Abstract
We describe an unusual cluster of Corynebacterium striatum infections in 21 patients with chronic obstructive pulmonary disease (COPD) admitted to a medium-size respiratory unit. Eleven isolates from eight patients occurred simultaneously within a month. C. striatum is a potentially pathogenic microorganism with the ability to produce nosocomial infectious outbreaks and respiratory colonization in patients with advanced COPD.
Corynebacterium species are found as colonizers of the skin and other tissues and in the environment (23, 27, 30). In addition to Corynebacterium diphtheriae, other Corynebacterium spp. have been reported to be pathogenic with some frequency, including C. amycolatum (24), C. jeikeium (formerly group JK), and C. urealyticum (formerly group D2) (8). Although C. striatum is one of the most frequently isolated coryneforms identified, there is little evidence linking C. striatum with infections in most locations (16, 21, 22, 25, 29, 31). The role of C. striatum as a potential cause of respiratory infections is difficult to establish. The clinical relevance of the isolation of Corynebacterium species from respiratory samples must be balanced by obtaining their correct identification and studying their abundance, their isolation as a single microorganism or their predominance when they are found in association with other microorganisms, and the repetition of positivity (23). In our hospital environment, C. striatum is occasionally isolated from cultures of sputum. The unusual clustering of patients in our respiratory ward produced a sentinel signal that justified the study of a possible outbreak.
The Hospital Joan March in Bunyola, Mallorca, Spain, is a secondary health care center that hosts a convalescence and rehabilitation department and a ward with 26 beds aimed at delivering care to patients with severe, chronic respiratory disease referred from tertiary-care hospitals within our catchment area. The Microbiology Laboratory based at Hospital Son Llàtzer is responsible for processing of the samples.
All positive samples reported here were obtained from the microbiological study of spontaneous sputum specimens obtained on admission from patients with an infectious exacerbation of chronic obstructive pulmonary disease (COPD), defined according to the of criteria Anthonisen et al. (1); during the follow-up of a patient's respiratory infection; or after hospital admission from patients with a newly identified infection that needed to be studied. The quality of samples was assessed by use of the scoring system of Murray and Washington (18) and current international guidelines (17). Identification of the isolates as C. striatum was based on colony morphology and pigmentation, Gram staining, motility, the catalase reaction, and the results obtained with the RapID CB Plus system (Remel, Lenexa, KS), which offers results within 4 h. In all cases the identification was confirmed within 24 h by use of the API Coryne system (BioMèrieux, l'Etoile, France), with 100% agreement achieved between both methods (10, 12, 14).
Antibiotic susceptibility was tested by the disk diffusion method (Oxoid SA, Spain) in Mueller-Hinton agar supplemented with 5% blood for all antibiotics tested except penicillin and ampicillin, for which the Etest system (AB Biodisk, Solna, Sweden) was used. The antibiotics tested included penicillin (10 U), ampicillin (10 μg), tetracycline (30 μg), gentamicin (10 μg), cefazolin (30 μg), vancomycin (30 μg), erythromycin (15 μg), imipenem (10 μg), ciprofloxacin (5 μg), and rifampin (30 μg).
The susceptibility criteria of the CLSI (formerly the NCCLS) (19) for Staphylococcus spp. were used for all antibiotics tested except penicillin and ampicillin, for which thresholds for Listeria spp. were used.
Twenty-one patients were admitted to Hospital Joan March within a period of 18 months (January 2004 to June 2005) due to an infectious respiratory exacerbation. The demographic and clinical characteristics of the patients indicated that they all had severe COPD (5), 18 were males and 3 were females, the mean age was 72 years (age range, 57 to years 88), and the patients had significant tobacco exposure (mean, 55.6 pack-years of cigarette smoking, where pack-year values are calculated as the number of cigarettes smoked per day divided by 20 and multiplied by the number of years the person has smoked). COPD was labeled as predominantly emphysema in nine patients (42.9%) and not specified in the rest of the patients. The severities of the cases of COPD, according to the spirometry thresholds of the current ATS/ERS guidelines (5), were 0% mild, 35.7% moderate, 35.7% severe, and 28.6% very severe. Many of the patients required home care assisted technologies, including long-term oxygen therapy (47.6%) and aerosolized therapy (23.8%). The mean Charlson comorbidy index was 2.76, and the mean number of admissions due to COPD exacerbation in the previous year was 2.48 (range, 0 to 7 admissions).
During the study period of 18 months, the 21 patients had 49 admissions-readmissions in our hospital only, that is, a mean per patient of 2.33 (range 1 to 4), with a mean duration of admission of 44 days (range, 5 to 176 days).
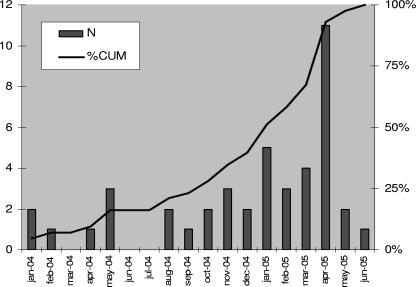
Figure 1 shows the epidemic curve of the outbreak. The observed slow growth curve is suggestive of a nosocomial infection, with transmission from person to person. Table 1 shows the chronology of all 43 positive sputum samples and the respective patient room and bed, a record of the organisms isolated before the identification of C. striatum, the antibiotics that had been prescribed within the 10 days before the isolation of C. striatum, sputum sample quality, the associated microorganisms, and the clinical response after treatment according to the antibiogram.
FIG. 1.
Epidemic curve for C. striatum over time. N, number of isolates; %CUM, cumulative percent.
TABLE 1.
C. striatum outbreak in COPD patients
| Patient no. (sample no.)a | Date (mo/day/yr) | Room and bed | Organism(s) previously isolated in sputumb | Antibiotic treatment 10 days earlierc | Sputum qualityd | Associated organism(s) in sputum culturee | Follow-up posttreatmentf |
|---|---|---|---|---|---|---|---|
| 1 (1) | 1/23/04 | 5a | AlcNi | TOB, Inh | G5 | CStr | Improved |
| 1 (2) | 1/27/04 | 5a | AlcNi | TOB, Inh | G5 | CStr | Improved |
| 2 (1) | 2/10/04 | 7a | LVX | G5 | CStr | Improved | |
| 1 (3) | 4/30/04 | 22a | CStr | G5 | CStr, StM | Improved | |
| 3 (1) | 5/17/04 | 33 | MRSA, HIn, PsA | CAZ, TOB | LQ | CStr | Improved |
| 3 (2) | 5/17/04 | 33 | MRSA, HIn, PsA | CAZ, TOB | LQ | CStr | Improved |
| 1 (4) | 5/17/04 | 11a | StM, CStr | CAZ, VAN | LQ | CStr | Improved |
| 1 (5) | 8/19/04 | 19a | StM, CStr | TZP, SXT, DOX, COL | G5 | CStr, StM, AFu, Ca | Improved |
| 1 (6) | 8/27/04 | 19a | CSt, StM, AFu, Ca | ITC, SXT | G5 | CStr, StM | Improved |
| 4 (1) | 9/8/04 | 19b | MRSA, PsA | LQ | CStr | Worsening | |
| 5 (1) | 10/5/04 | 11a | StM, SrrM | CIP | G5 | CStr, StM | Improved |
| 1 (7) | 10/11/04 | 19a | StM, CStr | SXT, MIN | LQ | CStr | Improved |
| 5 (2) | 11/3/04 | 11a | CStr, StM | IPM, SXT | G5 | CStr | Improved |
| 1 (8) | 11/5/04 | 19a | StM, CStr | LQ | CStr | Improved | |
| 5 (3) | 11/24/04 | 11a | CStr | IPM, CLR | G5 | CStr | Transferred |
| 6 (1) | 12/14/04 | 9a | LVX | LQ | CStr | Improved | |
| 7 (1) | 12/21/04 | 21 | StM, AFu, HIn | LVX | LQ | CStr | Deceased |
| 5 (4) | 1/3/05 | 13b | AFu, CStr | ITC, CIP, SXT | G5 | CStr | Same |
| 1 (9) | 1/14/05 | 5b | CStr, MoraxC | CIP, CAZ | G5 | CStr, StM | Deceased |
| 5 (5) | 1/14/05 | 13b | CStr | ITC, CIP, AMX | LQ | CStr | Improved |
| 5 (6) | 1/27/05 | 13b | CStr | ITC, AMP | G5 | CStr | Improved |
| 8 (1) | 1/28/05 | 1a | PsA, StM | SXT, MIN | LQ | CStr, PsA | Improved |
| 9 (1) | 2/8/05 | 9b | LVX, FEP, TOB | G5 | CStr, MRSA | Improved | |
| 10 (1) | 2/9/05 | 5b | AFlv | G5 | CStr | Improved | |
| 11 (1) | 2/25/05 | 5a | PsA | CAF, TOB | LQ | CStr | Deceased |
| 5 (7) | 3/3/05 | 23 | CSt, Klp, StM, AFu | ITC, MIN | G5 | CStr, StM | Same |
| 5 (8) | 3/14/05 | 23 | StM, CStr | ITC, CIP, AMX | G5 | CStr, StM, Klp | Improved |
| 12 (1) | 3/16/05 | 3b | PsA | LVX, TOB | G5 | CStr | Improved |
| 13 (1) | 3/18/05 | 13b | AchXy, CoryJk | SXT | LQ | CStr | Improved |
| 14 (1) | 4/6/05 | 17b | ITC, IPM, LVX | G5 | CStr | Deceased | |
| 15 (1) | 4/7/05 | 19b | StM, StPn | LQ | CSt, MRSA | Deceased | |
| 16 (1) | 4/13/05 | 11b | LVX | LQ | CStr | Improved | |
| 17 (1) | 4/14/05 | 17a | MRSA, StM, AsFu | CAF, LVX, ITC | G5 | CStr | Improved |
| 5 (9) | 4/16/05 | 23 | Klp, StM, CStr | ITC, IPM | G5 | CStr, StM | Improved |
| 2 (2) | 4/16/05 | 27 | CStr, PsA, MRSA | SXT | G5 | CStr, PsA | Improved |
| 12 (2) | 4/19/05 | 3b | CStr | AMC | LQ | CStr | Same |
| 5 (10) | 4/21/05 | 23 | StM, CStr | ITC | G5 | CStr, StM, Klp | Worsening |
| 5 (11) | 4/22/05 | 23 | Klp, StM, CStr | ITC, AMC | G5 | CStr, StM, Klp | Deceased |
| 18 (1) | 4/25/05 | 21b | PsA | FEP, TOB | G5 | CStr, StM | Improved |
| 17 (2) | 4/28/05 | 17a | StM, AFu, CStr | ITC, CIP, AMX | LQ | CStr | Improved |
| 19 (1) | 5/23/05 | 5a | LVX | G5 | CStr, PsA | Improved | |
| 20 (1) | 5/25/05 | 21b | AMC | G4 | CStr | Improved | |
| 21 (1) | 6/28/05 | 13a | MRSA, StPn | G4 | CStr, EAu | Improved |
Numbers of patients and numbers of samples positive for C. striatum by culture of sputum, in chronological order. Overall, there were 21 patients and 43 samples. Patient 1 had 9 positive samples; patient 5 had 11 positive samples; patients 2, 3, 12, and 17 had 2 positive samples; and the remaining 15 patients had only 1 positive sample each.
History of isolation of microorganisms in sputum up to 1 year before the isolation of C. striatum. The abbreviations for the microorganisms are as follows: AlcNi, Alcaligenes denitrificans; CStr, Corynebacterium striatum; MRSA, methicillin-resistant Staphylococcus aureus; HIn, Haemophilus influenzae; PsA, Pseudomonas aeruginosa; StM, Stenotrophomonas maltophilia; Afu, Aspergillus fumigatus; Ca, Candida albicans; SrrM, Serratia marcescens; MoraxC, Moraxella catarrhalis; Aflv, Aspergillus flavus; Klp, Klebsiella pneumoniae; AchrXy, Achromobacter xylosoxidans; CoryJk, Corynebacterium jeikeium; EAu, Staphylococcus aureus.
History of exposure to antibiotics and antifungals within the 10 days before the isolation of C. striatum. Abbreviations for antibiotics and antifungals: TOB, tobramycin; LVX, levofloxacin; CAZ, ceftazidime; VAN, vancomycin; TZP, piperacillin-tazobactam; SXT, trimethoprim-sulfamethoxazole; DOX, doxycycline; COL, colistin; ITC, itraconazole; CIP, ciprofloxacin; MIN, minocycline; IPM, imipenem; CLR, clarithromycin; AMX, amoxicillin; AMP, ampicillin; FEP, cefepime; AMC, amoxicillin-clavulanic acid.
G5, <10 epithelial cells per field and >25 polymorphic nuclear leukocytes per field; G4, 10 to 25 epithelial cells per field and >25 polymorphic nuclear leukocytes per field; LQ, low quality (<10 epithelial cells per field and <10 polymorphic nuclear leukocytes per field).
Microorganisms isolated in association with C. striatum in each of the positive samples. See footnote b for the definitions of the abbreviations.
Clinical follow-up after the completion of treatment with antibiotics according to antibiogram. Overall, 6 patients died, 31 improved, 2 remained the same, 2 worsened, and 1 was transferred.
To date, published reports identifying C. striatum isolates in respiratory samples as causal agents of disease are scarce. Until 1993 there were only three individual case reports of the confirmed pathogenicity of C. striatum (2, 3, 6). Since 1993, the isolation of C. striatum appears to have become more common (4, 7, 15, 20, 28). Initially, in two of these series (4, 15), a genotype study of the strains was conducted and confirmed patient-to-patient transmission. Brandenburg et al. (4) obtained samples from patients and from the hands of their caretakers and suggested that caretakers could have collaborated in the transmission. Recently, Otsuka et al. (20) reported 48 isolations of C. striatum from 1994 to 1998, with 75% of these samples being of respiratory origin and with all of them obtained from patients who had had long hospital admissions and who had received several courses of antibiotics. Genotyping identified 14 different patterns of C. striatum, with types A, D, and E associated with nosocomial outbreaks of respiratory origin and, in particular, with subtypes A1, A2, D2, and E associated with resistance to a broad range of antibiotics.
The outbreak reported here is unprecedented in the medical literature because it includes a large number of cases of C. striatum infection detected in sputum samples from patients with chronic respiratory disease in a hospital ward clustered in time and space; additionally, 11 cases were clustered in a single month (April 2005), and the outbreak affected one-third of the patients admitted to the ward. Several determinant factors may explain this outbreak of nosocomial infection, in which transmission was from patients and via caretakers: our hospital specializes in the care of patients with severe obstructive pulmonary disease who have many susceptibility factors (9, 13, 20, 26), high levels of use of health care resources (including multiple admissions), and repeated courses of antibiotic treatment; and the respiratory ward requires the generalized use of masks and glasses for oxygen delivery, inhalers, spacers, and nebulizers. Regrettably, without genotyping of the strains we cannot fully confirm this statement.
Most likely, factors that contributed to the end of this outbreak were the death of patient 5, who had 11 isolations until the time of his death in April 2005, and the reinforcement of implementation of universal preventative hygiene measures, both in the environment and by caretakers, after the identification of this outbreak. There were no further isolations of C. striatum in respiratory samples during the following 6 months.
Three of the six deaths during the study period occurred in patients from whom C. striatum was isolated in a pure culture and for whom no other independent cause of death was reported. Therefore, a causal link between death and C. striatum infection could be strongly hypothesized.
Previous authors (4, 11, 20, 31) noted that the rates of susceptibility of C. striatum to β-lactams and aminoglycosides are variable, with high levels of resistance to erythromycin, tetracycline, rifampin, and ciprofloxacin and with all strains sensitive to vancomycin. Our results on the antibiotic sensitivities of the C. striatum strains from this outbreak mirror the ones from previous publications: vancomycin, 100%; imipenem, 93%; cefazolin, 74.4%; penicillin and ampicillin, 67.4%; tetracycline, 23.2%; erythromycin, 18.6%; gentamicin, 9.3%; and rifampin and ciprofloxacin, 0%. According to the sensitivity patterns obtained by Otsuka et al. (20), by which C. striatum is considered and emerging, multidrug-resistant, nosocomial pathogen, we have observed in our samples that the criterion of multidrug resistance (resistance to three or more antibiotics of different families) applies to 100% of the strains isolated in our nosocomial outbreak, 65% of which were resistant to four or five different antibiotic groups, 6.9% were sensitive only to imipenem and vancomycin, and 11% were sensitive only to vancomycin.
We conclude that C. striatum is an emerging multidrug-resistant, potentially pathogenic microorganism that is able to cause nosocomial infections and respiratory colonization in patients with advanced, severe COPD. It can be transmitted between patients, from person to person, and via caretakers; and C. striatum infections should be treated according to the results of the antibiogram. Once the organism is identified, universal hygiene measures should be observed to avoid further spread and outbreaks.
Acknowledgments
We are grateful for the collaboration given by the Comisión de Infecciones del Complex Hospitalari de Mallorca, especially Matías Poblador (president), Eugenia Barceló (the nurse in charge of infection control), and Margalida Fortuny and Antoni Bennassar (research nurses).
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Anthonisen, N. R., J. Manfreda, C. P. Warren, E. S. Hershfield, G. K. Harding, and N. A. Nelson. 1987. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann. Intern. Med. 106:196-204. [DOI] [PubMed] [Google Scholar]
- 2.Barr, J. G., and P. G. Murphy. 1986. Corynebacterium striatum: an unusual organism isolated on pure culture from sputum. J. Infect. 13:297-298. [DOI] [PubMed] [Google Scholar]
- 3.Bowstead, T. T., and S. M. Santiago. 1980. Pleuropulmonary infection due to Corynebacterium striatum. Br. J. Dis. Chest 74:198-200. [DOI] [PubMed] [Google Scholar]
- 4.Brandenburg, A. H., A. Van Belkum, C. Van Pelt, H. A. Bruining, J. W. Mouton, and H. A. Verbrugh. 1996. Patient-to-patient spread of a single strain of Corynebacterium striatum causing infections in a surgical intensive care unit. J. Clin. Microbiol. 34:2089-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celli, B. R., and W. MacNee. 2004. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur. Respir. J. 23:932-946. [DOI] [PubMed] [Google Scholar]
- 6.Cowling, P., and L. Hall. 1993. Corynebacterium striatum: a clinically significant isolate from sputum in chronic obstructive airways disease. J. Infect. 26:335-336. [DOI] [PubMed] [Google Scholar]
- 7.Creagh, R., J. M. Saavedra, F. J. Rodríguez, P. Rodríguez, and D. Merino. 2000. Pneumonia caused by Corynebacterium striatum in a patient with AIDS. Enferm. Infecc. Microbiol. Clin. 18:297-298. [PubMed] [Google Scholar]
- 8.De Briel, D., J. C. Langs, G. Rougeron, P. Chabot, and A. Le Faou. 1991. Multiresistant corynebacteria in bacteriuria: a comparative study of the role of Corynebacterium group D-2 and Corynebacterium jeikeium. J. Hosp. Infect. 17:35-43. [DOI] [PubMed] [Google Scholar]
- 9.Decramer, M., R. Gosselink, T. Troosters, M. Vevschveren, and G. Evers. 1997. Muscle weakness is related to utilization of health care resources in COPD patients. Eur. Respir. J. 10:417-423. [DOI] [PubMed] [Google Scholar]
- 10.Freney, J., M. T. Duperron, C. Courtier, W. Hansen, F. Allard, J. M. Boeufgras, D. Monget, and J. Fleurette. 1991. Evaluation of API Coryne in comparison with conventional methods for identifying coryneform bacteria. J. Clin. Microbiol. 29:38-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funke, G., A. Von Graevenitz, J. E. Clarridge III, and K. A. Bernard. 1997. Clinical microbiology of coryneform bacteria. Clin. Microbiol. Rev. 10:125-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funke, G., K. K. Peters, and M. Aravena-Roman. 1998. Evaluation of the RapID CB Plus system for identification of coryneform bacteria and Listeria spp. J. Clin. Microbiol. 36:2439-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Rodríguez, J. A., and E. García. 1997. El resurgimiento de los grampositivos: razones, significado clínico y posibilidades de control. Rev. Clin. Esp. 197(Suppl. 2):3-11. [PubMed] [Google Scholar]
- 14.Hudspeth, M. K., S. Hunt Gerardo, D. M. Citron, and E. J. C. Goldstein. 1998. Evaluation of the RapID CB Plus System for identification of Corynebacterium species and other gram-positive rods. J. Clin. Microbiol. 36:543-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard, R. B., D. J. Nowowiejski, J. J. Warren, D. J. Finn, and M. B. Coyle. 1994. Molecular evidence of person-to-person transmission of a pigmented strain of Corynebacterium striatum in intensive care units. J. Clin. Microbiol. 32:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melero-Bascones, M., P. Muñoz, M. Rodríguez-Creixems, and E. Bouza. 1996. Corynebacterium striatum: an undescribed agent of pacemaker-related endocarditis. Clin. Infect. Dis. 22:576-577. [DOI] [PubMed] [Google Scholar]
- 17.Miravitlles, M. 2002. Exacerbations of chronic obstructive pulmonary disease: when are bacteria important? Eur. Respir. J. 20(Suppl. 36):9s-19s. [DOI] [PubMed] [Google Scholar]
- 18.Murray, P. R., and J. A. Washington. 1975. Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin. Proc. 50:339-344. [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 20.Otsuka, Y., K. Ohkusu, Y. Kawamura, S. Baba, T. Ezaki, and S. Kimura. 2006. Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagn. Microbiol. Infect. Dis. 54:109-114. [DOI] [PubMed] [Google Scholar]
- 21.Peiris, V., S. Fraser, C. Knowles, S. Norris, and C. Bennett. 1994. Isolation of Corynebacterium striatum from three hospital patients. Eur. J. Clin. Microbiol. Infect. Dis. 13:36-38. [DOI] [PubMed] [Google Scholar]
- 22.Prada, J. L., J. L. Villanueva, J. Torre-Cisneros, M. Anguita, J. Escauriaza, and P. Sánchez Guijo. 1993. Endocarditis por Corynebacterium no diphteriae. Presentación de 7 casos y revisión. Enferm. Infecc. Microbiol. Clin. 11:536-542. [PubMed] [Google Scholar]
- 23.Riegel, P. 1998. Les corynébactéries, aspects bactériologiques et cliniques. Ann. Biol. Clin. 56:285-296. [PubMed] [Google Scholar]
- 24.Riegel, P., R. Ruimy, R. Christen, and H. Monteil. 1996. Species identities and antimicrobial susceptibilities of corynebacteria isolated from various clinical resources. Eur. J. Clin. Microbiol. Infect. Dis. 15:657-662. [DOI] [PubMed] [Google Scholar]
- 25.Rufael, D. W., and S. E. Cohn. 1994. Native valve endocarditis due to Corynebacterium striatum: case report and review. Clin. Infect. Dis. 19:1054-1061. [DOI] [PubMed] [Google Scholar]
- 26.Soler, J. J., L. Sánchez, M. Latorre, J. Alamar, P. Román, and M. Perpiñá. 2001. Impacto asistencial hospitalario de la EPOC. Peso específico del paciente con EPOC de alto consumo sanitario. Arch. Bronconeumol. 37:375-381. [DOI] [PubMed] [Google Scholar]
- 27.Soriano, F., J. L. Rodríguez-Tudela, R. Fernández-Roblas, J. M. Aguado, and M. Santamaría. 1998. Skin colonization by Corynebacterium groups D2 and JK in hospitalized patients. J. Clin. Microbiol. 26:1878-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarr, P. E., F. Stock, R. H. Cooke, D. P. Fedorko, and D. R. Lucey. 2003. Multidrug resistant Corynebacterium striatum pneumonia in a heart trasplant recipient. Transplant. Infect. Dis. 5:53-58. [DOI] [PubMed] [Google Scholar]
- 29.Tumbarello, M., E. Tacconelli, A. del Forno, S. Caponera, and R. Cauda. 1994. Corynebacterium striatum bacteremia in patient with AIDS. Clin. Infect. Dis. 18:1007-1008. [DOI] [PubMed] [Google Scholar]
- 30.von Graevenitz, A., V. Pünter-Streit, P. Riegel, and G. Funke. 1998. Coryneform bacteria in throat cultures of healthy individuals. J. Clin. Microbiol. 36:2087-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins, D. A., A. Chahine, R. J. Creger, M. R. Jacobs, and H. M. Lazarus. 1993. Corynebacterium striatum: a diphtheroid with pathogenic potential. Clin. Infect. Dis. 17:21-25. [DOI] [PubMed] [Google Scholar]