang, H. S. Wang, and C. Y. Lee. 1999. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 354:1682-1686. [DOI] [PubMed] [Google Scholar]
- 6.Chang, L. Y., T. Y. Lin, Y. C. Huang, K. C. Tsao, S. R. Shih, M. L. Kuo, H. C. Ning, P. W. Chung, and C. M. Kang. 1999. Comparison of enterovirus 71 and coxsackie-virus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr. Infect. Dis. J. 18:1092-1096. [DOI] [PubMed] [Google Scholar]
- 7.Chung, P. W., Y. C. Huang, L. Y. Chang, T. Y. Lin, and H. C. Ning. 2001. Duration of enterovirus shedding in stool. J. Microbiol. Immunol. Infect. 34:167-170. [PubMed] [Google Scholar]
- 8.Fujimoto, T., M. Chikahira, S. Yoshida, H. Ebira, A. Hasegawa, A. Totsuka, and O. Nishio. 2002. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol. Immunol. 46:621-627. [DOI] [PubMed] [Google Scholar]
- 9.Ho, M., E. R. Chen, K. H. Hsu, S. J. Twu, K. T. Chen, S. F. Tsai, J. R. Wang, S. R. Shih, et al. 1999. An epidemic of enterovirus 71 infection in Taiwan. N. Engl. J. Med. 341:929-935. [DOI] [PubMed] [Google Scholar]
- 10.Kohler, K. A., W. G. Hlady, K. Banerjee, and R. W. Sutter. 2003. Outbreak of poliomyelitis due to type 3 poliovirus, northern India, 1999-2000: injections a major contributing factor. Int. J. Epidemiol. 32:272-277. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu, H., Y. Shimizu, Y. Takeuchi, H. Ishiko, and H. Takada. 1999. Outbreak of severe neurologic involvement associated with Enterovirus 71 infection. Pediatr. Neurol. 20:17-23. [DOI] [PubMed] [Google Scholar]
- 12.Lina, B., B. Pozzetto, L. Andreoletti, E. Beguier, T. Bourlet, E. Dussaix, L. Grangeot-Keros, B. Gratacap-Cavallier, C. Henquell, M. C. Legrand-Quillien, A. Novillo, P. Palmer, J. Petitjean, K. Sandres, P. Dubreuil, H. Fleury, F. Freymuth, I. Leparc-Goffart, D. Hober, J. Izopet, H. Kopecka, Y. Lazizi, H. Lafeuille, P. Lebon, A. Roseto, E. Marchadier, B. Masquelier, B. Picard, J. Puel, J. M. Seigneurin, P. Wattre, and M. Aymard. 1996. Multicenter evaluation of a commercially available PCR assay for diagnosing enterovirus infection in a panel of cerebrospinal fluid specimens. J. Clin. Microbiol. 34:3002-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMinn, P., K. Lindsay, D. Perera, H. M. Chan, K. P. Chan, and M. J. Cardosa. 2001. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J. Virol. 75:7732-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMinn, P., I. Stratov, and G. Dowse. 1999. Enterovirus 71 outbreak in Western Australia associated with acute flaccid paralysis. Preliminary report. Commun. Dis. Intell. 23:199. [DOI] [PubMed] [Google Scholar]
- 15.McMinn, P., I. Stratov, L. Nagarajan, and S. Davis. 2001. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin. Infect. Dis. 32:236-242. [DOI] [PubMed] [Google Scholar]
- 16.McMinn, P. C. 2002. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 26:91-107. [DOI] [PubMed] [Google Scholar]
- 17.Mizuta, K., C. Abiko, T. Murata, Y. Matsuzaki, T. Itagaki, K. Sanjoh, M. Sakamoto, S. Kongo, S. Murayama, and K. Hayasaka. 2005. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J. Clin. Microbiol. 43:6171-6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan, M. A., M. E. Craig, M. M. Lahra, W. D. Rawlinson, P. C. Prager, G. D. Williams, A. M. Bye, and P. I. Andrews. 2003. Survival after pulmonary edema due to enterovirus 71 encephalitis. Neurology 60:1651-1656. [DOI] [PubMed] [Google Scholar]
- 19.Ooi, M. H., S. C. Wong, D. Clear, D. Perera, S. Krishnan, T. Preston, P. H. Tio, H. J. Willison, B. Tedman, R. Kneen, M. J. Cardosa, and T. Solomon. 2003. Adenovirus-21 associated acute flaccid paralysis during a hand foot and mouth disease outbreak in Sarawak, Malaysia. Clin. Infect. Dis. 36:550-559. [DOI] [PubMed] [Google Scholar]
- 20.Pallansch, M. A., and R. P. Roos. 2001. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses, p. 723-775. In D. M. Knipe and P. M. Howley (ed.), Fields Virology, vol. 1. Lippincott Williams & Wilkins, Philadelphia, USA [Google Scholar]
- 21.Prager, P., M. Nolan, I. P. Andrews, and G. D. Williams. 2003. Neurogenic pulmonary edema in enterovirus 71 encephalitis is not uniformly fatal but causes severe morbidity in survivors. Pediatr. Crit. Care Med. 4:377-381. [DOI] [PubMed] [Google Scholar]
- 22.Rotbart, H. A. 1995. Enteroviral infections of the central nervous system. Clin. Infect. Dis. 20:971-981. [DOI] [PubMed] [Google Scholar]
- 23.Rotbart, H. A., A. Ahmed, S. Hickey, R. Dagan, G. H. McCracken Juniorperiod, R. J. Whitley, J. F. Modlin, M. Cascino, J. F. O'Connell, M. A. Menegus, and D. Blum. 1997. Diagnosis of enterovirus infection by polymerase chain reaction of multiple specimen types. Pediatr. Infect. Dis. J. 16:409-411. [DOI] [PubMed] [Google Scholar]
- 24.Rotbart, H. A., and J. R. Romero. 1995. Laboratory diagnosis of enteroviral infection, p. 401-418. In H. A. Rotbart (ed.), Human Enterovirus Infection. American Society for Microbiology, Washington, DC.
- 25.Thivierge, B., and G. Delage. 1982. Enterovirus infections of the CNS: 223 cases seen in a hospital for children from 1973 to 1981. Can. Med. Assoc. J. 127:1097-1102. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, J. R., H. P. Tsai, P. F. Chen, Y. J. Lai, J. J. Yan, D. Kiang, K. H. Lin, C. C. Liu, and I. J. Su. 2000. An outbreak of enterovirus 71 infection in Taiwan, 1998. II. Laboratory diagnosis and genetic analysis. J. Clin. Virol. 17:91-99. [DOI] [PubMed] [Google Scholar]
- 27.Wang, S. M., C. C. Liu, H. W. Tseng, J. R. Wang, C. C. Huang, Y. J. Chen, Y. J. Yang, S. J. Lin, and T. F. Yeh. 1999. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin. Infect. Dis. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 28.Yerly, S., A. Gervaix, V. Simonet, M. Caflisch, L. Perrin, and W. Wunderli. 1996. Rapid and sensitive detection of enteroviruses in specimens from patients with aseptic meningitis. J. Clin. Microbiol. 34:199-201. [DOI] [PMC free article] [PubMed] [Google Scholar]